A Sensitive Sandwich-Type Electrochemical Immunosensor for Carbohydrate Antigen 19-9 Based on Covalent Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
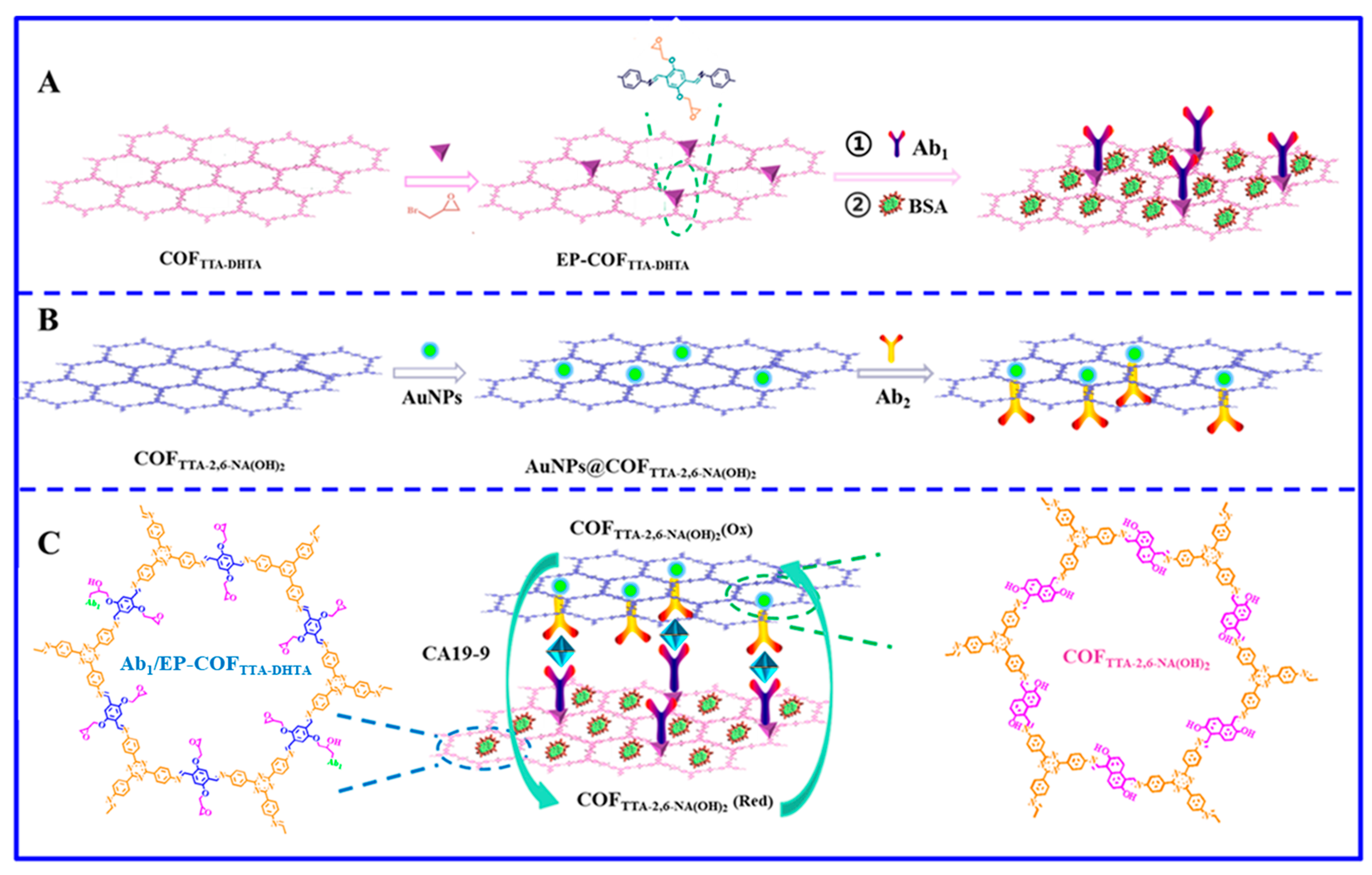
2.1. Synthesis of COFTTA-DHTA and COFTTA-2,6-NA(OH)2
2.2. Synthesis of EP-COFTTA-DHTA
2.3. Construction of Ab2/AuNPs@COFTTA-2,6-NA(OH)2 Signal Probe
2.4. Detection of CA 19-9
3. Results and Discussion
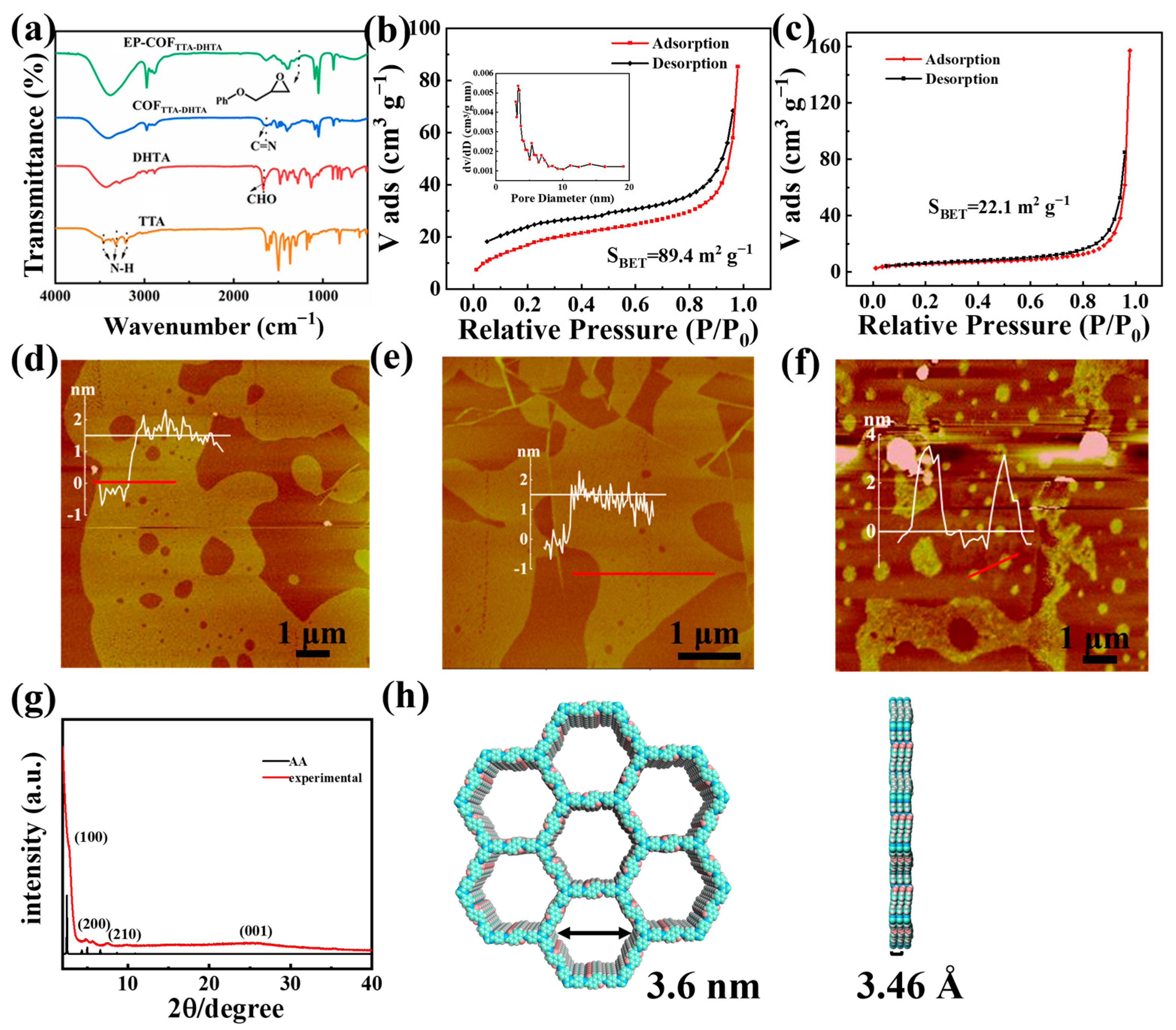
3.1. Characterization of Ab1/EP-COFTTA-DHTA
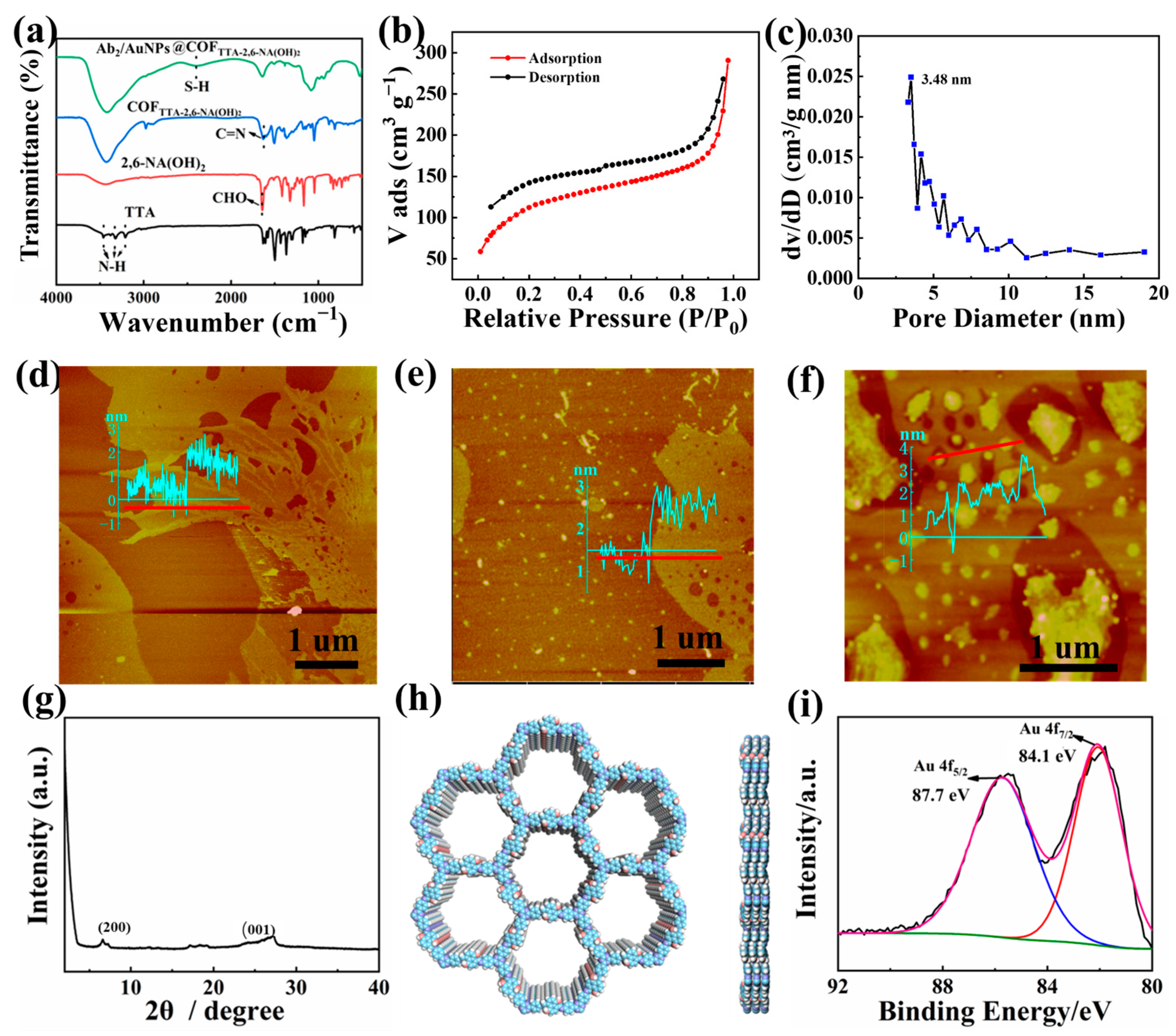
3.2. Characterization of Ab2/AuNPs@COFTTA-2,6-NA(OH)2
3.3. Electrochemical Behaviors
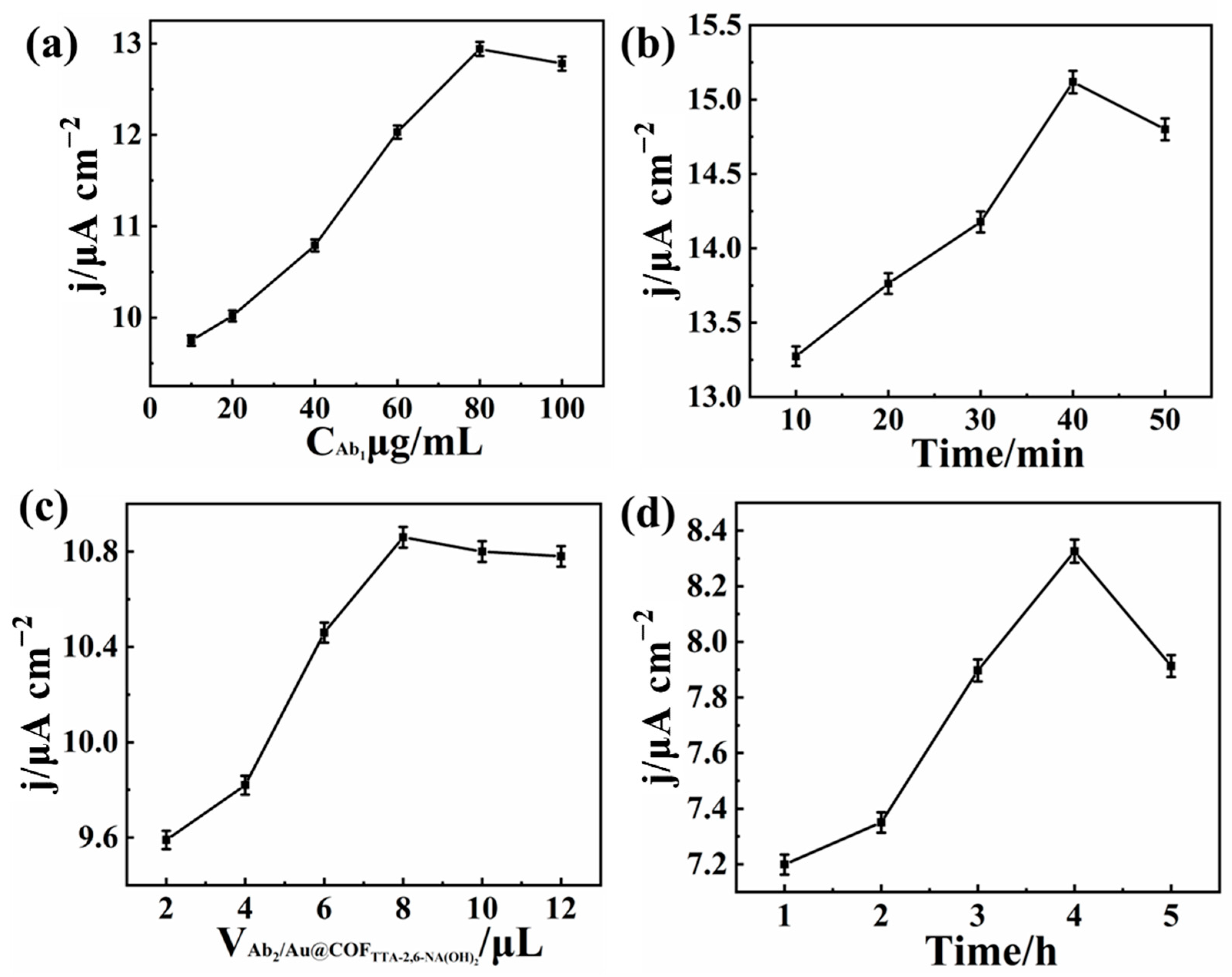
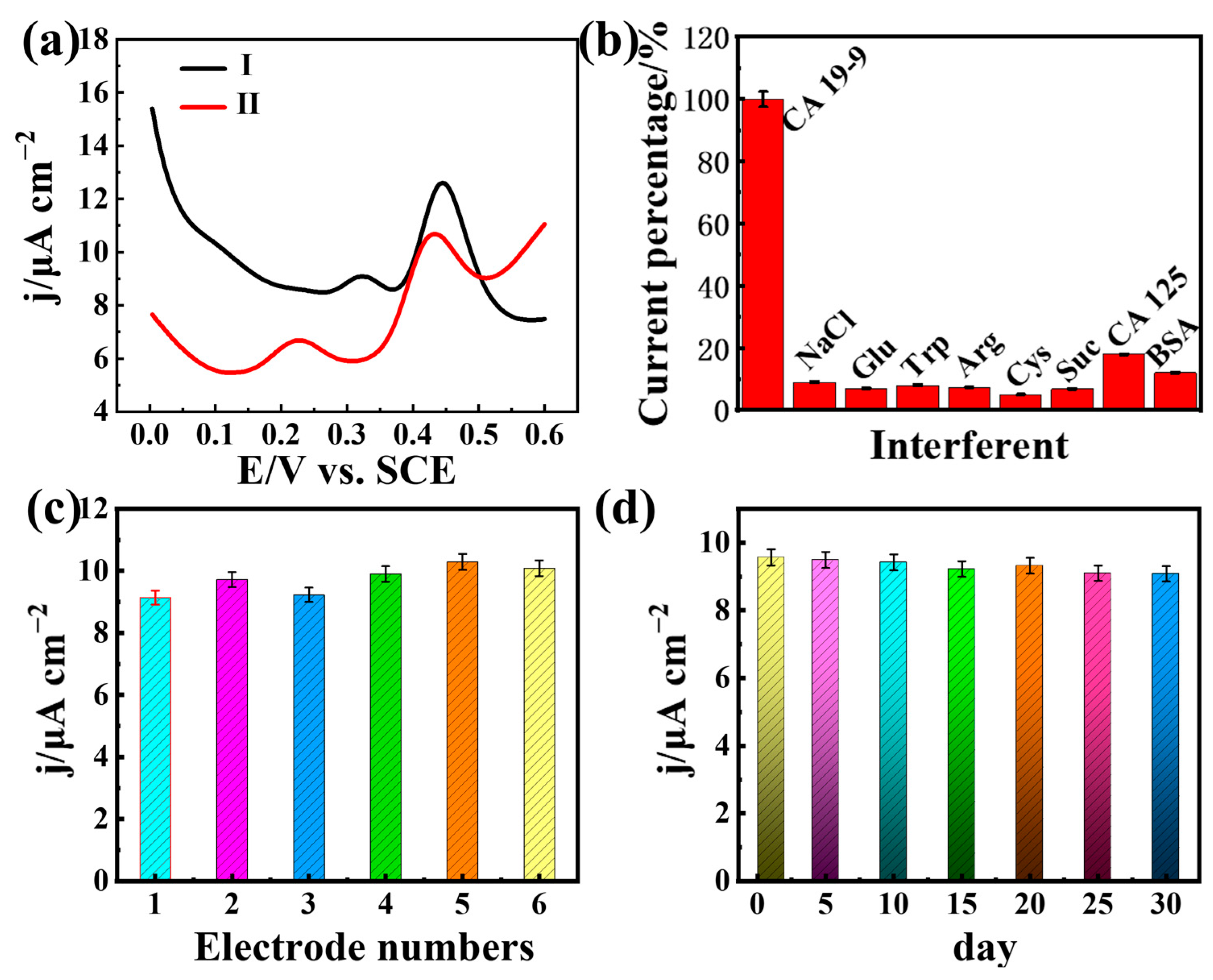
3.4. Electrochemical Detection CA 19-9 Based on AuNPs@COFTTA-2,6-NA(OH)2/Ab2/CA 19-9/Ab1/EP-COFTTA-DHTA/GCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Hu, M.; Hu, B.; Guo, C.; Song, Y.; Jia, Q.; He, L.; Zhang, Z.; Fang, S. Bimetallic cerium and ferric oxides nanoparticles embedded within mesoporous carbon matrix: Electrochemical immunosensor for sensitive detection of carbohydrate antigen 19-9. Biosens. Bioelectron. 2019, 135, 22–29. [Google Scholar] [CrossRef]
- An, Y.; Song, L.; Chen, X.; Ni, C.; Mao, K.; Zhu, L.; Gu, Y.; Miao, Y.; Song, B.; Ma, H. 3D Biomimetic hydrangea-like biOCl and PtNi nanocube-based electrochemical immunosensor for quantitative detection of CA19-9. J. Electrochem. Soc. 2022, 169, 056520. [Google Scholar] [CrossRef]
- Chang, J.; Lv, W.; Wu, J.; Li, H.; Li, F. Simultaneous photoelectrochemical detection of dual microRNAs by capturing CdS quantum dots and methylene blue based on target-initiated strand displaced amplification. Chin. Chem. Lett. 2021, 32, 775–778. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Gao, X.; Ge, L.; Sun, X.; Li, F. A universal paper-based electrochemical sensor for zero-background assay of diverse biomarkers. ACS Appl. Mater. Interfaces 2019, 11, 15381–15388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiang, J.; Cheng, H.; Liu, X.; Li, F. Flexible photoelectrochemical biosensor for ultrasensitive microRNA detection based on concatenated multiplex signal amplification. Biosens. Bioelectron. 2021, 194, 113581. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, C.; Luo, Y.; Pan, C.; Li, J. Electrochemical sensor for the simultaneous detection of CA72-4 and CA19-9 tumor markers using dual recognition via glycosyl imprinting and lectin-specific binding for accurate diagnosis of gastric cancer. Biosens. Bioelectron. 2022, 216, 114672. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Li, C.; Yang, J.; Liu, D.; Wang, H.; Xu, R.; Zhang, Y.; Wei, Q. An ultrasensitive split-type electrochemical immunosensor based on controlled-release strategy for detection of CA19-9. Biosens. Bioelectron. 2023, 227, 115180. [Google Scholar] [CrossRef]
- Li, H.; Shao, M.; Fang, J.; Li, Y.; Sun, X.; Ren, X.; Wei, Q.; Ju, H.; Ma, H. Metal-Organic framework incorporated luminescent PTCA combined with novel co-reactant accelerator for ultra-sensitive electrochemiluminescence detection of CA 19-9. Chem. Eng. J. 2024, 495, 153315. [Google Scholar] [CrossRef]
- Mo, G.; He, X.; Qin, D.; Meng, S.; Wu, Y.; Deng, B. Spatially-resolved dual-potential sandwich electrochemiluminescence immunosensor for the simultaneous determination of carbohydrate antigen 19-9 and carbohydrate antigen 24-2. Biosens. Bioelectron. 2021, 178, 113024. [Google Scholar] [CrossRef]
- Lee, H.S.; Jang, C.Y.; Kim, S.A.; Park, S.B.; Jung, D.E.; Kim, B.O.; Kim, H.Y.; Chung, M.J.; Park, J.Y.; Bang, S. Combined use of CEMIP and CA 19-9 enhances diagnostic accuracy for pancreatic cancer. Sci. Rep. 2018, 8, 3383. [Google Scholar] [CrossRef]
- Satake, K.; Takeuchi, T. Comparison of CA19-9 with other tumor markers in the diagnosis of cancer of the pancreas. Pancreas 1994, 9, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Ilie-Mihai, R.-M.; Stefan-van Staden, R.-I.; van Staden, J.K.F. Progress in electroanalysis of p53, CEA, and CA19-9. J. Electrochem. Soc. 2022, 169, 037518. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhang, Y.; Wang, P.; Qiu, B.; Guo, L.; Lin, Z.; Yang, H.-H. Exonuclease-catalyzed target recycling amplification and immobilization-free electrochemical aptasensor. Anal. Chem. 2015, 87, 11826–11831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Sha, F.; Xie, H.; Son, F.A.; Kim, K.S.; Gong, W.; Su, S.; Ma, K.; Wang, X.; Wang, X.; Farha, O.K. Rationally tailored mesoporous hosts for optimal protein encapsulation. J. Am. Chem. Soc. 2023, 145, 16383–16390. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Zhou, B.; Ian, H.; Shao, H. Advanced sensitivity amplification strategies for voltammetric immunosensors of tumor marker: State of the art. Biosens. Bioelectron. 2021, 178, 113021. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Wang, Z.; Newbigging, A.M.; Reid, M.S.; Li, X.-F.; Le, X.C. Aptamers facilitating amplified detection of biomolecules. Anal. Chem. 2015, 87, 274–292. [Google Scholar] [CrossRef]
- Gu, C.; Kong, X.; Liu, X.; Gai, P.; Li, F. Enzymatic biofuel-cell-based self-powered biosensor integrated with DNA amplification strategy for ultrasensitive detection of single-nucleotide polymorphism. Anal. Chem. 2019, 91, 8697–8704. [Google Scholar] [CrossRef]
- Ning, S.; Zhou, M.; Liu, C.; Waterhouse, G.I.; Dong, J.; Ai, S. Ultrasensitive electrochemical immunosensor for avian leukosis virus detection based on a β-cyclodextrin-nanogold-ferrocene host-guest label for signal amplification. Anal. Chim. Acta 2019, 1062, 87–93. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Song, Y.; Liu, F.; Deng, D.; Zhu, X.; He, H.; Yan, X.; Luo, L. A sandwich-type electrochemical immunosensor based on spherical nucleic acids-templated Ag nanoclusters for ultrasensitive detection of tumor biomarker. Biosens. Bioelectron. 2023, 223, 115029. [Google Scholar] [CrossRef]
- Liang, H.; Luo, Y.; Xiao, Y.; Xiong, J.; Chen, R.; Song, Y.; Wang, L. Immunosensing of neuron-specific enolase based on dual signal amplification strategy via electrocatalytic oxygen reduction by iron-porphyrin covalent organic framework. Chem. Eng. J. 2023, 460, 141740. [Google Scholar] [CrossRef]
- Naberezhnyi, D.; Park, S.; Li, W.; Westphal, M.; Feng, X.; Dong, R.; Dementyev, P. Mass transfer in boronate ester 2D COF single crystals. Small 2021, 17, 2104392. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, W.; Xu, H.; Liu, S.; Huang, W.; Zhao, Q. Fabrication of ultra-thin 2D covalent organic framework nanosheets and their application in functional electronic devices. Coord. Chem. Rev. 2021, 429, 213616. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Xie, Y.; Chu, H.; Wang, Y.; Wang, Y. In-situ anchoring bimetallic nanoparticles on covalent organic framework as an ultrasensitive electrochemical sensor for levodopa detection. Talanta 2021, 225, 122072. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent organic polymers and frameworks for fluorescence-based sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Zhang, K.; Kirlikovali, K.O.; Varma, R.S.; Jin, Z.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Covalent organic frameworks: Emerging organic solid materials for energy and electrochemical applications. ACS Appl. Mater. Interfaces 2020, 12, 27821–27852. [Google Scholar] [CrossRef]
- Babu, H.V.; Bai, M.M.; Rajeswara Rao, M. Functional π-conjugated two-dimensional covalent organic frameworks. ACS Appl. Mater. Interfaces 2019, 11, 11029–11060. [Google Scholar] [CrossRef]
- Pan, F.; Tong, C.; Wang, N.; Wang, Y.; Pan, D.; Zhu, R. Adsorbent-assisted in situ electrocatalysis: Highly sensitive and stable electrochemical sensor based on AuNF/COF-SH/CNT nanocomposites for the determination of trace Cu (II). ACS Sustain. Chem. Eng. 2022, 10, 16027–16036. [Google Scholar] [CrossRef]
- Sahoo, R.; Mondal, S.; Pal, S.C.; Mukherjee, D.; Das, M.C. Covalent-organic frameworks (COFs) as proton conductors. Adv. Energy Mater. 2021, 11, 2102300. [Google Scholar] [CrossRef]
- Liu, T.-Z.; Hu, R.; Liu, Y.; Zhang, K.-L.; Bai, R.-Y.; Yang, Y.-H. Amperometric immunosensor based on covalent organic frameworks and Pt/Ru/C nanoparticles for the quantification of C-reactive protein. Microchim. Acta 2020, 187, 320. [Google Scholar] [CrossRef]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. Electrochemical immunosensor for cardiac troponin I detection based on covalent organic framework and enzyme-catalyzed signal amplification. Anal. Chem. 2021, 93, 13572–13579. [Google Scholar] [CrossRef]
- Bu, T.; Bai, F.; Sun, X.; Tian, Y.; Zhang, M.; Zhao, S.; He, K.; Wang, X.; Jia, P.; Wang, L. An innovative prussian blue nanocubes decomposition-assisted signal amplification strategy suitable for competitive lateral flow immunoassay to sensitively detect aflatoxin B1. Food Chem. 2021, 344, 128711. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ding, L.; Li, Y.; Xiong, S.; Fang, H.; Li, X.; Nie, L.; Xiong, Y.; Huang, X. Covalent organic framework-gold nanoparticle heterostructures amplified dynamic light scattering immunosensor for ultrasensitive detection of NT-proBNP in whole blood. Sens. Actuators B Chem. 2022, 364, 131872. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Li, J.; Yan, J.; Liu, P.; Fan, X.; Song, W. A novel TAPP-DHTA COF cathodic photoelectrochemical immunosensor based on CRISPR/Cas12a-induced nanozyme catalytic generation of heterojunction. Electrochim. Acta 2023, 441, 141771. [Google Scholar] [CrossRef]
- Xing, C.; Mei, P.; Mu, Z.; Li, B.; Feng, X.; Zhang, Y.; Wang, B. Enhancing enzyme activity by the modulation of covalent interactions in the confined channels of covalent organic frameworks. Angew. Chem. 2022, 134, e202201378. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wang, P.; Fan, L.; Shi, Y. Highly sensitive multiplex detection of foodborne pathogens using a SERS immunosensor combined with novel covalent organic frameworks based biologic interference-free Raman tags. Talanta 2022, 243, 123369. [Google Scholar] [CrossRef]
- Wu, L.; Chen, R.; Jin, W.; Peng, C.; Wang, L.; Miao, L.; Song, Y. An electrochemical immunosensor for carbohydrate antigen CA125 based on electroactive COFs as a signal probe. Talanta 2025, 286, 127546. [Google Scholar] [CrossRef]
- Jin, W.; Chen, R.; Wu, L.; Peng, C.; Song, Y.; Miao, L.; Wang, L. An “on-off” electrochemical immunosensor for the detection of the glycan antigen CA125 by amplification signals using electropositive COFs. Talanta 2025, 286, 127593. [Google Scholar] [CrossRef]
- Liang, H.; Xiao, Y.; Chen, R.; Li, Y.; Zhou, S.; Liu, J.; Song, Y.; Wang, L. Immunosensing of neuron-specific enolase based on signal amplification strategies via catalysis of ascorbic acid by heteropolysate COF. Biosens. Bioelectron. 2023, 238, 115593. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Xiong, J.; Wu, D.; Li, S.; Tao, Y.; Qin, Y.; Kong, Y. Facile synthesis of chitosan-grafted beta-cyclodextrin for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2019, 125, 941–947. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B Chem. 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.; Xie, Y.; Song, Y.; Wang, L. Ratiometric electrochemical sensing and biosensing based on multiple redox-active state COFDHTA-TTA. Sens. Actuators B Chem. 2019, 281, 1009–1015. [Google Scholar] [CrossRef]
- Yang, T.-L.; Chen, J.-Y.; Kuo, S.-W.; Lo, C.-T.; El-Mahdy, A.F.M. Hydroxyl-functionalized covalent organic frameworks as high-performance supercapacitors. Polymers 2022, 14, 3428. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Z.; Du, P.; Ning, X.; Wang, Y.; Zhang, D.; Liu, J.; Zhang, S.; Lu, X. Embedding ultrasmall Au clusters into the pores of a covalent organic framework for enhanced photostability and photocatalytic performance. Angew. Chem. 2020, 132, 6138–6145. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Weng, X.; Liu, Y.; Xue, Y.; Wang, A.-J.; Wu, L.; Feng, J.-J. L-Proline bio-inspired synthesis of AuPt nanocalliandras as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen 19-9. Sens. Actuators B Chem. 2017, 250, 61–68. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, S.; Huang, Y.; Zhao, L.; Liu, Y.-M. Signal amplification in capillary electrophoresis based chemiluminescent immunoassays by using an antibody–gold nanoparticle–DNAzyme assembly. Talanta 2014, 124, 14–20. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Ge, S.; Song, X.; Ge, L.; Yan, M.; Yu, J. Multiplex electrochemical origami immunodevice based on cuboid silver-paper electrode and metal ions tagged nanoporous silver–chitosan. Biosens. Bioelectron. 2014, 56, 167–173. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, H.; Wang, Z.; Guo, W.; Zhang, A.; Huang, C.; Jia, N. An ultrasensitive multi-walled carbon nanotube–platinum–luminol nanocomposite-based electrochemiluminescence immunosensor. Analyst 2017, 142, 2253–2260. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, D.; Chu, C.; Ma, Z. Label-assisted chemical adsorption triggered conversion of electroactivity of sensing interface to achieve the Ag/AgCl process for ultrasensitive detection of CA 19-9. Anal. Chim. Acta 2020, 1093, 43–51. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Materon, E.M.; Furuta, R.H.M.; Wilson, D.; do Nascimento, G.F.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Oliveira, O.N.; Gonçalves, D. Screen-printed electrodes modified with carbon black and polyelectrolyte films for determination of cancer marker carbohydrate antigen 19-9. Microchim. Acta 2020, 187, 417. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yan, F.; Hu, X.; Ju, H. Chemiluminescent immunosensor for CA19-9 based on antigen immobilization on a cross-linked chitosan membrane. J. Immunol. Methods 2004, 291, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Guo, Z.; Chen, B.; Wang, S.; Ge, G.; Qiu, B.; Jiang, X. A one-step electrochemiluminescence immunosensor preparation for ultrasensitive detection of carbohydrate antigen 19-9 based on multi-functionalized graphene oxide. Biosens. Bioelectron. 2015, 66, 468–473. [Google Scholar] [CrossRef] [PubMed]


| Materials | Analytical Technique | LOD (U/mL)) | Linear Range (U/mL) | Matrix | Refs |
|---|---|---|---|---|---|
| AuPt nanocalliandras | DPV | 0.03 | 0.05–50 | PBS serum | [46] |
| Antibody-AuNP-G-quadruplex/hemin | CLIA | 0.016 | 0.025–1.00 | PBS serum | [47] |
| Cuboid silver-modified paper working electrode | SWV | 0.00004 | 0.1–100 | PBS serum | [48] |
| MWCNT-Pt-Luminol | ECL | 0.046 × 10−3 | 0.01–10 | PBS serum | [49] |
| Au/GO-MA | DPV | 0.032 | 0.01–100 | PBS serum | [50] |
| CB | DPV | 0.07 | 0.01–40 | PBS serum | [51] |
| Chitosan | CL | 1 | 2.0–25 | PBS serum | [52] |
| Multi-functionalized graphene oxide | ECL | 0.0005 | 0.001–5 | serum urine | [53] |
| AuNPs@COFTTA-2,6-NA(OH)2/Ab2/CA 19-9/Ab1/EP-COFTTA-DHTA | DPV | 0.0003 | 0.0009–100 | PBS, serum | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Chen, R.; Duan, Y.; Miao, L.; Zhu, Y.; Wang, L. A Sensitive Sandwich-Type Electrochemical Immunosensor for Carbohydrate Antigen 19-9 Based on Covalent Organic Frameworks. Biosensors 2025, 15, 492. https://doi.org/10.3390/bios15080492
Wu T, Chen R, Duan Y, Miao L, Zhu Y, Wang L. A Sensitive Sandwich-Type Electrochemical Immunosensor for Carbohydrate Antigen 19-9 Based on Covalent Organic Frameworks. Biosensors. 2025; 15(8):492. https://doi.org/10.3390/bios15080492
Chicago/Turabian StyleWu, Ting, Rongfang Chen, Yaqin Duan, Longfei Miao, Yongmei Zhu, and Li Wang. 2025. "A Sensitive Sandwich-Type Electrochemical Immunosensor for Carbohydrate Antigen 19-9 Based on Covalent Organic Frameworks" Biosensors 15, no. 8: 492. https://doi.org/10.3390/bios15080492
APA StyleWu, T., Chen, R., Duan, Y., Miao, L., Zhu, Y., & Wang, L. (2025). A Sensitive Sandwich-Type Electrochemical Immunosensor for Carbohydrate Antigen 19-9 Based on Covalent Organic Frameworks. Biosensors, 15(8), 492. https://doi.org/10.3390/bios15080492





