Progress in Electrode Materials for the Detection of Nitrofurazone and Nitrofurantoin
Abstract
1. Introduction
2. Nitrofurazone (NFZ) Sensors
2.1. Metal–Organic Framework (MOF)-Based Materials for NFZ Sensing
2.2. Carbon Derivative-Based Materials for NFZ Sensors
2.3. Metal Oxide-Based Materials for NFZ Sensors
2.4. Other Material-Based NFZ Sensors
3. Nitrofurantoin (NFT) Sensors
3.1. Carbon Derivative-Based Materials for NFT Sensing
3.2. Metal Oxide-Based Materials for NFT Sensing
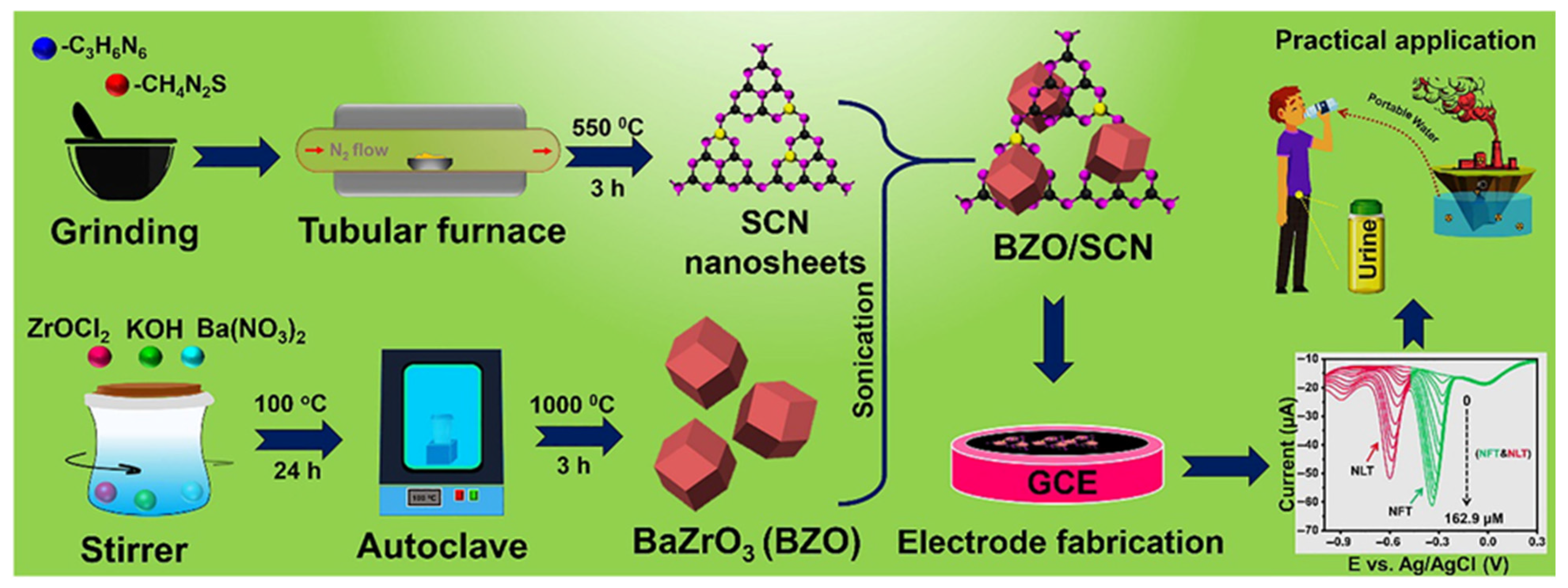
3.3. Polymers/Metal Sulfides/LDH/MXenes
3.4. Other Materials for NFT Sensing
4. Conclusions and Perspectives
- MXene-based materials are promising next-generation electrode materials, but have major concerns such as harsh conditions of HF for etching treatments.
- MOF-based materials have a larger surface area with high porosity, but their limited conductivity is one of the major concerns.
- The development of cost-effective and environmental friendly methods are of great significance to fabricate the novel hybrid electrode materials.
- LDH/Mene and MOF-based hybrid composites should be optimized for NFZ and NFT sensing applications.
- The long-term stability, real-time monitoring, and selectivity should be improved for practical applications.
- The depth mechanism for NFZ and NFT should be studied on MXene-based materials.
- The simultaneous detection of NFZ and NFT with appropriate differentiation should be studied.
- The fabricated NFZ and NFT sensors should be miniaturized and incorporated into portable devices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatima, S.; Hossain, M.E.; Alnour, M.; Kanwal, S.; Rehman, M.Z.; Esquivias, M.A. Assessing the Damage to Environmental Pollution: Discerning the Impact of Environmental Technology, Energy Efficiency, Green Energy and Natural Resources. Sustainability 2024, 16, 9307. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Porter, N.; Collins, R. Fungicides in English Rivers: Widening the Understanding of the Presence, Co-Occurrence and Implications for Risk Assessment. Environments 2025, 12, 45. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Scaccia, N.; Barbosa Segundo, I.; Diniz, D.d.S.; Diniz, J.U.; Mierzwa, J.C. Evaluation of the Pathway of Contaminants in the Environment: A Case Study of Different Aquatic Environmental Compartments. Sustainability 2024, 16, 3927. [Google Scholar] [CrossRef]
- Samrot, A.V.; Wilson, S.; Sanjay Preeth, R.S.; Prakash, P.; Sathiyasree, M.; Saigeetha, S.; Shobana, N.; Pachiyappan, S.; Rajesh, V.V. Sources of Antibiotic Contamination in Wastewater and Approaches to Their Removal—An Overview. Sustainability 2023, 15, 12639. [Google Scholar] [CrossRef]
- Burnham, J.P. The Antimicrobial Resistance–Water–Corporate Interface: Exploring the Connections Between Antimicrobials, Water, and Pollution. Trop. Med. Infect. Dis. 2025, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Kulik, K.; Lenart-Boroń, A.; Wyrzykowska, K. Impact of Antibiotic Pollution on the Bacterial Population within Surface Water with Special Focus on Mountain Rivers. Water 2023, 15, 975. [Google Scholar] [CrossRef]
- Wu-Wu, J.W.F.; Guadamuz-Mayorga, C.; Oviedo-Cerdas, D.; Zamora, W.J. Antibiotic Resistance and Food Safety: Perspectives on New Technologies and Molecules for Microbial Control in the Food Industry. Antibiotics 2023, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Musuka, G.; Machakwa, J.; Mano, O.; Iradukunda, P.G.; Gashema, P.; Moyo, E.; Nsengimana, A.; Manhokwe, S.; Dhliwayo, T.; Dzinamarira, T. Antimicrobial Resistance and Its Impact on Food Safety Determinants Along the Beef Value Chain in Sub-Saharan Africa—A Scoping Review. Trop. Med. Infect. Dis. 2025, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Adenaya, A.; Adeniran, A.A.; Ugwuoke, C.L.; Saliu, K.; Raji, M.A.; Rakshit, A.; Ribas-Ribas, M.; Könneke, M. Environmental Risk Factors Contributing to the Spread of Antibiotic Resistance in West Africa. Microorganisms 2025, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Niu, C.; Xie, Z.; Tan, N. Fluorescence sensor for nitrofurazone using 4-methyl-7-allyloxynaphtho[1,2-b]pyran-2-ketone as sensing carrier. J. Anal. Chem. 2010, 65, 260–266. [Google Scholar] [CrossRef]
- Vercelli, C.; Amadori, M.; Gambino, G.; Re, G. Does Nitrofurantoin Improve the Portfolio of Vets against Resistant Bacteria in Companion Animals? Antibiotics 2023, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Machado, J.; Peixe, L. Illegal use of nitrofurans in food animals: Contribution to human salmonellosis? Clin. Microbiol. Infect. 2006, 12, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, X.L.; Feng, D.W.; Xie, L.H.; Zhang, J.; Li, M.; Xie, Y.B.; Li, J.R.; Zhou, H.C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.W.; Liu, Z.; Lian, G.; Wang, L.; Wang, Q.L.; Zhan, J.H. High reliable and robust ultrathin-layer gold coating porous silver substrate via galvanic-free deposition for solid phase microextraction coupled with surface enhanced Raman spectroscopy. Anal. Chim. Acta 2017, 994, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.Q.; Chen, M.M.; Chen, S.S.; Du, N.N.; Liu, Z.-D.; Song, C.-F.; Qiao, R. High-performance liquid chromatography with fluorescence detection for the determination of nitrofuran metabolites in pork muscle. Food Addit. Contam. Part A 2013, 30, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Q.L.; Wang, B.L.; Li, D.D.; Yu, J.H. Fluorescent sensors based on AIEgen-functionalised mesoporous silica nanoparticles for the detection of explosives and antibioticsm. Inorg. Chem. Front. 2018, 5, 2183–2188. [Google Scholar] [CrossRef]
- Frigoli, M.; Krupa, M.P.; Hooyberghs, G.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Electrochemical Sensors for Antibiotic Detection: A Focused Review with a Brief Overview of Commercial Technologies. Sensors 2024, 24, 5576. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Li, J.; Xu, L.; Yao, Y.; Liu, Y. Construction of a voltammetric sensor based on MIL-101 hollow cages for electrocatalytic oxidation and sensitive determination of nitrofurazone. J. Electroanal. Chem. 2019, 848, 113287. [Google Scholar] [CrossRef]
- Wang, H.; Bo, X.; Zhou, M.; Guo, L. DUT-67 and tubular polypyrrole formed a cross-linked network for electrochemical detection of nitrofurazone and ornidazole. Anal. Chim. Acta 2020, 1109, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, Y.; Zhong, J.; Lu, Z.; Wang, G.; Sun, M.; Jiang, Y.; Zou, P.; Wang, X.; Zhao, Q.; et al. Molecularly imprinted electrochemical sensor based on biomass carbon decorated with MOF-derived Cr2O3 and silver nanoparticles for selective and sensitive detection of nitrofurazone. Chem. Eng. J. 2020, 398, 125664. [Google Scholar] [CrossRef]
- Rani, R.; Deep, A.; Mizaikoff, B.; Singh, S. Zirconium metal organic framework based opto-electrochemical sensor for nitrofurazone detection. J. Electroanal. Chem. 2022, 909, 116124. [Google Scholar] [CrossRef]
- Leelasree, T.; Aggarwal, H. Design and investigation of a new two-dimensional cobalt metal–organic framework for highly sensitive electrochemical detection of the nitrofurazone drug in food and biological samples. Mater. Adv. 2025, 6, 1423–1430. [Google Scholar] [CrossRef]
- He, B.; Li, M. Rapid Detection of Nitrofuran and Its Metabolites by Using Carboxylic Multi-walled Carbon Nanotubes Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2018, 13, 4171–4181. [Google Scholar] [CrossRef]
- Brito, C.L.; Ferreira, E.I.; La-Scalea, M.A. Application of multi-walled carbon nanotubes functionalized with hemin to evaluate the electrochemical behavior of nitrofurazone in aqueous media. Electrochim. Acta 2023, 459, 142486. [Google Scholar] [CrossRef]
- Cai, S.; Jiao, T.; Wang, L.; Wang, F.; Chen, Q. Electrochemical sensing of nitrofurazone on Ru(bpy)32+ functionalized polyoxometalate combined with graphene modified electrode. Food Chem. 2022, 378, 132084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Sheng, X.; Xie, H.; Zhou, S.; Huang, L.; Zhang, Z.; Zhu, Y.; Zhong, M. Molecularly Imprinted Electrochemistry Sensor Based on AuNPs/RGO Modification for Highly Sensitive and Selective Detection of Nitrofurazone. Food Anal. Methods 2023, 16, 709–720. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, J.; Liu, L.; Wu, K. Electrochemical detection of nitrofurazone using laser-engraved three-electrode graphene array. Anal. Chim. Acta 2024, 1317, 342898. [Google Scholar] [CrossRef] [PubMed]
- Kokulnathan, T.; Vishnuraj, R.; Wang, T.-J.; Pullithadathil, B. Multidimensional nanoarchitectures of TiO2/Au nanofibers with O-doped C3N4 nanosheets for electrochemical detection of nitrofurazone. Appl. Surf. Sci. 2022, 604, 154474. [Google Scholar] [CrossRef]
- Keyan, A.K.; Sakthinathan, S.; Vasu, D.; Yu, C.-L.; Vinothini, S.; Chiu, T.-W. Gadolinium molybdate decorated graphitic carbon nitride composite: Highly visualized detection of nitrofurazone in water samples. RSC Adv. 2022, 12, 34066–34079. [Google Scholar] [CrossRef] [PubMed]
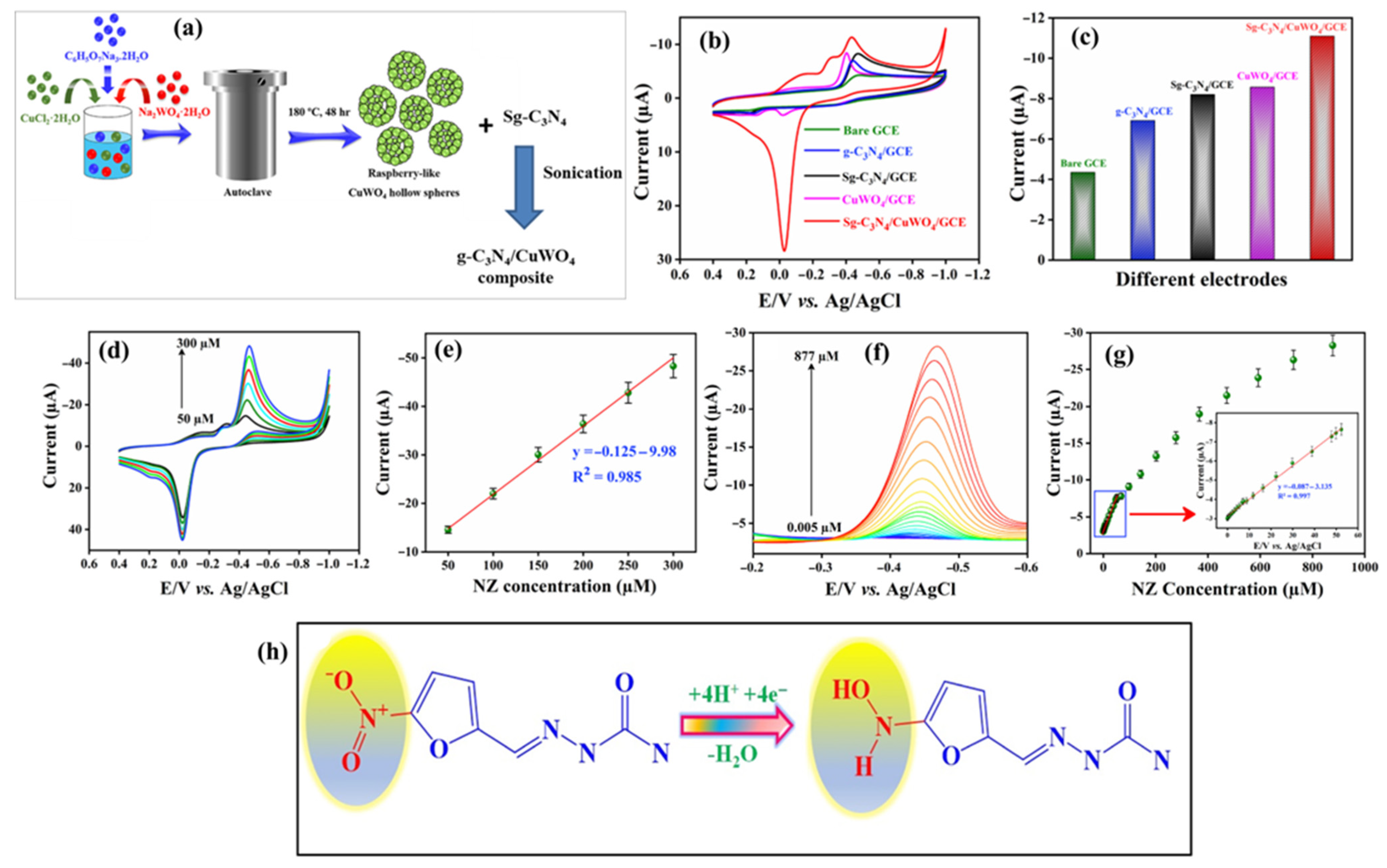
- Anupriya, J.; Rajakumaran, R.; Chen, S.M.; Karthik, R.; Kumar, J.V.; Shim, J.-J.; Shafi, P.M.; Lee, J.-W. Raspberry-like CuWO4 hollow spheres anchored on sulfur-doped g-C3N4 composite: An efficient electrocatalyst for selective electrochemical detection of antibiotic drug nitrofurazone. Chemosphere 2022, 296, 133997. [Google Scholar] [CrossRef] [PubMed]
- Srinithi, S.; Anupriya, J.; Chen, S.-M.; Balakumar, V. Ultrasonic fabrication of neodymium oxide@titanium carbide modified glassy carbon electrode: An efficient electrochemical detection of antibiotic drug nitrofurazone. J. Taiwan Inst. Chem. Eng. 2022, 139, 104522. [Google Scholar] [CrossRef]
- Nair, V.S.; Kokulnathan, T.; Wang, T.J.; Vishnuraj, R.; Dinesh, H.; Rangarajan, M. Hydrothermal synthesis of iron titanate hexagonal nanoplates for electrochemical detection of nitrofurazone. Microchim. Acta 2024, 191, 245. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, R.; Chen, S.-M. Orthorhombic dysprosium iron oxide decorated sulfur-doped graphitic carbon nitride for the selective electrochemical sensing of nitrofurazone in water samples. J. Alloy. Compd. 2025, 1011, 178469. [Google Scholar] [CrossRef]
- Liu, C.; Lv, L.; Sun, Y.; Di, X. Specific temperature-modulated crab shell-derived porous carbon as a typical recycling material for nitrofurazone electrochemical sensor. Microporous Mesoporous Mater. 2024, 374, 113143. [Google Scholar] [CrossRef]
- Rahi, A.; Sattarahmady, N.; Vais, R.D.; Heli, H. Sonoelectrodeposition of gold nanorods at a gold surface—Application for electrocatalytic reduction and determination of nitrofurazone. Sens. Actuators B Chem. 2015, 210, 96–102. [Google Scholar] [CrossRef]
- Zoubir, J.; Assabbane, A.; Bakas, I. Electroanalysis of nirofurazone at silver particles and graphite powder composite electrode. Carbon Lett. 2022, 32, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.-Y.; Liu, Y.-R.; Huang, T.-H.; Chen, Y.-H.; Hsieh, Y.-T. Electrochemical Sensing of Nitrofurazone and Semicarbazide on an Au-Ag Film Fabricating from a Deep Eutectic Solvent. J. Electrochem. Soc. 2022, 169, 107502. [Google Scholar] [CrossRef]
- Lu, H.; Liu, M.; Cui, H.; Huang, Y.; Li, L.; Ding, Y. An advanced molecularly imprinted electrochemical sensor based bifunctional monomers for highly sensitive detection of nitrofurazone. Electrochim. Acta 2022, 427, 140858. [Google Scholar] [CrossRef]
- Zoubir, J.; Radaa, C.; Bougdour, N.; Idlahcen, A.; El Hayaoui, W.; Tajat, N.; El Mouhri, W.; Nadif, I.; Qourzal, S.; Tamimi, M.; et al. A sensor based on silver nanoparticles synthesized on carbon graphite sheets for the electrochemical detection of nitrofurazone: Application: Tap water, commercial milk and human urine. J. Indian Chem. Soc. 2022, 99, 100590. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Sriram, B.; Muthukutty, B.; Zheng, L.; Wang, S.-F.; Choe, H.; Kwon, K. Layered metal chalcogenide of SnSe nanosheets integrated with 2D-hexagonal boron nitride for accurate and low-level detection of nitrofurazone. Chem. Eng. J. 2023, 455, 140521. [Google Scholar] [CrossRef]
- Yazdani, Z.; Vais, R.D.; Heli, H. Synthesis of nanoflakes-covered microparticles of nickel-Application for electrocatalytic oxidation and quantitation of nitrofurazone. Mater. Chem. Phys. 2024, 322, 129549. [Google Scholar] [CrossRef]
- Silva, F.W.L.; Bernardino, C.A.R.; Ferreira, J.H.A.; Mahler, C.F.; Santelli, R.E.; Canevari, T.C.; Cincotto, F.H. Disposable electrochemical sensor: Highly sensitive determination of nitrofurazone antibiotic in environmental samples and pharmaceutical formulations. Chemosphere 2024, 361, 142481. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Liu, H. Electrochemical determination of nitrofuran residues at gold nanoparticles/graphene modified thin film gold electrode. Microchem. J. 2019, 150, 104108. [Google Scholar] [CrossRef]
- Velmurugan, S.; Palanisamy, S.; Yang, T.C.-K.; Gochoo, M.; Chen, S.-W. Ultrasonic assisted functionalization of MWCNT and synergistic electrocatalytic effect of nano-hydroxyapatite incorporated MWCNT-chitosan scaffolds for sensing of nitrofurantoin. Ultrason. Sonochem. 2020, 62, 104863. [Google Scholar] [CrossRef] [PubMed]
- Hwa, K.Y.; Sharma, T.S.K. Nano assembly of NiFe spheres anchored on f-MWCNT for electrocatalytic reduction and sensing of nitrofurantoin in biological samples. Sci. Rep. 2020, 10, 12256. [Google Scholar] [CrossRef] [PubMed]
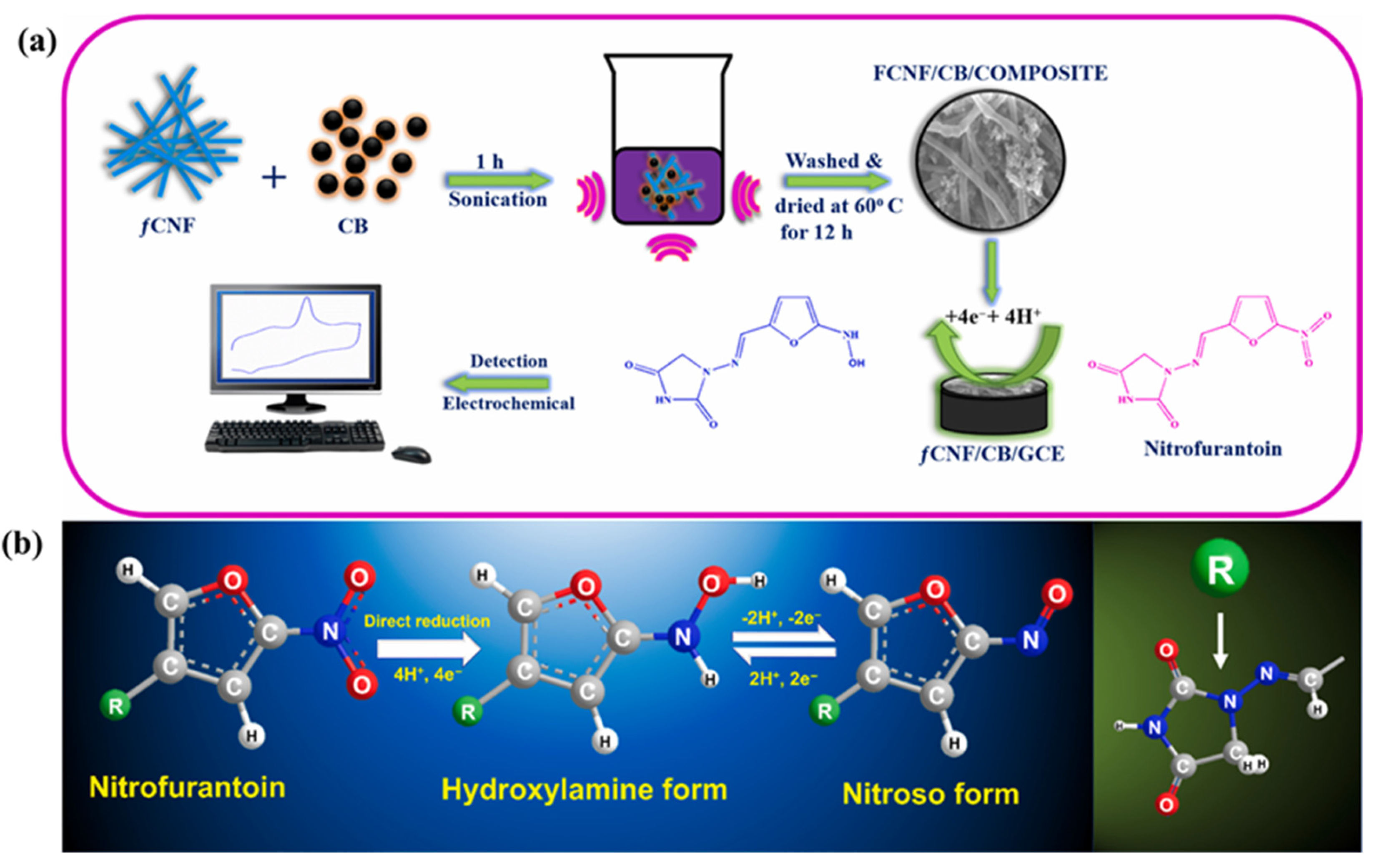
- Haidyrah, A.S.; Sundaresan, P.; Venkatesh, K.; Ramaraj, S.K.; Thirumalraj, B. Fabrication of functionalized carbon nanofibers/carbon black composite for electrochemical investigation of antibacterial drug nitrofurantoin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127112. [Google Scholar] [CrossRef]
- Prado, N.S.; Silva, L.A.J.; Takeuchi, R.M.; Richter, E.M.; dos Santos, A.L.; Falcão, E.H.L. Graphite sheets modified with poly(methylene blue) films: A cost-effective approach for the electrochemical sensing of the antibiotic nitrofurantoin. Microchem. J. 2022, 177, 107289. [Google Scholar] [CrossRef]
- Li, M.; Zhe, T.; Li, R.; Bai, F.; Jia, P.; Xu, Z.; Wang, X.; Bu, T.; Wu, H.; Wang, L. ZIF-derived Co nanoparticles embedded into N-doped carbon nanotube composites for highly efficient electrochemical detection of nitrofurantoin in food. Food Chem. 2023, 418, 135948. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.X.T.; Dang, T.-D.; Cao, H.H.; La, D.C. Self-Assembled porphyrin on Fe/Graphene: A Versatile electrode for Nitrofurantoin antibiotic sensing. Microchem. J. 2025, 212, 113418. [Google Scholar] [CrossRef]
- Qian, X.; Li, C.; Zheng, M.; Wang, J.; Huang, H.; Deng, K. Bimetallic MOF derived electrocatalyst with magnetic alloy nanoparticles confined in S, N-doped carbon nanotubes: A disposable magnetic sensor for simultaneous determination of antibiotics in food. Food Chem. 2025, 465, 142141. [Google Scholar] [CrossRef] [PubMed]
- Selvi, S.V.; Rajakumaran, R.; Chen, S.-M.; Rady, A.M.; Veerasankar, S.; Chen, T.-W.; Rwei, S.-P.; Lou, B.-S. Graphene/Tungsten trioxide (Gr/WO3) composite modified screen-printed carbon electrode for the sensitive electrochemical detection of nitrofurantoin in biological samples. Int. J. Electrochem. Sci. 2019, 14, 6454–6467. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J. Synthesis and characterization of 3D flower-like nickel oxide entrapped on boron doped carbon nitride nanocomposite: An efficient catalyst for the electrochemical detection of nitrofurantoin. Compos. Part B Eng. 2019, 174, 106914. [Google Scholar] [CrossRef]
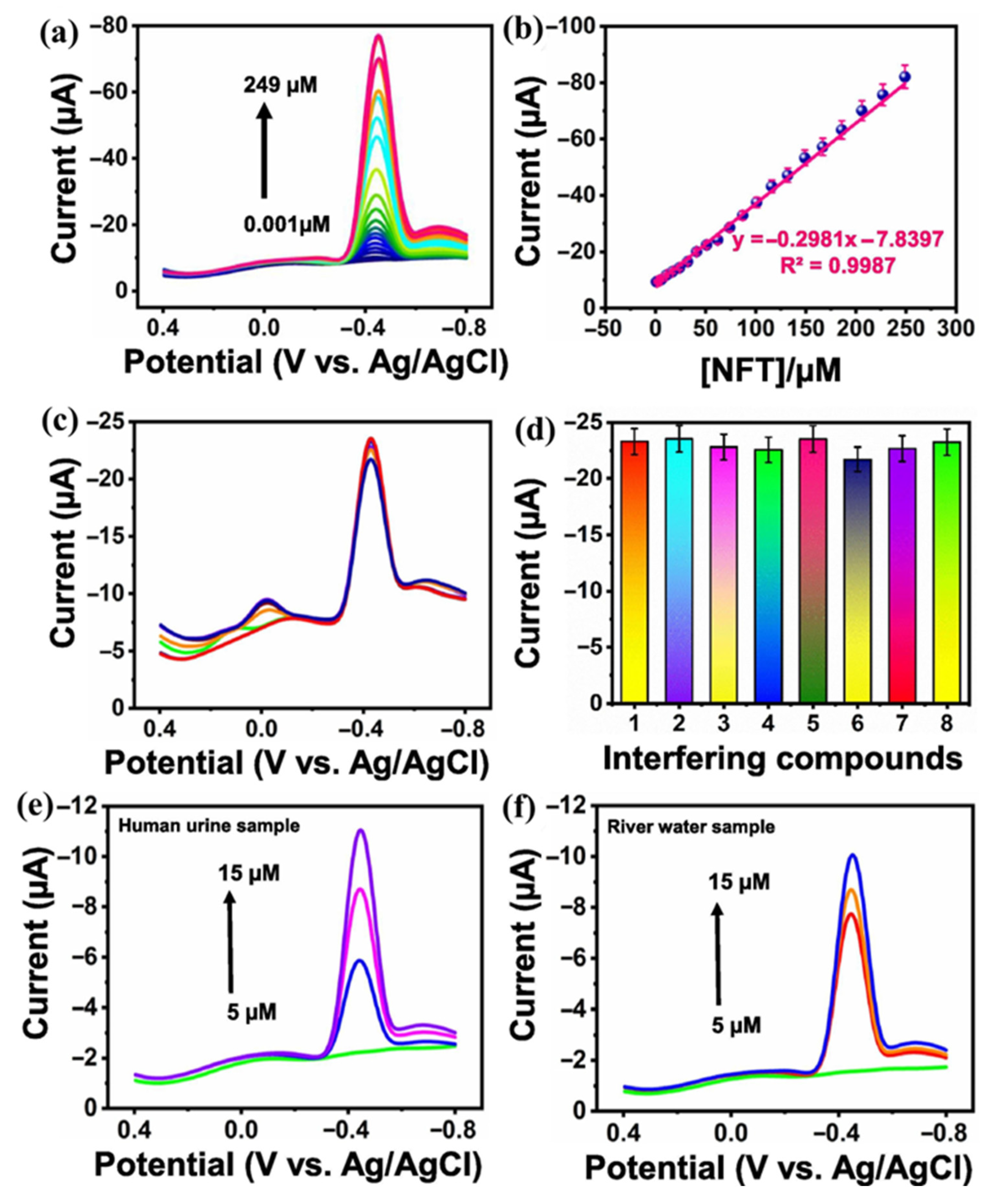
- Mariyappan, V.; Keerthi, M.; Chen, S.-M.; Jeyapragasam, T. Nanostructured perovskite type gadolinium orthoferrite decorated RGO nanocomposite for the detection of nitrofurantoin in human urine and river water samples. J. Colloid Interface Sci. 2021, 600, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, N.; Chen, S.-M. An electrochemical assay for the detection of nitrofurantoin based on bismuth titanate enclosed carbon nanofiber in environmental and biological samples. J. Electroanal. Chem. 2021, 887, 115152. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Hsu, Y.-F.; Wang, S.-F.; George, M.; Veerakumar, P.; Lin, K.-C. Electrochemical sensor-based barium zirconate on sulphur-doped graphitic carbon nitride for the simultaneous determination of nitrofurantoin (antibacterial agent) and nilutamide (anticancer drug). J. Electroanal. Chem. 2021, 901, 115782. [Google Scholar] [CrossRef]
- Sudha, K.; Elangovan, A.; Senthilkumar, S.; Jeevika, A.; Arivazhagan, G. Electrocatalytic reduction of nitrofurantoin in biological sample based on assembly of ScMo anchored f-MCNNcs modified GCE. Microchem. J. 2022, 172, 106943. [Google Scholar] [CrossRef]
- Li, M.; Zhe, T.; Li, F.; Li, R.; Bai, F.; Jia, P.; Bu, T.; Xu, Z.; Wang, L. Hybrid structures of cobalt-molybdenum bimetallic oxide embedded in flower-like molybdenum disulfide for sensitive detection of the antibiotic drug nitrofurantoin. J. Hazard. Mater. 2022, 435, 129059. [Google Scholar] [CrossRef] [PubMed]
- Babulal, S.M.; Koventhan, C.; Chen, S.M.; Hung, W. Construction of sphere like samarium vanadate nanoparticles anchored graphene nanosheets for enhanced electrochemical detection of nitrofurantoin in biological fluids. Compos. Part B Eng. 2022, 237, 109847. [Google Scholar] [CrossRef]
- Sridharan, G.; Godwin, C.J.T.; Atchudan, R.; Arya, S.; Govindasamy, M.; Osman, S.M.; Sundramoorthy, A.K. Iron oxide decorated hexagonal boron nitride modified electrochemical sensor for the detection of nitrofurantoin in human urine samples. J. Taiwan Inst. Chem. Eng. 2024, 163, 105320. [Google Scholar] [CrossRef]
- Alagumalai, K.; Munusamy, N.; Chen, S.M.; Vivekanandan, A.K.; Chen, S.-H.; Sivakumar, M.; Kim, S.-C.; Prakash, B. High-performance electrochemical sensors with SnBi2O3/Graphene oxide nanocomposite for selective antibiotic detection. J. Taiwan Inst. Chem. Eng. 2025, 171, 106033. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthik, R.; Chen, S.-M.; Chen, K.-H.; Sakthinathan, S.; Muthuraj, V.; Chiu, T.-W. Design of novel 3D flower-like neodymium molybdate: An efficient and challenging catalyst for sensing and destroying pulmonary toxicity antibiotic drug nitrofurantoin. Chem. Eng. J. 2018, 346, 11–23. [Google Scholar] [CrossRef]
- Dechtrirat, D.; Yingyuad, P.; Prajongtat, P.; Chuenchom, L.; Sriprachuabwong, C.; Tuantranont, A.; Tang, I.-M. A screen-printed carbon electrode modified with gold nanoparticles, poly(3,4-ethylenedioxythiophene), poly(styrene sulfonate) and a molecular imprint for voltammetric determination of nitrofurantoin. Microchim. Acta 2018, 185, 261. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, V.; Kesavan, G.; Chen, S.-M. Graphitic carbon nitride nanosheets incorporated with polypyrrole nanocomposite: A sensitive metal-free electrocatalyst for determination of antibiotic drug nitrofurantoin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127433. [Google Scholar] [CrossRef]
- Vilian, A.E.; Hwang, S.-K.; Bhaskaran, G.; Alhammadi, M.; Kim, S.; Tiwari, J.N.; Huh, Y.S.; Han, Y.-K. Polypyrrole-MXene supported gold nanoparticles for the trace-level detection of nitrofurantoin. Chem. Eng. J. 2023, 454, 139980. [Google Scholar] [CrossRef]
- Tumrani, S.H.; Neiber, R.R.; Pitafi, Z.; Ahmed, I.A.; Soomro, R.A.; Ibrahim, M.M.; Karakuş, S.; El-Bahy, Z.M. Synergistic integration of few-layer thick MXenes and small Pd nanocubes for enhanced electrochemical nitrofurantoin detection: Implications in pharmaceutical pollutant monitoring. J. Environ. Chem. Eng. 2023, 11, 111152. [Google Scholar] [CrossRef]
- Shui, X.; Ma, H.; Zhang, Y.; Zeng, T.; Yang, J.; Wu, Z.; Zhang, X.; Yang, N. Multi-core–shell nanoboxes comprising polydopamine-derived C coated NiCo@C and FeCo@C nanohybrid for enhanced electrochemical quantification of nitrofurantoin. Chem. Eng. J. 2024, 500, 156714. [Google Scholar] [CrossRef]
- Liu, M.; Zhe, T.; Li, F.; Zhu, L.; Ouyang, S.; Wang, L. An ultrasensitive electrochemical sensor based on NiFe-LDH-MXene and ruthenium nanoparticles composite for detection of nitrofurantoin in food samples. Food Chem. 2024, 461, 140915. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhe, T.; Zhu, L.; Li, F.; Ma, K.; Xuan, C.; Feng, Q.; Ouyang, S.; Wang, L. Interfacial engineering of VS2/Ti3C2Tx MXene heterojunctions for portable electrochemical sensing of nitrofurantoin residues in food and water samples. Food Chem. 2025, 486, 144624. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Rahmati, Z. Development of electrochemical sensor based on molecularly imprinted copolymer for detection of nitrofurantoin. J. Iran. Chem. Soc. 2019, 16, 999–1006. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Annalakshmi, M.; Chen, S.-M.; Sathesh, T.; Balamurugan, T.S.T. Ultrasonic energy-assisted preparation of β-cyclodextrin-carbon nanofiber composite: Application for electrochemical sensing of nitrofurantoin. Ultrason. Sonochem. 2019, 52, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ezazi, M.; Asadpour-Zeynali, K.; Saeb, E. Synergistic incorporation of Ag into nickel hydroxide nanostructure to enhance the electroctrocatalytic determination of nitrofurantoin. Inorg. Chem. Commun. 2024, 160, 111913. [Google Scholar] [CrossRef]
- Adane, W.D.; Chandravanshi, B.S.; Tessema, M. A pioneering electrochemical sensor for the simultaneous determination of nitrofurantoin and furazolidone residues in food and municipal wastewater samples. Sens. Bio-Sens. Res. 2024, 45, 100678. [Google Scholar] [CrossRef]
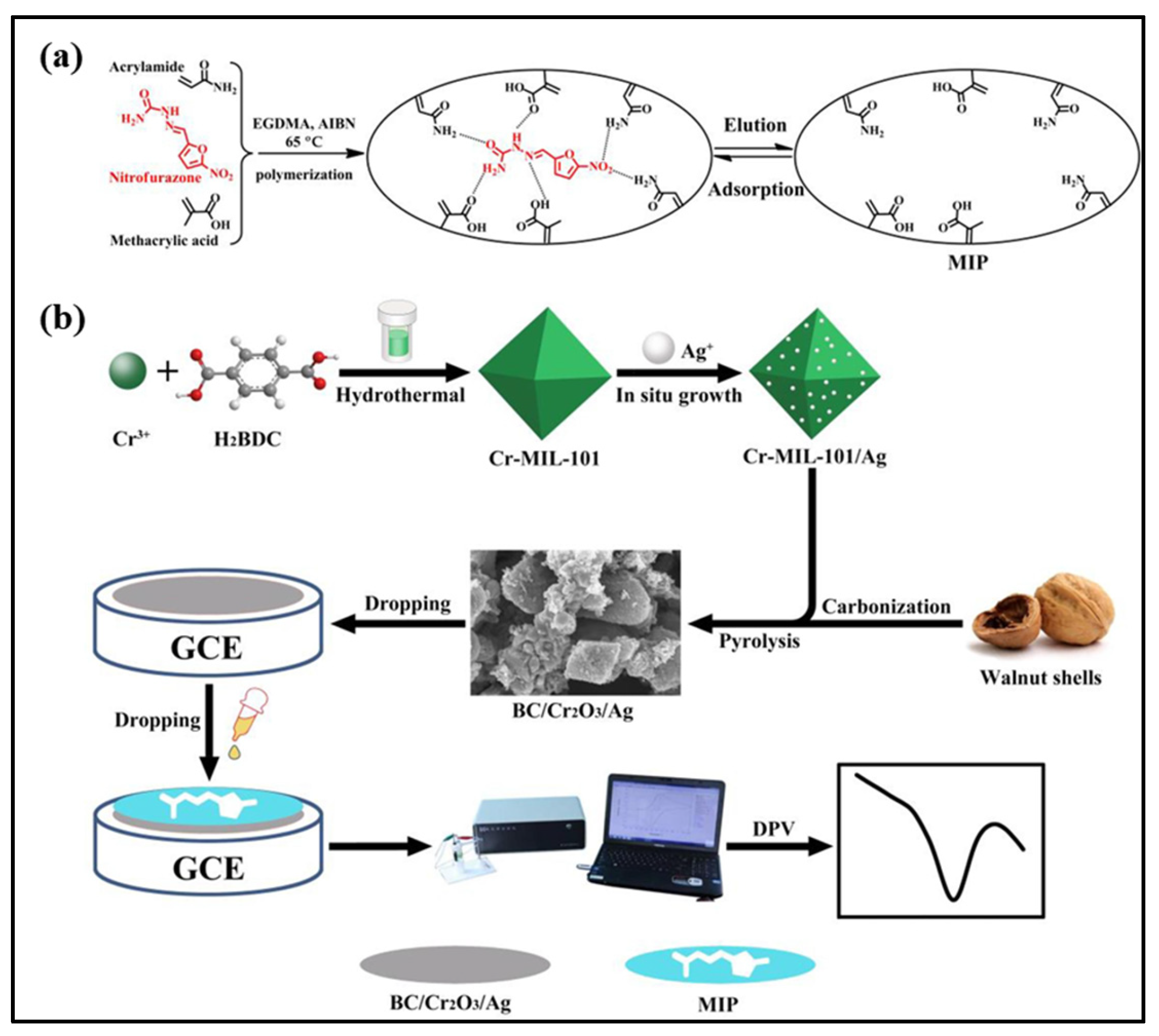
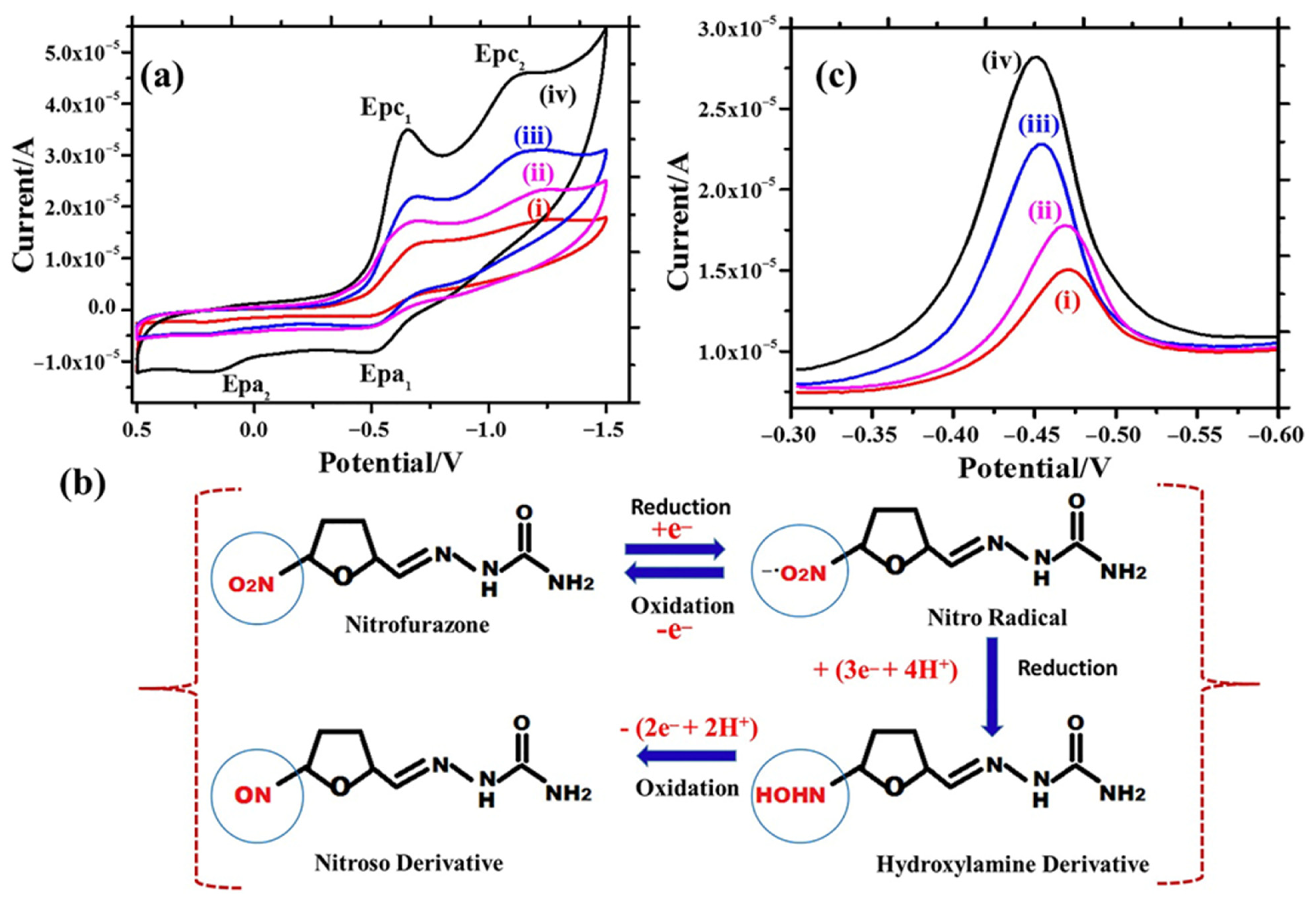
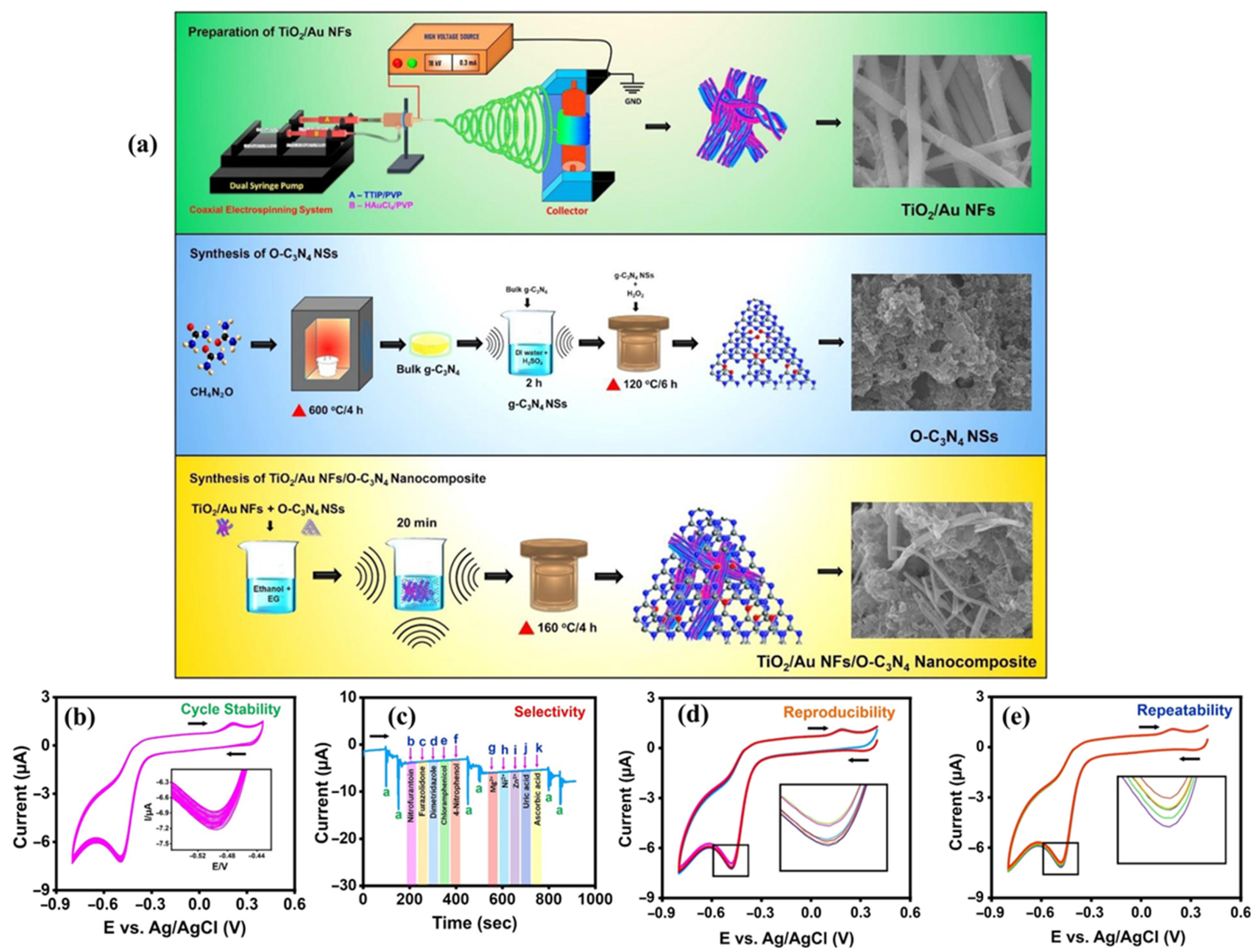

| Materials/Electrode | Synthesis Method | LD (µM) | LDR (µM) | Sensitivity | Detection technique | Real Sample | References |
|---|---|---|---|---|---|---|---|
| Hollow MIL-101/GCE | Crystal growth/acid etching | 0.01 | 0.030 to 55 | - | DPV | Milk, honey, and crayfish | [21] |
| DUT-67/T-PPY-2/GCE | In situ growth | 8.7 | 9.08 to 354.08; 354.08 to 1004.04 | 0.175 μA/μM | DPV | Milk and lake water | [22] |
| BC/Cr2O3/Ag/MIP/GCE | Precipitation polymerization/sonication | 0.003 | 0.005 to 10 | - | DPV | Blood and urine | [23] |
| AuNPs/UiO-66-NH2/SPCE | Solvothermal | 0.003 | 0.01 to 50 | - | DPV | Lake water | [24] |
| 2D-Co MOF/GCE | Slow evaporation (80 °C; 5 days) | 0.04 | 0 to 25 | - | SWV | Food and biological samples | [25] |
| MWCNTs/COOH/GCE | Mixed acid phase oxidation | 0.224 | - | - | AMP (i-t) | Pork liver sample | [26] |
| [Ru-PMo12/PDDA-GO]3 | Electrodeposition | 0.08952 | - | - | Chronoamperometry | Crayfish | [28] |
| Au NPs/rGO/MIP | Electropolymerization | 0.00018 | 0.005 to 1 | - | DPV | Milk | [29] |
| LIG-50 array | Laser engraving technique | 0.035 | 0.2 to 8 | - | LSV | Fish meat | [30] |
| TiO2/Au-NFs/O-C3N4/GCE | Solvothermal | 0.001 | 0.008 to 105 | 1.40 µA/µM.cm2 | AMP | Human urine and river water | [31] |
| g-C3N4/Gd2MoO6/GCE | Co-precipitation/ultrasonication | 0.006 | 0.02 to 2000 | 2.057 µA/µM.cm2 | DPV | Milk and human urine | [32] |
| Sg–C3N4/CuWO4/GCE | Hydrothermal/sonication | 0.003 | 0.005 to 52.305 | 1.24 µA/µM.cm2 | DPV | Urine and blood serum samples | [33] |
| NdO@TC/GCE | Ultrasonication | 0.0027 | 0.01 to 2231 | 0.1914 µA/µM.cm2 | DPV | Urine, blood serum, and tap water | [34] |
| FeTiO3/GCE | Hydrothermal | 0.002 | 0.01 to 162.2 | 0.551 µA/µM.cm2 | - | - | [35] |
| DFO/S-g-C3N4/GCE | Reflux/sonication | 0.0071 | 0.025 to 180.13 | 2.929 µA/µM.cm2 | AMP (i-t) | Lake and river water | [36] |
| Crab shell (CS)/GCE | High temperature heating | 0.11 | 0.40 to 80 | 0.55 μA/μM | DPV | Compound cod liver ointment | [37] |
| Au-AuNR | Sonoelectrodeposition | 0.18 | 3 to 500 | - | DPV | Urine and blood serum | [38] |
| Ag-NPs@CPE | - | 0.01 | - | - | - | Urine and tap water | [39] |
| Au-Ag modified electrode | Electrodeposition | 0.2 | 1.99 to 643.49 | - | - | - | [40] |
| Ag-NPs@CPE | Calcination | 0.012 | 0.2 to 100 | - | DPV | Urine, tap water and milk | [42] |
| SnSe/h-BN/GCE | Solvothermal | 0.00034 | 0.001 to 12.12; 15.2 to 342.2 | 1.927 µA/µM.cm2 | AMP (i-t) | Urine and water | [43] |
| NMCN-CPE | Hydrothermal | 4.3 | 30 to 570 | - | AMP | Ointments | [44] |
| Materials | Synthesis Method | LD (µM) | LDR (µM) | Sensitivity | Detection Technique | Real Sample | References |
|---|---|---|---|---|---|---|---|
| HA NPs/MWCNT-CHI/GCE | Hydrothermal/ultrasonication | 0.0013 | 0.005 to 982.1 | - | AMP | Water and pharmaceutical tablet | [47] |
| NiFe/f-MWCNT/SPCE | Hydrothermal/ultrasonication | 0.03 | - | 11.45 µA/µM.cm2 | DPV | Human serum and urine | [48] |
| f-CNF/CB/GCE | Sonication | 0.016 | 0.05 to 104.66 | 15.25 µA/µM.cm2 | AMP | - | [49] |
| GS/PMB | Electropolymerization | 0.055 | 5 to 100 | 0.297 µA/µM | DPV | Urine and tap water | [50] |
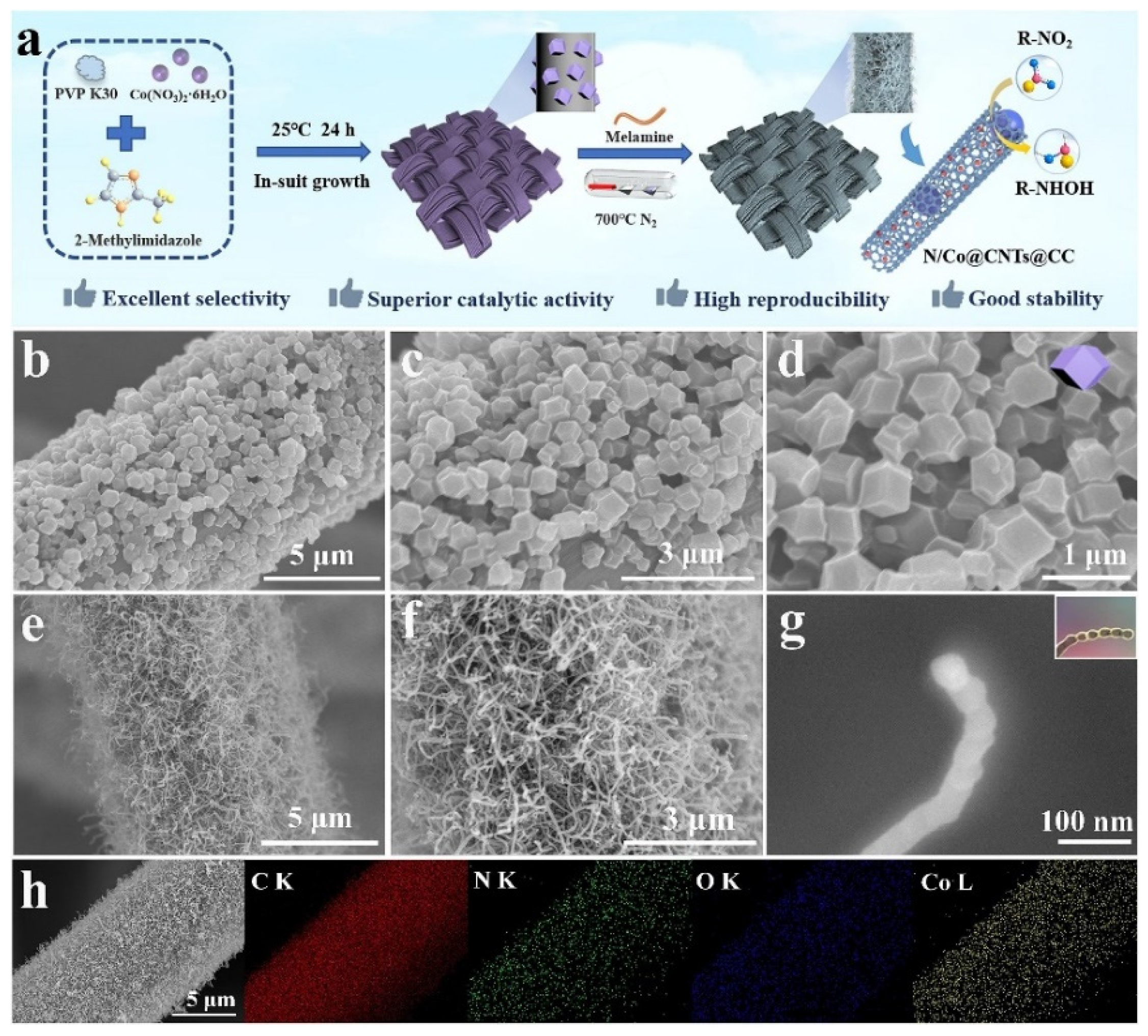
| N/Co@CNTs@CC-II | in-situ growth and sublimation-gas phase | 0.01841 | - | 8.19 µA/µM.cm2 | LSV | Milk and tap water | [51] |
| Fe/Graphene/Porphyrin/GCE | Self-assembled method | 0.246 | 0.5 to 200 | 0.9808 µA/µM.cm2 | DPV | Milk | [52] |
| FeCo@S,N-CNTs | Thermal treatment | 0.0035 | 0.01 to 75.0 | - | DPV | Honey, egg, fish, chicken, and milk | [53] |
| Gr/WO3/SPCE | Calcination | 0.002 | 0.01 to 234 | 2.18 µA/µM.cm2 | LSV | Urine and tap water | [54] |
| NiO/BCN | Polycondensation/hydrothermal | 0.01 | 0.05 to 230 | - | AMP | Urine | [55] |
| GdFeO3/RGO | Hydrothermal | 0.0153 | 0.001 to 249 | 4.1985 µA/µM.cm2 | DPV | Urine and river water | [56] |
| BT/CNF | Hydrothermal | 0.005 | 0.06 to 450 | 1.76 µA/µM.cm2 | AMP (i-t) | Urine, river water, and blood serum | [57] |
| BZO/SCN/GCE | Hydrothermal/sonication | 0.002 | 0.09 to 260.9 | 35.73 µA/µM.cm2 | DPV | Water | [58] |
| ScMo@f-MCN Ncs/GCE | Ultrasonication | 0.0093 | 0.01 to 180 | 0.5136 µA/µM.cm2 | DPV | Blood serum, urine, and lake water | [59] |
| Co2Mo3O8/MoS2@CC | Hydrothermal | 0.0119 | 100 to 700 | 27.6 µA/µM.cm2 | LSV | Milk, honey, and tap water | [60] |
| SVG-2/GCE | Co-precipitation/Ultrasonication | 0.0087 | 0.0035 to 672.3 | - | AMP (i-t) | Blood serum and urine | [61] |
| α-Fe2O3/h-BN | Hydrothermal | 0.015 | 0.025 to 22.95 | 2.36 µA/µM.cm2 | AMP | Urine | [62] |
| SnBi2O3/GO/GCE | Hydrothermal/sonication | 0.0124 | 0.023 to 814.36 | 2.857 µA/µM.cm2 | DPV | Urine and tap water | [63] |
| NdM/SPCE | Sol-gel | 0.016 | 0.1 to 21; 28 to 481 | µA/µM.cm2 | DPV | River water, lake water, and urine | [64] |
| g-C3N4/PPy | Sonochemical | 0.005 | 0.04 to 585.2 | 7.813 µA/µM.cm2 | DPV | Urine and blood serum | [66] |
| Au-PPy-MXene-GCE | Sonication | 0.00026 | 6 to 172 | - | AMP | Pond water, honey, and hospital wastewater | [67] |
| Pd–Ti3C2Tx–P | - | 0.00001 | 0.001 to 0.14 | - | DPV/AMP | Urine and hospital waste effluent | [68] |
| NiCo@C/FeCo@C@C/GCE | Polymerization/pyrolysis | 0.014 | 0.05 to 100 | - | DPV | Lake water | [69] |
| Ru/NiFe-LDH-MXene/SPCE | Electrodeposition | 0.0022 | 0.01 to 275 | 152.44 µA/µM.cm2 | LSV | Honey and milk | [70] |
| VS2/Ti3C2Tx/SPCE | Liquid phase mixing/annealing | 0.0047 | 0.01 to 400 | - | LSV | Milk, lake water, honey, and tap water | [71] |
| β-CD/CNF | Ultrasonication | 0.0018 | 0.004 to 308 | - | AMP | Blood serum, tablet, and urine sample | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.; Ali, S.; Ahmad, K.; Danishuddin. Progress in Electrode Materials for the Detection of Nitrofurazone and Nitrofurantoin. Biosensors 2025, 15, 482. https://doi.org/10.3390/bios15080482
Aslam M, Ali S, Ahmad K, Danishuddin. Progress in Electrode Materials for the Detection of Nitrofurazone and Nitrofurantoin. Biosensors. 2025; 15(8):482. https://doi.org/10.3390/bios15080482
Chicago/Turabian StyleAslam, Mohammad, Saood Ali, Khursheed Ahmad, and Danishuddin. 2025. "Progress in Electrode Materials for the Detection of Nitrofurazone and Nitrofurantoin" Biosensors 15, no. 8: 482. https://doi.org/10.3390/bios15080482
APA StyleAslam, M., Ali, S., Ahmad, K., & Danishuddin. (2025). Progress in Electrode Materials for the Detection of Nitrofurazone and Nitrofurantoin. Biosensors, 15(8), 482. https://doi.org/10.3390/bios15080482





