Simultaneous Routing with Washing Droplets Based on Shape-Dependent Velocity Model in MEDA Biochips
Abstract
1. Introduction
- 1.
- We developed a simultaneous routing method for multiple droplets, including washing droplets, on a MEDA biochip.
- 2.
- We realized a routing method that assumes that washing will make pathways previously blocked due to contamination available for droplet routing.
- 3.
- We solved a routing time minimization problem by considering the difference in droplet moving velocity, including washing with different volume ratios of functional and washing droplets.
2. Related Works
3. Simultaneous Routing with Washing Droplets
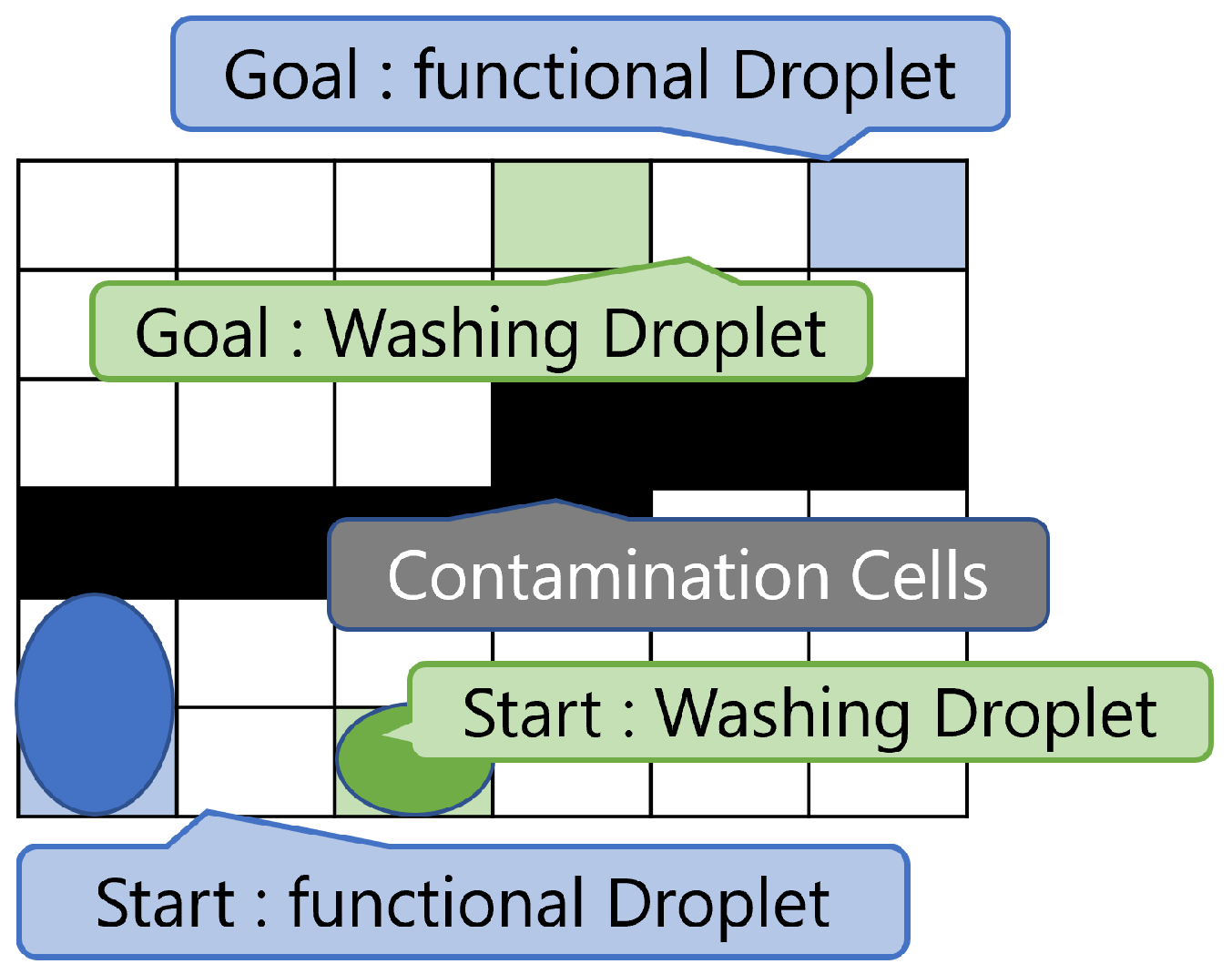
3.1. Problem Description
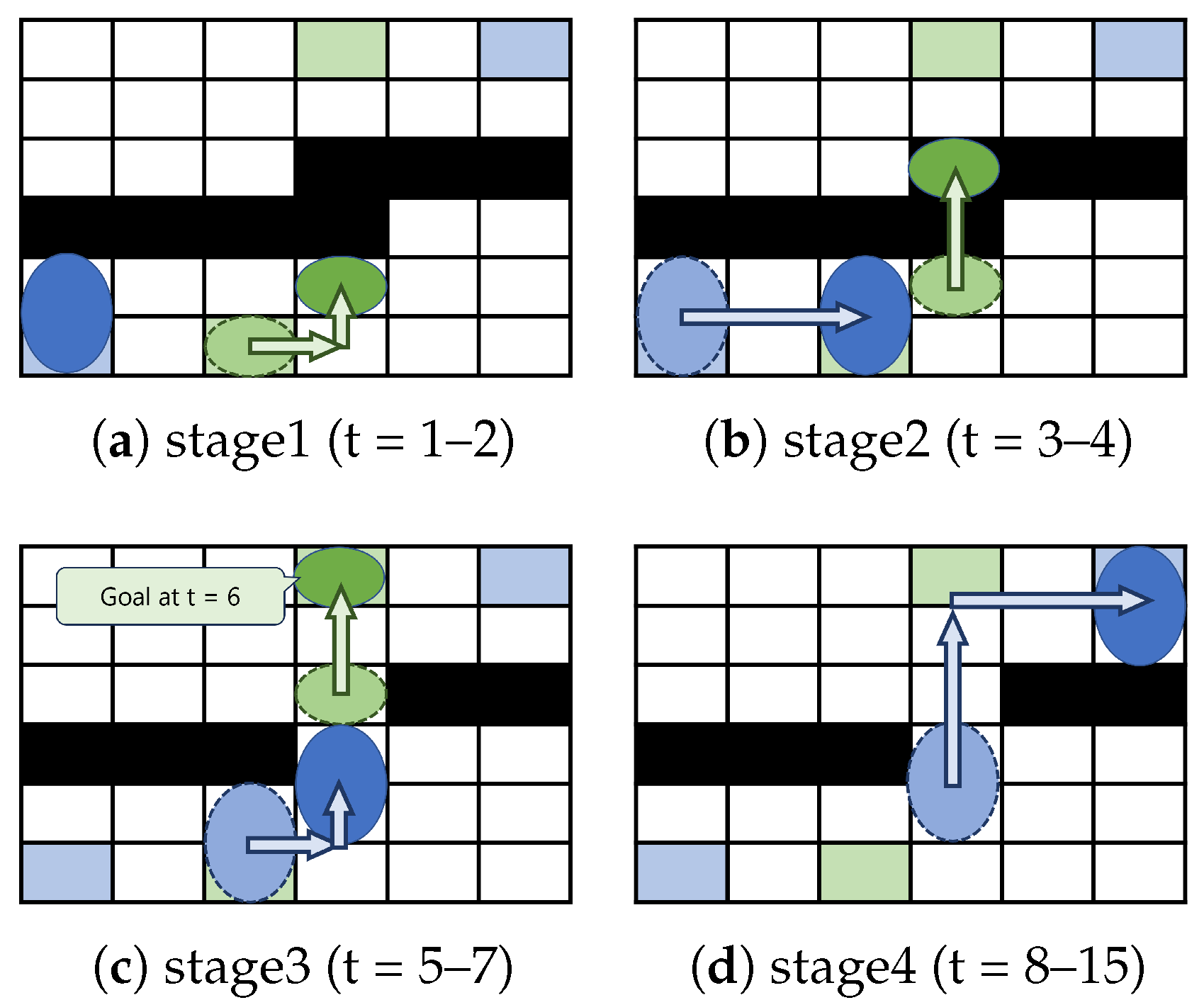
3.2. Example
- 1.
- Moving along the chip edges in the direction where movement takes less time with only one shape-morphing operation;
- 2.
- Repeating diagonal movements and morphing operations only.
3.3. Formulation
- 1.
- When , the washing or functional droplet does not move in step s.
- 2.
- When , the droplet moves one cell in the horizontal direction.
- 3.
- When , the droplet moves one cell in the vertical direction.
- 1.
- When , the functional droplet morphs at step s.
- 2.
- When , the functional droplet moves one cell in the diagonal direction at step s.
- 1.
- represents the time required when no operation is performed (always one time step).
- 2.
- represents the time required when the droplet moves in the x-axis (horizontal) direction.
- 3.
- represents the time required when the droplet moves in the y-axis (vertical) direction.
- 4.
- represents the time required when the droplet moves diagonally.
- 5.
- represents the time required when the droplet undergoes shape deformation.
4. Experiments
4.1. Setup
- Existing Method (Wait): The functional droplet starts moving only after the washing droplet is done moving [23].
- Existing Method (Ignoring Velocity Differences): The washing and functional droplets move simultaneously, without using a shape-dependent velocity model.
- Proposed Method (Exhaustive Search): The washing and functional droplets move simultaneously, with a shape-dependent velocity model used.
- Proposed Method (Partial Constraints): A variation of the exhaustive search method that restricts the functional droplet’s movement directions to reduce the solution space.
- The washing droplet size was set to 1, with only one such droplet present on the biochip.
- The functional droplet size was set to 2, with only one present on the biochip.
- The biochip size is assumed to be .
- Experiments were conducted for two contamination ratios: 10% and 20% of the total biochip cells.
- Contaminated cells were assigned randomly in each scenario.
- The start and goal cells of the washing droplet were randomly assigned for each scenario with certain constraints: the start cell’s y-coordinate was 1, and its goal cell’s y-coordinate was H.
4.2. Results
- 1.
- 2.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azizipour, N.; Avazpour, R.; Rosenzweig, D.H.; Sawan, M.; Ajji, A. Evolution of biochip technology: A review from lab-on-a-chip to organ-on-a-chip. Micromachines 2020, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Van Hoof, V.; Barglazan, D.; Blairon, L.; Braekevelt, B.; Debois, R.; De Vos, N.V.J.; Gruson, D.; Jonckheere, J.; Lanckmans, K.; Moens, M.; et al. Organisation and quality monitoring for point-of-care testing (POCT) in Belgium: Proposal for an expansion of the legal framework for POCT into primary health care. Acta Clin. Belg. 2022, 77, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.L.; Fahey, N.M.; Hafer, N.; Buchholz, B.; Dunlap, D.R.; Murphy, R.L.; Achenbach, C.; Stone, C.; Cleeton, R.; O’Neal, J.; et al. The RADx Tech Clinical Studies Core: A model for academic based clinical studies. IEEE Open J. Eng. Med. Biol. 2021, 2, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, Z.; Chakrabarty, K.; Ho, T.Y.; Lee, C.Y. Micro-electrode-dot-array digital microfluidic biochips: Technology, design automation, and test techniques. IEEE Trans. Biomed. Circuits Syst. 2018, 13, 292–313. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chakrabarty, K. Integrated droplet routing in the synthesis of microfluidic biochips. In Proceedings of the 44th Annual Design Automation Conference, San Diego, NA, USA, 4–8 June 2007; pp. 948–953. [Google Scholar]
- Lu, G.R.; Kuo, C.H.; Chiang, K.C.; Banerjee, A.; Bhattacharya, B.B.; Ho, T.Y.; Chen, H.M. Flexible droplet routing in active matrix–based digital microfluidic biochips. Acm Trans. Des. Autom. Electron. Syst. (TODAES) 2018, 23, 1–25. [Google Scholar] [CrossRef]
- Chen, Z.; Teng, D.H.Y.; Wang, G.C.J.; Fan, S.K. Droplet routing in high-level synthesis of configurable digital microfluidic biochips based on microelectrode dot array architecture. Biochip J. 2011, 5, 343–352. [Google Scholar] [CrossRef]
- Lai, K.Y.T.; Yang, Y.T.; Lee, C.Y. An intelligent digital microfluidic processor for biomedical detection. J. Signal Process. Syst. 2015, 78, 85–93. [Google Scholar] [CrossRef]
- Li, Z.; Lai, K.Y.T.; Chakrabarty, K.; Ho, T.Y.; Lee, C.Y. Droplet Size-Aware High-Level Synthesis for Micro-Electrode-Dot-Array Digital Microfluidic Biochips. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Shiro, C.; Nishikawa, H.; Kong, X.; Tomiyama, H.; Yamashita, S.; Roy, S. Shape-dependent velocity based droplet routing on meda biochips. IEEE Access 2022, 10, 122423–122430. [Google Scholar] [CrossRef]
- Zhao, Y.; Chakrabarty, K. Cross-contamination avoidance for droplet routing in digital microfluidic biochips. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2012, 31, 817–830. [Google Scholar] [CrossRef]
- Huang, Z.; Bai, X.; Lan, T.; Li, X.; Lin, G. Unified contamination-aware routing method considering realistic washing capacity constraint in digital microfluidic biochips. IEEE Access 2020, 8, 192867–192879. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Q.; Shen, Y.; Ho, T.Y.; Cai, Y. Integrated functional and washing routing optimization for cross-contamination removal in digital microfluidic biochips. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2015, 35, 1283–1296. [Google Scholar] [CrossRef]
- Joshi, K.; Velasco, V.; Esfandyarpour, R. A low-cost, disposable and portable inkjet-printed biochip for the developing world. Sensors 2020, 20, 3593. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Pan, D.Z. A high-performance droplet routing algorithm for digital microfluidic biochips. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2008, 27, 1714–1724. [Google Scholar]
- Keszocze, O.; Wille, R.; Chakrabarty, K.; Drechsler, R. A general and exact routing methodology for digital microfluidic biochips. In Proceedings of the 2015 IEEE/ACM International Conference on Computer-Aided Design (ICCAD), Austin, NA, USA, 2–6 November 2015; pp. 874–881. [Google Scholar]
- Keszocze, O.; Li, Z.; Grimmer, A.; Wille, R.; Chakrabarty, K.; Drechsler, R. Exact routing for micro-electrode-dot-array digital microfluidic biochips. In Proceedings of the 2017 22nd Asia and South Pacific Design Automation Conference (ASP-DAC), Chiba, Japan, 16–19 January 2017; pp. 708–713. [Google Scholar]
- Chakraborty, S.; Chakraborty, S. Routing performance optimization for homogeneous droplets on MEDA-based digital microfluidic biochips. In Proceedings of the 2019 IEEE Computer Society Annual Symposium on VLSI (ISVLSI), Miami, NA, USA, 15–17 July 2019; pp. 419–424. [Google Scholar]
- Roy, P.; Banerjee, A.; Wille, R.; Bhattacharya, B.B. Harnessing the granularity of micro-electrode-dot-array architectures for optimizing droplet routing in biochips. Acm Trans. Des. Autom. Electron. Syst. (TODAES) 2019, 25, 1–37. [Google Scholar] [CrossRef]
- Liang, T.C.; Chan, Y.S.; Ho, T.Y.; Chakrabarty, K.; Lee, C.Y. Multitarget sample preparation using MEDA biochips. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2019, 39, 2682–2695. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Fair, R.B.; Zeng, J. Design tools for digital microfluidic biochips: Toward functional diversification and more than moore. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2010, 29, 1001–1017. [Google Scholar] [CrossRef]
- Li, Z.; Lai, K.Y.T.; Chakrabarty, K.; Ho, T.Y.; Lee, C.Y. Droplet size-aware and error-correcting sample preparation using micro-electrode-dot-array digital microfluidic biochips. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Ghoshal, S.; Rahaman, H.; Chakrabarty, K.; Bhattacharya, B.B. Automated path planning for washing in digital microfluidic biochips. In Proceedings of the 2012 IEEE International Conference on Automation Science and Engineering (CASE), Seoul, Republic of Korea, 20–24 August 2012; pp. 115–120. [Google Scholar]
- Mori, K.; Shiro, C.; Nishikawa, H.; Kong, X.; Tomiyama, H.; Yamashita, S. A Fast Approach to Droplet Routing with Shape-Dependent Velocity on MEDA Biochips. In Proceedings of the 2023 21st IEEE Interregional NEWCAS Conference (NEWCAS), Edinburgh, UK, 26–28 June 2023; pp. 1–5. [Google Scholar]
- Kawakami, T.; Shiro, C.; Nishikawa, H.; Kong, X.; Tomiyama, H.; Yamashita, S. A deep reinforcement learning approach to droplet routing for erroneous digital microfluidic biochips. Sensors 2023, 23, 8924. [Google Scholar] [CrossRef] [PubMed]






| Character | Meaning | Ranges |
|---|---|---|
| Volume of functional droplet | Given | |
| Number of washing droplets | Given | |
| Number of functional droplets | Given | |
| Start cell of washing droplet i | Given, | |
| Goal cell of washing droplet i | Given, | |
| Start cell of functional droplet i | Given, | |
| Goal cell of functional droplet i | Given, | |
| Coordinates of contaminated cell | Given | |
| Reference point of washing droplet i at step s | ||
| Reference point of functional droplet i at step s | ||
| Width and height of functional droplet i at step s | ||
| Operation of washing droplet i at step s | ||
| Operation of functional droplet i at step s | ||
| Elapsed time of washing droplet i until step s | ||
| Elapsed time of functional droplet i until step s | ||
| Routing time of washing droplet i | ||
| Routing time of functional droplet i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiro, C.; Nishikawa, H.; Kong, X.; Tomiyama, H.; Yamashita, S. Simultaneous Routing with Washing Droplets Based on Shape-Dependent Velocity Model in MEDA Biochips. Biosensors 2025, 15, 533. https://doi.org/10.3390/bios15080533
Shiro C, Nishikawa H, Kong X, Tomiyama H, Yamashita S. Simultaneous Routing with Washing Droplets Based on Shape-Dependent Velocity Model in MEDA Biochips. Biosensors. 2025; 15(8):533. https://doi.org/10.3390/bios15080533
Chicago/Turabian StyleShiro, Chiharu, Hiroki Nishikawa, Xiangbo Kong, Hiroyuki Tomiyama, and Shigeru Yamashita. 2025. "Simultaneous Routing with Washing Droplets Based on Shape-Dependent Velocity Model in MEDA Biochips" Biosensors 15, no. 8: 533. https://doi.org/10.3390/bios15080533
APA StyleShiro, C., Nishikawa, H., Kong, X., Tomiyama, H., & Yamashita, S. (2025). Simultaneous Routing with Washing Droplets Based on Shape-Dependent Velocity Model in MEDA Biochips. Biosensors, 15(8), 533. https://doi.org/10.3390/bios15080533







