Microfluidic Platforms for Ex Vivo and In Vivo Gene Therapy
Abstract
1. Introduction
2. Microfluidic Platforms for Ex Vivo Gene Therapy
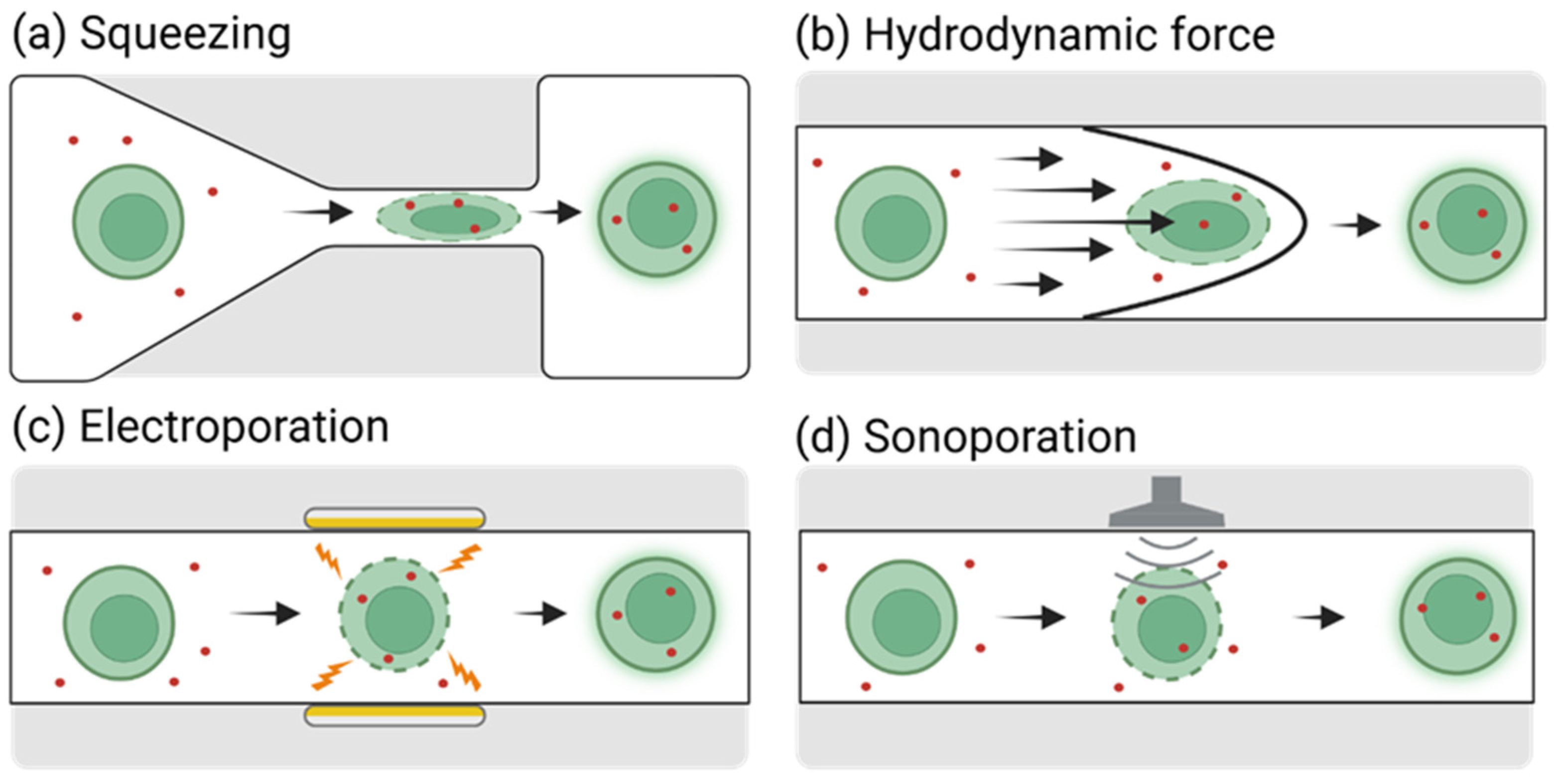
2.1. Cell Deformation-Based Gene Delivery to Ex Vivo Cells in Microfluidic Platforms
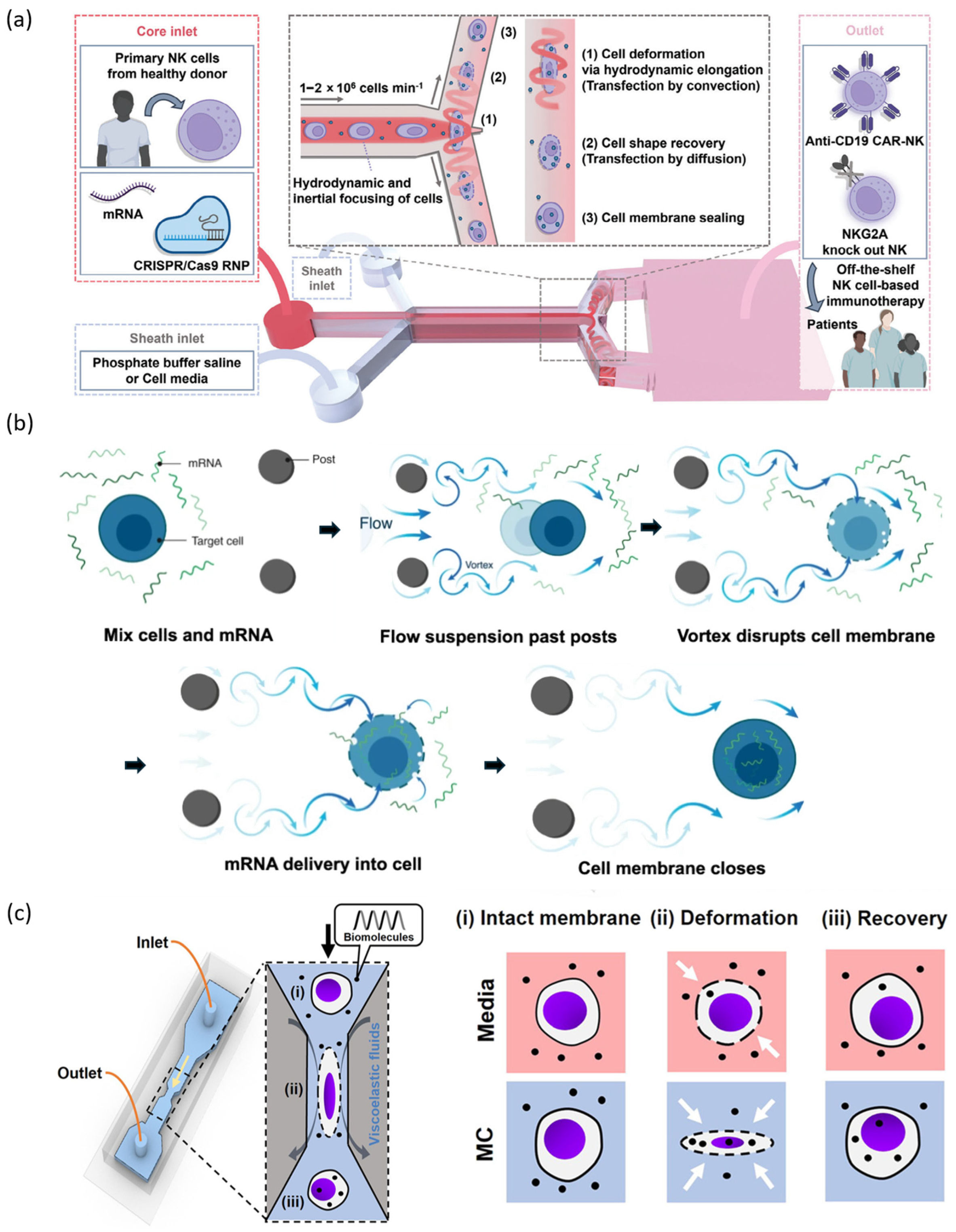
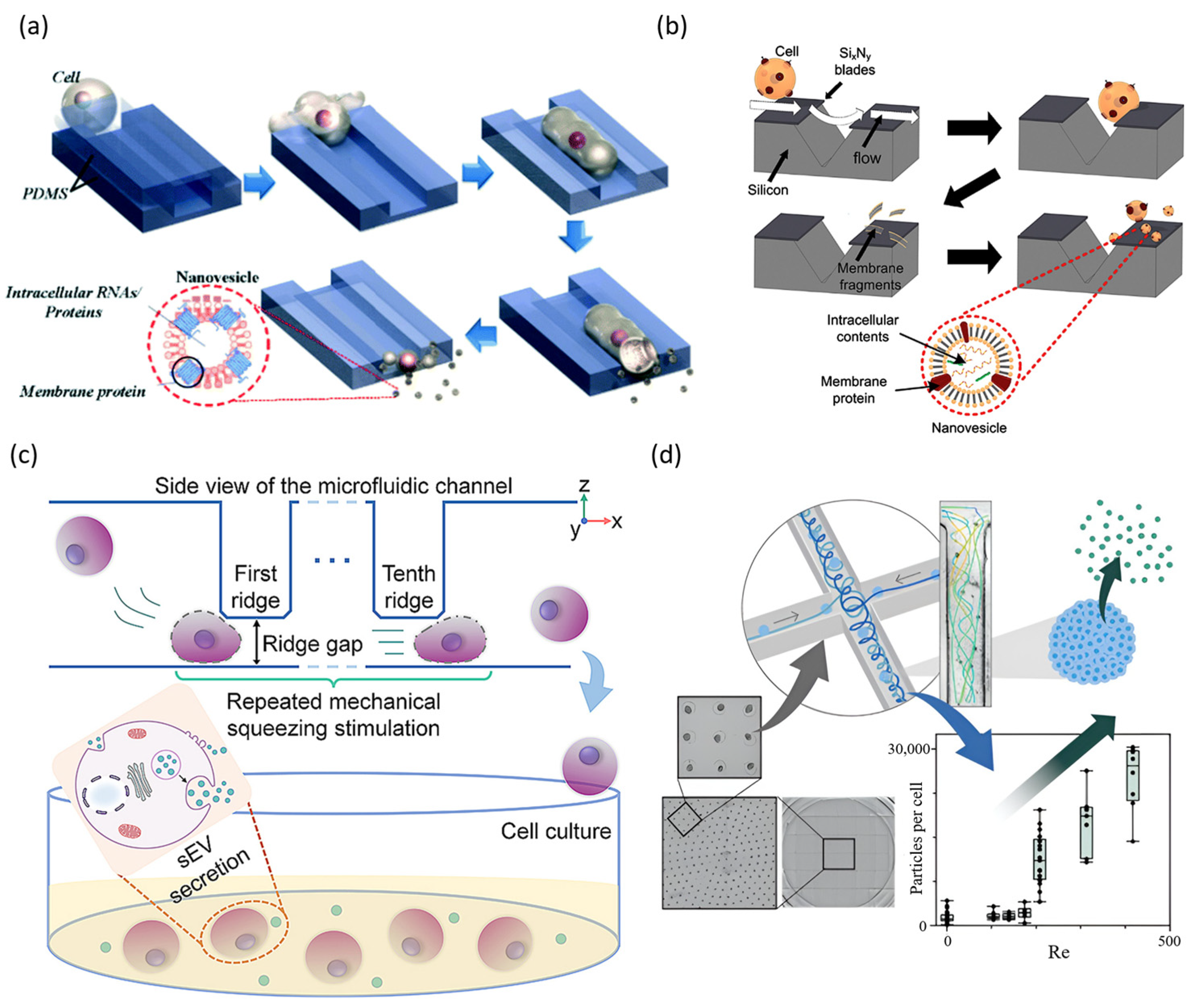
2.1.1. Mechanical Constriction and Squeezing-Based Gene Delivery
2.1.2. Hydrodynamic Flow-Based Gene Delivery
2.2. Electroporation-Based Gene Delivery to Ex Vivo Cells Using Microfluidic Platforms
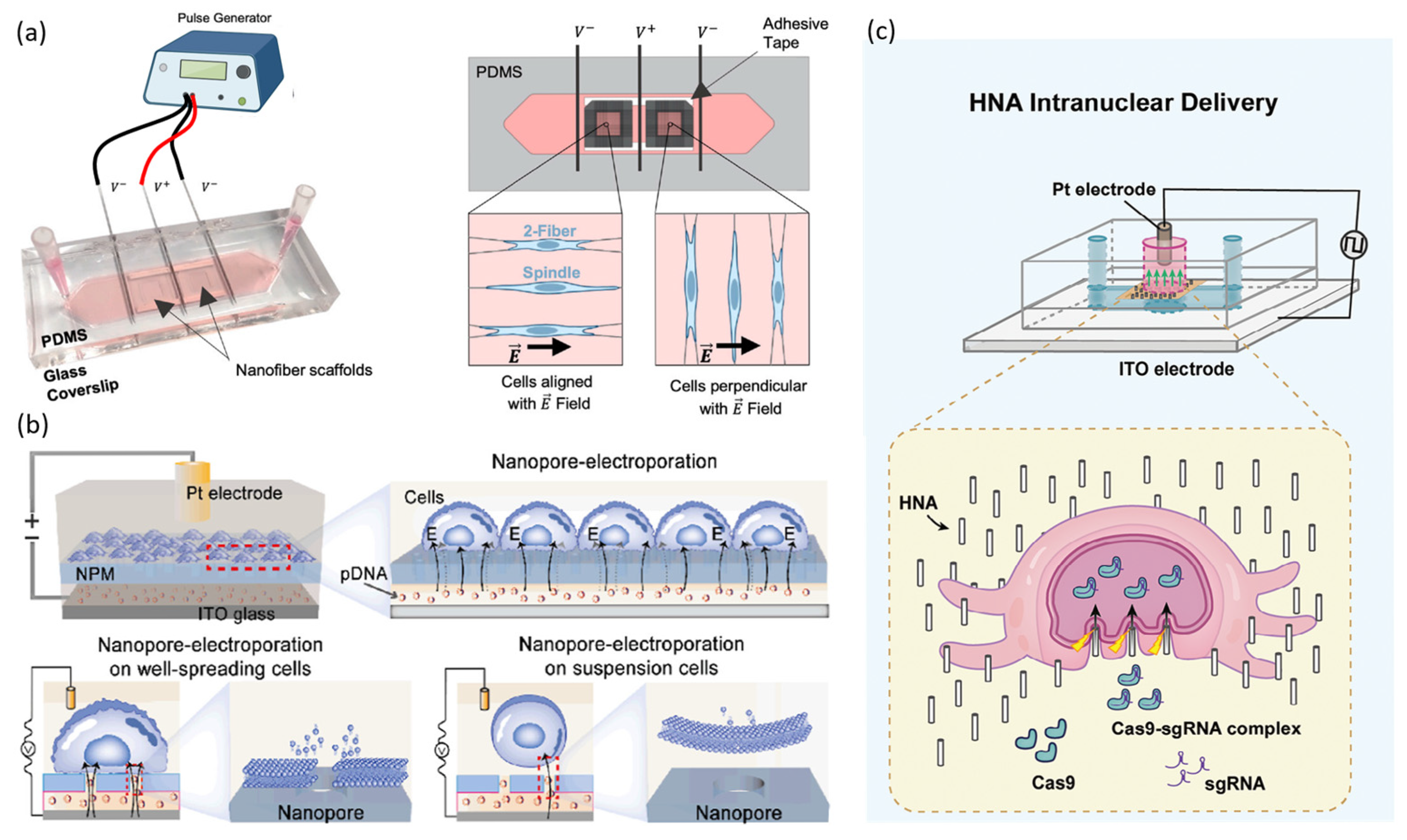
2.2.1. Static Microfluidic Electroporation Platforms
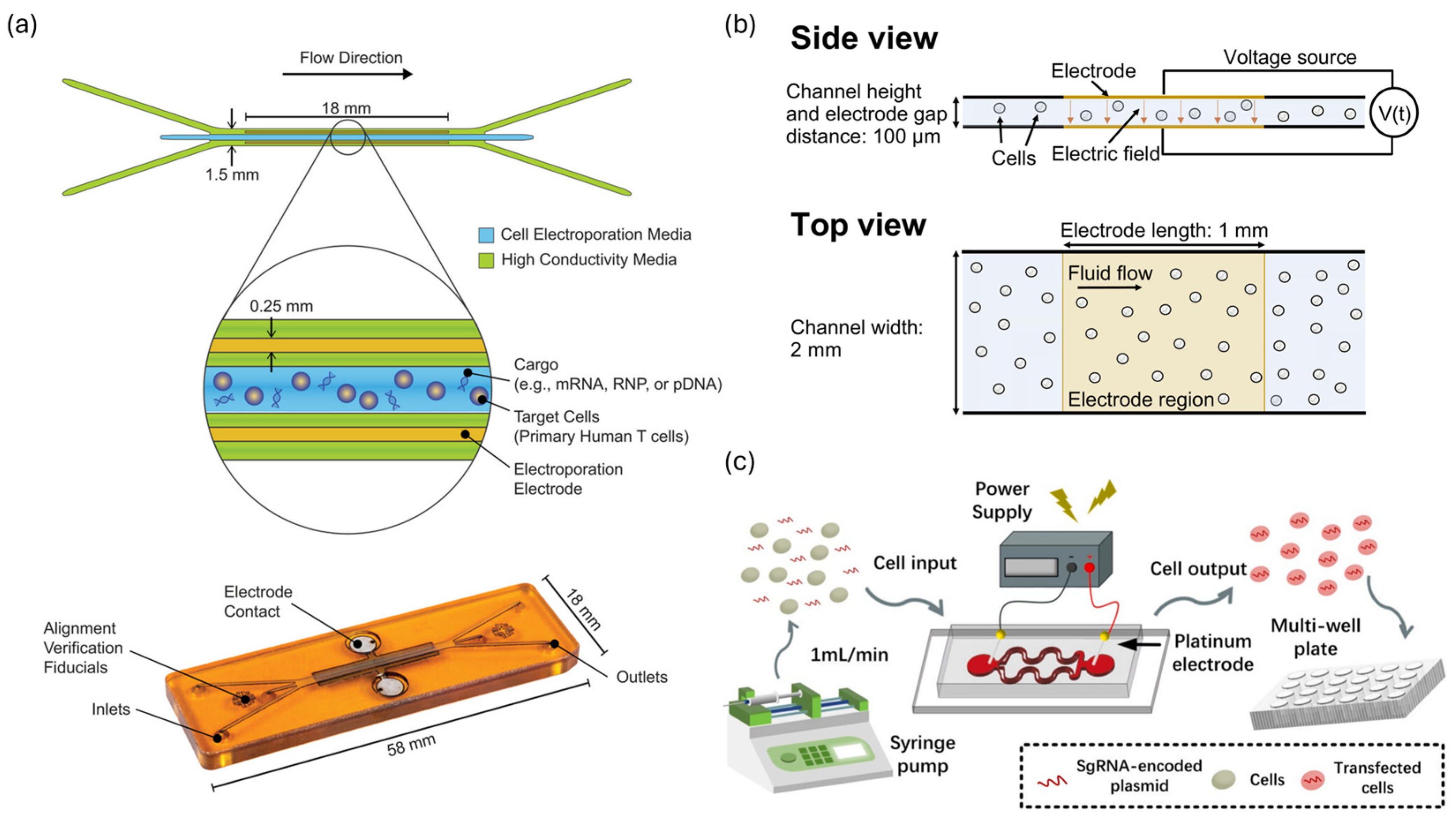
2.2.2. Continuous-Flow Microfluidic Electroporation Platforms
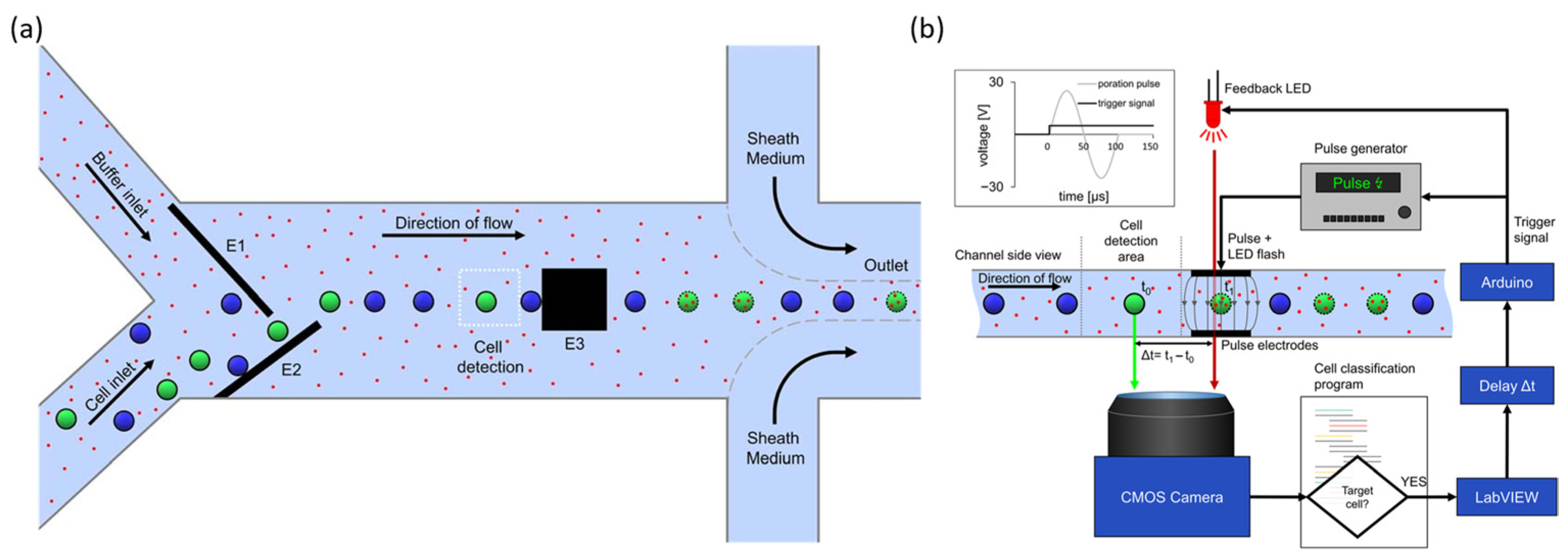
2.2.3. Target Selective Electroporation Platforms
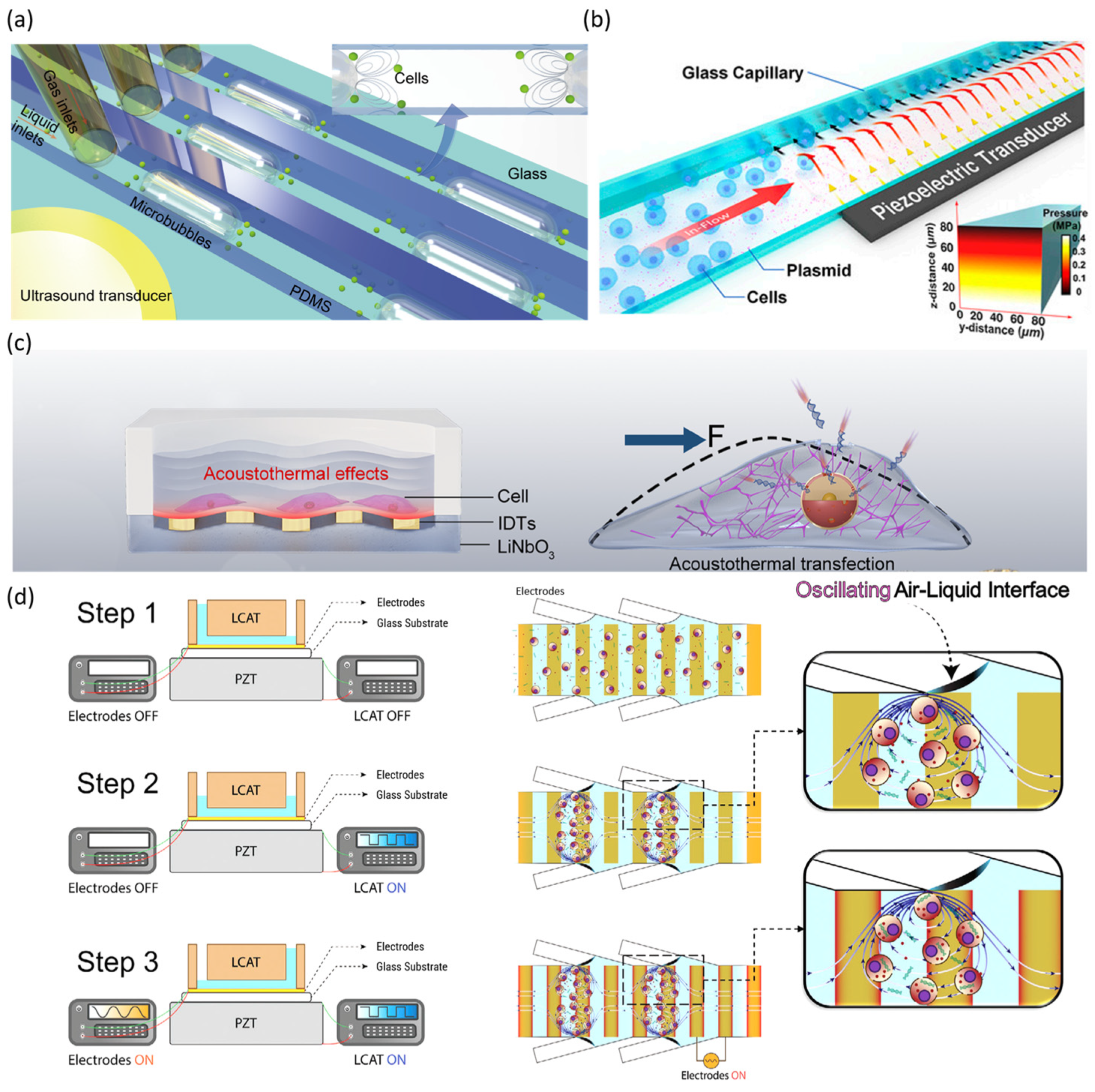
2.3. Sonoporation-Based Gene Delivery Using Microfluidic Platforms
3. Microfluidic Fabricated Nano-Carriers for In Vivo Gene Therapy
3.1. Microfluidic Platforms for Nanoparticle Synthesis
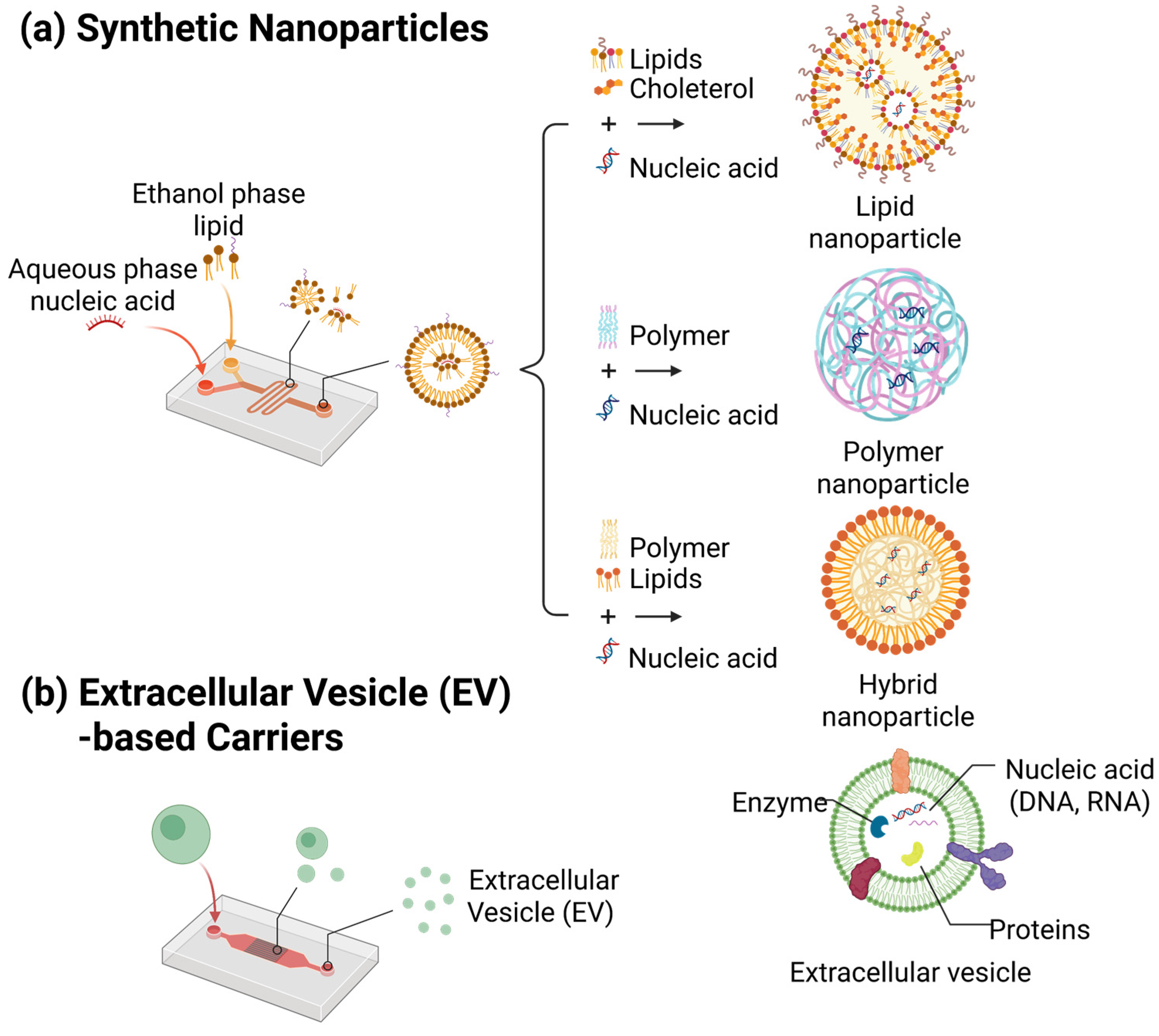
3.1.1. Types of Synthetic Nanoparticles
- Lipid Nanoparticles (LNPs)
- Polymeric Nanoparticles
- Hybrid nanoparticles
3.1.2. Key Parameters for the Fabrication of Nanoparticles
3.1.3. Optimizing Particle Size and Uniformity Control of Nanoparticles
3.1.4. Tissue-Specific Targeting Enabled by Microfluidic Platform
3.2. Extracellular Vesicles (EVs) and EV–Nanoparticle Hybridization
3.2.1. EV Production and Purification via Microfluidic Platforms
3.2.2. Gene Encapsulation in EVs via Microfluidic Platforms
3.2.3. Functional Enhancement via EV–Nanoparticle Hybridization
4. Integrated Microfluidic Strategies: From Intracellular Engineering to Therapeutic Carrier Development
4.1. Engineering of Cells for EV-Mediated Gene Therapy
4.2. Hydrogel-Integrated Stem Cell Systems for Targeted Therapeutic Release
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prazeres, D.; Monteiro, G. Plasmid biopharmaceuticals. Microbiol. Spectr. 2014, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puente, D.H.; Pérez-Trujillo, J.J.; Zavala-Flores, L.M.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Valdés, J.; Saucedo-Cárdenas, O.; Montes de Oca-Luna, R.; Loera-Arias, M.d.J. Plasmid DNA for therapeutic applications in cancer. Pharmaceutics 2022, 14, 1861. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; Dipiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Robb, G.B.; Rana, T.M. RNA helicase A interacts with RISC in human cells and functions in RISC loading. Mol. Cell 2007, 26, 523–537. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Fernández, E.F.; Gaul, R.; Vencken, S.; Glasgow, A.; Oglesby, I.K.; Hurley, K.; Hawkins, F.; Mitash, N.; Mu, F.; et al. Precise targeting of miRNA sites restores CFTR activity in CF bronchial epithelial cells. Mol. Ther. 2020, 28, 1190–1199. [Google Scholar] [CrossRef]
- Sun, Q.; Hang, M.; Guo, X.; Shao, W.; Zeng, G. Expression and significance of miRNA-21 and BTG2 in lung cancer. Tumor Biol. 2013, 34, 4017–4026. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Li, Y.-J.; Zhang, S.; Li, Z.-L.; Yue, Z.; Xie, N.; Xie, S.-Y. Regulating A549 cells growth by ASO inhibiting miRNA expression. Mol. Cell. Biochem. 2010, 339, 163–171. [Google Scholar] [CrossRef]
- Aung-Htut, M.T.; McIntosh, C.S.; Ham, K.A.; Pitout, I.L.; Flynn, L.L.; Greer, K.; Fletcher, S.; Wilton, S.D. Systematic approach to developing splice modulating antisense oligonucleotides. Int. J. Mol. Sci. 2019, 20, 5030. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef] [PubMed]
- Tomanin, R.; Scarpa, M. Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr. Gene Ther. 2004, 4, 357–372. [Google Scholar] [CrossRef]
- Cesana, D.; Ranzani, M.; Volpin, M.; Bartholomae, C.; Duros, C.; Artus, A.; Merella, S.; Benedicenti, F.; Sergi, L.S.; Sanvito, F.; et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014, 22, 774–785. [Google Scholar] [CrossRef]
- Vanderburgh, J.A.; Corso, G.T.; Levy, S.L.; Craighead, H.G. A multiplexed microfluidic continuous-flow electroporation system for efficient cell transfection. Biomed. Microdevices 2024, 26, 10. [Google Scholar] [CrossRef]
- Schmiderer, L.; Subramaniam, A.; Žemaitis, K.; Bäckström, A.; Yudovich, D.; Soboleva, S.; Galeev, R.; Prinz, C.N.; Larsson, J.; Hjort, M. Efficient and nontoxic biomolecule delivery to primary human hematopoietic stem cells using nanostraws. Proc. Natl. Acad. Sci. USA 2020, 117, 21267–21273. [Google Scholar] [CrossRef] [PubMed]
- Lissandrello, C.A.; Santos, J.A.; Hsi, P.; Welch, M.; Mott, V.L.; Kim, E.S.; Chesin, J.; Haroutunian, N.J.; Stoddard, A.G.; Czarnecki, A.; et al. High-throughput continuous-flow microfluidic electroporation of mRNA into primary human T cells for applications in cellular therapy manufacturing. Sci. Rep. 2020, 10, 18045. [Google Scholar] [CrossRef]
- Layzer, J.M.; McCaffrey, A.P.; Tanner, A.K.; Huang, Z.; Kay, M.A.; Sullenger, B.A. In vivo activity of nuclease-resistant siRNAs. RNA 2004, 10, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Querbes, W.; Alabi, C.; Eltoukhy, A.; Sarkar, S.; Zurenko, C.; Karagiannis, E.; Love, K.; Chen, D.; Zoncu, R.; et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013, 31, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Hornung, V.; Guenthner-Biller, M.; Bourquin, C.; Ablasser, A.; Schlee, M.; Uematsu, S.; Noronha, A.; Manoharan, M.; Akira, S.; de Fougerolles, A.; et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 2005, 11, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Sago, C.D.; Monaco, C.M.; Hudson, W.H.; Castro, M.G.; Rudoltz, T.G.; Kalathoor, S.; Vanover, D.A.; Santangelo, P.J.; Ahmed, R.; et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 2018, 18, 2148–2157. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics With Endogenous and Exogenous Ligand-Based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Hur, J.; Chung, A.J. Microfluidic and Nanofluidic Intracellular Delivery. Adv. Sci. 2021, 8, 2004595. [Google Scholar] [CrossRef]
- Duckert, B.; Vinkx, S.; Braeken, D.; Fauvart, M. Single-cell transfection technologies for cell therapies and gene editing. J. Control. Release 2021, 330, 963–975. [Google Scholar] [CrossRef]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Z.; Tong, L.; Wang, R.; Yao, S.; Chen, D.; Hu, H. Microfluidic Mechanoporation: Current Progress and Applications in Stem Cells. Biosensors 2024, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Harberts, J.; Assadi, A.; Chen, Y.; Spatz, J.P.; Duan, W.; Nisbet, D.R.; Voelcker, N.H.; Elnathan, R. The Roles of Micro-and Nanoscale Materials in Cell-Engineering Systems. Adv. Mater. 2024, 36, 2410908. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2022, 2, 24. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Wang, S.; Zhang, L.; Jiang, X. Microfluidics-Assembled Nanovesicles for Nucleic Acid Delivery. Acc. Chem. Res. 2025, 58, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, J.; Normak, K.; Gerlt, M.; Makasewicz, K.; Seiffert, C.; Capasso Palmiero, U.; Ye, S.; González Gómez, M.A.; Piñero, Y.; Rivas, J.; et al. Microfluidics-Driven Manufacturing and Multiscale Analytical Characterization of Nanoparticle-Vesicle Hybrids. Adv. Healthc. Mater. 2025, 14, 2403264. [Google Scholar] [CrossRef] [PubMed]
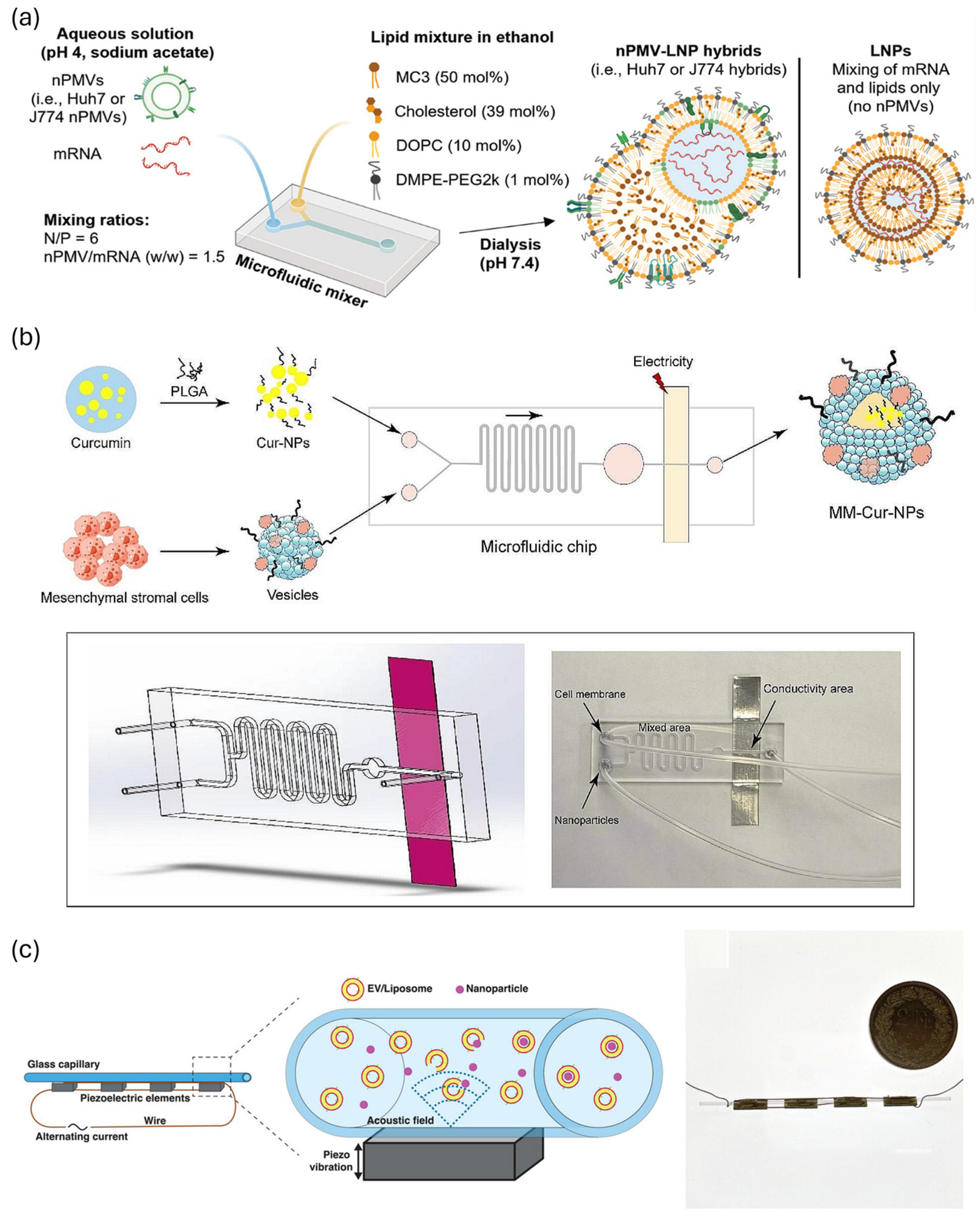
- Alter, C.L.; Lotter, C.; Puligilla, R.D.; Bolten, J.S.; Sedzicki, J.; Marchese, J.; Schittny, V.; Rucci, F.; Beverly, M.; Palivan, C.G.; et al. Nano Plasma Membrane Vesicle-Lipid Nanoparticle Hybrids for Enhanced Gene Delivery and Expression. Adv. Healthc. Mater. 2025, 14, e2401888. [Google Scholar] [CrossRef]
- Mehraji, S.; Devoe, D.L. Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities. Lab A Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Sharei, A.; Cho, N.; Mao, S.; Jackson, E.; Poceviciute, R.; Adamo, A.; Zoldan, J.; Langer, R.; Jensen, K.F. Cell Squeezing as a Robust, Microfluidic Intracellular Delivery Platform. J. Vis. Exp. 2013, 7, 50980. [Google Scholar] [CrossRef]
- Ebstrup, M.L.; Dias, C.; Heitmann, A.S.B.; Sønder, S.L.; Nylandsted, J. Actin Cytoskeletal Dynamics in Single-Cell Wound Repair. Int. J. Mol. Sci. 2021, 22, 10886. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Park, I.; Lim, K.M.; Doh, J.; Cho, S.-G.; Chung, A.J. Microfluidic Cell Stretching for Highly Effective Gene Delivery into Hard-to-Transfect Primary Cells. ACS Nano 2020, 14, 15094–15106. [Google Scholar] [CrossRef]
- Ditommaso, T.; Cole, J.M.; Cassereau, L.; Buggé, J.A.; Hanson, J.L.S.; Bridgen, D.T.; Stokes, B.D.; Loughhead, S.M.; Beutel, B.A.; Gilbert, J.B.; et al. Cell engineering with microfluidic squeezing preserves functionality of primary immune cells in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E10907–E10914. [Google Scholar] [CrossRef]
- Belling, J.N.; Heidenreich, L.K.; Tian, Z.; Mendoza, A.M.; Chiou, T.-T.; Gong, Y.; Chen, N.Y.; Young, T.D.; Wattanatorn, N.; Park, J.H.; et al. Acoustofluidic sonoporation for gene delivery to human hematopoietic stem and progenitor cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10976–10982. [Google Scholar] [CrossRef]
- Han, X.; Liu, Z.; Jo, M.C.; Zhang, K.; Li, Y.; Zeng, Z.; Li, N.; Zu, Y.; Qin, L. CRISPR-Cas9 delivery to hard-to-transfect cells via membrane deformation. Sci. Adv. 2015, 1, e1500454. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Juba, B.M.; Vazquez, M.; Kortum, S.W.; Pierce, B.S.; Pacheco, M.; Roberts, L.; Strohbach, J.W.; Jones, L.H.; et al. Microfluidic-Enabled Intracellular Delivery of Membrane Impermeable Inhibitors to Study Target Engagement in Human Primary Cells. ACS Chem. Biol. 2017, 12, 2970–2974. [Google Scholar] [CrossRef]
- Saung, M.T.; Sharei, A.; Adalsteinsson, V.A.; Cho, N.; Kamath, T.; Ruiz, C.; Kirkpatrick, J.; Patel, N.; Mino-Kenudson, M.; Thayer, S.P.; et al. A Size-Selective Intracellular Delivery Platform. Small 2016, 12, 5873–5881. [Google Scholar] [CrossRef]
- Lam, K.H.; Fernandez-Perez, A.; Schmidtke, D.W.; Munshi, N.V. Functional cargo delivery into mouse and human fibroblasts using a versatile microfluidic device. Biomed. Microdevices 2018, 20, 52. [Google Scholar] [CrossRef]
- Uvizl, A.; Goswami, R.; Gandhi, S.D.; Augsburg, M.; Buchholz, F.; Guck, J.; Mansfeld, J.; Girardo, S. Efficient and gentle delivery of molecules into cells with different elasticity via Progressive Mechanoporation. Lab A Chip 2021, 21, 2437–2452. [Google Scholar] [CrossRef]
- Alhmoud, H.; Alkhaled, M.; Kaynak, B.E.; Hanay, M.S. Leveraging the elastic deformability of polydimethylsiloxane microfluidic channels for efficient intracellular delivery. Lab A Chip 2023, 23, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, S.; Chen, C.; Zhang, Y.; Lai, S.; Gu, R.; Xie, W.; Feng, Y.; Lang, J.; Huang, J.; et al. Flexible Mechanoporation Chips for High-Throughput Intracellular Delivery Based on Controlled Pneumatic Microvalve Array. ACS Nano 2025, 19, 22017–22031. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jhita, N.; Shankles, P.; Fedanov, A.; Kramer, N.; Raikar, S.S.; Sulchek, T. Development of a microfluidic cell transfection device into gene-edited CAR T cell manufacturing workflow. Lab A Chip 2023, 23, 4804–4820. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yun, D.; Lee, J.K.; Jung, C.; Chung, A.J. Highly efficient CRISPR-mediated genome editing through microfluidic droplet cell mechanoporation. Nat. Commun. 2024, 15, 8099. [Google Scholar] [CrossRef]
- Joo, B.; Hur, J.; Kim, G.-B.; Yun, S.G.; Chung, A.J. Highly Efficient Transfection of Human Primary T Lymphocytes Using Droplet-Enabled Mechanoporation. ACS Nano 2021, 15, 12888–12898. [Google Scholar] [CrossRef]
- Hallow, D.M.; Seeger, R.A.; Kamaev, P.P.; Prado, G.R.; Laplaca, M.C.; Prausnitz, M.R. Shear-induced intracellular loading of cells with molecules by controlled microfluidics. Biotechnol. Bioeng. 2008, 99, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Sevenler, D.; Toner, M. High throughput intracellular delivery by viscoelastic mechanoporation. Nat. Commun. 2024, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Sytsma, B.J.; Allain, V.; Bourke, S.; Faizee, F.; Fathi, M.; Ferreira, L.M.R.; Brewer, W.J.; Li, L.; Pan, F.L.; Rothrock, A.G.; et al. Scalable intracellular delivery via microfluidic vortex shedding enhances the function of chimeric antigen receptor T-cells. Sci. Rep. 2025, 15, 5749. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.; Han, B.; Kim, J.; Cho, D.; Doh, J.; Chung, A.J. Advancing Allogeneic NK Cell Immunotherapy through Microfluidic Gene Delivery. Adv. Sci. 2025, 12, 2412544. [Google Scholar] [CrossRef]
- Deng, Y.; Kizer, M.; Rada, M.; Sage, J.; Wang, X.; Cheon, D.-J.; Chung, A.J. Intracellular Delivery of Nanomaterials via an Inertial Microfluidic Cell Hydroporator. Nano Lett. 2018, 18, 2705–2710. [Google Scholar] [CrossRef]
- Jarrell, J.A.; Twite, A.A.; Lau, K.H.W.J.; Kashani, M.N.; Lievano, A.A.; Acevedo, J.; Priest, C.; Nieva, J.; Gottlieb, D.; Pawell, R.S. Intracellular delivery of mRNA to human primary T cells with microfluidic vortex shedding. Sci. Rep. 2019, 9, 3214. [Google Scholar] [CrossRef]
- Jarrell, J.A.; Sytsma, B.J.; Wilson, L.H.; Pan, F.L.; Lau, K.H.W.J.; Kirby, G.T.S.; Lievano, A.A.; Pawell, R.S. Numerical optimization of microfluidic vortex shedding for genome editing T cells with Cas9. Sci. Rep. 2021, 11, 11818. [Google Scholar] [CrossRef]
- Kwon, C.; Chung, A.J. Highly efficient mRNA delivery with nonlinear microfluidic cell stretching for cellular engineering. Lab A Chip 2023, 23, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Maremonti, M.I.; Panzetta, V.; Netti, P.A.; Causa, F. HiViPore: A highly viable in-flow compression for a one-step cell mechanoporation in microfluidics to induce a free delivery of nano- macro-cargoes. J. Nanobiotechnol. 2024, 22, 441. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef]
- Park, I.; Choi, S.; Gwak, Y.; Kim, J.; Min, G.; Lim, D.; Lee, S.W. Microfluidic Electroporation Arrays for Investigating Electroporation-Induced Cellular Rupture Dynamics. Biosensors 2024, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.; Flusberg, D.A.; Hsi, P.; Haroutunian, N.J.; Santos, J.A.; Kim, E.S.; Markovic, S.; Coppeta, J.R.; Lissandrello, C.A.; Balestrini, J.L.; et al. High-Throughput CRISPR/Cas9 Mediated Gene Editing of Primary Human T Cells in a Microfluidic Device for Cellular Therapy Manufacturing. Adv. Mater. Technol. 2023, 8, 2300275. [Google Scholar] [CrossRef]
- Pfisterer, F.; Godino, N.; Gerling, T.; Kirschbaum, M. Continuous microfluidic flow-through protocol for selective and image-activated electroporation of single cells. RSC Adv. 2023, 13, 19379–19387. [Google Scholar] [CrossRef]
- Ye, Y.; Luan, X.; Zhang, L.; Zhao, W.; Cheng, J.; Li, M.; Zhao, Y.; Huang, C. Single-Cell Electroporation with Real-Time Impedance Assessment Using a Constriction Microchannel. Micromachines 2020, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, X.; Chiang, C.-L.; Ma, Y.; Cheng, J.; Bertani, P.; Lu, W.; Lee, L.J. Joule heating and electroosmotic flow in cellular micro/nano electroporation. Lab A Chip 2024, 24, 819–831. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, J.; Wang, J.; Xie, X.; Chen, H.-J.; He, G. Interrogation on the Cellular Nano-Interface and Biosafety of Repeated Nano-Electroporation by Nanostraw System. Biosensors 2022, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Patino, C.A.; Mukherjee, P.; Berns, E.J.; Moully, E.H.; Stan, L.; Mrksich, M.; Espinosa, H.D. High-Throughput Microfluidics Platform for Intracellular Delivery and Sampling of Biomolecules from Live Cells. ACS Nano 2022, 16, 7937–7946. [Google Scholar] [CrossRef] [PubMed]
- Jacobs Iv, E.J.; Graybill, P.M.; Jana, A.; Agashe, A.; Nain, A.S.; Davalos, R.V. Engineering high post-electroporation viabilities and transfection efficiencies for elongated cells on suspended nanofiber networks. Bioelectrochemistry 2023, 152, 108415. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; He, M.; Chen, J.; Huang, S.; Liu, Z.; Yao, C.; Chen, H.-j.; Xie, X.; Wang, J. Nanopore Electroporation Device for DNA Transfection into Various Spreading and Nonadherent Cell Types. ACS Appl. Mater. Interfaces 2023, 15, 50015–50033. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Liu, J.; Yang, H.; Huang, Z.; Deng, C.; Li, Y.; Shang, L.; Wang, X.; Xie, X.; et al. Nanoneedle Array-Electroporation Facilitates Intranuclear Ribonucleoprotein Delivery and High Throughput Gene Editing. Adv. Healthc. Mater. 2024, 13, 2400645. [Google Scholar] [CrossRef]
- Wang, S.; Lee, L.J. Micro-/nanofluidics based cell electroporation. Biomicrofluidics 2013, 7, 011301. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Lin, Y.; Zhang, Y.; Zhang, A.; Wu, X.; Jiyu, X.; Li, Q.; Wei, Z. Expanding the cell quantity of CRISPR/Cas9 gene editing by continuous microfluidic electroporation chip. Bioelectrochemistry 2025, 161, 108840. [Google Scholar] [CrossRef]
- Song, K.-H.; Fan, A.C.; Brlansky, J.T.; Trudeau, T.; Gutierrez-Hartmann, A.; Calvisi, M.L.; Borden, M.A. High Efficiency Molecular Delivery with Sequential Low-Energy Sonoporation Bursts. Theranostics 2015, 5, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Blöck, J.; Li, H.; Collado-Lara, G.; Kooiman, K.; Rix, A.; Chen, J.; Hark, C.; Radermacher, H.; Porte, C.; Kiessling, F. The Compression-Dominated Ultrasound Response of Poly(n-butyl cyanoacrylate) Hard-Shelled Microbubbles Induces Significant Sonoporation and Sonopermeation Effects In Vitro. ACS Appl. Bio Mater. 2025, 8, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lin, L.; Liu, Q.; Wu, S.; Chen, J.; Zhu, R.; You, H.; Sun, C. Three-dimensional array of microbubbles sonoporation of cells in microfluidics. Front. Bioeng. Biotechnol. 2024, 12, 1353333. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, X.; Wang, Y.; Zhang, W.; Zhou, W.; Cai, F.; Li, F.; Wu, J.; Xu, L.; Niu, L.; et al. Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array. Adv. Sci. 2019, 6, 1900557. [Google Scholar] [CrossRef]
- Huang, G.; Sun, C.; Wei, X.; Tang, S.; Wu, S.; He, W.; Dai, P.; Cai, Y.; You, H. Ultrasound-Mediated Plasmid Delivery by Stable Oscillation of Linear Array Microbubbles in Microfluidic Channels. IEEE Sens. J. 2025, 25, 144–151. [Google Scholar] [CrossRef]
- Liu, X.; Rong, N.; Tian, Z.; Rich, J.; Niu, L.; Li, P.; Huang, L.; Dong, Y.; Zhou, W.; Zhang, P.; et al. Acoustothermal transfection for cell therapy. Sci. Adv. 2024, 10, eadk1855. [Google Scholar] [CrossRef]
- Aghaamoo, M.; Chen, Y.H.; Li, X.; Garg, N.; Jiang, R.; Yun, J.T.H.; Lee, A.P. High-Throughput and Dosage-Controlled Intracellular Delivery of Large Cargos by an Acoustic-Electric Micro-Vortices Platform. Adv. Sci. 2022, 9, 2102021. [Google Scholar] [CrossRef]
- Strelkova Petersen, D.M.; Chaudhary, N.; Arral, M.L.; Weiss, R.M.; Whitehead, K.A. The mixing method used to formulate lipid nanoparticles affects mRNA delivery efficacy and organ tropism. Eur. J. Pharm. Biopharm. 2023, 192, 126–135. [Google Scholar] [CrossRef]
- Sousa De Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Zöller, K.; Haddadzadegan, S.; Lindner, S.; Veider, F.; Bernkop-Schnürch, A. Design of charge converting lipid nanoparticles via a microfluidic coating technique. Drug Deliv. Transl. Res. 2024, 14, 3173–3185. [Google Scholar] [CrossRef]
- Pershina, A.G.; Demin, A.M.; Perekucha, N.A.; Brikunova, O.Y.; Efimova, L.V.; Nevskaya, K.V.; Vakhrushev, A.V.; Zgoda, V.G.; Uimin, M.A.; Minin, A.S.; et al. Peptide ligands on the PEGylated nanoparticle surface and human serum composition are key factors for the interaction between immune cells and nanoparticles. Coll. Surf. B Biointerfaces 2023, 221, 112981. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Thouvenot, E.; Charnay, L.; Burshtein, N.; Guigner, J.M.; Dec, L.; Loew, D.; Silva, A.K.A.; Lindner, A.; Wilhelm, C. High-Yield Bioproduction of Extracellular Vesicles from Stem Cell Spheroids via Millifluidic Vortex Transport. Adv. Mater. 2024, 37, e2412498. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Frolova, L.; Li, I. Targeting Capabilities of Native and Bioengineered Extracellular Vesicles for Drug Delivery. Bioengineering 2022, 9, 496. [Google Scholar] [CrossRef]
- Cholkar, S.S.; Gawade, A.R.; Kuchekar, A.B. Lipid nanoparticles: Key facilitators of mrna vaccine development. Biosci. Biotechnol. Res. Asia 2022, 19, 199–213. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ishihara, H. Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid nanoparticle–mRNA formulations for therapeutic applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; Van Der Meel, R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef]
- McLendon, J.M.; Joshi, S.R.; Sparks, J.; Matar, M.; Fewell, J.G.; Abe, K.; Oka, M.; McMurtry, I.F.; Gerthoffer, W.T. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J. Control. Release 2015, 210, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Shalosky, E.M.; Barnes, R.; Shukla, V.C.; Xu, S.; Ballinger, M.N.; Farkas, L.; Lee, R.J.; Ghadiali, S.N.; Englert, J.A. Macrophage-targeted lipid nanoparticle delivery of microRNA-146a to mitigate hemorrhagic shock-induced acute respiratory distress syndrome. ACS Nano 2023, 17, 16539–16552. [Google Scholar] [CrossRef]
- Yang, L.; Ma, F.; Liu, F.; Chen, J.; Zhao, X.; Xu, Q. Efficient delivery of antisense oligonucleotides using bioreducible lipid nanoparticles in vitro and in vivo. Mol. Ther. Nucleic Acids 2020, 19, 1357–1367. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, L.; Satterlee, A.; Huang, L. Delivery of oligonucleotides with lipid nanoparticles. Adv. Drug Deliv. Rev. 2015, 87, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Myhre, J.L.; Chen, S.; Tam, Y.Y.C.; Danescu, A.; Richman, J.M.; Cullis, P.R. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1377–1387. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J. Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Feng, Q.; Wang, N.; Chen, Z.; Huang, Y.; Zheng, W.; Jiang, X. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017, 9, e441. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Wu, D.-C.; Li, Z.-J.; Chen, G.-Q. Polymer nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 299–323. [Google Scholar]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.Ø.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef]
- Maeki, M.; Uno, S.; Sugiura, K.; Sato, Y.; Fujioka, Y.; Ishida, A.; Ohba, Y.; Harashima, H.; Tokeshi, M. Development of polymer–lipid hybrid nanoparticles for large-sized plasmid DNA transfection. ACS Appl. Mater. Interfaces 2023, 16, 2110–2119. [Google Scholar] [CrossRef]
- Danaeifar, M.; Negahdari, B.; Eslam, H.M.; Zare, H.; Ghanaat, M.; Koushali, S.S.; Malekshahi, Z.V. Polymeric nanoparticles for DNA vaccine-based cancer immunotherapy: A review. Biotechnol. Lett. 2023, 45, 1053–1072. [Google Scholar] [CrossRef]
- Patil, Y.; Panyam, J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int. J. Pharm. 2009, 367, 195–203. [Google Scholar] [CrossRef]
- Suberi, A.; Grun, M.K.; Mao, T.; Israelow, B.; Reschke, M.; Grundler, J.; Akhtar, L.; Lee, T.; Shin, K.; Piotrowski-Daspit, A.S.; et al. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci. Transl. Med. 2023, 15, eabq0603. [Google Scholar] [CrossRef]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-generation vaccines: Nanoparticle-mediated DNA and mRNA delivery. Adv. Healthc. Mater. 2021, 10, 2001812. [Google Scholar] [CrossRef]
- Ashok, B.; Peppas, N.A.; Wechsler, M.E. Lipid-and polymer-based nanoparticle systems for the delivery of CRISPR/Cas9. J. Drug Deliv. Sci. Technol. 2021, 65, 102728. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J.; Park, J.H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Zhao, Y. Engineered hybrid nanoparticles for on-demand diagnostics and therapeutics. Acc. Chem. Res. 2015, 48, 3016–3025. [Google Scholar] [CrossRef]
- He, C.; Lu, J.; Lin, W. Hybrid nanoparticles for combination therapy of cancer. J. Control. Release 2015, 219, 224–236. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid− polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Cheng, S.-H.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; Lo, L.-W. Theranostic applications of mesoporous silica nanoparticles and their organic/inorganic hybrids. J. Mater. Chem. B 2013, 1, 3128–3135. [Google Scholar] [CrossRef]
- Shetty, A.; Chandra, S. Inorganic hybrid nanoparticles in cancer theranostics: Understanding their combinations for better clinical translation. Mater. Today Chem. 2020, 18, 100381. [Google Scholar] [CrossRef]
- Haque, S.T.; Chowdhury, E.H. Recent progress in delivery of therapeutic and imaging agents utilizing organic-inorganic hybrid nanoparticles. Curr. Drug Deliv. 2018, 15, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Hengelbrock, A.; Schmidt, A.; Strube, J. Formulation of Nucleic Acids by Encapsulation in Lipid Nanoparticles for Continuous Production of mRNA. Processes 2023, 11, 1718. [Google Scholar] [CrossRef]
- Young, H.; He, Y.; Joo, B.; Ferguson, S.; Demko, A.; Butterfield, S.K.; Lowe, J.; Mjema, N.F.; Sheth, V.; Whitehead, L.; et al. Toward the Scalable, Rapid, Reproducible, and Cost-Effective Synthesis of Personalized Nanomedicines at the Point of Care. Nano Lett. 2024, 24, 920–928. [Google Scholar] [CrossRef]
- Singh, V.; Chernatynskaya, A.; Qi, L.; Chuang, H.-Y.; Cole, T.; Jeyalatha, V.M.; Bhargava, L.; Yeudall, W.A.; Farkas, L.; Yang, H. Liposomes-Encapsulating Double-Stranded Nucleic Acid (Poly I:C) for Head and Neck Cancer Treatment. ACS Pharmacol. Transl. Sci. 2024, 7, 1612–1623. [Google Scholar] [CrossRef]
- Lin, W.-Z.S.; Bostic, W.K.V.; Malmstadt, N. 3D-printed microfluidic device for high-throughput production of lipid nanoparticles incorporating SARS-CoV-2 spike protein mRNA. Lab A Chip 2024, 24, 162–170. [Google Scholar] [CrossRef]
- Ali-Jerman, H.; Al-Quraishi, Z.; Muglikar, A.; Perrie, Y.; Tate, R.; Mullin, M.; McNeill, G.; Mackenzie, G.; Dufès, C. Enhancing Transfection Efficacy in Glioma Cells: A Comparison of Microfluidic versus Manual Polypropylenimine Dendriplex Formation. Int. J. Nanomed. 2024, 19, 12189–12203. [Google Scholar] [CrossRef]
- Bovone, G.; Cousin, L.; Steiner, F.; Tibbitt, M.W. Solvent Controls Nanoparticle Size during Nanoprecipitation by Limiting Block Copolymer Assembly. Macromolecules 2022, 55, 8040–8048. [Google Scholar] [CrossRef]
- Santhanes, D.; Zhang, H.; Wilkins, A.; Aitken, R.J.; Gannon, A.-L.; Liang, M. Precise control of microfluidic flow conditions is critical for harnessing the in vitro transfection capability of pDNA-loaded lipid-Eudragit nanoparticles. Drug Deliv. Transl. Res. 2024, 14, 3055–3069. [Google Scholar] [CrossRef]
- Hong, J.; Lee, S.; Park, H.; Ahn, D.; Lee, J.M.; Choe, H.; Kim, D.; Kim, J.H.; Chon, C.H. Size-Controllable and Monodispersed Lipid Nanoparticle Production with High mRNA Delivery Efficiency Using 3D-Printed Ring Micromixers. ACS Appl. Mater. Interfaces 2024, 16, 46044–46052. [Google Scholar] [CrossRef]
- Palanki, R.; Han, E.L.; Murray, A.M.; Maganti, R.; Tang, S.; Swingle, K.L.; Kim, D.; Yamagata, H.; Safford, H.C.; Mrksich, K.; et al. Optimized microfluidic formulation and organic excipients for improved lipid nanoparticle mediated genome editing. Lab A Chip 2024, 24, 3790–3801. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sasaki, K.; Sato, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Three-dimensional, symmetrically assembled microfluidic device for lipid nanoparticle production. RSC Adv. 2021, 11, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Jang, S.; Park, D.; Bae, N.H.; Han, C.S.; Ryu, S.; Lim, E.-K.; Lee, K.G. Automated Microfluidic Systems Facilitating the Scalable and Reliable Production of Lipid Nanoparticles for Gene Delivery. BioChip J. 2025, 19, 79–90. [Google Scholar] [CrossRef]
- Maharjan, R.; Hada, S.; Lee, J.E.; Han, H.K.; Kim, K.H.; Seo, H.J.; Foged, C.; Jeong, S.H. Comparative study of lipid nanoparticle-based mRNA vaccine bioprocess with machine learning and combinatorial artificial neural network-design of experiment approach. Int. J. Pharm. 2023, 640, 123012. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Kim, K.H.; Lee, K.; Han, H.K.; Jeong, S.H. Machine learning-driven optimization of mRNA-lipid nanoparticle vaccine quality with XGBoost/Bayesian method and ensemble model approaches. J. Pharm. Anal. 2024, 14, 100996. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Han, X.; Mukalel, A.J.; El-Mayta, R.; Thatte, A.S.; Wu, J.; Padilla, M.S.; Alameh, M.-G.; Srikumar, N.; Lee, D.; et al. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proc. Natl. Acad. Sci. USA 2023, 120, e2303567120. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, Y.; Zhao, X.; Wang, Y.; Zhang, Z.; Zhang, X.; Li, J.; Zheng, H.; Zhang, Y.; Shi, H.; et al. Pathologic Tissue Injury and Inflammation in Mice Immunized with Plasmid DNA-Encapsulated DOTAP-Based Lipid Nanoparticles. Bioconjug. Chem. 2024, 35, 2015–2026. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef]
- Naidu, G.S.; Yong, S.B.; Ramishetti, S.; Rampado, R.; Sharma, P.; Ezra, A.; Goldsmith, M.; Hazan-Halevy, I.; Chatterjee, S.; Aitha, A.; et al. A Combinatorial Library of Lipid Nanoparticles for Cell Type-Specific mRNA Delivery. Adv. Sci. 2023, 10, 2301929. [Google Scholar] [CrossRef]
- Kim, J.; Jozić, A.; Bloom, E.; Jones, B.; Marra, M.; Murthy, N.T.V.; Eygeris, Y.; Sahay, G. Microfluidic Platform Enables Shearless Aerosolization of Lipid Nanoparticles for mRNA Inhalation. ACS Nano 2024, 18, 11335–11348. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Parada, N.; Romero-Trujillo, A.; Georges, N.; Alcayaga-Miranda, F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J. Adv. Res. 2021, 31, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.-U.; Allen, T.; Shen, D.; et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y. Separation and aggregation of extracellular vesicles by microfluidics. Biomed. Microdevices 2025, 27, 31. [Google Scholar] [CrossRef]
- Abreu, C.M.; Costa-Silva, B.; Reis, R.L.; Kundu, S.C.; Caballero, D. Microfluidic platforms for extracellular vesicle isolation, analysis and therapy in cancer. Lab A Chip 2022, 22, 1093–1125. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z.; Chen, Y.; Chang, Y.; Yang, M.; Zhong, W. Enhancing Analysis of Extracellular Vesicles by Microfluidics. Anal. Chem. 2025, 97, 6922–6937. [Google Scholar] [CrossRef]
- Liew, W.J.M.; Alkaff, S.A.; Leong, S.Y.; Yee, M.Z.L.; Hou, H.W.; Czarny, B. Cell Membrane- and Extracellular Vesicle-Coated Chitosan Methacrylate-Tripolyphosphate Nanoparticles for RNA Delivery. Int. J. Mol. Sci. 2024, 25, 13724. [Google Scholar] [CrossRef]
- Jo, W.; Jeong, D.; Kim, J.; Cho, S.; Jang, S.C.; Han, C.; Kang, J.Y.; Gho, Y.S.; Park, J. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab A Chip 2014, 14, 1261–1269. [Google Scholar] [CrossRef]
- Yoon, J.; Jo, W.; Jeong, D.; Kim, J.; Jeong, H.; Park, J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials 2015, 59, 12–20. [Google Scholar] [CrossRef]
- Hao, R.; Hu, S.; Zhang, H.; Chen, X.; Yu, Z.; Ren, J.; Guo, H.; Yang, H. Mechanical stimulation on a microfluidic device to highly enhance small extracellular vesicle secretion of mesenchymal stem cells. Mater. Today Bio 2023, 18, 100527. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
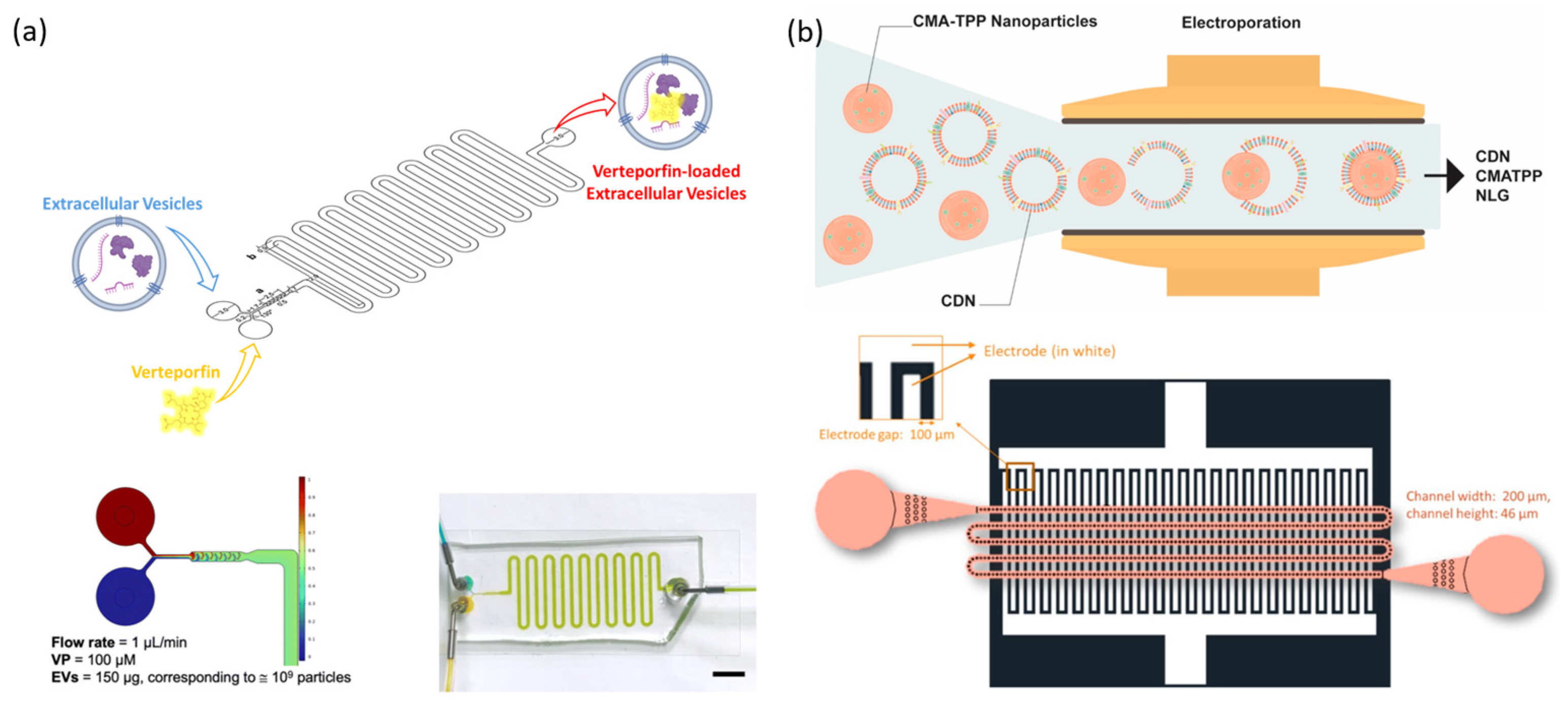
- Piunti, C.; Micheli, S.; Giancaterino, S.; Fusco, P.; Boi, C.; Cimetta, E. Microfluidic loading of verteporfin into extracellular vesicles for neuroblastoma therapy. Lab A Chip 2025, 25, 1718–1727. [Google Scholar] [CrossRef]
- Itakura, S.; Shohji, A.; Amagai, S.; Kitamura, M.; Takayama, K.; Sugibayashi, K.; Todo, H. Gene knockdown in HaCaT cells by small interfering RNAs entrapped in grapefruit-derived extracellular vesicles using a microfluidic device. Sci. Rep. 2023, 13, 3102. [Google Scholar] [CrossRef]
- Lei, T.; Li, C.; Liu, Y.; Cui, Z.; Deng, S.; Cao, J.; Yang, H.; Chen, P. Microfluidics-enabled mesenchymal stem cell derived Neuron like cell membrane coated nanoparticles inhibit inflammation and apoptosis for Parkinson’s Disease. J. Nanobiotechnol. 2024, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Pareja Tello, R.; Lamparelli, E.P.; Ciardulli, M.C.; Hirvonen, J.; Barreto, G.; Mafulli, N.; Della Porta, G.; Santos, H.A. Hybrid lipid nanoparticles derived from human mesenchymal stem cell extracellular vesicles by microfluidic sonication for collagen I mRNA delivery to human tendon progenitor stem cells. Biomater. Sci. 2025, 13, 2066–2081. [Google Scholar] [CrossRef]
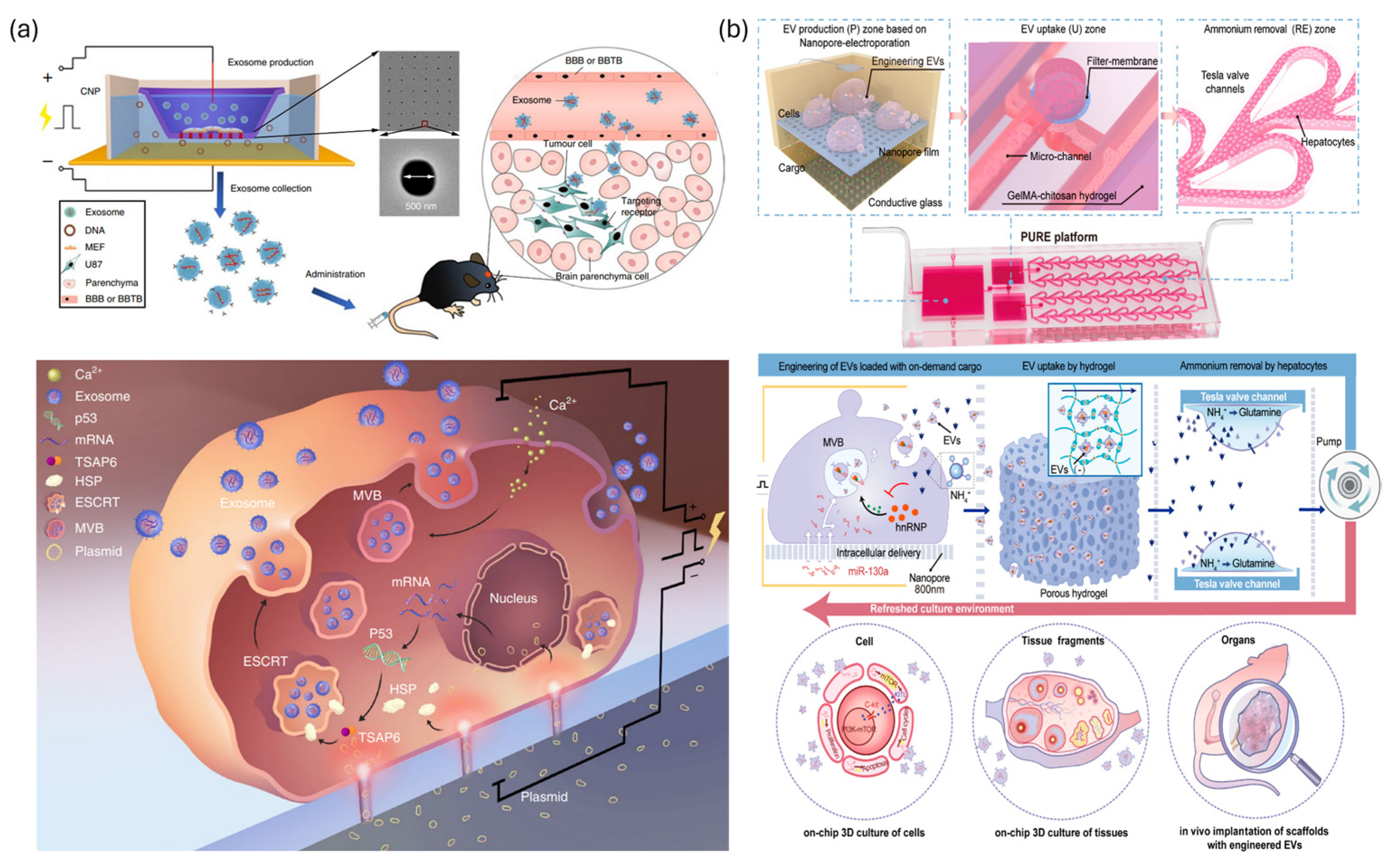
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, K.; Huang, Z.; Wang, Y.; Xiao, A.; Jiang, X.; Liu, F.; Wang, Z.; Sun, H.; Hu, Y.; et al. Efficient, High-Quality Engineering of Therapeutic Extracellular Vesicles on an Integrated Nanoplatform. ACS Nano 2024, 18, 32421–32437. [Google Scholar] [CrossRef]
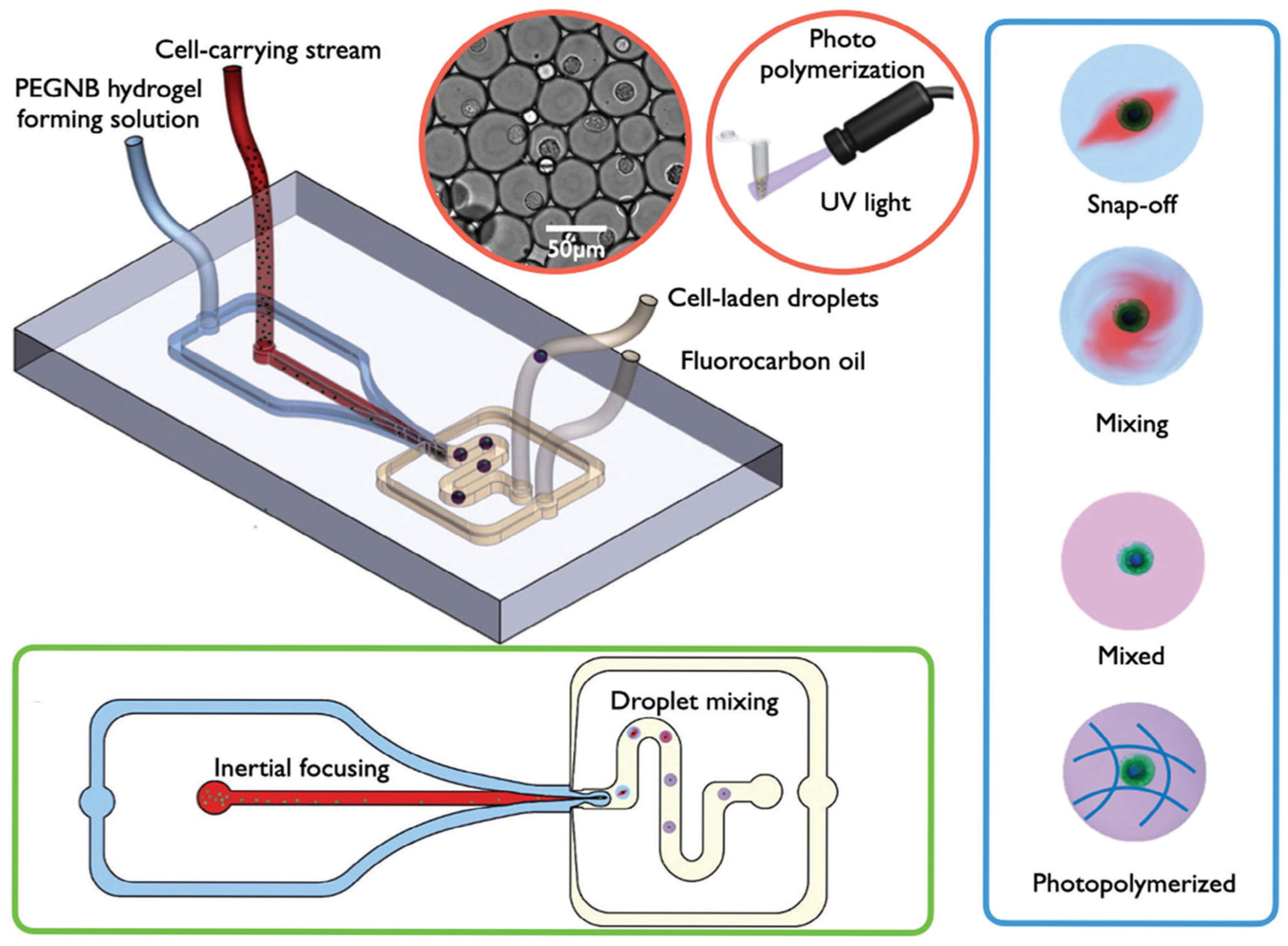
- Si, H.; Chen, Y.; Jiang, K.; Ma, K.; Ramsey, E.; Oakey, J.; Sun, M.; Jiang, Z. Deterministic Single-Cell Encapsulation in PEG Norbornene Microgels for Promoting Anti-Inflammatory Response and Therapeutic Delivery of Mesenchymal Stromal Cells. Adv. Healthc. Mater. 2024, 13, 2304386. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.O.; Theruvath, A.J.; Nejadnik, H.; Liu, A.; Xing, L.; Sulchek, T.; Daldrup-Link, H.E.; Pratx, G. Mechanoporation enables rapid and efficient radiolabeling of stem cells for PET imaging. Sci. Rep. 2022, 12, 2955. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Hou, Y.; Liang, L.; Wu, J.; Fan, K.; Lian, L.; Qiu, J.; Miao, Y.; Ravanbakhsh, H.; Xu, M.; et al. Microgels for Cell Delivery in Tissue Engineering and Regenerative Medicine. Nano Micro Lett. 2024, 16, 218. [Google Scholar] [CrossRef]
- Fang, A.; Wang, Y.; Guan, N.; Zuo, Y.; Lin, L.; Guo, B.; Mo, A.; Wu, Y.; Lin, X.; Cai, W.; et al. Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury. Nat. Commun. 2023, 14, 4011. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Fritz, Z.; Novik, E.M.; Yarmush, G.M.; Schloss, R.S.; Zahn, J.D.; Yarmush, M.L. Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification. Technology 2018, 6, 1–23. [Google Scholar] [CrossRef]
- Kerk, Y.J.; Jameel, A.; Xing, X.H.; Zhang, C. Recent advances of integrated microfluidic suspension cell culture system. Eng. Biol. 2021, 5, 81–97. [Google Scholar] [CrossRef]
- Kundacina, I.; Kundacina, O.; Miskovic, D.; Radonic, V. Advancing microfluidic design with machine learning: A Bayesian optimization approach. Lab A Chip 2025, 25, 657–672. [Google Scholar] [CrossRef]
- Sato, S.; Sano, S.; Muto, H.; Kubara, K.; Kondo, K.; Miyazaki, T.; Suzuki, Y.; Uemoto, Y.; Ukai, K. Understanding the Manufacturing Process of Lipid Nanoparticles for mRNA Delivery Using Machine Learning. Chem. Pharm. Bull. 2024, 72, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y. Enhancing single-cell biology through advanced AI-powered microfluidics. Biomicrofluidics 2023, 17, 051301. [Google Scholar] [CrossRef]
- Liang, X.; Gupta, D.; Xie, J.; Van Wonterghem, E.; Van Hoecke, L.; Hean, J.; Niu, Z.; Ghaeidamini, M.; Wiklander, O.P.B.; Zheng, W.; et al. Engineering of extracellular vesicles for efficient intracellular delivery of multimodal therapeutics including genome editors. Nat. Commun. 2025, 16, 4028. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, S.; Lee, H.; Yu, D.; Jeon, T.-J.; Kim, S.M.; Ryu, H. Microfluidic Platforms for Ex Vivo and In Vivo Gene Therapy. Biosensors 2025, 15, 504. https://doi.org/10.3390/bios15080504
Kwak S, Lee H, Yu D, Jeon T-J, Kim SM, Ryu H. Microfluidic Platforms for Ex Vivo and In Vivo Gene Therapy. Biosensors. 2025; 15(8):504. https://doi.org/10.3390/bios15080504
Chicago/Turabian StyleKwak, Sungjun, Hyojeong Lee, Dongjun Yu, Tae-Joon Jeon, Sun Min Kim, and Hyunil Ryu. 2025. "Microfluidic Platforms for Ex Vivo and In Vivo Gene Therapy" Biosensors 15, no. 8: 504. https://doi.org/10.3390/bios15080504
APA StyleKwak, S., Lee, H., Yu, D., Jeon, T.-J., Kim, S. M., & Ryu, H. (2025). Microfluidic Platforms for Ex Vivo and In Vivo Gene Therapy. Biosensors, 15(8), 504. https://doi.org/10.3390/bios15080504






