Recent Advances in Hydrogel-Promoted Photoelectrochemical Sensors
Abstract
1. Introduction
2. Hydrogel Types and Functional Roles in PEC Sensors
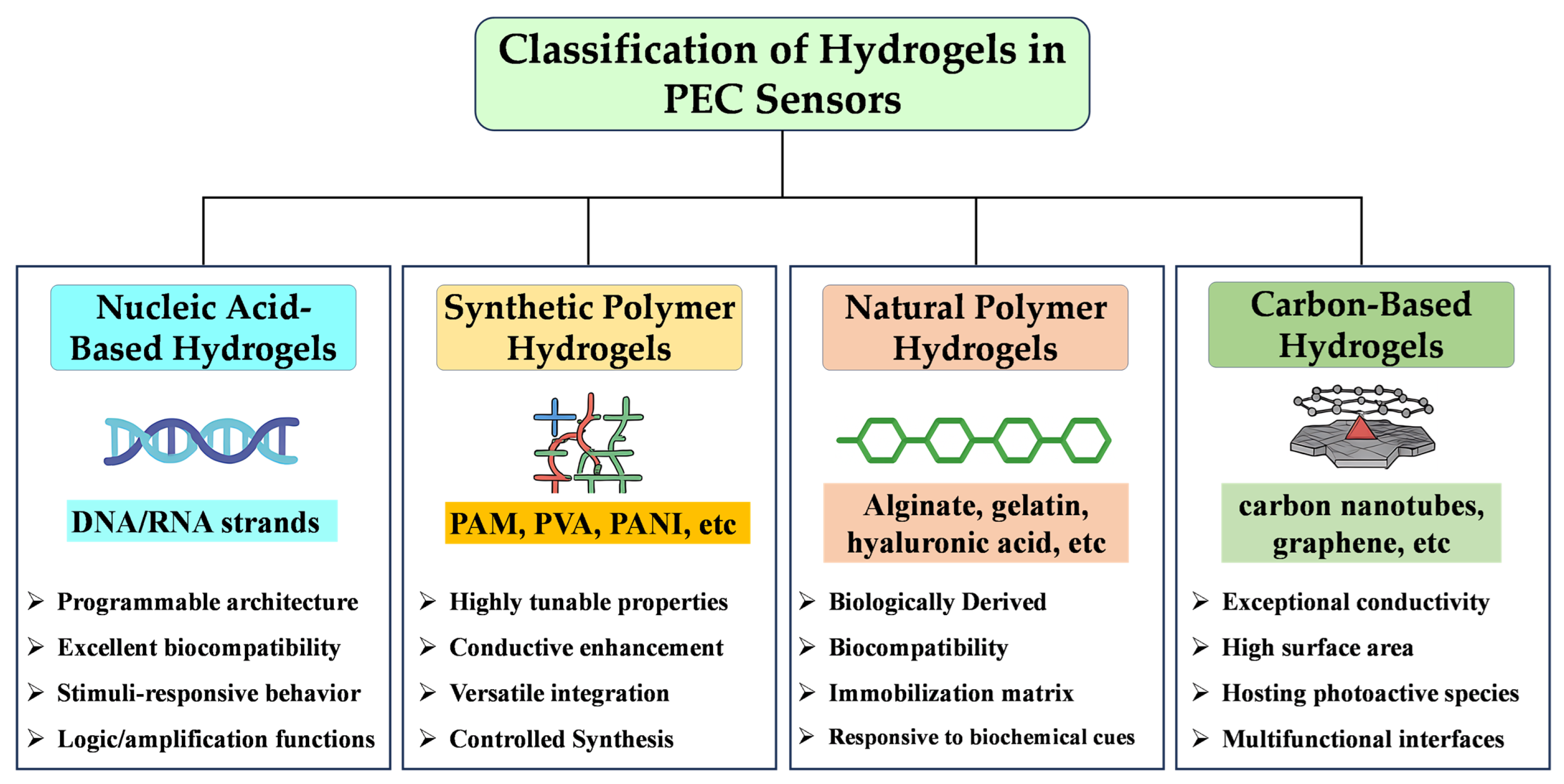
2.1. Classification of Hydrogels in PEC Sensors
2.1.1. Nucleic Acid-Based Hydrogels
2.1.2. Synthetic Polymer Hydrogels
2.1.3. Natural Polymer Hydrogels
2.1.4. Carbon-Based Hydrogels
2.2. Functional Roles of Hydrogels in PEC Systems
2.2.1. Structural Scaffold and Conductivity Enhancement
2.2.2. Stimuli-Responsive Release and Signal Amplification
2.2.3. Antifouling and Selectivity Control
2.2.4. In Situ Formation of Insulating Hydrogel for Signal-Off Regulation
2.2.5. Flexible Electrolyte and Polarization Medium
2.2.6. Visual Output and Multi-Functional Integration
3. Applications of Hydrogel-Based PEC Sensors
3.1. Detection of Ions
3.2. Detection of Small Molecules
3.2.1. Biomolecules
3.2.2. Drug and Pesticide Molecules
3.2.3. Mycotoxins and Pollutants
3.3. Detection of Biomacromolecules
3.3.1. microRNAs
3.3.2. Proteins
3.3.3. Enzymes
3.4. Other Applications
3.4.1. Detection of Escherichia coli
3.4.2. Photodetectors
3.4.3. Flexible Bioelectronic Interfaces
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4NP | 4-Nitrophenol |
| ACP | Acetamiprid |
| Alg | Alginate |
| ATZ | Atrazine |
| CBZ | Carbendazim |
| C-dots | Carbon dots |
| CMC | Carboxymethyl cellulose |
| CEA | Carcinoembryonic antigen |
| cTnI | Cardiac troponin I |
| CLIA | Chemiluminescent immunoassay |
| CAP | Chloramphenicol |
| cDNA | Complementary DNA |
| CV | Crystal violet |
| DBCO | Dibenzocyclooctyne |
| E. coli | Escherichia coli |
| Fc | Ferrocene |
| GCE | Glassy carbon electrode |
| GOx | Glucose oxidase |
| AuNPs | Gold nanoparticles |
| GH | Graphene hydrogel |
| g-C3N4 | Graphitic carbon nitride |
| HRP | Horseradish peroxidase |
| HER2 | Human epidermal growth factor receptor 2 |
| HIgG | Human immunoglobulin G |
| HA | Hyaluronic acid |
| HAase | Hyaluronidase |
| H2ase | Hydrogenase |
| IPCE | Incident photon-to-current efficiency |
| ITO | Indium tin oxide |
| LDH | Layered double hydroxide |
| LOD | Limit of detection |
| LSPR | Localized surface plasmon resonance |
| MIL-125 | Materials of Institute Lavoisier-125, a titanium-based metal–organic framework. |
| MC-LR | Microcystin-LR |
| miRNA | MicroRNA |
| MIPs | Molecularly imprinted polymers |
| NGH | Nitrogen-doped graphene hydrogel |
| OTA | Ochratoxin A |
| OPD | o-Phenylenediamine |
| OPECT | Organic photoelectrochemical transistor |
| PPF | Paired-pulse facilitation |
| PCP | Pentachlorophenol |
| PEC | Photoelectrochemical |
| PSI | Photosystem I |
| PGH | Platinum nanocube-embedded gelatin hydrogel |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PAMAM | Poly(amidoamine) |
| PEGMA | Poly(ethylene glycol) methacrylate |
| PTB7-Th | Poly[[2,6′-4,8-di(5-ethylhexylthienyl) benzo[1,2-b;3,3-b] dithiophene][3-fluoro-2-(2-ethylhexy) carbonyl-thieno[3,4-b]thiophenediyl]] |
| PAM | Polyacrylamide |
| PAA | Polyacrylic acid |
| PANI | Polyaniline |
| PEG | Polyethylene glycol |
| PEGDA | Polyethylene glycol diacrylate |
| PEGDGE | Polyethylene glycol diglycidyl ether |
| PEI | Polyethyleneimine |
| PVA | Polyvinyl alcohol |
| RSD | Relative standard deviation |
| SWCNTs | Single-walled carbon nanotubes |
| SA | Sodium alginate |
| Tc | Tetracycline |
| Ti2CTx | Transition metal carbides and nitrides (Ti2CTx) |
| UV | Ultraviolet |
| XOD | Xanthine oxidase |
| Z-MOFs | Zwitterionic metal–organic frameworks |
References
- Sun, B.; Ai, S. Fabrication and Application of Photoelectrochemical Sensor. Prog. Chem. 2014, 26, 834–845. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical DNA Biosensors. Chem. Rev. 2014, 114, 7421–7441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, Z.; Dai, Z. Selective photoelectrochemical architectures for biosensing: Design, mechanism and responsibility. TrAC Trends Anal. Chem. 2018, 105, 470–483. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Li, T.; Dong, H.; Hao, Y.; Zhang, Y.; Chen, S.; Xu, M.; Zhou, Y. Near-infrared Responsive Photoelectrochemical Biosensors. Electroanalysis 2022, 34, 956–965. [Google Scholar] [CrossRef]
- Abdi, G.; Alluhaibi, L.; Kowalewska, E.; Mazur, T.; Mech, K.; Podborska, A.; Sławek, A.; Tanaka, H.; Szaciłowski, K. Reservoir computing and photoelectrochemical sensors: A marriage of convenience. Coord. Chem. Rev. 2023, 487, 215155. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, Z.; Zhu, Z.-J. Advanced analytical and informatic strategies for metabolite annotation in untargeted metabolomics. TrAC Trends Anal. Chem. 2023, 158, 116903. [Google Scholar] [CrossRef]
- Lu, M.-J.; Zhao, K.-H.; Zhang, S.-Q.; Cai, X.-B.; Kandegama, W.; Chen, M.-X.; Sun, Y.; Li, X.-Y. Research Progress of Biosensor Based on Organic Photoelectrochemical Transistor. J. Agric. Food. Chem. 2024, 72, 17746–17761. [Google Scholar] [CrossRef]
- Luo, Y.; Hao, Y.; Zhang, P.; Zhang, Y.; Zeng, R.; Chen, S.; Xu, M. Chemiluminescence-driven photoelectrochemical sensor: A mini review. Electroanalysis 2024, 36, e202300257. [Google Scholar] [CrossRef]
- Yang, P.; Hou, X.; Gao, X.; Peng, Y.; Li, Q.; Niu, Q.; Liu, Q. Recent Trends in Self-Powered Photoelectrochemical Sensors: From the Perspective of Signal Output. ACS Sens. 2024, 9, 577–588. [Google Scholar] [CrossRef]
- Hou, X.; Li, S.; Gao, X.; Peng, Y.; Liu, Q.; Wang, K. Photoactive gate material-based organic photoelectrochemical transistor sensors: Working principle and representative applications. Chem. Commun. 2025, 61, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Jiang, H.; Yang, P.; Luo, L.; Niu, Q.; You, T. Photoactivities regulating of inorganic semiconductors and their applications in photoelectrochemical sensors for antibiotics analysis: A systematic review. Biosens. Bioelectron. 2022, 216, 114634. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Shaikh, T.; Niazi, J.H. Semiconductor quantum dots in photoelectrochemical sensors from fabrication to biosensing applications. Analyst 2023, 148, 1633–1652. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Fan, J.; Ju, Y.; Xue, H.; Pang, H. Current Advances in Semiconductor Nanomaterial-Based Photoelectrochemical Biosensing. Chem. Eur. J. 2018, 24, 14010–14027. [Google Scholar] [CrossRef]
- Blaskievicz, S.F.; Mascaro, L.H.; Zhao, Y.; Marken, F. Semiconductor photoelectroanalysis and photobioelectroanalysis: A perspective. TrAC Trends Anal. Chem. 2021, 135, 116154. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Chen, S.; Zhang, P.; Xu, M.; Hao, Y. Recent Advances in Single-Atom Materials Prompted Photoelectrochemical Sensing. Electroanalysis 2025, 37, e70000. [Google Scholar] [CrossRef]
- Guo, L.; Li, Z.; Marcus, K.; Navarro, S.; Liang, K.; Zhou, L.; Mani, P.D.; Florczyk, S.J.; Coffey, K.R.; Orlovskaya, N.; et al. Periodically Patterned Au-TiO2 Heterostructures for Photoelectrochemical Sensor. ACS Sens. 2017, 2, 621–625. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, Z.; Zhu, D.; Harberts, J.; Blick, R.H.; Zierold, R.; Lisdat, F.; Parak, W.J. Quantum Dot/TiO2 Nanocomposite-Based Photoelectrochemical Sensor for Enhanced H2O2 Detection Applied for Cell Monitoring and Visualization. Small 2024, 20, 2401703. [Google Scholar] [CrossRef]
- Silva, M.d.A.; Effting, L.M.; da Silva, P.R.C.; Segatelli, M.G.; Ogatta, S.F.Y.; Dall’Antonia, L.H.; Tarley, C.R.T. Photoelectrochemical sensor based on imprinted WO3 and graphene oxide/MIP-iniferter nanocomposite for creatinine determination in urine samples. Electrochim. Acta 2025, 524, 145941. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Wu, F.; Guan, Y.; Xu, G. TiO2 Nanomaterials in Photoelectrochemical and Electrochemiluminescent Biosensing. Top. Curr. Chem. 2020, 378, 28. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Tang, Y.; Wang, C.; Zhao, F.; Zeng, B. Recent advances in bismuth oxyhalide-based functional materials for photoelectrochemical sensing. TrAC Trends Anal. Chem. 2020, 131, 116020. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, Z. Rationally Engineered Photonic-Plasmonic Synergistic Resonators in Second Near-Infrared Window for in Vivo Photoelectrochemical Biodetection. Nano Lett. 2019, 19, 9069–9074. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, Z.; Yao, H.; Dou, X.; Zhang, R.; Wang, H.; Miao, Y.; Zhang, Z. Oxygen Vacancies-Induced Antifouling Photoelectrochemical Aptasensor for Highly Sensitive and Selective Determination of α-Fetoprotein. Anal. Chem. 2024, 96, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Hong, Q.; Li, H.; Liu, C.; Li, F. Direct-Laser-Writing of Metal Sulfide-Graphene Nanocomposite Photoelectrode toward Sensitive Photoelectrochemical Sensing. Adv. Funct. Mater. 2019, 29, 1904000. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Gaur, R.; Shahabuddin, S.; Mukherjee, N.; Chandra, P. Recent advances in nanostructured transition metal sulfide based sensors for environmental applications. Mater. Today Proc. 2022, 62, 6945–6949. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Sun, X.; Deng, K.; Li, X.; Xie, Y.; Guo, H.; Zhao, P.; Fei, J. Square-shaped Cu2MoS4 loaded on three-dimensional flower-like AgBiS2 to form S-scheme heterojunction as a light-driven photoelectrochemical sensor for efficient detection of serotonin in biological samples. Talanta 2025, 290, 127774. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Zhang, J.; Yang, Z.; Li, J.; Cao, Y.; Wu, K.; Liu, Z.; Hao, J.; Ye, X. NIR-Excitable POM-Encapsulated Yb-Bi2S3 Decorated Graphene for Wearable Photoelectrochemical Sensing. Adv. Funct. Mater. 2024, 34, 2315917. [Google Scholar] [CrossRef]
- Tan, A.Y.S.; Awan, H.T.A.; Cheng, F.; Zhang, M.; Tan, M.T.T.; Manickam, S.; Khalid, M.; Muthoosamy, K. Recent advances in the use of MXenes for photoelectrochemical sensors. Chem. Eng. J. 2024, 482, 148774. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Pan, G.; Xu, W.; Zhu, J.; Zhou, D.; Li, D.; Chen, C.; Lu, G.; Song, H. Ti3C2 MXene quantum dots/TiO2 inverse opal heterojunction electrode platform for superior photoelectrochemical biosensing. Sens. Actuators B Chem. 2019, 289, 131–137. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.-C.; Wang, S.; Sillanpaa, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Wang, B.; Cao, J.-T.; Liu, Y.-M. Recent progress of heterostructure-based photoelectrodes in photoelectrochemical biosensing: A mini review. Analyst 2020, 145, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, J.; Ju, H. Z-Scheme Heterojunction of Hierarchical Cu2S/CdIn2S4 Hollow Cubes to Boost Photoelectrochemical Performance. Small 2024, 20, 2405712. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Venkateswara Raju, C.; Majid Ghoreishian, S.; Mohana Rani, G.; Kumar, K.; Oh, M.-H.; Pil Park, J.; Suk Huh, Y. Recent advances in the use of graphitic carbon nitride-based composites for the electrochemical detection of hazardous contaminants. Coord. Chem. Rev. 2022, 470, 214708. [Google Scholar] [CrossRef]
- Idris, A.O.; Oseghe, E.O.; Msagati, T.A.M.; Kuvarega, A.T.; Feleni, U.; Mamba, B. Graphitic Carbon Nitride: A Highly Electroactive Nanomaterial for Environmental and Clinical Sensing. Sensors 2020, 20, 5743. [Google Scholar] [CrossRef]
- Malik, R.; Joshi, N.; Tomer, V.K. Functional graphitic carbon (IV) nitride: A versatile sensing material. Coord. Chem. Rev. 2022, 466, 214611. [Google Scholar] [CrossRef]
- Nemati, S.S.; Dehghan, G. Photoelectrochemical biosensors: Prospects of graphite carbon nitride-based sensors in prostate-specific antigen diagnosis. Anal. Biochem. 2025, 696, 115686. [Google Scholar] [CrossRef]
- Rezapour, R.; Arvand, M.; Habibi, M.F. A “signal-on” photoelectrochemical sensor based on hierarchical titanium dioxide nanowires/microflowers decorated graphite-like carbon nitride quantum dots for glutathione detection. Talanta 2025, 285, 127448. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Han, D.; Yang, H.; Hong, Q.; Fang, Y.; Zhou, Z.; Shen, Y.; Liu, S.; Huang, C.; et al. Carbon Nitride-Based Heterojunction Photoelectrodes with Modulable Charge-Transfer Pathways toward Selective Biosensing. Anal. Chem. 2023, 95, 13716–13724. [Google Scholar] [CrossRef]
- Hu, T.; Zheng, Y.-N.; Li, M.-J.; Liang, W.-B.; Chai, Y.-Q.; Yuan, R. A Highly Sensitive Photoelectrochemical Assay with Donor–Acceptor-Type Material as Photoactive Material and Polyaniline as Signal Enhancer. Anal. Chem. 2018, 90, 6096–6101. [Google Scholar] [CrossRef]
- Zheng, Y.-N.; Liang, W.-B.; Xiong, C.-Y.; Yuan, Y.-L.; Chai, Y.-Q.; Yuan, R. Self-Enhanced Ultrasensitive Photoelectrochemical Biosensor Based on Nanocapsule Packaging Both Donor–Acceptor-Type Photoactive Material and Its Sensitizer. Anal. Chem. 2016, 88, 8698–8705. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, M.; Shen, G.; Wang, Q.; Tang, J. PTB7-Th sensitized by AA-CDs for photoelectrochemical protein assay. Microchem. J. 2024, 207, 112173. [Google Scholar] [CrossRef]
- Tan, A.Y.S.; Cheng, F.; Zhang, M.; Tan, M.T.T.; Manickam, S.; Muthoosamy, K. Graphitic carbon nitride/1-pyrenebutyric acid N-hydroxysuccinimide/polythiophene nanocomposite photoelectrochemical biosensor for CA 19-9 detection. Talanta 2025, 293, 128065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Chen, Q.; Tan, X.; Hu, X.; An, Y.; Liu, M. Aptamer molecular gate functionalized mesoporous SiO2@MB controlled-release system for pollutant detection using Ti(III) self-doped TiO2 NTs as active photoanode coupled with electrostatic modulation. Talanta 2024, 277, 126409. [Google Scholar] [CrossRef]
- Zeng, R.; Xu, J.; Liang, T.; Li, M.; Tang, D. Photocurrent-Polarity-Switching Photoelectrochemical Biosensor for Switching Spatial Distance Electroactive Tags. ACS Sens. 2023, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, M.; Lin, X.; Zong, C.; Wang, H.; Li, C. Dye-Sensitized NiO photocathode based on rhodamine B-Appended Iridium(III) complex for Photoelectrochemical assay. Appl. Surf. Sci. 2022, 599, 153913. [Google Scholar] [CrossRef]
- Deng, X.; Wu, S.; Li, Z.; Zhao, Y.; Duan, C. Ratiometric Detection of DNA and Protein in Serum by a Universal Tripyridinyl RuII Complex–Encapsulated SiO2@Polydopamine Fluorescence Nanoplatform. Anal. Chem. 2020, 92, 15908–15915. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Gao, X.; Lin, J.; Zeng, B.; Gong, C.; Zhao, F. Porphyrin and molecularly imprinted polymer double sensitized cathode photoelectrochemical sensor for chlortetracycline. Microchem. J. 2023, 193, 109094. [Google Scholar] [CrossRef]
- Yuan, Z.; Chai, H.; Huang, Y.; Zhang, Z.; Tan, W.; Sun, Y.; Ma, J.; Zhang, G. Porphyrin-engineered metal–organic frameworks for photo/electrochemical sensing: Preparation and mechanisms. Coord. Chem. Rev. 2025, 527, 216385. [Google Scholar] [CrossRef]
- Neven, L.; Barich, H.; Ching, H.Y.V.; Khan, S.U.; Colomier, C.; Patel, H.H.; Gorun, S.M.; Verbruggen, S.; Van Doorslaer, S.; De Wael, K. Correlation between the Fluorination Degree of Perfluorinated Zinc Phthalocyanines, Their Singlet Oxygen Generation Ability, and Their Photoelectrochemical Response for Phenol Sensing. Anal. Chem. 2022, 94, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Xu, Y.-T.; Wang, B.; Yu, S.-Y.; Shi, X.-M.; Zhao, W.-W.; Jiang, D.; Chen, H.-Y.; Xu, J.-J. A Photoelectrochemical Nanoreactor for Single-Cell Sampling and Near Zero-Background Faradaic Detection of Intracellular microRNA. Angew. Chem. Int. Ed. 2022, 61, e202212752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Luo, Y.; Hao, Y.; Han, H.; Hu, X.; Yang, Y.; Long, X.; He, J.; Zhang, P.; Zeng, R.; et al. A responsive organic probe based photoelectrochemical sensor for hydrazine detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123463. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, T.; Luo, L.; Fan, S.; Chen, S.; Zhang, Y.; Tang, Z.; Xu, M.; Zeng, R.; Chen, S. A reaction based dual-modal probe for fluorescent and photoelectrochemical determination of thiophenol. Sens. Actuators B Chem. 2022, 369, 132405. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Y.; Peng, Y.; Wang, X.; Zhou, Y.; Chen, S.; Xu, M. Responsive photoactive probe based photoelectrochemical sensor for sulfur dioxide. Electroanalysis 2024, 36, e202400041. [Google Scholar] [CrossRef]
- Luo, L.; Yang, Y.; Chen, S.; Zhang, P.; Zeng, R. A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer. Molecules 2024, 29, 614. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Z.; Qu, C.; Han, Q.; Xu, L. Organic Molecules as a Bridge Connecting Photoelectrochemistry and Fluorescence for Dual-Signal Assay. Anal. Chem. 2025, 97, 7842–7850. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical detection of metal ions. Analyst 2016, 141, 4262–4271. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Xiao, C.; Luo, Y.; Zhong, N.; Xie, Q.; Chang, H.; Zhong, D.; Xu, Y.; Zhao, M.; et al. Recent advances in photoelectrochemical sensors for detection of ions in water. Chin. Chem. Lett. 2023, 34, 107904. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, J.; Liu, Z. A Laser-Induced Photoelectrochemical Sensor for Natural Sweat Cu2+ Detection. Chemosensors 2022, 10, 169. [Google Scholar] [CrossRef]
- Liu, L.; Hao, J.; Wu, K. Potential-Driven Porous Red Phosphorus-Powered Fully Integrated Arrays for High-Throughput Photoelectrochemical Aptasensing. Adv. Funct. Mater. 2025, 35, 2414738. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Wang, Y.; Xu, R.; Gao, X.; Shang, C.; Lei, P.; Zeng, Q.; Zhou, Y.; Chen, B.; et al. A Solution-Processable Porphyrin-Based Hydrogen-Bonded Organic Framework for Photoelectrochemical Sensing of Carbon Dioxide. Angew. Chem. Int. Ed. 2023, 62, e202311482. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Kong, Y.; Dai, M.; Han, D.; Liu, Z. FRET Modulated Signaling: A Versatile Strategy to Construct Photoelectrochemical Microsensors for In Vivo Analysis. Angew. Chem. Int. Ed. 2021, 60, 11774–11778. [Google Scholar] [CrossRef]
- Lin, D.; Lu, T.; Wang, X.; Ye, X.; Liu, Z. A reversible photoelectrochemical microsensor for dynamically monitoring sulfur dioxide in the epileptic brain. Chem. Sci. 2024, 15, 4824–4832. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, L.; Zheng, H.; Li, Q.; Huang, J.; Zhang, L.; Yang, H.; Cui, K.; Yu, J. Bifunctional Strategy toward Constructing Perovskite/Upconversion Lab-on-Paper Photoelectrochemical Device for Sensitive Detection of Malathion. ACS Nano 2023, 17, 13418–13429. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, D.; Lu, S.; Zhou, X.; Yang, S.; Zhang, Z. Atomically dispersed recognition unit for selective in vivo photoelectrochemical medicine detection. Nat. Commun. 2024, 15, 8827. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, D.; Zhou, X.; Huang, J.; Gu, S.; Zhang, Z. Implantable photoelectrochemical-therapeutic methotrexate monitoring system with dual-atomic docking strategy. Nat. Commun. 2025, 16, 1747. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Q.-Q.; Chen, M.-H.; Xu, J.-J.; Chen, H.-Y.; Zhao, W.-W. Organic photoelectrochemical memtransistor. eScience 2025, 5, 100374. [Google Scholar] [CrossRef]
- Hu, J.; Jing, M.-J.; Huang, Y.-T.; Kou, B.-H.; Li, Z.; Xu, Y.-T.; Yu, S.-Y.; Zeng, X.; Jiang, J.; Lin, P.; et al. A Photoelectrochemical Retinomorphic Synapse. Adv. Mater. 2024, 36, 2405887. [Google Scholar] [CrossRef]
- Chen, F.; Wei, X.; Hao, Y.; Wan, Y.; Zhang, Y.; Xu, M. Recent advances in photoelectrochemical detection of hydrogen sulfide. Int. J. Electrochem. Sci. 2025, 20, 101046. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, Y.; Wang, W.; Gu, H.; Chen, W.; Li, C.; Zhang, P.; Zeng, R.; Xu, M.; Chen, S. Development of a Photoelectrochemical Microelectrode Using an Organic Probe for Monitoring Hydrogen Sulfide in Living Brains. Anal. Chem. 2024, 96, 19822–19832. [Google Scholar] [CrossRef]
- Li, T.; Hao, Y.; Dong, H.; Li, C.; Liu, J.; Zhang, Y.; Tang, Z.; Zeng, R.; Xu, M.; Chen, S. Target-Induced In Situ Formation of Organic Photosensitizer: A New Strategy for Photoelectrochemical Sensing. ACS Sens. 2022, 7, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Yu, H.; Liu, M.; Xu, W.; Qin, Y.; Tan, R.; Chen, Y.; Wen, J.; Peng, X.; Gu, W.; et al. Selective Chemical Reactivity of Non-Fullerene Acceptor for Photoelectrochemical Bioassay of Urease Activity. Adv. Funct. Mater. 2023, 33, 2304915. [Google Scholar] [CrossRef]
- Qin, Y.; Xiao, R.; Xu, W.; Yu, H.; Liu, M.; Yang, W.; Tan, R.; Chen, Y.; Wen, J.; Peng, X.; et al. Near-Infrared Light Driven Reversible Photoelectrochemical Bioassay by S-Scheme All-Polymer Blends for Acetylcholinesterase Activity Monitoring. Nano Lett. 2025, 25, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.T.; Steijlen, A.; Laurijssen, D.; Campos, R.; Steckel, J.; Daems, W.; Bassini, S.; Daems, E.; De Wael, K. A 96-Well LED Array for Multiplexed Photoelectrochemical Detection of Nucleic Acids. Anal. Chem. 2024, 96, 15091–15096. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, L.; Cai, T.; Li, Z.; Dai, Z. Integrating multiple probes for simplifying signal-on photoelectrochemical biosensing of microRNA with ultrasensitivity and wide detection range based on biofunctionalized porous ferroferric oxide and hypotoxic quaternary semiconductor. Biosens. Bioelectron. 2024, 243, 115781. [Google Scholar] [CrossRef]
- Kong, W.; Zhu, D.; Zhang, Y.; Luo, R.; Ma, J.; Lei, J.; Ju, H. Electron Donor Coordinated Metal-Organic Framework to Enhance Photoelectrochemical Performance. Angew. Chem. Int. Ed. 2023, 62, e202308514. [Google Scholar] [CrossRef]
- Chen, F.-Z.; Hou, L.; Gao, Y.; Zhou, J.-Y.; Kong, F.-Y.; Han, D.-M.; Zhao, W.-W. Polyoxometalates: Cascading Functionality to Unique On-Off Organic Photoelectrochemical Transistor Operation. Adv. Funct. Mater. 2024, 34, 2408186. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Ruan, Y.-F.; Zhang, T.-Y.; Shi, X.-M.; Wang, H.-Y.; Zhao, W.-W.; Chen, H.-Y.; Xu, J.-J. Ion current rectification-nanopipette technique for single-cell analysis. TrAC Trends Anal. Chem. 2023, 167, 117217. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Zhang, Y.; Jiang, S.; Lin, Q.; Zhang, H.; Xiong, X.; Wang, Y.; Deng, H.; Li, C. Photoelectrochemical aptasensing and fluorescence imaging co-joint detecting MCF-7 cells in whole blood via an inertial separation microfluidic chip. Talanta 2025, 286, 127488. [Google Scholar] [CrossRef]
- Luo, J.; Zeng, Q.; Liu, S.; Wei, Q.; Wang, Z.; Yang, M.; Zou, Y.; Lu, L. Highly sensitive photoelectrochemical sensing platform based on PM6:Y6 p-n heterojunction for detection of MCF-7 cells. Sens. Actuators B 2022, 363, 131814. [Google Scholar] [CrossRef]
- Wei, J.-J.; Li, H.-B.; Wang, G.-Q.; Zheng, J.-Y.; Wang, A.-J.; Mei, L.-P.; Zhao, T.; Feng, J.-J. Novel Ultrasensitive Photoelectrochemical Cytosensor Based on Hollow CdIn2S4/In2S3 Heterostructured Microspheres for HepG2 Cells Detection and Inhibitor Screening. Anal. Chem. 2022, 94, 12240–12247. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Zhang, T.-Y.; Li, Z.; Liu, X.-N.; Zhu, Y.-C.; Zhao, W.-W.; Chen, H.-Y.; Xu, J.-J. Photoelectrochemical Cytosensors. Electroanalysis 2022, 34, 947–955. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Li, X.; Gong, J.P. Design principles for strong and tough hydrogels. Nat. Rev. Mater. 2024, 9, 380–398. [Google Scholar] [CrossRef]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent Progress in Biomedical Sensors Based on Conducting Polymer Hydrogels. ACS Appl. Bio Mater. 2023, 6, 1720–1741. [Google Scholar] [CrossRef] [PubMed]
- Chenani, H.; Saeidi, M.; Rastkhiz, M.A.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and Advances of Hydrogel-Based Wearable Electrochemical Biosensors for Real-Time Monitoring of Biofluids: From Lab to Market. A Review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef]
- Jiang Luo, J.; Rui Zhu, L.; Guo, Z.; Pi, N.; Li, X.; Lin Zou, H.; Qun Luo, H.; Bing Li, N.; Li, B.L. Hydrogel-innovated nanotechnologies for chemical and biological analysis. Coord. Chem. Rev. 2024, 511, 215874. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized hydroxypropyl cellulose/carboxymethyl chitosan hydrogels permit pH-responsive, targeted drug release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef] [PubMed]
- Das, I.J.; Bal, T. pH factors in chronic wound and pH-responsive polysaccharide-based hydrogel dressings. Int. J. Biol. Macromol. 2024, 279, 135118. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Wang, Y.; Feng, Y.; Duan, X.; Zhang, Q.; Wan, A.; Xia, Z.; Shou, W.; Fan, J. PNIPAAm-based temperature responsive ionic conductive hydrogels for flexible strain and temperature sensing. J. Colloid Interface Sci. 2025, 678, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Song, W.; Liu, T.; Xin, J.; Hiscox, W.C.; Zhang, J.; Liu, G.; Kong, Z. Temperature and pH Responsive Hydrogels Using Methacrylated Lignosulfonate Cross-Linker: Synthesis, Characterization, and Properties. ACS Sustain. Chem. Eng. 2018, 6, 1763–1771. [Google Scholar] [CrossRef]
- Nakamura, K.; Di Caprio, N.; Burdick, J.A. Engineered Shape-Morphing Transitions in Hydrogels Through Suspension Bath Printing of Temperature-Responsive Granular Hydrogel Inks. Adv. Mater. 2024, 36, 2410661. [Google Scholar] [CrossRef]
- Lee, M.J.; Shrotriya, D.R.; Espinosa-Marzal, R.M. Responsiveness of Charged Double Network Hydrogels to Ionic Environment. Adv. Funct. Mater. 2024, 34, 2402279. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Y.; Chen, D.; Zhao, Q. Reversible Shape-Shifting of an Ionic Strength Responsive Hydrogel Enabled by Programmable Network Anisotropy. ACS Appl. Mater. Interfaces 2022, 14, 40344–40350. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.-F.; Zhao, C.-S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Y.; Liu, P.; Luo, D. Bioresponsive DNA Hydrogels: Beyond the Conventional Stimuli Responsiveness. Acc. Chem. Res. 2017, 50, 733–739. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Wu, H.; Lin, X.; Cai, L. Stimuli-responsive hydrogel dressing for wound healing. APL Mater. 2025, 13, 010601. [Google Scholar] [CrossRef]
- Shafique, H.; de Vries, J.; Strauss, J.; Khorrami Jahromi, A.; Siavash Moakhar, R.; Mahshid, S. Advances in the Translation of Electrochemical Hydrogel-Based Sensors. Adv. Healthc. Mater. 2023, 12, 2201501. [Google Scholar] [CrossRef]
- hanjai; Sinha, A.; Kalambate, P.K.; Mugo, S.M.; Kamau, P.; Chen, J.; Jain, R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. TrAC Trends Anal. Chem. 2019, 118, 488–501. [Google Scholar] [CrossRef]
- Marcisz, K.; Kaniewska, K.; Karbarz, M. Smart functionalized thin gel layers for electrochemical sensors, biosensors and devices. Curr. Opin. Electrochem. 2020, 23, 57–64. [Google Scholar] [CrossRef]
- Song, M.; Zhang, J.; Shen, K.; Hu, Y.; Shen, W.; Tang, S.; Lee, H.K. Application of smart-responsive hydrogels in nucleic acid and nucleic acid-based target sensing: A review. Biosens. Bioelectron. 2025, 267, 116803. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wan, J.; Jiang, S.; Wu, Y.; Wang, W.; Gong, X.; Wang, H. Quantum dots-based hydrogels for sensing applications. Chem. Eng. J. 2021, 408, 127351. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, Z. Biocompatible AgInS2@hydrogel microelectrode with enhanced photoelectrochemical sensitivity for real-time in vivo dopamine monitoring. Analyst 2025, 150, 2350–2355. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Huang, Y.-T.; Jing, M.-J.; Yuan, C.; Zeng, X.; Zhao, W.-W.; Lin, P. Nanocomposite Hydrogel Enables Color-Gated Organic Photoelectrochemical Transistor Biodetection. Adv. Funct. Mater. 2025, 35, 2412928. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Zhang, H.; Li, S.; Wang, C.; Wang, P.; Sha, J.; Zhou, X. Flexible Self-Powered Photoelectrochemical Photodetectors Based on Cellulose-Based Hydrogel Electrolytes and Bi2O2Se Nanosheets. ACS Appl. Nano Mater. 2024, 7, 594–605. [Google Scholar] [CrossRef]
- Hu, J.; Liao, R.; Mao, J.; Chen, J.; Chen, C.; Deng, W.; Tan, Y.; Xie, Q. Novel “Signal-On” photoelectrochemical assay by regulating the release of cationic dyes with the utilization of target-specific enzymolysis hydrogels. Microchem. J. 2024, 206, 111350. [Google Scholar] [CrossRef]
- Liu, M.; Ma, W.; Zhou, Y.; Liu, B.; Zhang, X.; Zhang, S. A Label-Free Photoelectrochemical Biosensor Based on CRISPR/Cas12a System Responsive Deoxyribonucleic Acid Hydrogel and “Click” Chemistry. ACS Sens. 2022, 7, 3153–3160. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, L.; Wang, H.; Cheng, H.; Qin, J.; Zeng, Z.; Lin, Y.; Tang, D.; Pu, S. In situ generated PANI promoted flexible photoelectrochemical biosensor for ochratoxin A based on GOx-stuffed DNA hydrogel as enhancer. Microchim. Acta 2023, 190, 106. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Huang, Z.; Wang, Y.; Tang, D. Enzyme-Encapsulated DNA Hydrogel for Highly Efficient Electrochemical Sensing Glucose. ChemElectroChem 2020, 7, 1537–1541. [Google Scholar] [CrossRef]
- Yang, J.; Fu, S.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. Homogeneous photoelectrochemical biosensor for microRNA based on target-responsive hydrogel coupled with exonuclease III and nicking endonuclease Nb.BbvCI assistant cascaded amplification strategy. Microchim. Acta 2021, 188, 267. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, H.; Deng, H.; Xiong, X.; Li, C.; Li, W. Molecularly imprinted photoelectrochemical sensor for carcinoembryonic antigen based on polymerized ionic liquid hydrogel and hollow gold nanoballs/MoSe2 nanosheets. Anal. Chim. Acta 2019, 1090, 64–71. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Y.; Guo, Z.; Ji, Y.; Huang, Z.; Bo, Z.; Qiao, H.; Bao, Q.; Qi, X. Stretchable and self-healing photoelectrochemical photodetectors based on Ti2CTx nanosheets hydrogels. Appl. Mater. Today 2025, 42, 102577. [Google Scholar] [CrossRef]
- Qing, M.; Chen, S.L.; Han, L.; Yang, Y.Z.; Luo, H.Q.; Li, N.B. Three–dimensional donor–acceptor–type photoactive material/conducting polyaniline hydrogel complex for sensitive photocathodic enzymatic bioanalysis. Biosens. Bioelectron. 2020, 158, 112179. [Google Scholar] [CrossRef]
- Xu, X.; Lu, S.; Zhang, Z. Hydrogel/MOF Dual-Modified Photoelectrochemical Biosensor for Antibiofouling and Biocompatible Dopamine Detection. Langmuir 2024, 40, 10718–10725. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Wang, X.; Ge, S.; Yu, J. Construction of built-in correction photoelectrochemical sensing platform for diagnosis of Alzheimer’s disease. Biosens. Bioelectron. 2024, 249, 116020. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, D.; Shan, X.; Du, X.; Wei, M.; Zhang, Y.; Chen, Z. Visible light-driven self-powered aptasensors for ultrasensitive Microcystin-LR detection based on the carrier density effect of N-doped graphene hydrogel/hematite Schottky junctions. Analyst 2021, 146, 6220–6227. [Google Scholar] [CrossRef]
- Du, X.; Sun, J.; Jiang, D.; Du, W. Non-noble metal plasmonic enhanced photoelectrochemical sensing of chlorpyrifos based on 1D TiO2-x/3D nitrogen-doped graphene hydrogel heterostructure. Anal. Bioanal. Chem. 2021, 413, 5373–5382. [Google Scholar] [CrossRef]
- Hao, N.; Hua, R.; Chen, S.; Zhang, Y.; Zhou, Z.; Qian, J.; Liu, Q.; Wang, K. Multiple signal-amplification via Ag and TiO2 decorated 3D nitrogen doped graphene hydrogel for fabricating sensitive label-free photoelectrochemical thrombin aptasensor. Biosens. Bioelectron. 2018, 101, 14–20. [Google Scholar] [CrossRef]
- Yu, L.; Li, J.; Zhang, J.; Chen, X.; Yuan, M.; Zhang, Q.; Xu, B.; Yao, Z.; Xu, Q. Dual 3D Structural BiWO @Graphene Hydrogel Composites Modified Molecularly Imprinted Polymers: Application for Sensitive Photoelectrochemical Sensing of 4-Nitrophenol in PM. J. Appl. Polym. Sci. 2025, 142, e56699. [Google Scholar] [CrossRef]
- Ge, L.; Li, H.; Du, X.; Zhu, M.; Chen, W.; Shi, T.; Hao, N.; Liu, Q.; Wang, K. Facile one-pot synthesis of visible light-responsive BiPO4/nitrogen doped graphene hydrogel for fabricating label-free photoelectrochemical tetracycline aptasensor. Biosens. Bioelectron. 2018, 111, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Du, X.; Liu, Q.; Hao, N.; Wang, K. MoS2/nitrogen doped graphene hydrogels p-n heterojunction: Efficient charge transfer property for highly sensitive and selective photoelectrochemical analysis of chloramphenicol. Biosens. Bioelectron. 2019, 126, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Hao, N.; Lu, J.; Qian, J.; Liu, Q.; Li, H.; Wang, K. A sensitive Potentiometric resolved ratiometric Photoelectrochemical aptasensor for Escherichia coli detection fabricated with non-metallic nanomaterials. Biosens. Bioelectron. 2018, 106, 57–63. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, M.; Qian, Z.; Bi, H.; Lin, S. Hydrogel flexible photodetector based on polarization of free water molecules and image sensor application. Nano Energy 2025, 138, 110889. [Google Scholar] [CrossRef]
- Yang, Q.; Liao, J.; Feng, L.; Wang, S.; Zhao, Z.; Wang, J.; Bu, Y.; Zhuang, J.; Zhang, D.-W. One-step construction of multiplexed enzymatic biosensors using light-addressable electrochemistry on a single silicon photoelectrode. Biosens. Bioelectron. 2024, 253, 116194. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Yu, C.; Wang, X.; Wang, J. Swellable microneedle-coupled light-addressable photoelectrochemical sensor for in-situ tracking of multiple pesticides pollution in vivo. J. Hazard. Mater. 2024, 470, 134216. [Google Scholar] [CrossRef]
- Hu, J.; Lu, M.-J.; Chen, F.-Z.; Jia, H.-M.; Zhou, H.; Li, K.; Zeng, X.; Zhao, W.-W.; Lin, P. Multifunctional Hydrogel Hybrid-Gated Organic Photoelectrochemical Transistor for Biosensing. Adv. Funct. Mater. 2022, 32, 2109046. [Google Scholar] [CrossRef]
- Leng, D.; Yu, Z.; Liu, J.; Jin, W.; Wu, T.; Ren, X.; Ma, H.; Wu, D.; Ju, H.; Wei, Q. Multifunctional Supramolecular Hydrogel Modulated Heterojunction Interface Carrier Transport Engineering Facilitates Sensitive Photoelectrochemical Immunosensing. Anal. Chem. 2024, 96, 8814–8821. [Google Scholar] [CrossRef]
- Hao, N.; Dai, Z.; Meng, X.; Hua, R.; Lu, J.; Wang, K. A portable solar-driven ratiometric photo-electrochromic visualization biosensor for detection of ochratoxin A. Sens. Actuators B Chem. 2020, 306, 127594. [Google Scholar] [CrossRef]
- Hao, Q.; Zhao, C.; Sun, B.; Lu, C.; Liu, J.; Liu, M.; Wan, L.-J.; Wang, D. Confined Synthesis of Two-Dimensional Covalent Organic Framework Thin Films within Superspreading Water Layer. J. Am. Chem. Soc. 2018, 140, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Dai, S.; Wang, L. Optical aptasensors for quantitative detection of small biomolecules: A review. Biosens. Bioelectron. 2014, 59, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Cañeque, T.; Müller, S.; Rodriguez, R. Visualizing biologically active small molecules in cells using click chemistry. Nat. Rev. Chem. 2018, 2, 202–215. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease Detection with Molecular Biomarkers: From Chemistry of Body Fluids to Nature-Inspired Chemical Sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Șoldănescu, I.; Lobiuc, A.; Covașă, M.; Dimian, M. Detection of Biological Molecules Using Nanopore Sensing Techniques. Biomedicines 2023, 11, 1625. [Google Scholar] [CrossRef]
- Krämer, J.; Kang, R.; Grimm, L.M.; De Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef]
- Song, Z.; Hao, Y.; Long, Y.; Zhang, P.; Zeng, R.; Chen, S.; Chen, W. Luminescent Lanthanide Infinite Coordination Polymers for Ratiometric Sensing Applications. Molecules 2025, 30, 396. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, X.; Zhou, Z.; Qian, J.; Liu, Q.; Chen, S.; Zhang, Y.; Wang, K. Three-dimensional nitrogen-doped graphene porous hydrogel fabricated biosensing platform with enhanced photoelectrochemical performance. Sens. Actuators B Chem. 2017, 250, 476–483. [Google Scholar] [CrossRef]
- Zhao, F.; Conzuelo, F.; Hartmann, V.; Li, H.; Stapf, S.; Nowaczyk, M.M.; Rögner, M.; Plumeré, N.; Lubitz, W.; Schuhmann, W. A novel versatile microbiosensor for local hydrogen detection by means of scanning photoelectrochemical microscopy. Biosens. Bioelectron. 2017, 94, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Mohan, B.; Kumari, R.; Virender; Singh, G.; Singh, K.; Pombeiro, A.J.L.; Yang, X.; Ren, P. Covalent organic frameworks (COFs) and metal–organic frameworks (MOFs) as electrochemical sensors for the efficient detection of pharmaceutical residues. Environ. Int. 2023, 175, 107928. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, D.J.; Jo, A.; Yun, S.H.; Eun, J.-B.; Im, M.-H.; Shim, J.-H.; Abd El-Aty, A.M. Onsite/on-field analysis of pesticide and veterinary drug residues by a state-of-art technology: A review. J. Sep. Sci. 2021, 44, 2310–2327. [Google Scholar] [CrossRef]
- Jia, M.; E, Z.; Zhai, F.; Bing, X. Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs. Molecules 2020, 25, 3590. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Su, X. Review of optical sensors for pesticides. TrAC Trends Anal. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Hu, X.; Wei, W.; Li, X.; Yang, Y.; Zhou, B. Recent advances in ratiometric electrochemical sensors for food analysis. Food Chem. X 2024, 23, 101681. [Google Scholar] [CrossRef]
- Yang, Y.; Xin, M.; Huang, L.; Hao, Y.; Xu, M. A novel coumarin-incorporated lanthanide coordination nanoprobe for ratiometric sensing of tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 337, 126108. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wang, X.; Liu, X.; Wang, J.; Liu, Z. Support-Free Implantable Photoelectrochemical Hydrogel Fiber Enables Long-Term Monitoring in Free-Behaving Organisms. Anal. Chem. 2025, 97, 9501–9511. [Google Scholar] [CrossRef]
- Gao, P.; Mukherjee, S.; Zahid Hussain, M.; Ye, S.; Wang, X.; Li, W.; Cao, R.; Elsner, M.; Fischer, R.A. Porphyrin-based MOFs for sensing environmental pollutants. Chem. Eng. J. 2024, 492, 152377. [Google Scholar] [CrossRef]
- Jiang, Y.; Sima, Y.; Liu, L.; Zhou, C.; Shi, S.; Wan, K.; Chen, A.; Tang, N.; He, Q.; Liu, J. Research progress on portable electrochemical sensors for detection of mycotoxins in food and environmental samples. Chem. Eng. J. 2024, 485, 149860. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Y.; Ma, X.; Chen, S.; Xu, M. A dicyanoisophorone-based highly sensitive and selective near-infrared fluorescent probe for sensing thiophenol in water samples and living cells. Environ. Pollut. 2020, 265, 114958. [Google Scholar] [CrossRef] [PubMed]
- Chau, S.L.; Zhao, A.; Jia, W.; Wang, L. Simultaneous Determination of Pesticide Residues and Mycotoxins in Storage Pu-erh Tea Using Ultra-High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules 2023, 28, 6883. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hidaka, T.; Kumagai, Y.; Yamamoto, M. Environmental pollutants and the immune response. Nat. Immunol. 2020, 21, 1486–1495. [Google Scholar] [CrossRef]
- Han, T.; Cui, C.; Xing, Y.; Shi, J.; Xu, J.; Zhu, J.-J. Advances of signal amplification strategies and sensing formats in electrochemiluminescence sensors for mycotoxins. TrAC Trends Anal. Chem. 2024, 180, 117961. [Google Scholar] [CrossRef]
- Martins, C.S.M.; LaGrow, A.P.; Prior, J.A.V. Quantum Dots for Cancer-Related miRNA Monitoring. ACS Sens. 2022, 7, 1269–1299. [Google Scholar] [CrossRef]
- Ratre, P.; Nazeer, N.; Kumari, R.; Thareja, S.; Jain, B.; Tiwari, R.; Kamthan, A.; Srivastava, R.K.; Mishra, P.K. Carbon-Based Fluorescent Nano-Biosensors for the Detection of Cell-Free Circulating MicroRNAs. Biosensors 2023, 13, 226. [Google Scholar] [CrossRef]
- Dong, H.; Lei, J.; Ding, L.; Wen, Y.; Ju, H.; Zhang, X. MicroRNA: Function, Detection, and Bioanalysis. Chem. Rev. 2013, 113, 6207–6233. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Application of electrochemical biosensors for the detection of microRNAs (miRNAs) related to cancer. Coord. Chem. Rev. 2022, 464, 214565. [Google Scholar] [CrossRef]
- Ye, J.; Xu, M.; Tian, X.; Cai, S.; Zeng, S. Research advances in the detection of miRNA. J. Pharm. Anal. 2019, 9, 217–226. [Google Scholar] [CrossRef]
- Jet, T.; Gines, G.; Rondelez, Y.; Taly, V. Advances in multiplexed techniques for the detection and quantification of microRNAs. Chem. Soc. Rev. 2021, 50, 4141–4161. [Google Scholar] [CrossRef]
- Hao, N.; Lu, J.; Chi, M.; Xiong, M.; Zhang, Y.; Hua, R.; Wang, K. A universal photoelectrochemical biosensor for dual microRNA detection based on two CdTe nanocomposites. J. Mater. Chem. B 2019, 7, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Y.; Xianyu, Y.; Chen, W.; Zhao, Y.; Jiang, X. Nanomaterials for Ultrasensitive Protein Detection. Adv. Mater. 2013, 25, 3802–3819. [Google Scholar] [CrossRef] [PubMed]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N.S. Protein-Protein Interaction Detection: Methods and Analysis. Int. J. Biol. Macromol. 2014, 2014, 147648. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, H.; Tang, M.; Zhang, X.; Qing, G. Nanopore: Emerging for detecting protein post-translational modifications. TrAC Trends Anal. Chem. 2024, 173, 117658. [Google Scholar] [CrossRef]
- Luo, X.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 42, 5944–5962. [Google Scholar] [CrossRef]
- Guo, W.; Wang, J.; Guo, W.; Kang, Q.; Zhou, F. Interference-free photoelectrochemical immunoassays using carboxymethylated dextran-coated and gold-modified TiO2 nanotube arrays. Anal. Bioanal. Chem. 2021, 413, 4847–4854. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Kwon, N.; Ma, H.; Yoon, J. Activatable fluorescent probes for in situ imaging of enzymes. Chem. Soc. Rev. 2022, 51, 450–463. [Google Scholar] [CrossRef]
- Sun, H.; Wang, N.; Zhang, L.; Meng, H.; Li, Z. Aptamer-Based Sensors for Thrombin Detection Application. Chemosensors 2022, 10, 255. [Google Scholar] [CrossRef]
- Shaban, S.M.; Byeok Jo, S.; Hafez, E.; Ho Cho, J.; Kim, D.-H. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coord. Chem. Rev. 2022, 465, 214567. [Google Scholar] [CrossRef]
- Fatimi, A.; Damiri, F.; Berrada, M.; Musuc, A.M. Patent Overview of Innovative Hyaluronic Acid-Based Hydrogel Biosensors. Biosensors 2024, 14, 567. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Zhao, F.; Feng, Y.; Feng, W. Multi-functional and multi-responsive layered double hydroxide-reinforced polyacrylic acid composite hydrogels as ionic skin sensors. Adv. Compos. Hybrid Mater. 2023, 6, 65. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Hu, J.; Li, Z.; Xu, Y.-T.; Yuan, C.; Jiang, D.; Zhao, W.-W. Metal–Organic Framework Nanofluidic Synapse. J. Am. Chem. Soc. 2024, 146, 27022–27029. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.-M.; Xu, Y.-T.; Li, Z.; Pang, J.-X.; Xu, J.-J.; Zhao, W.-W. Neuromorphic Nanofluidic Sense Digitalization. Angew. Chem. Int. Ed. 2025, 64, e202420602. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-Q.; Li, Z.; Chen, M.-H.; Yuan, C.; Zhu, Y.-C.; Xu, J.-J.; Zhao, W.-W. Reticular Photoelectrochemical Transistor with Biochemical Metaplasticity. Adv. Mater. 2025, 37, 2504338. [Google Scholar] [CrossRef]
- Yu, Y.; Su, X.; Xing, T.; Zhao, X.; Zhang, Z.; Zhang, W.; Wang, X.; Zhao, W.; Li, M.; Zhao, F. Enhanced photocurrent generation of a bio-photocathode based on photosystem I integrated in solvated redox polymers films nanostructured by SWCNTs. Bioelectrochemistry 2025, 165, 108979. [Google Scholar] [CrossRef]


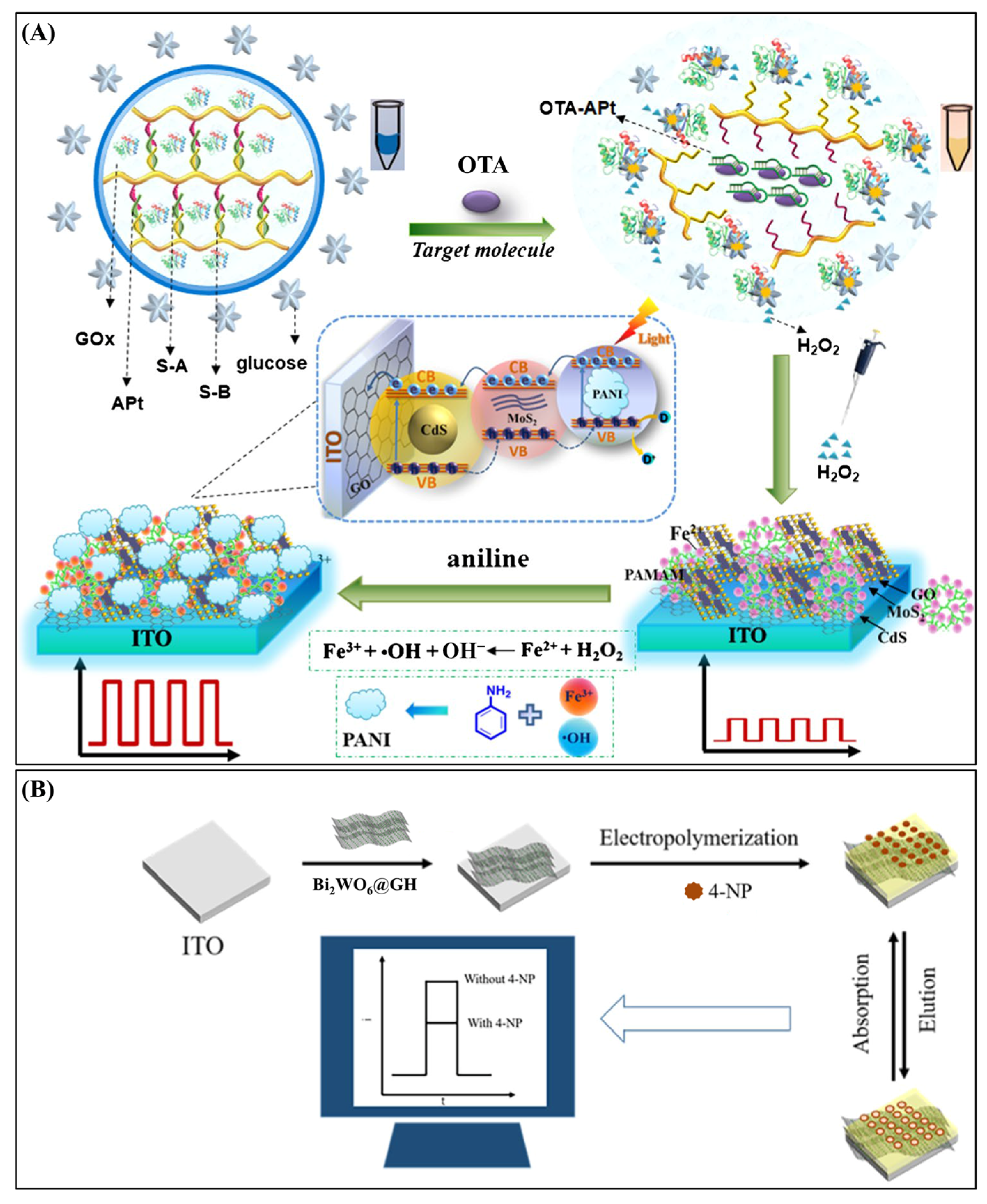
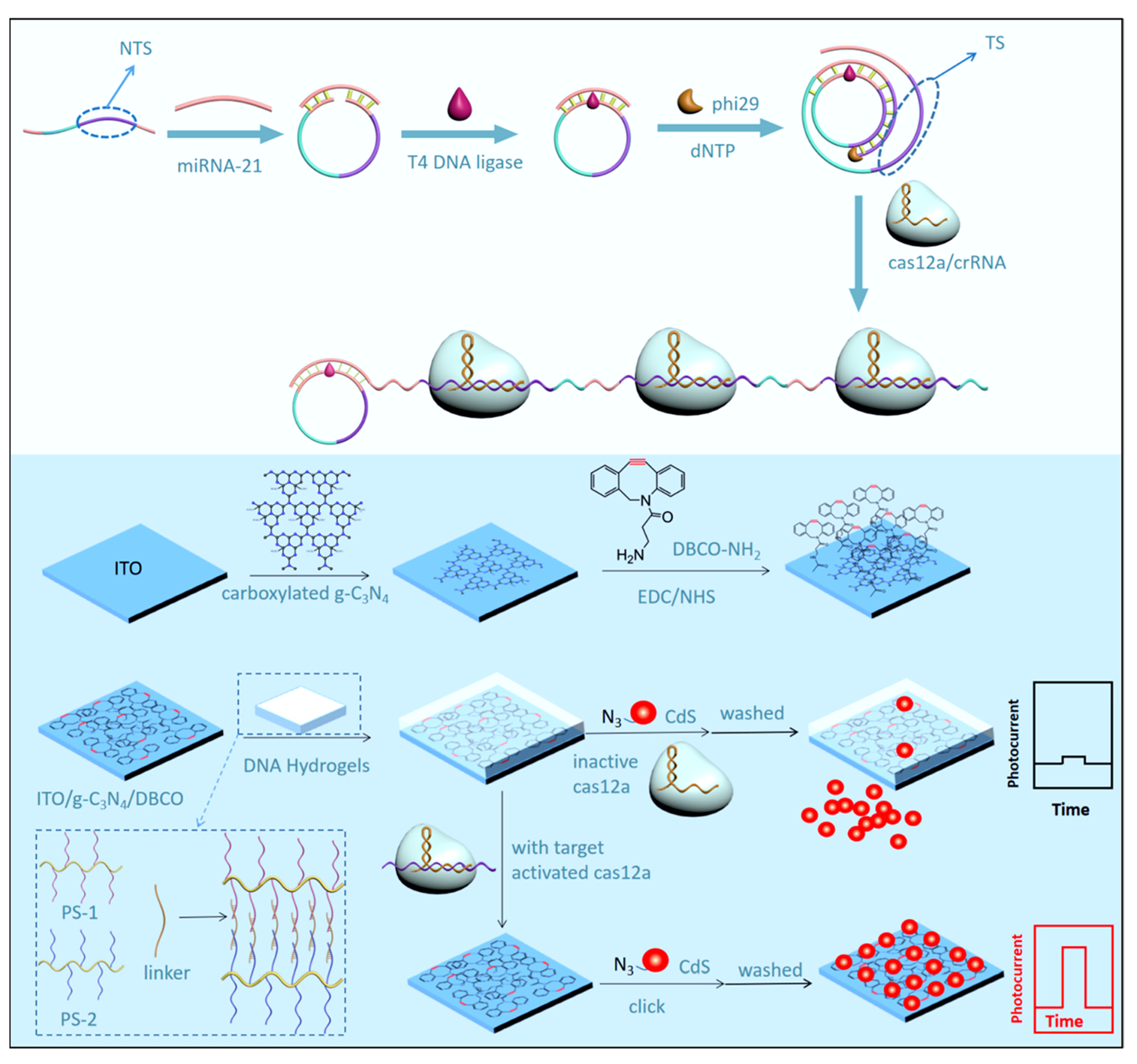

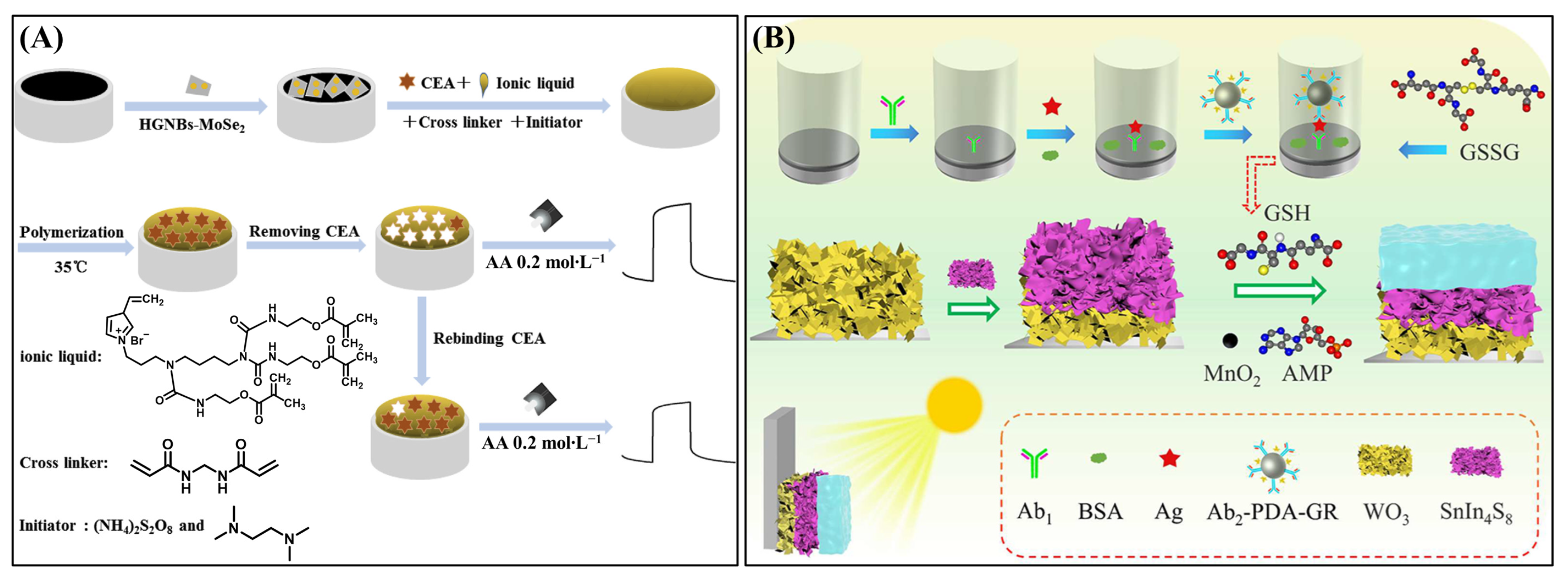
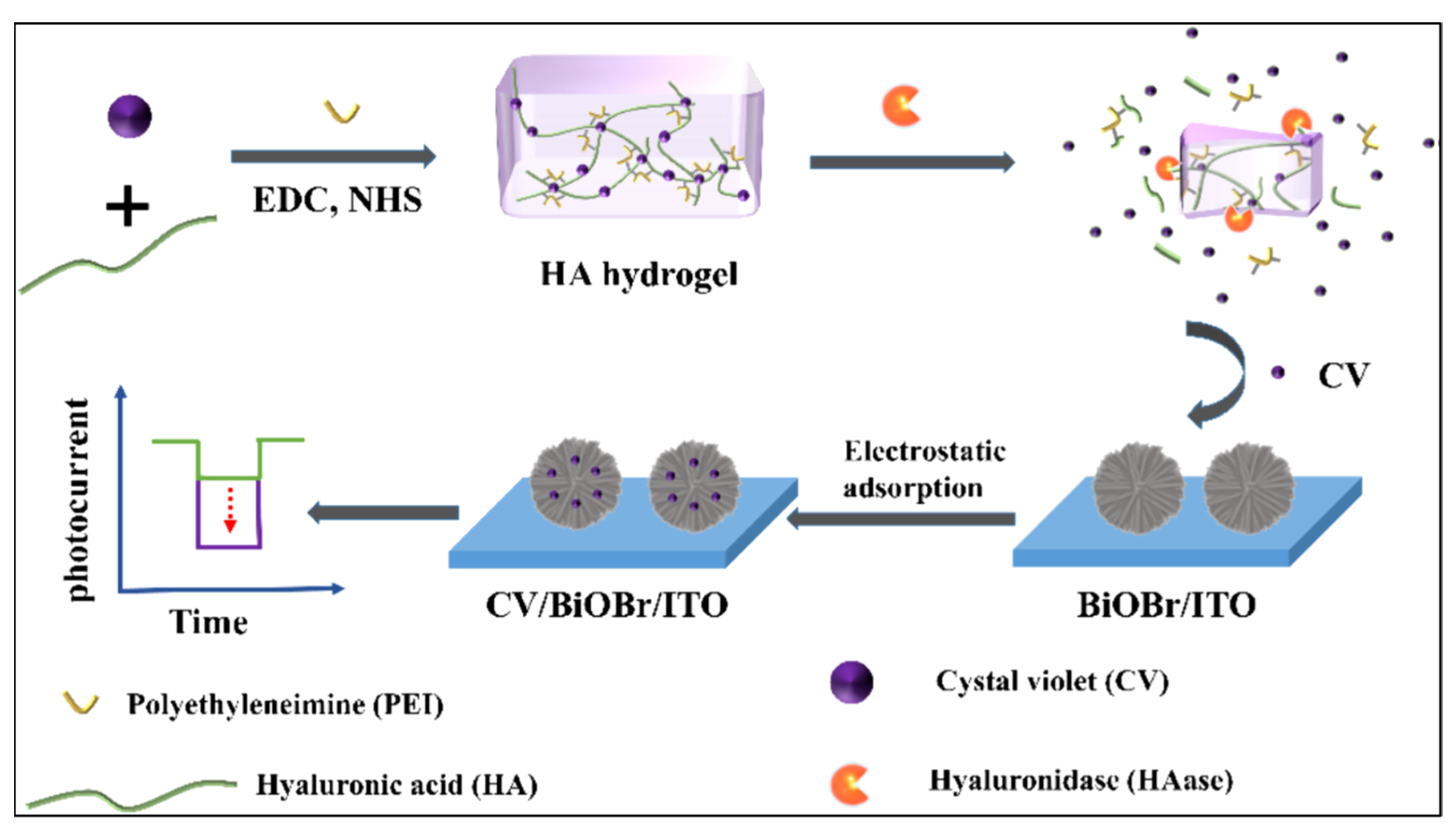
| Targets | Hydrogel | Functions of Hydrogel | Photoactive Materials | Dynamic Range | LOD | Applications | Ref. |
|---|---|---|---|---|---|---|---|
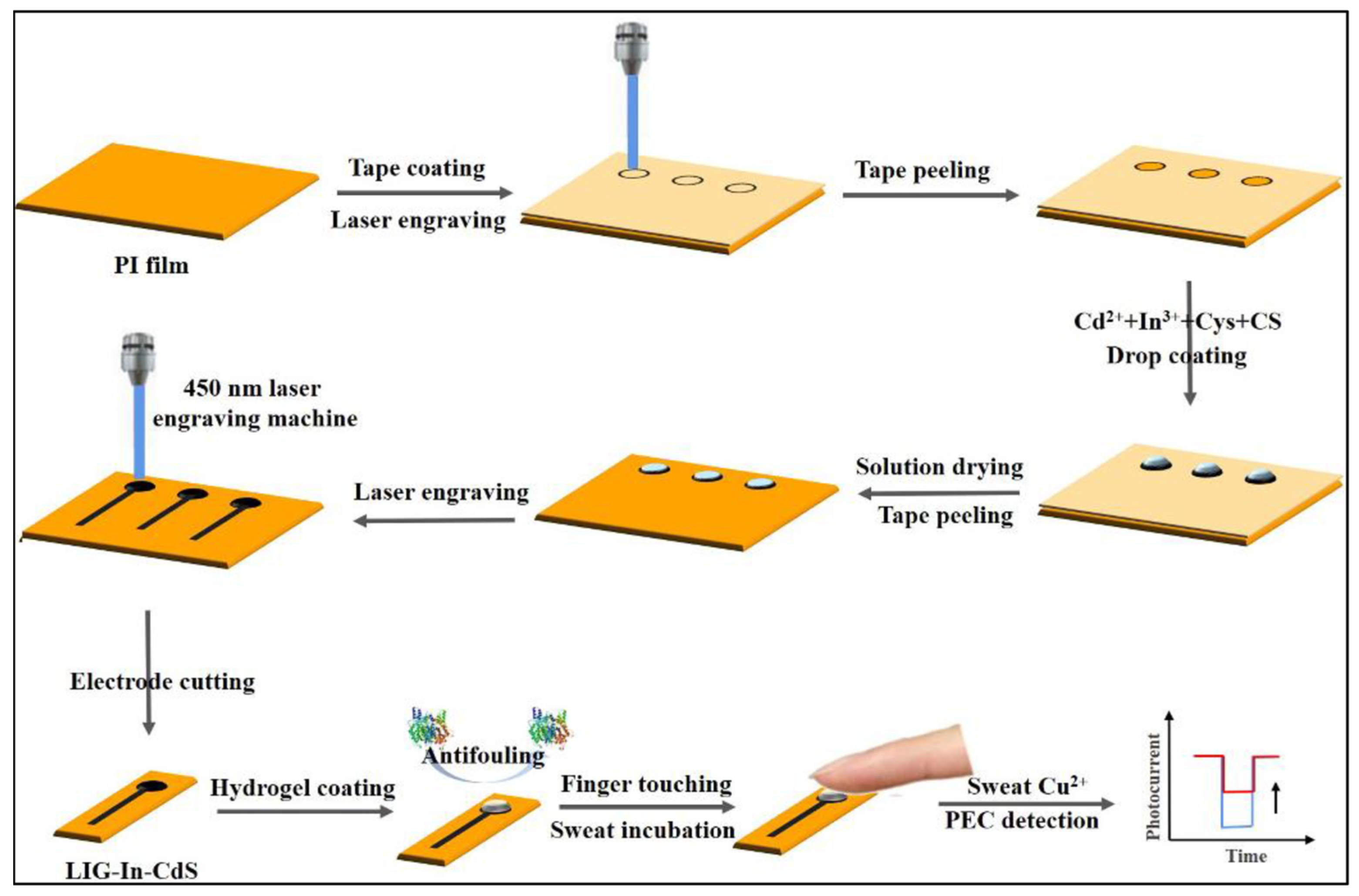
| Cu2+ | PVA hydrogel | Antifouling barrier | PI film/LIG-In-CdS/Hydrogel | 1.28 ng∙mL−1–5.12 μg∙mL−1 | -- | Sweat sample | [60] |
| Ru3+ | PAAm hydrogel | Confined reactor for 2D COF synthesis | ITO/COF(TTA-DHTA) | 0.3–30 μM | -- | -- | [133] |
| Dopamine | Acrylamide-based antibiofouling hydrogel | Antifouling interface | Ti/TiO2 NTPCs/Z-MOF/Hydrogel | 10–500 nM | 6.0 nM | -- | [118] |
| Dopamine | PEDOT/PSS/PR-PEGMA/AAm | Biocompatible and flexible interface | Hydrogel/AgInS2 | 0.2–4 μM | 64 nM | In vivo monitoring | [107] |
| Glucose | GOx & HRP DNA hydrogel | Conductive scaffold | FTO/In2O3–CdS | 10–500 nM | 9.62 nM | -- | [113] |
| H2O2, Glucose | Three-dimensional nitrogen-doped graphene hydrogel (3DNGH) | Biocompatible enzyme matrix | ITO/3DNGH@ZnO/HRP | H2O2: 0.001–5 mM Glucose: 0.002–8 mM | H2O2: 0.33 μM Glucose: 0.66 μM | Orange juice | [141] |
| Glucose, Sarcosine, Lactate | A Fc–PEG–SS–gelatin hydrogel (amide bond-based) | Conductive 3D matrix | Silicon/Fc-containing hydrogel | Glucose: 0.5–2.5 mM; Sarcosine: 0.3–1.5 mM; Lactate: 1.0–3.0 mM | Glucose: 179 μM; Sarcosine: 16 μM; Lactate: 780 μM | Sweat sample | [128] |
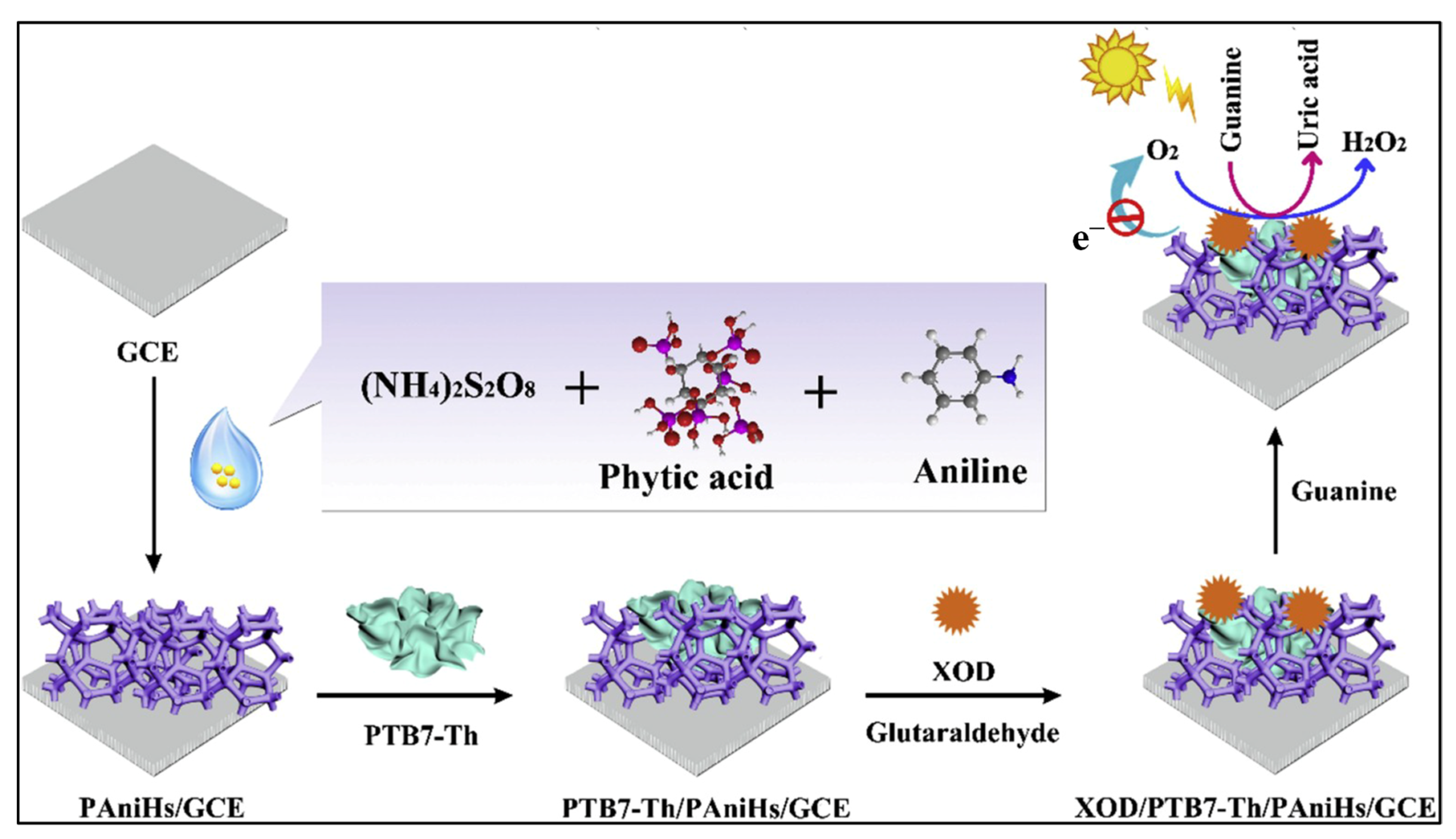
| Guanine | Polyaniline hydrogel (PAniHs) | Self-redox conductive hydrogel matrix | GCE/PAniHs/PTB7–Th/XOD | 0.1–80 μM | 0.02 μM | Aciclovir tablets | [117] |
| Hydrogen | Viologen-modified polyethyleneimine | Redox-active enzyme matrix | [NiFe]-H2ase/PS1–Pt | 1.6–24 µM | 0.81 µM | -- | [142] |
| Tetracycline | Three-dimensional nitrogen-doped graphene hydrogel (3DNGH) | Conductive 3D scaffold | ITO/3DNGH/BiPO4/Apt | 0.1 nM–1 μM | 0.033 nM | Milk sample | [124] |
| Chloramphenicol (CAP) | Three-dimensional nitrogen-doped graphene hydrogel (NGH) | Conductive matrix for p–n heterojunction | ITO/NGH/MoS2-Apt | 32.3 ng∙L−1–96.9 μg∙L−1 | 3.23 ng∙L−1 | Honey sample | [125] |
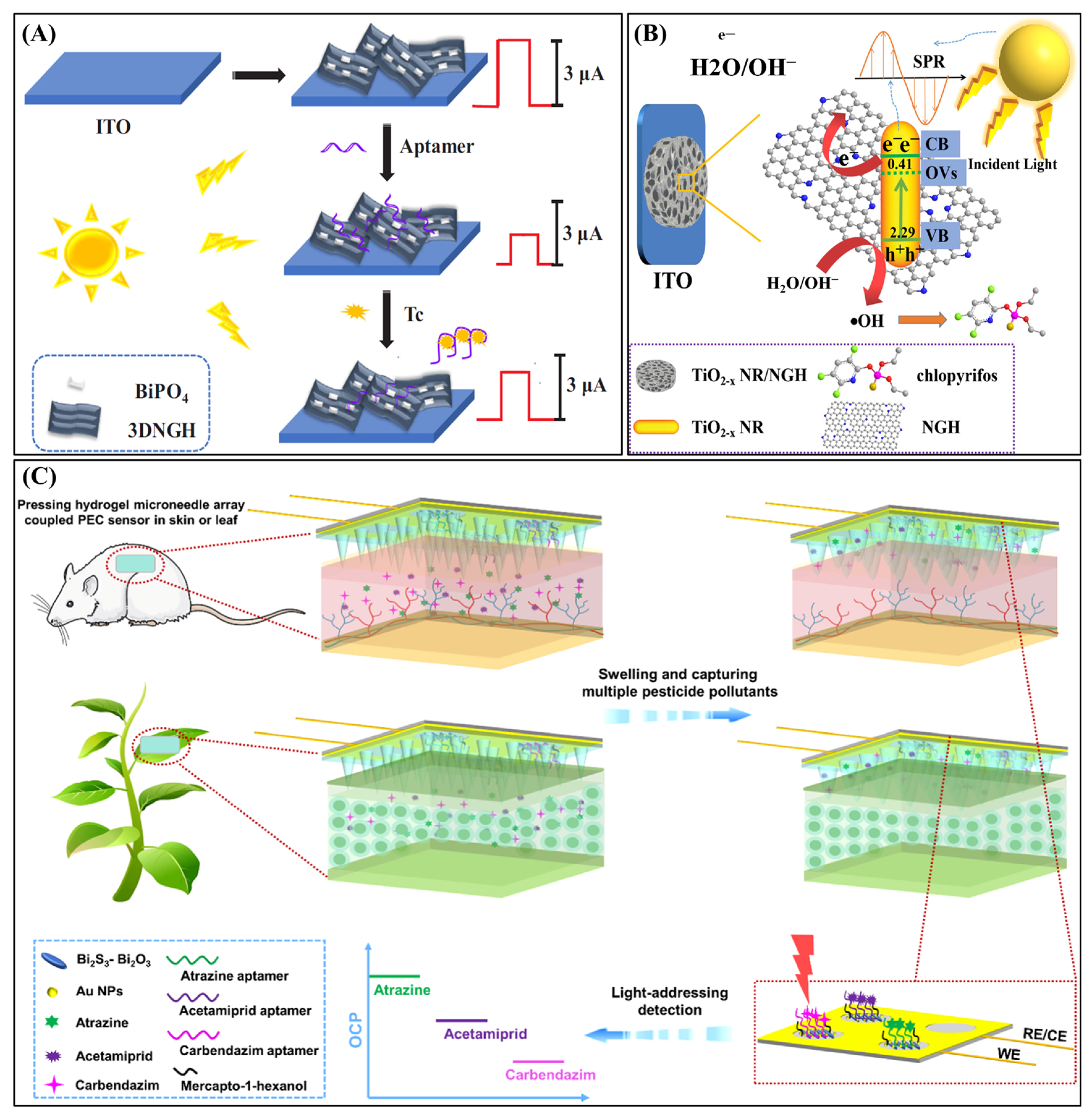
| Chlorpyrifos | 3D nitrogen-doped graphene hydrogel (NGH) | Plasmonic conductive matrix | ITO/TiO2-x | 0.05 ng∙mL−1–0.5 µg∙mL−1 | 0.017 ng∙mL−1 | Water sample | [121] |
| Pentachlorophenol (PCP) | PEGDA hydrogel coated with calcium alginate (CA) | Freestanding optical fiber matrix | PEGDA hydrogel fiber(Au@Ag NWs and PCN-224(Zn)@TiO2)/CA | PEC: 0.05–1000 ng∙mL−1; FL: 0.01 pg∙mL−1–1 μg∙mL−1 | PEC: 2.9 fg∙mL−1; FL: 0.11 fg∙mL−1 | In vivo monitoring | [150] |
| Atrazine(ATZ), Acetamiprid(ACP), Carbendazim (CBZ) | MeHA–HA Hydrogel | Swellable microneedle array | ITO/Bi2S3-Bi2O3/Au NPs-Apt/MeHA–HA Hydrogel | ATZ: 0.1 fg∙mL−1–0.1 ng∙mL−1 | ATZ: 0.029 fg∙mL−1; ACP: 5.5 fg∙mL−1; CBZ: 21 fg∙mL−1 | In vivo detection (mouse and leaves) | [129] |
| Ochratoxin A | Three-dimensional graphene hydrogel (3DGH) | Conductive scaffold | ITO/Co, N-co-doped TiO2@3DGH/PB | 1–500 ng∙mL−1 | 0.29 ng∙mL−1 | Corn juice | [132] |
| Ochratoxin A | Polyaniline hydrogel | In situ conductive PANI matrix for signal amplification | ITO/GO-MoS2-CdS/PAMAM | 0.0001–0.1 ng∙mL−1 | 0.05 pg∙mL−1 | Red wine | [112] |
| Microcystin-LR | Three-dimensional nitrogen-doped graphene hydrogel (NGH) | Conductive 3D NGH scaffold | ITO/Fe2O3/NGH/Apt | 1 pM–5 nM | 0.23 pM | Water sample | [120] |
| 4-Nitrophenol | 3D Graphene Hydrogel (GH) | 3D conductive scaffold | ITO/Bi2WO6@GH/PPy | 5.0 × 10−12–1.0 × 10−7 M | 5.78 × 10−13 M | Detection in PM2.5 | [123] |
| microRNA-141, microRNA-21 | 3D Graphene Hydrogel (3DGH) | 3D conductive scaffold | ITO/CdTe-3DGH | miRNA-141: 1.0–104 fM; miRNA-21: 1.0–105 fM | miRNA-141: 0.63 fM; miRNA-21: 0.29 fM | Serum sample | [163] |
| microRNA-155 | HA–PEI–DNA@TiO2 NPs hydrogel | Target-responsive DNA hydrogel for signal amplification | FTO/TiO2 NPs | 1.0 fM–100 pM | 0.41 fM | Cell lysates | [114] |
| microRNA-21 | DNA hydrogel | Stimuli-responsive DNA hydrogel for signal amplification | ITO/g-C3N4 & CdS | 10 aM–1 nM | 3.2 aM | Cell Lysates | [111] |
| HIgG | SA/GO-Ca2+ hydrogel | Ca2+-induced hydrogel gelation for signal modulation | ITO/CdS | 100 fg∙mL−1–100 ng∙mL−1 | 50 fg∙mL−1 | Serum sample | [130] |
| HIgG | Pt nanocube-embedded gelatin hydrogel (PGH) | Color-gated PEC signal modulation | FTO/CdIn2S4 | 10 fg∙mL−1–10 ng∙mL−1 | 10 fg∙mL−1 | Serum sample | [108] |
| CEA | Polymerized ionic liquid hydrogel | Imprinted recognition matrix and ion-conductive layer | GCE/MoSe2/HGNBs | 0.05–5.0 ng∙mL−1 | 11.2 pg∙mL−1 | Serum sample | [115] |
| cTnI | Carboxymethylated dextran | Stable support | Ti/TiO2 NTA/Au NPs | 0.22 pM–2.2 nM | 0.1 pM (2.2 pg/mL) | Human serum | [169] |
| Amyloid-β peptide (Aβ1-42) | Calcium alginate hydrogel | In Situ formation for signal-off regulation | Ti3C2@Bi2WO6 | 0.1 pg∙mL−1–100 ng∙mL−1 | 0.06 pg∙mL−1 | Artificial cerebrospinal fluid | [119] |
| HER2 | MnO2-doped/AMP supramolecular hydrogel | In Situ formation for signal-off regulation | ITO/WO3/SnIn4S8 | 0.1 pg∙mL−1–50 ng∙mL−1 | 0.037 pg∙mL−1 | Human serum | [131] |
| Thrombin | Ag/TiO2/3D nitrogen doped graphene hydrogel (3DNGH) | Conductive scaffold | ITO/Ag/TiO2/3DNGH-Apt | 0.01 pM–10 pM | 3 fM | Serum sample | [122] |
| Hyaluronidase | HA–PEI hydrogel | Target-responsive hydrogel for signal amplification | ITO/BiOBr & (crystal violet) | 0.10–120 U∙mL−1 | 0.034 U∙mL−1 | Urine sample | [110] |
| Escherichia coli | 3D graphene hydrogel loaded with carbon dots (C-dots/3DGH) | Conductive scaffold | ITO/C-dots/3DGH-Apt | 2.9–2.9 × 106 cfu∙mL−1 | 0.66 cfu∙mL−1 | Milk sample | [126] |
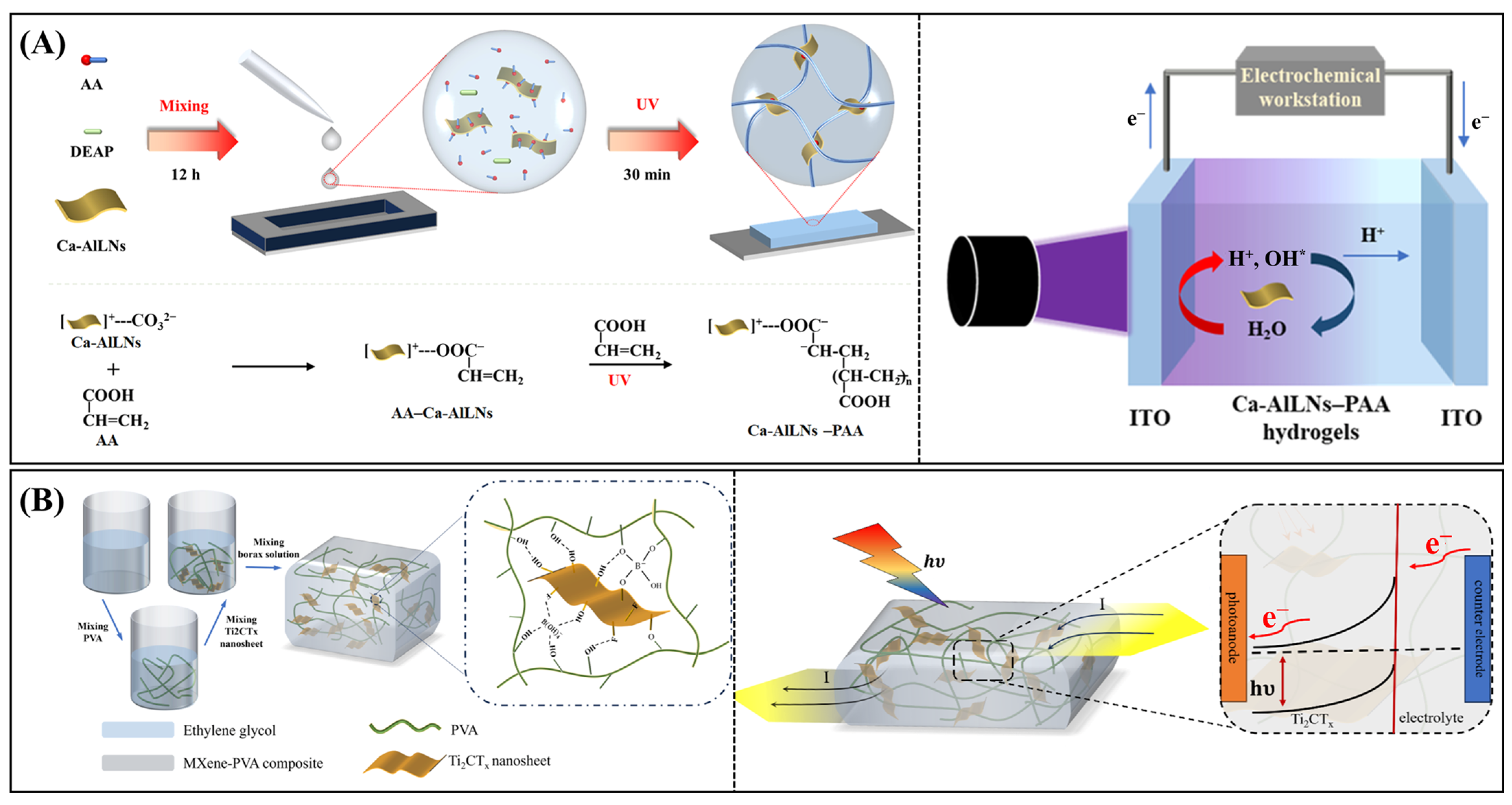
| Mechanical force, temperature, UV light | Ca-AlLNs–PAA | Multi-responsive | PEDOT/PSS/ZnO/agarose/PVA Ca-AlLNs–PAA | Temperature: 0–78 °C; UV: ≤405 nm | 1.33 × 107 Jones | Skin sensor | [174] |
| Photodetector | cellulose/CMC | Ionic conductivity and mechanical flexibility | PET/ITO/Bi2O2Se | 365–850 nm; 6.84–36.16 mW∙cm−2 | 2.44 × 108 Jones | Rotational speed measurement | [109] |
| Photodetector | PVA/Ti2CTx MXene | Electron-conductive matrix | PVA/Ti2CTx MXene | Light intensity: 60–150 mW∙cm−2 | -- | Wearable electronics | [116] |
| Photodetector | PEDOT/Alg(Fe3+) hydrogel | Electrochromic and conductive scaffold | PET/TiO2/hydrogel/Gr | 225–505 nm, 0–660 μW∙cm−2 | 9.09 × 109 Jones | Image acquisition | [127] |
| Photosystem I | (poly(vinyl)imidazole/Os(bipy)2Cl)/PEGDGE | Conductive scaffold | Au/SWCNTs/PSI-POs | Light intensity: 0.14–471 mW∙cm−2 | -- | solar-to-electricity conversion | [178] |
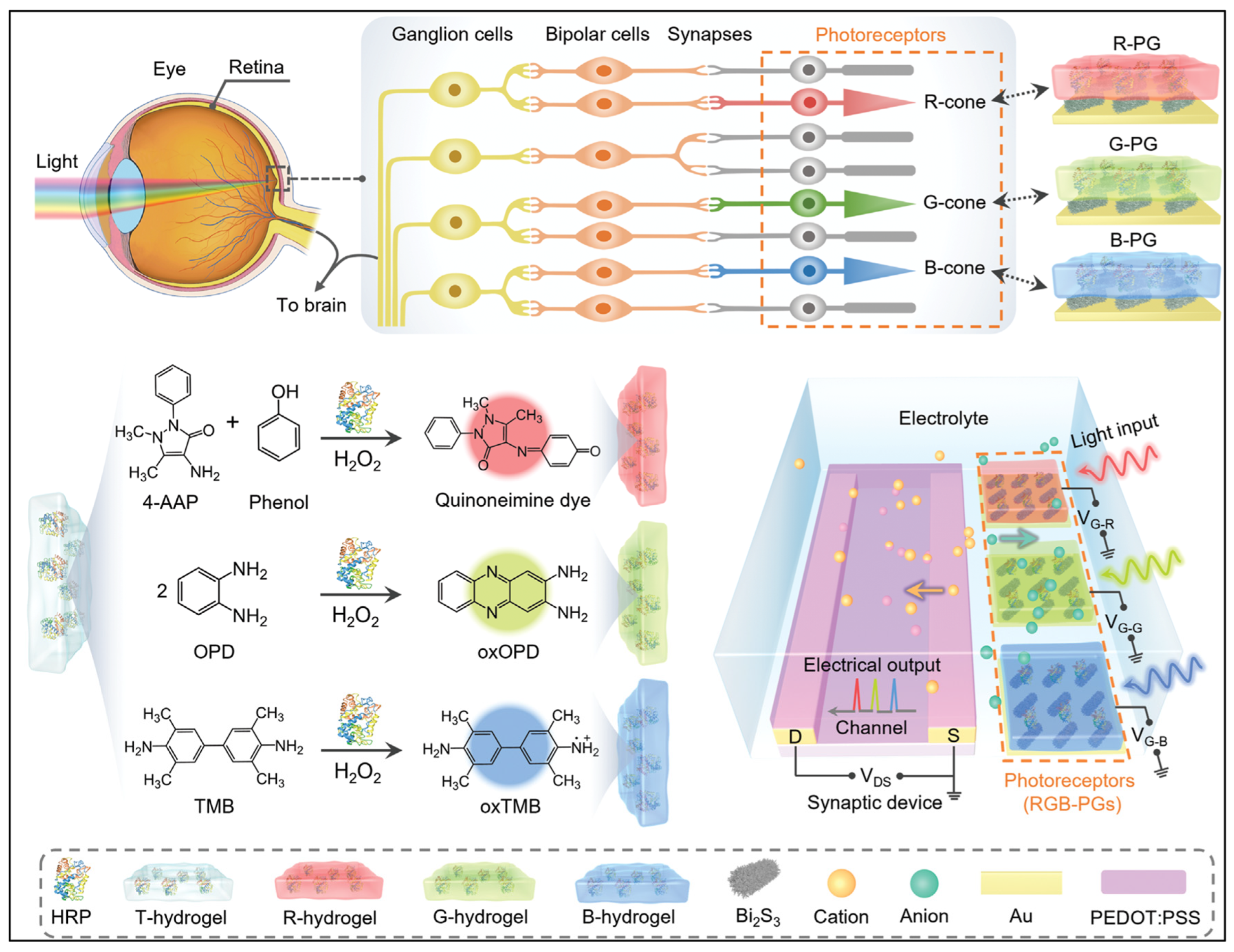
| Color-specific light signals | Gelatin hydrogels | As chromogenic reaction matrix | Au/Bi2S3 | Qualitative RGB color range, biomolecule concentration-dependent PSC modulation | Response to H2O2 down to 0.1 mM | Artificial visual synapse with RGB image | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhang, Y.; Wang, L.; Hao, Y. Recent Advances in Hydrogel-Promoted Photoelectrochemical Sensors. Biosensors 2025, 15, 524. https://doi.org/10.3390/bios15080524
Cui Y, Zhang Y, Wang L, Hao Y. Recent Advances in Hydrogel-Promoted Photoelectrochemical Sensors. Biosensors. 2025; 15(8):524. https://doi.org/10.3390/bios15080524
Chicago/Turabian StyleCui, Yali, Yanyuan Zhang, Lin Wang, and Yuanqiang Hao. 2025. "Recent Advances in Hydrogel-Promoted Photoelectrochemical Sensors" Biosensors 15, no. 8: 524. https://doi.org/10.3390/bios15080524
APA StyleCui, Y., Zhang, Y., Wang, L., & Hao, Y. (2025). Recent Advances in Hydrogel-Promoted Photoelectrochemical Sensors. Biosensors, 15(8), 524. https://doi.org/10.3390/bios15080524





