Capacitive Sensors for Label-Free Detection in High-Ionic-Strength Bodily Fluids: A Review
Abstract
1. Introduction
2. Basic Principles of Capacitive Detection
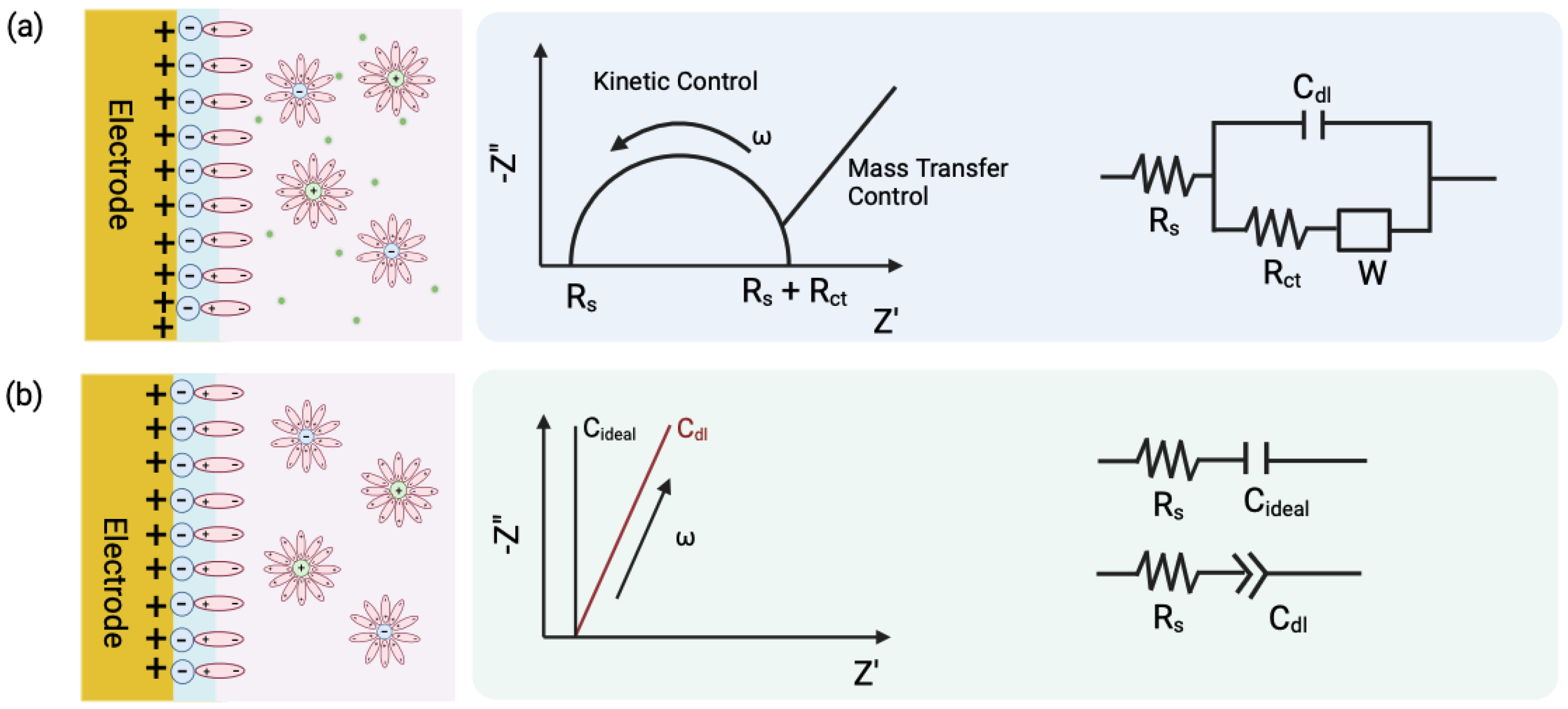

2.1. Electrodes Based on Potentiostatic Measurements


2.2. Interdigitated Electrodes (IDE)
2.3. Vertically Paired Electrodes (VPE)
3. Challenges in Deploying Capacitive Sensors in Biofluids
3.1. Debye Screening Limitation and Mitigation Strategies
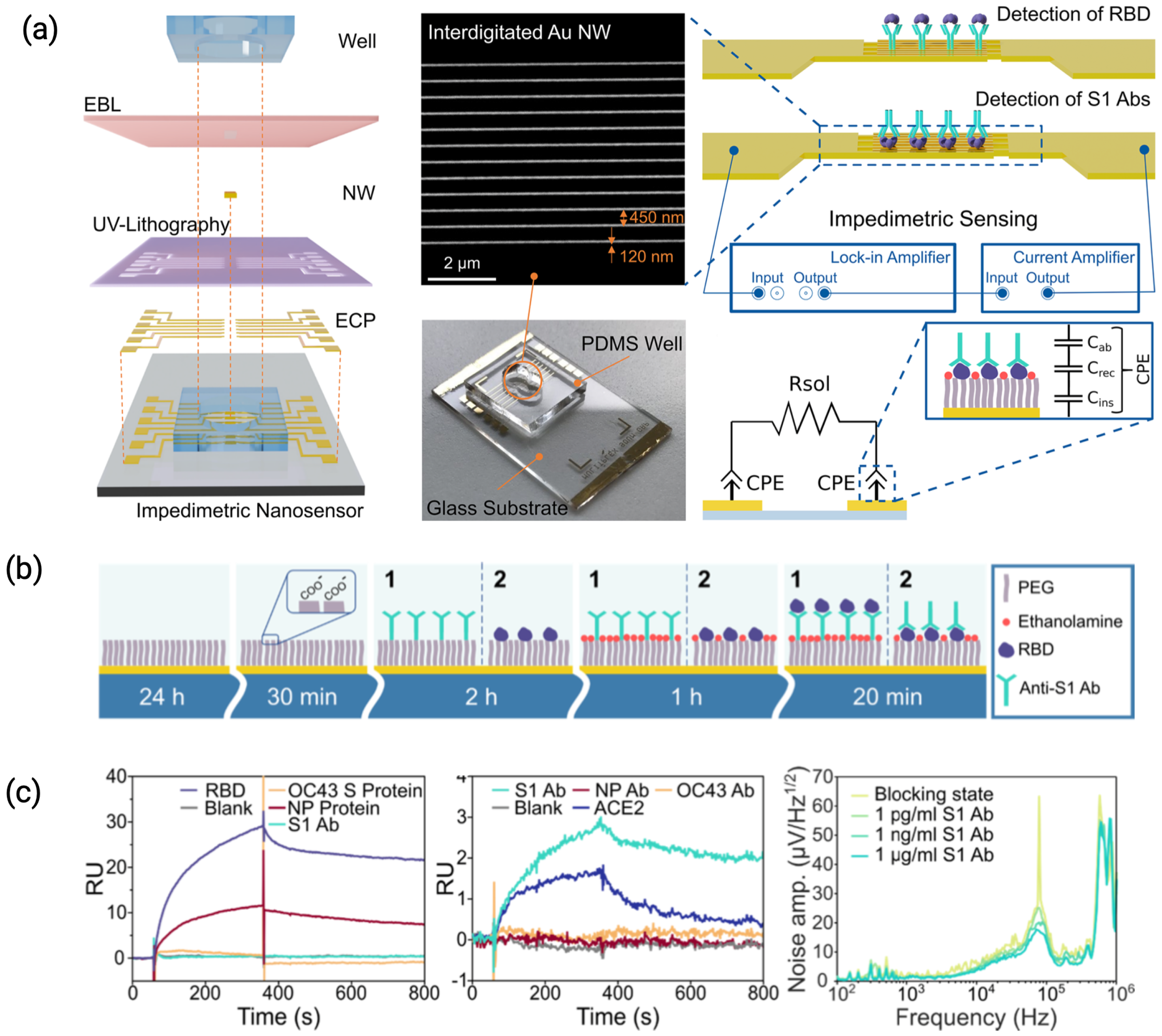
3.1.1. Electrode Nanostructures
| Nanostructure | Class | Mechanism | Sensitivity/LoD | Units | Ref. |
|---|---|---|---|---|---|
| Au Nanogap Arrays | Plasmonic (0D/2D) | LSPR | 1900 nm/RIU; 3.6 nM | RIU, nM | [39,40] |
| Silicon Nanowires | 1D Semiconductor | FET | 0.51 ag/mL | ag/mL | [41] |
| Carbon Nanotubes | 1D Conductor | – coupling | 10 fM | fM | [42] |
| 3D Graphene Foams | 3D Hierarchical | Electrochemical | 0.5 pM | pM | [43] |
| Metal Nanogaps (Pt, Au–Ti) | Electrical (0D) | Tunneling | 1–10 nA; 1 fM | nA, fM | [35,36,37] |
| Micro-gap PPE (EIS) | Electrical (2D) | Uniform field | 10 fg/mL | fg/mL | [44] |
| ZnO Nanoporous Electrodes | Semiconductor (3D) | Nanoconfinement | <10 fM | fM | [45,46] |
| Nanowell EIS Systems | Electrical (3D) | Confined pores | <1 fg/mL | fg/mL | [47] |
| Vertical Nanogaps | Electrical (3D) | Vertical field | 1 fg/mL; 20,000% G | fg/mL, % | [48] |
| Tubular 3D Nanochannels | Microfluidic (3D) | Biomimetic flow | Enhanced detection | — | [49] |
3.1.2. Reducing Ionic Strength of the Sample
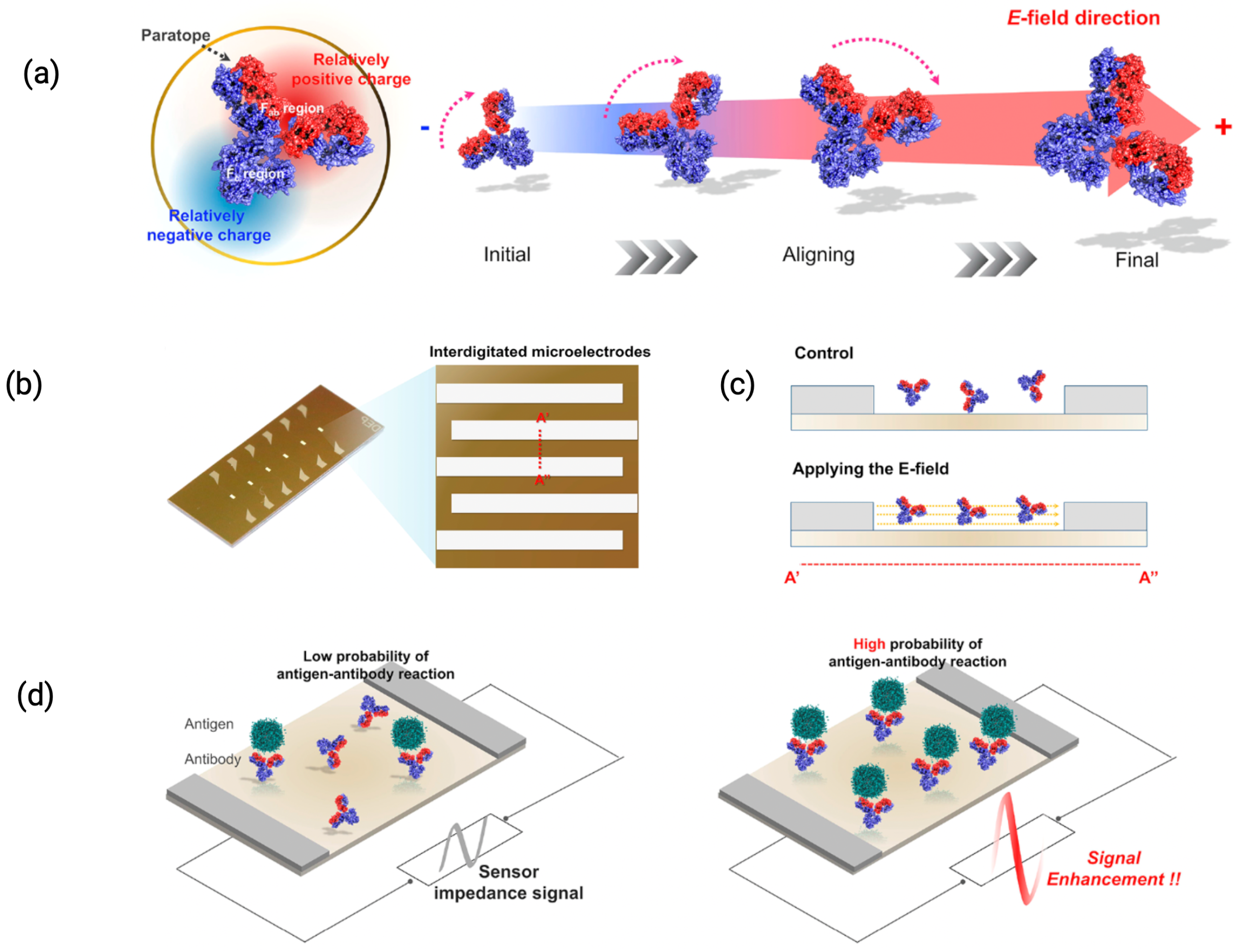
3.1.3. Electric Field Modulation at the Interface
3.2. Non-Specific Interactions and Sensitivity
3.3. Sensor Regeneration
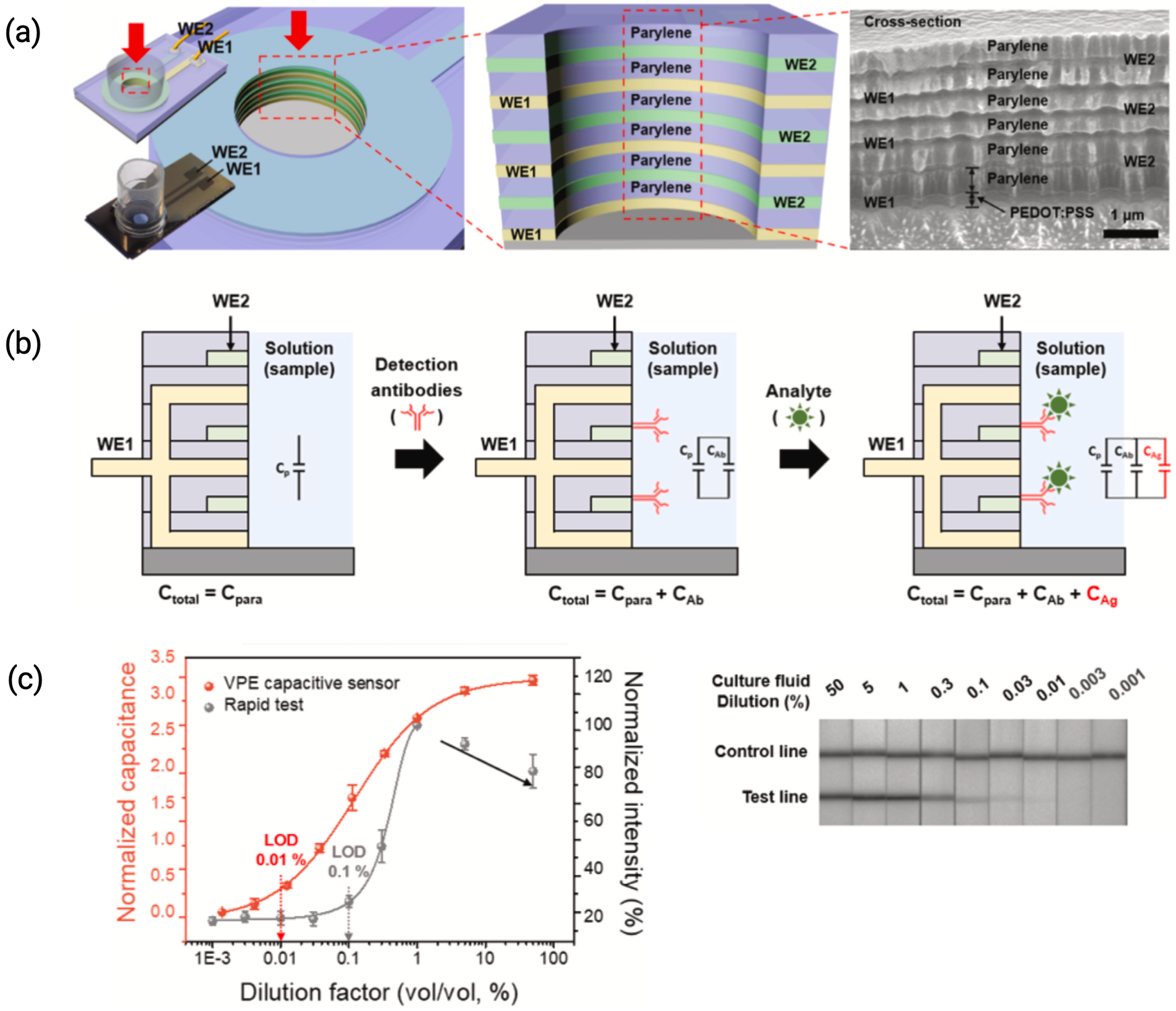
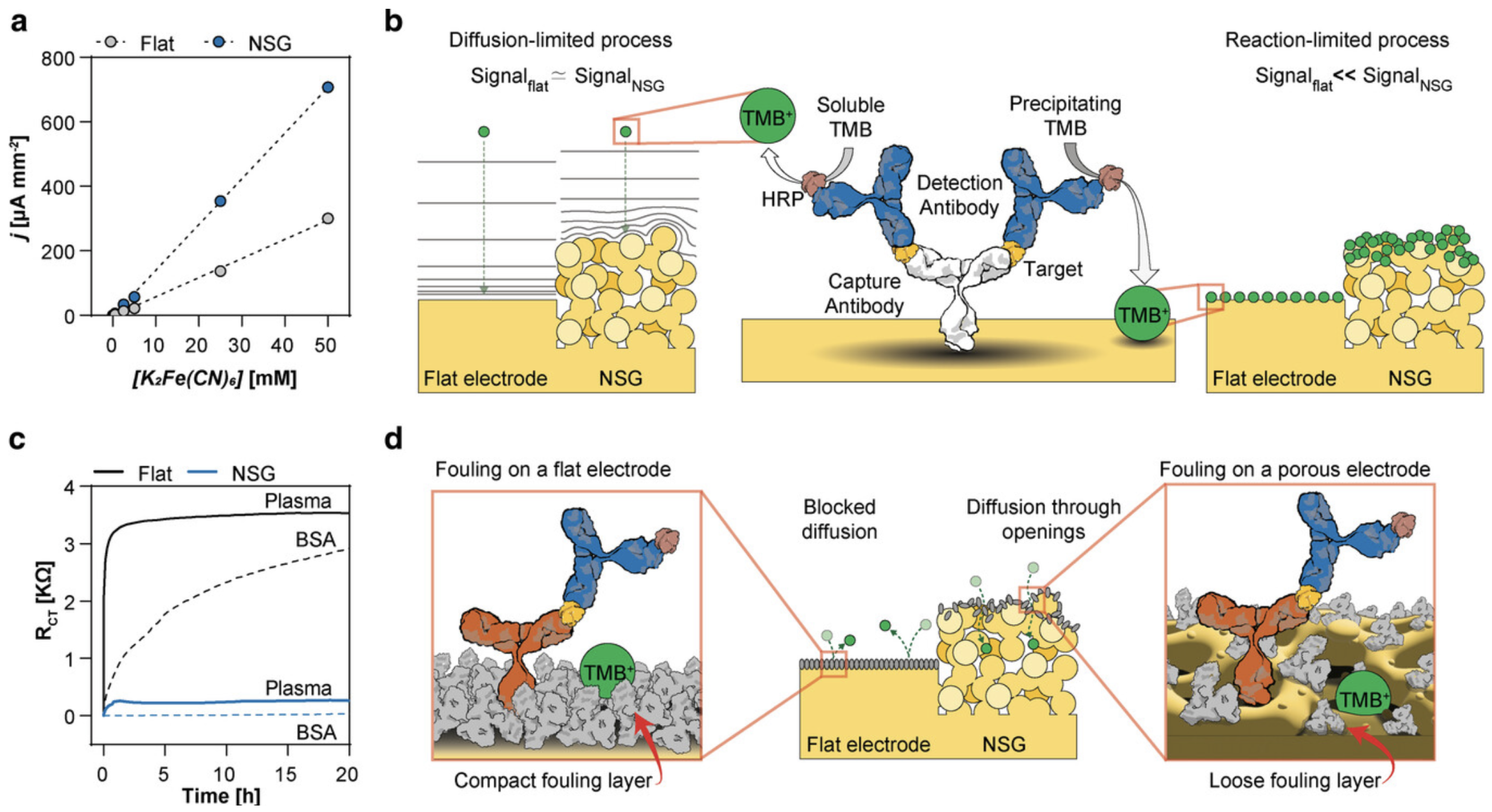
3.4. Sensor Longevity and Anti-Fouling Layers
4. Measurement Strategies
5. Advances in Surface Functionalization
5.1. Self-Assembled Monolayers (SAM)
5.2. Polymeric Films
5.3. Bioreceptor Immobilization
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EIS | Electrochemical Impedance Spectroscopy |
| f-EIS | Faradaic-Electrochemical Impedance Spectroscopy |
| nf-EIS | Non Faradaic-Electrochemical Impedance Spectroscopy |
| LoD | Limit of Detection |
| EDL | Electrical Double Layer |
| IDEs | Interdigitated Electrodes |
| VPsE | Vertically Paired Electrodes |
| MAIDEs | Microneedle Array Integrated with IDEs |
| SNR | Signal-to-Noise Ratio |
| ACEK | Alternating Current Electrokinetic |
| IDMEs | Interdigitated Microelectrodes |
| DEP | Dielectrophoretic |
| SAM | Self-Assembled Monolayer |
| MIPs | Molecularly Imprinted Polymers |
| ISF | Insterstitial Fluid |
| BSA | Bovine Serum Albumin |
References
- Luo, X.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 42, 5944–5962. [Google Scholar] [CrossRef] [PubMed]
- Garrote, B.L.; Santos, A.; Bueno, P.R. Perspectives on and Precautions for the Uses of Electric Spectroscopic Methods in Label-free Biosensing Applications. ACS Sens. 2019, 4, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Gai, Y.; Wu, Y.; Liu, Z.; Li, Z. Wearable mechanical and electrochemical sensors for real-time health monitoring. Commun. Mater. 2024, 5, 211. [Google Scholar] [CrossRef]
- Heerens, W.C. Application of capacitance techniques in sensor design. J. Phys. E Sci. Instrum. 1986, 19, 897. [Google Scholar] [CrossRef]
- Newman, A.L.; Hunter, K.W.; Stanbro, W.D. The capacitive affinity sensor: A new biosensor. In Proceedings of the Chemical Sensors: 2nd International Meeting, Bordeaux, France, 7–10 July 1986. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Voros, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, J.; Panda, S. Sensitivity Enhancement Mechanisms in Textured Dielectric based Electrolyte-Insulator-Semiconductor (EIS) Sensors. ECS J. Solid State Sci. Technol. 2015, 4, N18–N22. [Google Scholar] [CrossRef]
- Singh, K.; Tai, L.C.; Her, J.L.; Pan, T.M. Enhanced pH sensing with Ce-doped YTixOy sensing membrane in high-performance electrolyte–insulator–semiconductor devices. Mater. Chem. Phys. 2024, 311, 128563. [Google Scholar] [CrossRef]
- Wu, J. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- Luo, L.Q.; Zhang, Z.; Ding, Y.P.; Deng, D.M.; Zhu, X.L.; Wang, Z.X. Label-free electrochemical impedance genosensor based on 1-aminopyrene/graphene hybrids. Analyst 2013, 138, 2624–2630. [Google Scholar] [CrossRef]
- Bonanni, A.; Loo, A.H.; Pumera, M. Graphene for Impedimetric Biosensing. Trends Anal. Chem. 2012, 37, 12–21. [Google Scholar] [CrossRef]
- Singal, S.; Biradar, A.M.; Mulchandani, A.; Rajesh, M.; Malhotra, B.D. Ultrasensitive Electrochemical Immunosensor Based on Pt Nanoparticle–Graphene Composite. Appl. Biochem. Biotechnol. 2014, 174, 971–983. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, J.H.; Lee, S.K.; Lim, D.S.; Hwang, S.W.; Whang, D. Analytical Characteristics of Electrochemical Biosensor Using Pt-Dispersed Graphene on Boron Doped Diamond Electrode. Electroanalysis 2011, 23, 2443–2450. [Google Scholar] [CrossRef]
- Kramplová, Z.; Ferancová, A.; Maliar, T.; Purdešová, A. Tuneable Properties of Boron-Doped Diamond Working Electrodes and Their Advantages for the Detection of Pesticides. J. Electroanal. Chem. 2023, 949, 117846. [Google Scholar] [CrossRef]
- Baruah, S.; Mohanta, D.; Betty, C.A. Composite PEDOT-PSS Based Highly Sensitive Electrochemical Sensors for Sensing Glucose from Human Saliva. Microchem. J. 2024, 206, 111411. [Google Scholar] [CrossRef]
- Liu, D.; Rahman, M.M.; Ge, C.; Kim, J.; Lee, J.J. Highly Stable and Conductive PEDOT:PSS/Graphene Nanocomposites for Biosensor Applications in Aqueous Medium. New J. Chem. 2017, 41, 14810–14817. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, J.; Liu, X.; Travas Sejdic, J. Electrochemical Biosensing Platform Based on AuNWs/rGO-CMC-PEDOT:PSS Composite for the Detection of Superoxide Anion Released from Living Cells. Biosens. Bioelectron. 2024, 254, 116228. [Google Scholar] [CrossRef]
- Oziat, J.; Babin, T.; Gougis, M.; Malliaras, G.G.; Mailley, P. Electrochemical Detection of Redox Molecules Secreted by Pseudomonas aeruginosa—Part 2: Enhanced Detection Owing to PEDOT:PSS Electrode Structuration. Bioelectrochemistry 2023, 154, 108538. [Google Scholar] [CrossRef]
- Shen, F.; Ju, F.; Li, G.; Ma, L. Flexible and Highly Sensitive Electrochemical Sensor Based on PEDOT:PSS for Dopamine Detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef]
- Santos-Neto, I.; Carvalho, C.; Balby Araujo Filho, G.; Andrade, C.; Santos, G.; Barros Filho, A.; Neto, J.; Paucar, V.; Alencar, L.; Lopes, A.; et al. Interdigitated Electrode for Electrical Characterization of Commercial Pseudo-Binary Biodiesel–Diesel Blends. Sensors 2021, 21, 7288. [Google Scholar] [CrossRef]
- Yildiz, Y.; Kallempudi, S.; Altintas, Z.; Totthill, I.; Gurbuz, Y. Novel label-free capacitive sensor based on interdigitated electrode arrays for multiplex point-of-care testing. In Proceedings of the 20th Anniversary World Congress on Biosensors, Glasgow, UK, 26–28 May 2010. [Google Scholar]
- Lin, C.H.; Lee, Y.C.; Yang, C.M.; Lu, M.S. Detection of DNA Hybridization Beyond the Debye Screening Length by CMOS Capacitive Sensors. IEEE Electron. Device Lett. 2022, 43, 1319–1322. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Almansour, R.; Alessa, J.A.; Alhudaithy, S.; Fakhouri, A.S.; Alsaleh, A. Roles of Interdigitated Electrode Geometry in Non-Faradaic Impedimetric Biosensors. J. Electrochem. Soc. 2024, 171, 087515. [Google Scholar] [CrossRef]
- Alzahrani, K.E.; Almansour, M.J.; Qurayshan, S.M.; Albrithen, H.; Fakhouri, A.S.; Alhussaini, K.; Assaifan, A.K.; Alodhayb, A.N. Effect of Applied AC Voltage on the Performance of Non-Faradaic Impedimetric Biosensors. J. Electrochem. Soc. 2024, 171, 037516. [Google Scholar] [CrossRef]
- Hadiyan, M.; Salehi, A.; Mirzanejad, H. Gas sensing behavior of Cu2O and CuO/Cu2O composite nanowires synthesized by template-assisted electrodeposition. J. Korean Chem. Soc. 2020, 58, 94–105. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, G.Y.; Song, Z.; Bong, J.H.; Chang, Y.W.; Cho, S.; Kang, M.J.; Pyun, J.C. Capacitive biosensor based on vertically paired electrodes for the detection of SARS-CoV-2. Biosens. Bioelectron. 2022, 202, 113975. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Kargupta, R.; Ghoshal, D.; Li, Z.; Chande, C.; Feng, L.; Chatterjee, S.; Koratkar, N.; Motkuri, R.K.; Basuray, S. ESSENCE—A rapid, shear-enhanced, flow-through, capacitive electrochemical platform for rapid detection of biomolecules. Biosens. Bioelectron. 2021, 182, 113163. [Google Scholar] [CrossRef]
- Sandoval Bojórquez, D.I.; Janicíjevic, Z.; Palestina Romero, B.; Oliveros Mata, E.S.; Laube, M.; Feldmann, A.; Kegler, A.; Drewitz, L.; Fowley, C.; Pietzsch, J.; et al. Impedimetric Nanobiosensor for the Detection of SARS-CoV-2 Antigens and Antibodies. ACS Sens. 2023, 8, 576–586. [Google Scholar] [CrossRef]
- Mirzajani, H.; Urey, H. IDE-Integrated Microneedle Arrays as Fully Biodegradable Platforms for Wearable/Implantable Capacitive Biosensing. IEEE Sens. Lett. 2024, 8, 1–4. [Google Scholar] [CrossRef]
- Lee, G.Y.; Choi, Y.H.; Chung, H.W.; Ko, H.; Cho, S.; Pyun, J.C. Capacitive immunoaffinity biosensor based on vertically paired ring-electrodes. Biosens. Bioelectron. 2013, 40, 227–232. [Google Scholar] [CrossRef]
- Lee, G.; Chang, Y.; Ko, H.; Kang, M.; Pyun, J. Band-type microelectrodes for amperometric immunoassays. Anal. Chim. Acta 2016, 928, 39–48. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.; Chang, Y.; Kang, M.; Cho, S.; Pyun, J. Capacitive biosensor based on vertically paired electrode with controlled parasitic capacitance. Sens. Actuators B Chem. 2018, 273, 384–392. [Google Scholar] [CrossRef]
- Sathya, S.; Muruganand, S.; Manikandan, N.; Karuppasamy, K. Design of capacitance based on interdigitated electrode for BioMEMS sensor application. Mater. Sci. Semicond. Process. 2019, 101, 206–213. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Z.; Yang, G.M.; Li, J.; Li, M.Q.; Liu, J.H.; Huang, X.J. Electrical nanogap devices for biosensing. Mater. Today 2010, 13, 28–41. [Google Scholar] [CrossRef]
- Porath, D.; Bezryadin, A.; de Vries, S.; Dekker, C. Direct measurement of electrical transport through DNA molecules. Nature 2000, 403, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Hashioka, S.; Saito, M.; Tamiya, E.; Matsumura, H. Metal nanogap devices fabricated by conventional photolithography and their application to deoxyribose nucleic acid analysis. In Proceedings of the School of Materials Science, Japan Advanced Institute of Science and Technology, Tatsunokuchi, Ishikawa-ken, Japan, November/December 2003; Volume 21, pp. 2937–2940. [Google Scholar]
- Hashioka, S.; Saito, M.; Tamiya, E.; Matsumura, H. Deoxyribonucleic acid sensing device with 40-nm-gap-electrodes fabricated by low-cost conventional techniques. Appl. Phys. Lett. 2004, 85, 687–688. [Google Scholar] [CrossRef]
- Herne, T.M.; Tarlov, M.J. Characterization of DNA probes immobilized on gold surfaces. J. Am. Chem. Soc. 1997, 119, 8916–8920. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Li, S.; Ding, F.; Li, N.; Meng, S.; Li, R.; Qi, J.; Liu, Q.; Liu, G.L. Plasmonic nano-arrays for ultrasensitive bio-sensing. Nanophotonics 2018, 7, 1–17. [Google Scholar] [CrossRef]
- Aćimović, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR Chip for Parallel, Rapid, and Sensitive Detection of Cancer Markers in Serum. Anal. Chem. 2014, 86, 5005–5012. [Google Scholar] [CrossRef]
- Wu, C.-C. Polycrystalline Silicon Nanowire Field Effect Transistor Biosensors for SARS-CoV-2 Detection. Sens. Actuators B Chem. 2023, 376, 132838. [Google Scholar]
- Meskher, H.; Mustansar, H.C.; Thakur, A.K.; Sathyamurthy, R.; Lynch, I.; Singh, P.; Han, T.K.; Saidur, R. Recent trends in carbon nanotube (CNT)-based biosensors for the detection of human viruses. Biosens. Bioelectron. 2023, 200, 113911. [Google Scholar]
- Xu, S.; Zhang, C.; Jiang, S.; Hu, G.; Li, X.; Zou, Y.; Liu, H.; Li, J.; Li, Z.; Wang, X.; et al. Graphene foam field-effect transistor for ultra-sensitive label-free detection of ATP. Biosens. Bioelectron. 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Honda, R.; Nakatsuka, Y.; Miyahara, Y. Toward a practical impedimetric biosensor: A micro-gap parallel plate electrode structure that enables highly sensitive and reproducible measurements in complex media. Biosens. Bioelectron. 2022, 213, 114439. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Selvam, A.P.; Barrett, T.; Kazmierczak, S.; Rana, M.; Prasad, S. Portable nanoporous electrical biosensor for ultrasensitive detection of Troponin-T. Future Sci. OA 2015, 1, FSO24. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Muthukumar, S.; Selvam, A.P.; Prasad, S. Electrochemical nanostructured ZnO biosensor for ultrasensitive detection of cardiac troponin-T. Nanomedicine 2016, 11, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Selvam, A.P.; Prasad, S. Nanosensor electrical immunoassay for quantitative detection of NT-pro brain natriuretic peptide. Future Cardiol. 2013, 9, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Thavarungkul, P.; Dawan, S.; Kanatharana, P.; Asawatreratanakul, P. Detection of protein binding based on nanogap embedded FET biosensors. Biosens. Bioelectron. 2007, 23, 701–707. [Google Scholar] [CrossRef]
- Alnaimi, A.; Egunov, A.I.; Karnaushenko, D.; Schmidt, O.; Kanoun, O. Functionalization of an Inner Surface of 3D Self-Assembled Sensor-In-A-Tube for Cytokines Detection. In Proceedings of the 2024 International Workshop on Impedance Spectroscopy (IWIS), Chemnitz, Germany, 24–27 September 2024; pp. 94–97. [Google Scholar] [CrossRef]
- Sabaté del Río, J.; Woo, H.K.; Park, J.; Ha, H.K.; Kim, J.R.; Cho, Y.K. SEEDING to Enable Sensitive Electrochemical Detection of Biomarkers in Undiluted Biological Samples. Adv. Mater. 2022, 34, 2200981. [Google Scholar] [CrossRef]
- Wan, N.; Jiang, Y.; Huang, J.; Oueslati, R.; Eda, S.; Wu, J.; Lin, X. Rapid and Sensitive Detection of miRNA Based on AC Electrokinetic Capacitive Sensing for Point-of-Care Applications. Sensors 2021, 21, 3985. [Google Scholar] [CrossRef]
- Daniels, J.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef]
- Sappia, L.; Piccinini, E.; Marmisollé, W.; Santilli, N.; Maza, E.; Moya, S.; Battaglini, F.; Madrid, R.E.; Azzaroni, O. Integration of Biorecognition Elements on PEDOT Platforms through Supramolecular Interactions. Adv. Mater. Interfaces 2017, 4, 1700502. [Google Scholar] [CrossRef]
- Branzoi, F.; Brânzoi, V.; Muşină, A. Fabrication and characterisation of conducting composite films based on conducting polymers and functionalised carbon nanotubes. Surf. Interface Anal. 2012, 44, 1076–1080. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, Y.; Wu, J.J.; Eda, S.; Wan, N. An alternating current electrokinetics biosensor for rapid on-site serological screening of Taenia solium cysticercosis infection. Microchim. Acta 2022, 189, 476. [Google Scholar] [CrossRef]
- Goode, J.; Rushworth, J.; Millner, P. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2014, 31, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Ahn, J.H.; Kim, J.Y.; Lee, J.; Kim, J.H. Improvement of Sensitivity and Limit of Detection in a Nanogap Biosensor by Controlling Surface Wettability. BioNanoScience 2013, 3, 192–197. [Google Scholar] [CrossRef]
- Bakestani, R.M.; Wu, Y.; Glahn-Martínez, B.; Kippin, T.E.; Plaxco, K.W.; Kolkman, R.W. Carboxylate-Terminated Electrode Surfaces Improve the Performance of Electrochemical Aptamer-Based Sensors. ACS Appl. Mater. Interfaces 2025, 17, 8706–8714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Mei, Z.; Wang, Y.; Li, H.; Li, S.; Xia, F. Incorporating Hydrophobic Moieties into Self-Assembled Monolayers to Enable Electrochemical Aptamer-Based Sensors Deployed Directly in a Complex Matrix. ACS Sens. 2022, 7, 2615–2624. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, K.K.; Park, B.G.; Lee, K.H.; Kim, D.K. Capacitive Oil Detector Using Hydrophobic and Oleophilic PDMS Sponge. Int. J. Precis. Eng.-Manuf.-Green Technol. 2018, 5, 303–309. [Google Scholar] [CrossRef]
- Kim, Y.T.; Ito, Y.; Tadai, K.; Mitani, T.; Kim, U.S.; Kim, H.S.; Cho, B.W. Drastic Change of Electric Double Layer Capacitance by Surface Functionalization of Carbon Nanotubes. Appl. Phys. Lett. 2005, 87, 234106. [Google Scholar] [CrossRef]
- Vivod, D.; Voß, J.; Halik, M.; Zahn, D. Tailoring the Wetting Behavior of Self-Assembled Monolayers by Surface Charge. J. Phys. Chem. C 2024, 128, 8498–8504. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, Z.; Hoque, M.J.; Li, L.; Rabbi, K.F.; Ho, J.Y.; Braun, P.V.; Wang, P.; Miljkovic, N. A Lipid-Inspired Highly Adhesive Interface for Durable Superhydrophobicity in Wet Environments and Stable Jumping Droplet Condensation. ACS Nano 2022, 16, 4251–4262. [Google Scholar] [CrossRef]
- Mousavi, S.; Pitchumani, R. Long-Term Static and Dynamic Corrosion Stability of Nonwetting Surfaces. Langmuir 2022, 38, 6911–6922. [Google Scholar] [CrossRef]
- Otsuka, H.; Matsukuma, D. Stimuli-Responsive Polymer Materials for Creation of Biointerfaces. In Polymer Science: A Comprehensive Reference; Ise, N., Hashimoto, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 351–370. [Google Scholar] [CrossRef]
- Li, W.S.; Chuang, K.C.; Li, Y.S.; Luo, J.D.; Chang, M.L.; Cheng, H.C. Enhancement on the Characteristics of Supercapacitors Using Surface Modification of Sprayed-Carbon Nanotube Thin Film Electrodes with Oxygen Plasma Treatment. Jpn. J. Appl. Phys. 2019, 58, 056502. [Google Scholar] [CrossRef]
- Brothers, M.; Moore, D.; Lawrence, M.; Harris, J.; Joseph, R.M.; Ratcliff, E.; Ruiz, O.N.; Glavin, N.; Kim, S.S. Impact of Self-Assembled Monolayer Design and Electrochemical Factors on Impedance-Based Biosensing. Sensors 2020, 20, 2246. [Google Scholar] [CrossRef] [PubMed]
- Saateh, A.; Ansaryan, S.; Gao, J.; de Miranda, L.O.; Zijlstra, P.; Altug, H. Long-Term and Continuous Plasmonic Oligonucleotide Monitoring Enabled by Regeneration Approach. Angew. Chem. Int. Ed. 2024, 63, e202410076. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Kilic, T.; Zhang, Y.S.; Avci, H.; Hu, N.; Kim, D.; Branco, C.; Aleman, J.; Massa, S.; Silvestri, A.; et al. Label-Free and Regenerative Electrochemical Microfluidic Biosensors for Continual Monitoring of Cell Secretomes. Adv. Sci. 2017, 4, 1600522. [Google Scholar] [CrossRef]
- Alemán, J.; Kilic, T.; Mille, L.S.; Shin, S.R.; Zhang, Y.S. Microfluidic Integration of Regeneratable Electrochemical Affinity-Based Biosensors for Continual Monitoring of Organ-on-a-Chip Devices. Nat. Protoc. 2021, 16, 2564–2593. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Murray, A.J.; Nagraj, N.; Pris, A.D.; Ashe, J.M.; Todorovic, M. Towards Maintenance-Free Biosensors for Hundreds of Bind/Release Cycles. Angew. Chem. Int. Ed. 2015, 54, 2174–2178. [Google Scholar] [CrossRef]
- Gupta, R.; Luan, J.; Chakrabartty, S.; Scheller, E.L.; Morrissey, J.; Singamaneni, S. Refreshable Nanobiosensor Based on Organosilica Encapsulation of Biorecognition Elements. ACS Appl. Mater. Interfaces 2020, 12, 5420–5428. [Google Scholar] [CrossRef]
- Bharti, A.M.; Kumar, R.K.R.; Chuang, C.H.; Shaikh, M.O. Universal Nanocomposite Coating with Antifouling and Redox Capabilities for Electrochemical Affinity Biosensing in Complex Biological Fluids. Nanoscale Horizons 2024, 9, 843–852. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, S.Y.; Song, J.; Jang, H.; Kim, M.; Kim, H.; Choi, S.Q.; Kim, S.; Jolly, P.; Kang, T.; et al. Micrometer-Thick and Porous Nanocomposite Coating for Electrochemical Sensors with Exceptional Antifouling and Electroconducting Properties. Nat. Commun. 2024, 15, 711. [Google Scholar] [CrossRef]
- Sharkey, C.; Twiddy, J.; Peterson, K.L.; Aroche, A.F.; Menegatti, S.; Daniele, M.A. Towards Electrochemical Control of pH for Regeneration of Biosensors. In Proceedings of the 2023 IEEE BioSensors Conference (BioSensors), London, UK, 30 July–1 August 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Masaki, M.; Akimoto, Y.; Shetty, V. Recyclable Cardiac Biosensor Based on Light-Triggered Antibody Regeneration Method. ECS Meet. Abstr. 2024, MA2024-02, 4291. [Google Scholar] [CrossRef]
- Hensel, R.C.; Pereira-da Silva, M.; Riul, A.; Rodrigues, V. Dielectric Permittivity and Surface Charge Density in Layer-by-Layer Poly(diallyldimethylammonium chloride)/Poly(styrenesulfonate) Nanostructured Films: Implications for Biosensing. ACS Appl. Nano Mater. 2020, 3, 1749–1754. [Google Scholar] [CrossRef]
- Young, T.; Clark, V.; Arroyo, N.; Heikenfeld, J. Perspective—The Feasibility of Continuous Protein Monitoring in Interstitial Fluid. ECS Sens. Plus 2023, 2, 027001. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, L.; Huang, L.h.; Zuo, Z.; Ho, V.; Jin, L.; Lu, Y.; Chen, X.; Zhao, J.; Qian, D.; et al. Microfluidic integrated capacitive biosensor for C-reactive protein label-free and real-time detection. Analyst 2021, 146, 5380–5388. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, D.; Park, Y.; Kim, D.H.; Kim, J. Electric-Field-Mediated In-Sensor Alignment of Antibody’s Orientation to Enhance the Antibody–Antigen Binding for Ultrahigh Sensitivity Sensors. Nano Lett. 2022, 22, 6537–6544. [Google Scholar] [CrossRef]
- Chen, D.; Dong, G.; Hao, Z.; Feng, Z.; Li, G.; Tong, X. An enhanced measurement method with voltage-frequency-voltage conversion of EISCAP sensor. IEEE Trans. Instrum. Meas. 2024, 73, 1–11. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Chen, Y.; Tan, J.; Zhao, W.; Yang, Y.; Hui, C.; Zheng, Y.; Tan, Y.; Li, G.; et al. Optimization of Electrolyte-Insulator-Semiconductor Capacitor Sensor by Differential Design. Electroanalysis 2024, 37, e202400201. [Google Scholar] [CrossRef]
- Baeuscher, M.; Reinicke, O.; Henke, M.; Mackowiak, P.; Schiffer, M.; Schneider-Ramelow, M.; Lang, K.D.; Ngo, H.D. Investigation of IDC Structures for Graphene Based Biosensors Using Low Frequency EIS Method. In Proceedings of the 2019 12th International Conference on Developments in eSystems Engineering (DeSE), Kazan, Russia, 7–10 October 2019; pp. 939–942. [Google Scholar] [CrossRef]
- Mondal, S.; Subramaniam, C. Scalable approach towards specific and ultrasensitive cation sensing under harsh environmental conditions by engineering the analyte–transducer interface. Nanoscale Adv. 2020, 2, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Namhil, Z.G.; Kemp, C.; Verrelli, E.; Iles, A.; Pamme, N.; Adawi, A.; Kemp, N. A label-free aptamer-based nanogap capacitive biosensor with greatly diminished electrode polarization effects. Phys. Chem. Chem. Phys. 2019, 21, 681–691. [Google Scholar] [CrossRef]
- Yusof, Y.; Yanagimoto, Y.; Uno, S.; Nakazato, K. Electrical Characteristics of Biomodified Electrodes using Nonfaradaic Electrochemical Impedance Spectroscopy. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2011, 5, 29–33. [Google Scholar]
- Strakosas, X.; Sessolo, M.; Hama, A.; Rivnay, J.; Stavrinidou, E.; Malliaras, G.; Owens, R. A facile biofunctionalisation route for solution processable conducting polymer devices. J. Mater. Chem. B 2014, 2, 2537–2545. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Ramos, N.C.; Medlin, J.W.; Holewinski, A. Electrochemical Stability of Thiolate Self-Assembled Monolayers on Au, Pt, and Cu. ACS Appl. Mater. Interfaces 2023, 15, 14470–14480. [Google Scholar] [CrossRef]
- Assaifan, A.K. Thiol-SAM Concentration Effect on the Performance of Interdigitated Electrode-Based Redox-Free Biosensors. Micromachines 2024, 15, 1254. [Google Scholar] [CrossRef]
- Berezhetska, O.; Liberelle, B.; De Crescenzo, G.; Cicoira, F. A simple approach for protein covalent grafting on conducting polymer films. J. Mater. Chem. B 2015, 3, 5087–5094. [Google Scholar] [CrossRef]
- Kondyurin, A.; Tsoutas, K.; Latour, Q.X.; Higgins, M.; Moulton, S.; Mckenzie, D.; Bilek, M. Structural Analysis and Protein Functionalization of Electroconductive Polypyrrole Films Modified by Plasma Immersion Ion Implantation. ACS Biomater. Sci. Eng. 2017, 3, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Rossignatti, B.C.; Vieira, A.P.; Barbosa, M.; Abegão, L.; Mello, H.J.N.P.D. Thin Films of Polyaniline-Based Nanocomposites with CeO2 and WO3 Metal Oxides Applied to the Impedimetric and Capacitive Transducer Stages in Chemical Sensors. Polymers 2023, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Gornall, D.D.; Collyer, S.; Higson, S. Evaluation of Poly(o-phenylenediamine) Films for Application as Insulating Layers upon Carbon Substrates for Use within Sonochemically Fabricated Microelectrode Arrays. Electroanalysis 2010, 22, 384–392. [Google Scholar] [CrossRef]
- Baltierra-Uribe, S.L.; Chanona-Pérez, J.J.; Méndez-Méndez, J.V.; Perea-Flores, M.d.J.; Sánchez-Chávez, A.C.; García-Pérez, B.E.; Moreno-Lafont, M.C.; López-Santiago, R. Detection of Brucella abortus by a Platform Functionalized with Protein A and Specific Antibodies IgG. Microsc. Res. Tech. 2019, 82, 586–595. [Google Scholar] [CrossRef]
- Liao, B.Y.; Chang, C.J.; Wang, C.F.; Lu, C.H.; Chen, J.K. Controlled Antibody Orientation on Fe3O4 Nanoparticles and CdTe Quantum Dots Enhanced Sensitivity of a Sandwich-Structured Electrogenerated Chemiluminescence Immunosensor for the Determination of Human Serum Albumin. Sens. Actuators B Chem. 2021, 336, 129710. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef]
- Supraja, P.; Tripathy, S.; Singh, R.; Gangwar, R.; Singh, S.G. A novel cleanroom-free technique for simultaneous electrodeposition of polypyrrole onto array of IDuEs: Towards low-cost, stable and accurate point-of-care TBI diagnosis without trained manpower. Biosens. Bioelectron. 2025, 267, 116824. [Google Scholar] [CrossRef]
- Panigrahi, A.R.; Nisha; Yadav, P.; Beura, S.K.; Singh, J.; Maurya, A.K. Development of an Impedimetric Immunosensor for D-Dimer Detection Using Reduced Graphene Oxide, Gold and Thionine Based Nanocomposite. Anal. Lett. 2025, in press. [Google Scholar] [CrossRef]
- Duke, K.; Dhungana, P.; Richards, C.; Preusser, K.; Romeo, A.; Gonzalez-Garcia, J.; Mummareddy, B.; Cortes, P.; Li, F.; Park, B.W. Label-Free Impedimetric Determination of Cortisol Using Gold Nanoparticles Functionalized Laser-Induced Graphene Interdigitated Electrodes. Adv. Mater. Technol. 2025, 10, 2401040. [Google Scholar] [CrossRef]
- Harma, P.; Kim, N.; Ganbold, E.; Seong, R.; Kim, Y.; Park, J.; Shin, Y.; Han, H.; Kim, E.; Kim, S. SARS-CoV-2 detection in COVID-19 patients’ sample using Wooden quoit conformation structural aptamer (WQCSA)-Based electronic bio-sensing system. Biosens. Bioelectron. 2025, 267, 116506. [Google Scholar]
- Tsai, P.C.; Chen, R.; Hsieh, B.C.; Cheng, T.J. Nitrocellulose/acrylic resin coated screen-printed carbon electrode to construct a capacitive immunosensor for anti-BSA. Biosens. Bioelectron. 2024, 258, 116376. [Google Scholar] [CrossRef] [PubMed]
- Liv, L.; Özerdem, Z. First DFT-supported point of care and novel electrochemical biosensing: Determination of yellow fever NS1 antibody in human plasma. Int. J. Biol. Macromol. 2024, 269, 132169. [Google Scholar] [CrossRef] [PubMed]
- Khorshed, A.A.; Jiang, T.; Chen, J. A Label-Free Point-of-Care Electrochemical Biosensor for Early and Accurate Detection of Monkeypox. Biosens. Bioelectron. 2025, 278, 117337. [Google Scholar] [CrossRef]
- Mehta, D.; Kaur, S.; Nagaiah, T.C. Realizing the label-free sensitive detection of carcinoembryogenic antigen (CEA) in blood serum via a MNC-decorated flexible immunosensor. Anal. Methods 2024, 16, 1473–1479. [Google Scholar] [CrossRef]
- Orzari, L.O.; Brazaca, L.C.; Janegitz, B.C. Parkinson Biomarker Determination with an Au Microflower–Enhanced Electrochemical Immunosensor Using Non-Faradaic Capacitance Measurements. Microchim. Acta 2024, 191, 663. [Google Scholar] [CrossRef]
- Shoute, L.C.; Charlton, C.L.; Kanji, J.N.; Babiuk, S.; Babiuk, L.; Chen, J. Faradaic Impedimetric Immunosensor for Label-Free Point-of-Care Detection of COVID-19 Antibodies Using Gold-Interdigitated Electrode Array. Biosensors 2024, 14, 6. [Google Scholar] [CrossRef]
- Bachour, B., Jr.; Batistuti Sawazaki, M.R.; Mulato, M. Electrochemical capacitive dengue aptasensor using NS1 in undiluted human serum. Mikrochim. Acta 2024, 191, 72. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, D.; Calucho, E.; Marrugo-Ramírez, J.; Álvarez Diduk, R.; Orozco, J.; Merkoçi, A. Capacitive immunosensing at gold nanoparticle-decorated reduced graphene oxide electrodes fabricated by one-step laser nanostructuration. Biosens. Bioelectron. 2024, 252, 116142. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, A.; Kastrati, G.; Pekarkova, J.; Metelka, R.; Drbohlavova, J.; Bilkova, Z.; Selesovska, R.; Korecka, L. Biosensor with Electrochemically Active Nanocomposites for Signal Amplification and Simultaneous Detection of Three Ovarian Cancer Biomarkers. Electrochim. Acta 2023, 469, 143213. [Google Scholar] [CrossRef]
- Vora, K.; Kordas, N.; Seidl, K. Label-Free, Impedance-Based Biosensor for Kidney Disease Biomarker Uromodulin. Sensors 2023, 23, 9696. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Solanki, P.R. Enhanced Electrochemical Biosensing of the Sp17 Cancer Biomarker in Serum Samples via Engineered Two-Dimensional MoS2 Nanosheets on the Reduced Graphene Oxide Interface. ACS Appl. Mater. Interfaces 2023, 15, 43645–43656. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de Los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Corrigan, D.K. Comparing nanobody and aptamer-based capacitive sensing for detection of interleukin-6 (IL-6) at physiologically relevant levels. Anal. Bioanal. Chem. 2023, 415, 7035–7045. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsystems Nanoeng. 2023, 9, 3. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Sadeghi, M.; Ehzari, H.; Derakhshankhah, H. Label-Free Electrochemical Immunosensor Based on Antibody-Immobilized Fe-Cu Layered Double Hydroxide Nanosheets as an Electrochemical Probe for the Detection of Ultra Trace Amount of Prostate Cancer Biomarker (PSA). Microchem. J. 2023, 195, 109460. [Google Scholar] [CrossRef]
- Ruankham, W.; Morales Frías, I.A.; Phopin, K.; Tantimongcolwat, T.; Bausells, J.; Zine, N.; Errachid, A. One-Step Impedimetric NT-ProBNP Aptasensor Targeting Cardiac Insufficiency in Artificial Saliva. Talanta 2023, 256, 124280. [Google Scholar] [CrossRef]
- Schuck, A.; Kim, H.E.; Kang, M.; Kim, Y.S. Rapid Detection of Inflammation-Related Biomarkers Using an Electrochemical Sensor Modified with a PBNC-AuNS-GO-Based Nanocomposite. ACS Appl. Electron. Mater. 2022, 4, 4587–4596. [Google Scholar] [CrossRef]
- Ganguly, A.; Gunda, V.; Thai, K.; Prasad, S. Inflammatory Stimuli Responsive Non-Faradaic, Ultrasensitive Combinatorial Electrochemical Urine Biosensor. Sensors 2022, 22, 7757. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kim, E.S.; Mishra, S.; Ganbold, E.; Seong, R.S.; Kaushik, A.K.; Kim, N.Y. Ultrasensitive probeless capacitive biosensor for amyloid beta (Aβ1-42) detection in human plasma using interdigitated electrodes. Biosens. Bioelectron. 2022, 212, 114365. [Google Scholar] [CrossRef] [PubMed]
- Georgas, A.; Lampas, E.; Houhoula, D.P.; Skoufias, A.; Patsilinakos, S.; Tsafaridis, I.; Patrinos, G.P.; Adamopoulos, N.; Ferraro, A.; Hristoforou, E. ACE2-based capacitance sensor for rapid native SARS-CoV-2 detection in biological fluids and its correlation with real-time PCR. Biosens. Bioelectron. 2022, 202, 114021. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.N.; Kim, D.; Phan, L.M.T.; Cho, S. Ultrasensitive capacitance sensor to detect amyloid-beta 1-40 in human serum using supramolecular recognition of β-CD/RGO/ITO micro-disk electrode. Talanta 2022, 237, 122907. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kim, E.S.; Mishra, S.; Ganbold, E.; Seong, R.S.; Kaushik, A.K.; Kim, N.Y. Ultrasensitive and Reusable Graphene Oxide-Modified Double-Interdigitated Capacitive (DIDC) Sensing Chip for Detecting SARS-CoV-2. ACS Sens. 2021, 6, 3468–3476. [Google Scholar] [CrossRef]
- Hwang, C.; Park, N.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.; Kim, N.Y.; Kim, J.H. Ultra-fast and recyclable DNA biosensor for point-of-care detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177. [Google Scholar] [CrossRef]
- Piccoli, J.P.; Soares, A.C.; Oliveira, O.N., J.; Cilli, E.M. Nanostructured functional peptide films and their application in C-reactive protein immunosensors. Bioelectrochemistry 2021, 138, 107692. [Google Scholar] [CrossRef]
- Huang, Y.W.; Wu, C.S.; Chuang, C.; Pang, S.; Pan, T.; Yang, Y.S.; Ko, F. Real-time and label-free detection of the prostate-specific antigen in human serum by a polycrystalline silicon nanowire field-effect transistor biosensor. Anal. Chem. 2013, 85, 7912–7918. [Google Scholar] [CrossRef]
- Abouzar, M.H.; Poghossian, A.; Siqueira, J.R.; Oliveira, O.N.; Moritz, W.; Schöning, M. Capacitive electrolyte–insulator–semiconductor structures functionalised with a polyelectrolyte/enzyme multilayer: New strategy for enhanced field-effect biosensing. Phys. Status Solidi A 2010, 207, 884–890. [Google Scholar] [CrossRef]
- Moraes, A.C.M.; Kubota, L. Recent Trends in Field-Effect Transistors-Based Immunosensors. Chemosensors 2016, 4, 20. [Google Scholar] [CrossRef]
- Chu, C.; Sarangadharan, I.; Regmi, A.; Chen, Y.W.; Hsu, C.P.; Chang, W.H.; Lee, G.Y.; Chyi, J.; Chen, C.; Shiesh, S.; et al. Beyond the Debye length in high ionic strength solution: Direct protein detection with field-effect transistors (FETs) in human serum. Sci. Rep. 2017, 7, 5256. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chen, Y.W.; Lu, M.S. CMOS Biosensors for the Detection of DNA Hybridization in High Ionic-Strength Solutions. IEEE Sens. J. 2021, 21, 4135–4142. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Z.; Lv, X.; Chen, H.; Geng, Y.; Geng, Z. Sensitivity-Enhanced Nanoplasmonic Biosensor Using Direct Immobilization of Two Engineered Nanobodies for SARS-CoV-2 Spike Receptor-Binding Domain Detection. Sens. Actuators B Chem. 2023, 383, 133575. [Google Scholar] [CrossRef]
- Yan, H.; Fu, J.; Tang, X.; Wang, D.; Zhang, Q.; Li, P. Sensitivity Enhancement of Paper-Based Sandwich Immunosensor via Nanobody Immobilization Instead of IgG Antibody, Taking Aflatoxigenic Fungi as an Analyte Example. Sens. Actuators B Chem. 2022, 373, 132760. [Google Scholar] [CrossRef]
- Wen, P.; Su, H.; Yin, W.J.; Hu, J.C.; Wang, Y.; Yang, J.Y.; Xiao, Z.L.; Xu, Z.L.; Shen, Y.D.; Wang, H.; et al. Fabrication of a High-Sensitivity Electrochemical Immuno-Sensor by the Oriented Immobilization of Engineered Nanobody on Nanofibrous Membrane. SSRN Electron. J. 2024, 191, 712. [Google Scholar] [CrossRef]
- Rutten, I.; Daems, D.; Lammertyn, J. Boosting Biomolecular Interactions through DNA Origami Nano-Tailored Biosensing Interfaces. J. Mater. Chem. B 2020, 8, 3606–3615. [Google Scholar] [CrossRef]
- Poghossian, A.; Karschuck, T.L.; Wagner, P.H.; Schöning, M. Field-Effect Capacitors Decorated with Ligand-Stabilized Gold Nanoparticles: Modeling and Experiments. Biosensors 2022, 12, 334. [Google Scholar] [CrossRef]
- Koch, C.; Poghossian, A.; Schöning, M.; Wege, C. Penicillin Detection by Tobacco Mosaic Virus-Assisted Colorimetric Biosensors. Nanotheranostics 2018, 2, 184–196. [Google Scholar] [CrossRef]
- Nam, U.; Suh, H.N.; Sung, S.K.; Seo, C.; Lee, J.H.; Lee, J.Y.; Kim, S.; Lee, J. Rapid and High-Density Antibody Immobilization Using Electropolymerization of Pyrrole for Highly Sensitive Immunoassay. ACS Appl. Mater. Interfaces 2024, 16, 30611–30621. [Google Scholar] [CrossRef]
- Lin, P.H.; Huang, S.C.; Chen, K.P.; Li, B.R.; Li, Y.K. Effective Construction of a High-Capacity Boronic Acid Layer on a Quartz Crystal Microbalance Chip for High-Density Antibody Immobilization. Sensors 2019, 19, 28. [Google Scholar] [CrossRef]
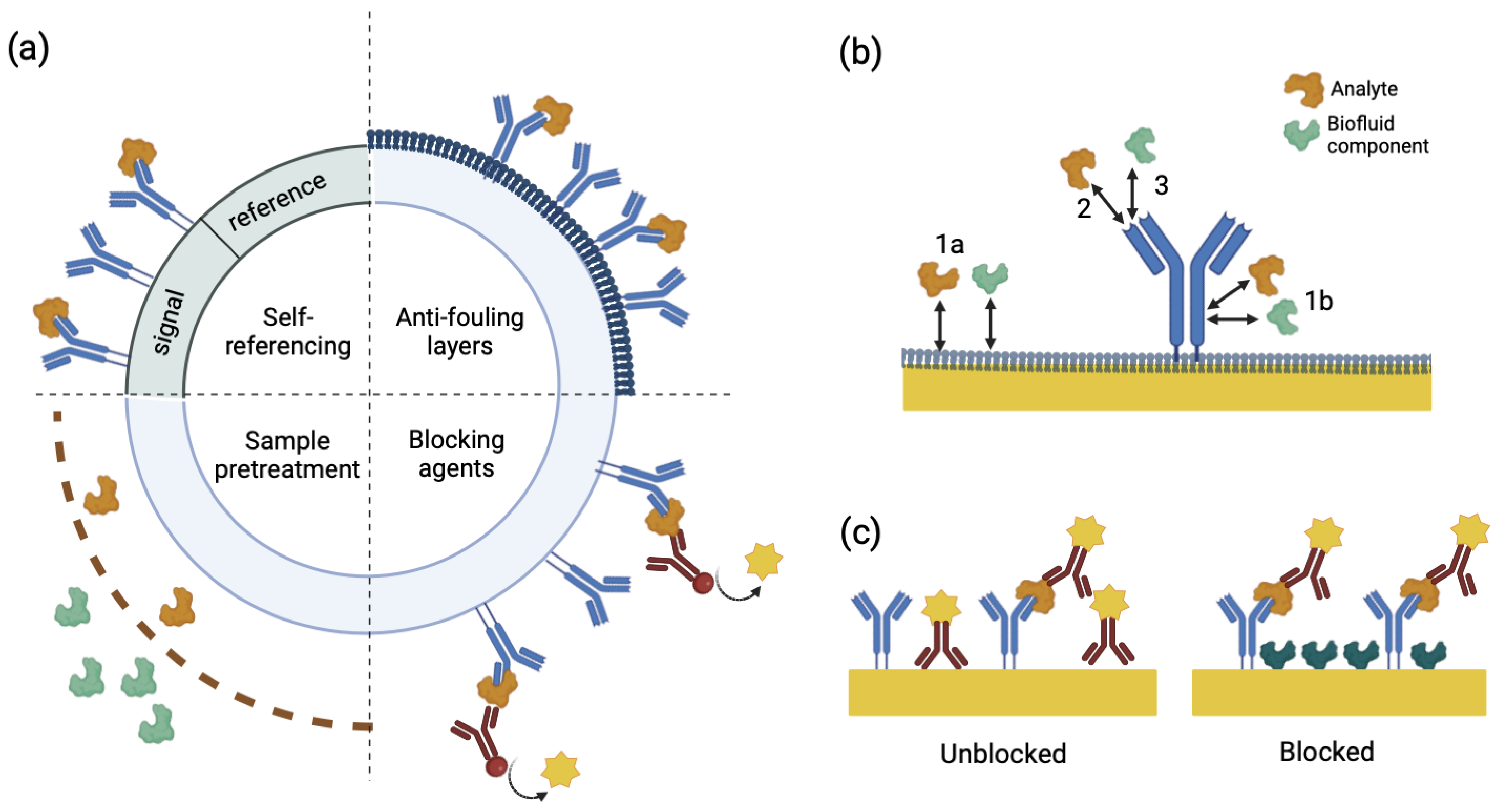



| Features | Specific Binding | Nonspecific Binding |
|---|---|---|
| Intermolecular forces | Short-range forces (van der Waals/ acid base interactions) | Long-range forces (electrostatic/hydrophobic) |
| Structural (interface architecture) |
|
|
|
| |
|
|
| Attribute | Criteria |
|---|---|
| Signal loss between interrogation cycles | <5% |
| Number of continual cycles achieved | >10 |
| Restoration of baseline signal | <±5% |
| Biosensor/transducer reconstruction | Avoided |
| Signal loss profile | Linear, allowing accurate calibration |
| Regeneration conditions | Explicitly listed with incubation time and full buffer components |
| Biofluid | Electrode Design | Insulating Layer | Recognition Element | Disease | LoD | Ref., Year |
|---|---|---|---|---|---|---|
| Human Plasma | IDEs | Polypyrrole | Antibody | Traumatic Brain Injury | 0.184, 0.339 fg/mL | [98], 2025 |
| Human Plasma | Glassy Carbon Electrode | rGO-AuNPs-Thionine | Antibody | Thrombotic Disorders | 0.184, 0.339 fg/mL | [99], 2025 |
| Synthetic Human Sweat | AuNP-functionalized LIG-IDE | MUA SAM Layer | Antibody | Cortisol | 0.0085 nM | [100], 2025 |
| Urine and Nasal Samples | IDEs | APTES | Aptamer | SARS-CoV-2 | 10 fg/mL | [101], 2025 |
| PBS | Carbon SPEs | Acrylic Resin, Chitosan and Nitrocellulose Membrane | BSA Molecule | Anti-BSA Antibodies | 2.5 mg/mL | [102], 2024 |
| Human Serum | Au SPEs | Cys/DCC & DMAP | Yellow Fever Antigen | Yellow Fever Antibodies | 96 ag/mL | [103], 2024 |
| Saliva (spiked) | Au Working Electrode | SAM | Anti-A29 Monoclonal Antibody | Monkeypox (A29 Protein) | 1.8 ng/mL | [104], 2024 |
| Blood Serum | SPCEs with MNC substrate | SAM | Anti-CEA Antibodies | Cancer | 9.04 pg/mL | [105], 2024 |
| Artificial CSF | Carbon SPEs with Au Microflowers | SAM | Antibody | Parkinson’s | 0.207 ng/mL | [106], 2024 |
| Human Serum | Au IDEs | SAM; EDC/NHS | SARS-CoV2 Spike Protein | COVID-19 | 21 ng/mL | [107], 2024 |
| Human Serum | Au SPEs | SAM | Aptamer | Zika Virus, Dengue | 41.8 fg/mL | [108], 2024 |
| Human Serum | rGO electrodes + AuNPs | Physiosorption | Antibody | Cancer | 8.9 U/mL | [109], 2024 |
| Serum | AuSPEs | Mesoporous Silica Nanoparticles (SiNPs) | Antibody | Ovarian Cancer | 0.02–20 pM (HE4) | [110], 2023 |
| Artificial Urine | IDEs | Ta2O5 + Biotin/ Streptavidin | Antibodies | Kidney Tubular Damage | 0.5 ng/mL–8 ng/mL | [111], 2023 |
| Serum | ITO electrode with APTES/nMoS2 NS@rGO Nanohybrid | APTES Silanization | Antibody | Cancer (Sp17 Biomarker) | 0.13 ng/mL (Amperometric), 0.23 ng/mL (EIS) | [112], 2023 |
| 10% Human Serum | Au MEAs | SAM, EDC/NHS; SAM (MCH) | Nanobody; Aptamers | Interleukin-6, IL-6 | 10 pg/mL | [113], 2023 |
| Human Serum | IMA | SAM (APTES, MUOH), EDC/NHS | (S-Protein) of SARS-CoV-2 | SARS-CoV-2 | 0.4 BAU/mL | [114], 2023 |
| Serum | Fe-Cu LDH/rGO Nanocomposite on PGE | Physiosorption | Anti-PSA Antibodies | Prostate Cancer | [115], 2023 | |
| Artificial Human Saliva | Au Microelectrode Array | MCH Co-immobilization | Aptamer | NT-proBNP | – pg/mL; LOD = pg/mL | [116], 2023 |
| Viral Culture Fluid | IDEs + Parylene | PEDOT:PSS | Antibody | SARS-CoV-2 Nucleoprotein | 4.1 ng/mL | [26], 2022 |
| Serum | Au Nanostars | Prussian Blue Nanocubes, Graphene Oxide | Antibodies | Inflammation/ Septic shock | Linear Response with R2 > 0.99 | [117], 2022 |
| Serum | IDME | Carboxylayed CNFs + EDC/NHS | Taenia Solium Antigen (rT24H) | Anti-rT24H Antibodies | 24.1 fg/mL | [55], 2022 |
| Urine | Au SPEs | DSP Crosslinker | Antibody | IL6, IL8 | 1 pg/mL | [118], 2022 |
| Human Plasma | IDEs | APTS-Pt/Ti–SiO2 IDCs | o-A Aptamer, Antibody | A42 Oligomers | 0.1 fg/ mL | [119], 2022 |
| Saliva | Au IDEs | SAM (L-Cys) | ACE2 protein | SARS-CoV-2 | 750 pg/L/mm2 | [120], 2022 |
| Serum | ITO microdisk | -CD/RGO nanohybrid | Antibody | A40 | 0.69 fg/mL | [121], 2022 |
| Blood | GrO-glazed DIDC | EDC-NHS | Antibody | SARS-CoV-2 | 1.0 fg/mL | [122], 2021 |
| Diluted Serum | Au IDEs | SAM | ssDNA | miRNA-16b | 1.0 fg/mL | [51], 2021 |
| DI Water | Pt/Ti IDEs | APTES | DNA Probe | SARS-CoV-2 | 10 ng/mL | [123], 2021 |
| PBS | Au electrodes | SAM (Fc-Glu-(Ala)n-Cys-NH2) | Antibody | Inflammation | 240 pg/mL | [124], 2021 |
| Immobilization Strategy | Advantages | Drawbacks |
|---|---|---|
| Covalent Binding | Strong and stable immobilization; low risk of receptor loss; avoids physical desorption | May reduce receptor conformational flexibility; steric hindrance potential |
| Physical Adsorption | Simple, low cost, and easily reversible | Weak attachment; high risk of desorption; unstable under high ionic strength conditions |
| Polyelectrolyte Multilayers | Enhances receptor density; maintains receptor hydration; improves stability and bioactivity | May lead to receptor aggregation; variability in reproducibility; complex fabrication processes |
| Self-Assembled Monolayers | Precise surface control; reduces nonspecific adsorption; improves receptor orientation | Requires optimized surface chemistries; fabrication can be time-intensive |
| Nanoparticle Assisted Immobilization | High receptor density; enhanced stability and signal amplification; potential for reusable surfaces | Additional functionalization required for nanomaterials; scalability challenges |
| Encapsulation Strategies (Zwitterionic Polymers) | Protects bioreceptors from harsh environmental factors; ensures hydration; extends receptor longevity | Potential diffusion limitations may reduce reaction kinetics; integration with sensors can be complex |
| Affinity Based (Protein A/G-Based Binding) | Exploits highly specific affinity interactions; ensures correct Fab orientation of antibodies | Primary reliance on affinity stability; not suitable for all bioreceptors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekhon, S.; Bayford, R.; Demosthenous, A. Capacitive Sensors for Label-Free Detection in High-Ionic-Strength Bodily Fluids: A Review. Biosensors 2025, 15, 491. https://doi.org/10.3390/bios15080491
Sekhon S, Bayford R, Demosthenous A. Capacitive Sensors for Label-Free Detection in High-Ionic-Strength Bodily Fluids: A Review. Biosensors. 2025; 15(8):491. https://doi.org/10.3390/bios15080491
Chicago/Turabian StyleSekhon, Seerat, Richard Bayford, and Andreas Demosthenous. 2025. "Capacitive Sensors for Label-Free Detection in High-Ionic-Strength Bodily Fluids: A Review" Biosensors 15, no. 8: 491. https://doi.org/10.3390/bios15080491
APA StyleSekhon, S., Bayford, R., & Demosthenous, A. (2025). Capacitive Sensors for Label-Free Detection in High-Ionic-Strength Bodily Fluids: A Review. Biosensors, 15(8), 491. https://doi.org/10.3390/bios15080491







