Microfluidic System Based on Flexible Structures for Point-of-Care Device Diagnostics with Electrochemical Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Reagents
2.3. Solutions
2.4. The Tox Gene Fragment Amplification in Standard PCR Mode
2.5. The Tox Gene Fragment Amplification in Asymmetric PCR Mode (aPCR)
3. Results and Discussion
3.1. PCR Microchip
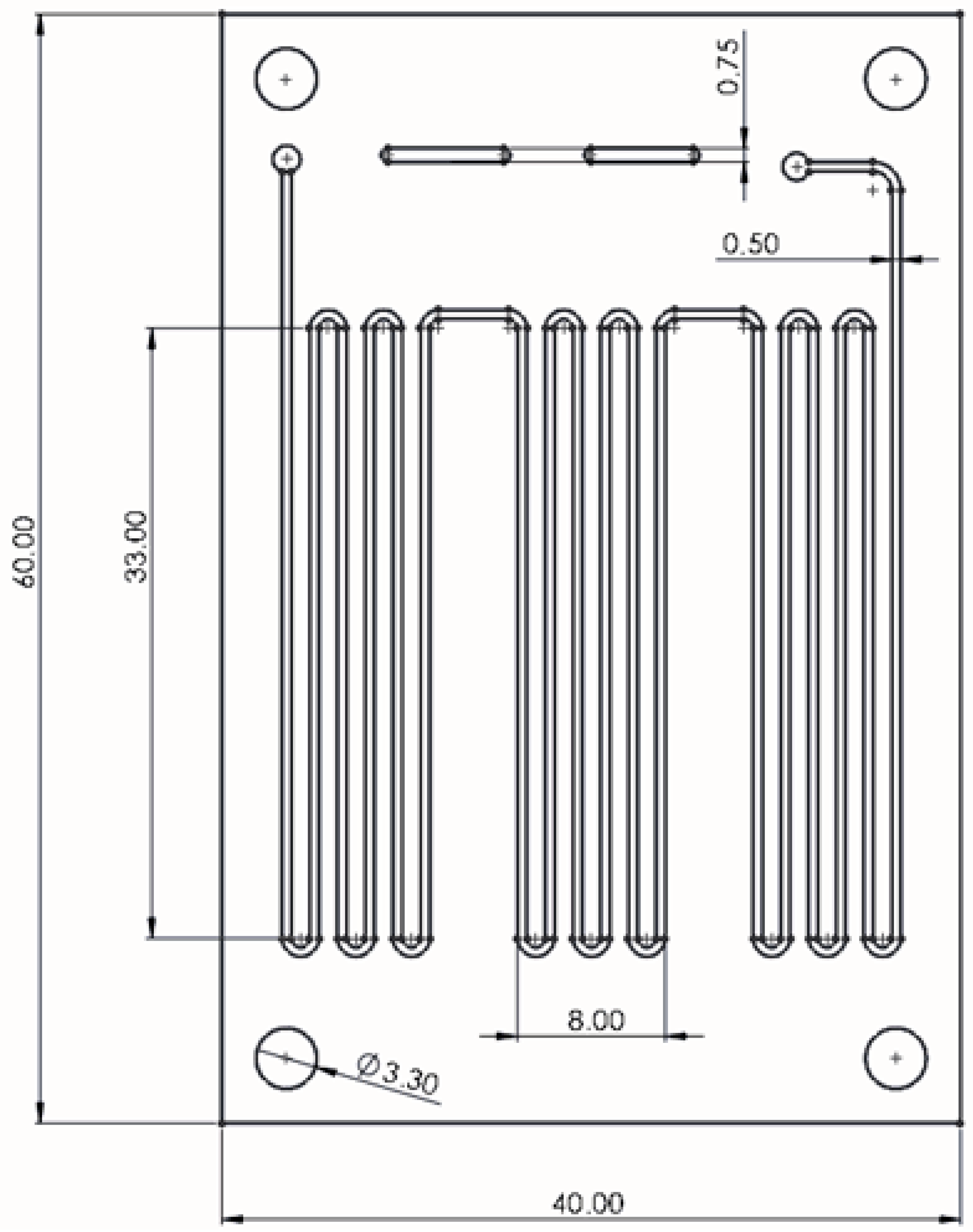
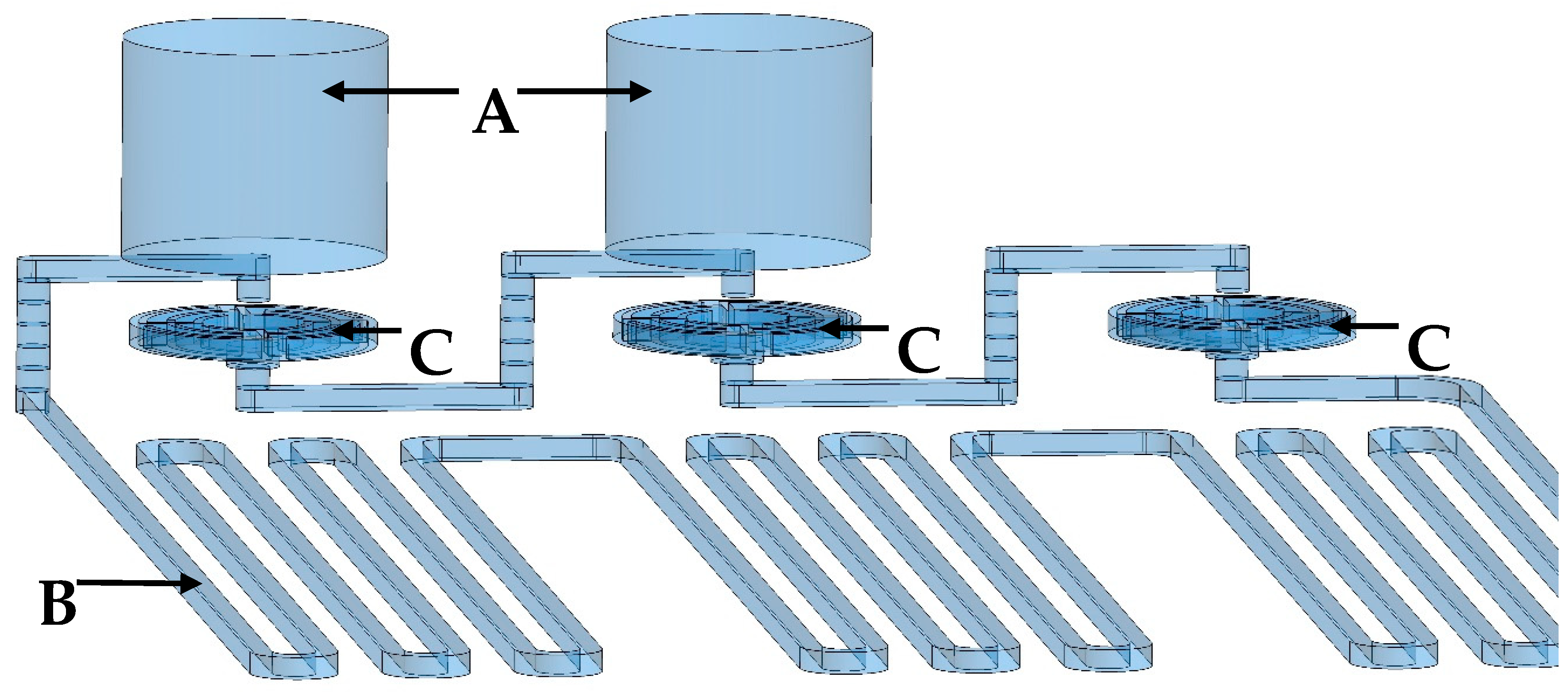
3.1.1. Design and Fabrication of PCR Microfluidic Device
3.1.2. Heating System and Temperature Control
3.1.3. Optimization of PCR Reaction
Flow Rate Optimization
Reaction Mixture Optimization
3.1.4. DNA and Polymerase Adsorption—Prevention Methods
3.1.5. Electrophoretic Separation and Detection of PCR Products
3.1.6. PCR Reaction Carried out in Thermocycler vs. in PCR Microfluidic Device
3.2. Electrochemical Sensor
3.2.1. Fabrication of Biosensor’s Transducers on a Flexible Substrate
3.2.2. Biosensor Receptor Layer Preparation
3.2.3. Rapid Detection of Tox Gene Fragment as a Product of Real Asymmetric PCR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://immunizationdata.who.int/global/wiise-detail-page/diphtheria-reported-cases-and-incidence?GROUP=WHO_REGIONS&YEAR= (accessed on 3 January 2025).
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S. A Comparison of Optical, Electrochemical, Magnetic, and Colorimetric Point-of-Care Biosensors for Infectious Disease Diagnosis. ACS Infect. Dis. 2018, 4, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.; Hu, C.; Wang, Y.; Wang, L.; Peng, F.; Geng, P.; Guan, M. Recent advancements in microfluidic chip biosensor detection of foodborne pathogenic bacteria: A review. Anal. Bioanal. Chem. 2022, 414, 2883–2902. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Cong, H.; Hassan, J.; Gonzalez, G.; Gilchrist, M.D.; Zhang, N. Pathogen detection on microfluidic platforms: Recent advances, challenges, and prospects. Biosens. Bioelectron. X 2022, 10, 100134. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Rauch, C.B.; Stevens, R.L.; Liu, R.H.; Lenigk, R.; Grodzinski, P. High sensitivity PCR assay in plastic micro reactors. Lab Chip 2002, 2, 179. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Duarte, G.R.; Poe, B.L.; Riehl, P.S.; dos Santos, F.M.; Martin-Didonet, C.C.; Carrilho, E.; Landers, J.P. A disposable laser print-cut-laminate polyester microchip for multiplexed PCR via infra-red-mediated thermal control. Anal. Chim. Acta 2015, 901, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Liao, M.H.; Li, K.T.; Shen, C.M. One-heater flow-through polymerase chain reaction device by heat pipes cooling. Biomicrofluidics 2015, 9, 014107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Lee, J.Y.; Seong, S.; Cha, S.H.; Lee, S.H.; Kim, J.J.; Park, T.H. Fabrication and characterization of a PDMS–glass hybrid continuous-flow PCR chip. Biochem. Eng. J. 2006, 29, 91–97. [Google Scholar] [CrossRef]
- Schaerli, Y.; Wootton, R.C.; Robinson, T.; Stein, V.; Dunsby, C.; Neil, M.A.; French, P.M.; DeMello, A.J.; Abell, C.; Hollfelder, F. Continuous-Flow Polymerase Chain Reaction of Single-Copy DNA in Microfluidic Microdroplets. Anal. Chem. 2009, 81, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Ball, C.S.; Renzi, R.F.; Priye, A.; Meagher, R.J. A simple check valve for microfluidic point of care diagnostics. Lab Chip 2016, 16, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, N.; Warkiani, M.E.; Gong, T.H. Acetylated bovine serum albumin differentially inhibits polymerase chain reaction in microdevices. Biomicrofluidics 2017, 11, 034110, Published 17 May 2017. [Google Scholar] [CrossRef] [PubMed]
- Marchlewicz, K.; Ostrowska, I.; Oszwałdowski, S.; Zasada, A.; Ziółkowski, R.; Malinowska, E. Molecular diagnostic of toxigenic Corynebacterium diphtheriae strain by DNA sensor potentially suitable for electrochemical point-of-care diagnostic. Talanta 2021, 227, 122161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]







| Temperature of the Heating Surfaces [°C] | Measurement of Temperature by Pt100 Sensors [°C] |
|---|---|
| 101 | 97 |
| 75 | 73 |
| 56 | 55 |
| Reaction Mixture | Concentration |
|---|---|
| Primer F (5′-CAA TCA TCG TCA TAA TTT CCT TGT GTA CC-3′) | 1250 nM |
| Primer R (5′-GAA AAC TTT TCT TCG TAC CAC GGG ACT AA-3′) | 5000 nM |
| HotStartTaq Polymerase | X * |
| dNTP | 200 µM |
| MgCl2 | 1.5 mM |
| Template DNA | ≈0.023 ng/µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchlewicz, K.; Ziółkowski, R.; Żukowski, K.; Krzemiński, J.; Malinowska, E. Microfluidic System Based on Flexible Structures for Point-of-Care Device Diagnostics with Electrochemical Detection. Biosensors 2025, 15, 483. https://doi.org/10.3390/bios15080483
Marchlewicz K, Ziółkowski R, Żukowski K, Krzemiński J, Malinowska E. Microfluidic System Based on Flexible Structures for Point-of-Care Device Diagnostics with Electrochemical Detection. Biosensors. 2025; 15(8):483. https://doi.org/10.3390/bios15080483
Chicago/Turabian StyleMarchlewicz, Kasper, Robert Ziółkowski, Kamil Żukowski, Jakub Krzemiński, and Elżbieta Malinowska. 2025. "Microfluidic System Based on Flexible Structures for Point-of-Care Device Diagnostics with Electrochemical Detection" Biosensors 15, no. 8: 483. https://doi.org/10.3390/bios15080483
APA StyleMarchlewicz, K., Ziółkowski, R., Żukowski, K., Krzemiński, J., & Malinowska, E. (2025). Microfluidic System Based on Flexible Structures for Point-of-Care Device Diagnostics with Electrochemical Detection. Biosensors, 15(8), 483. https://doi.org/10.3390/bios15080483







