Recent Advances in Liquid Metal-Based Stretchable and Conductive Composites for Wearable Sensor Applications
Abstract
1. Introduction
2. Fundamentals of LMs and Their Particles
2.1. Types and Physical Properties of LMs
| Property | Ga | EGaIn | Galinstan |
|---|---|---|---|
| Melting point (°C) | 29.8 | 15.5 | 10.9 |
| Boiling point (°C) | 2402 | 2000 | >1300 |
| Vapor pressure (Pa) | ≈10−35 at 29.9 °C | <1.33 × 10−10 at 300 °C | <1.33 × 10−6 at 500 °C |
| Viscosity (mPa s) | 1.969 | 1.99 | 2.09 |
| Density (g cm−3) | 5.91 | 6.25 | 6.44 |
| Surface tension (mN m−1) | 750 | 632 | 718 |
| Electrical conductivity (S cm−1) | 6.73 × 104 | 3.4 × 104 | 3.46 × 104 |
| Thermal conductivity (W m−1 K−1) | 30.5 | 26.4 | 25.4 |

2.2. Characteristics of LMPs
3. Fabrication Methods of LM-Based Composites
3.1. LMP Formation Methods
3.2. The Use of an LM as a Single Filler
3.3. The Use of an LM as a Hybrid Filler
4. Applications of LM Composites in Wearable Sensors
4.1. Physical Motion Monitoring
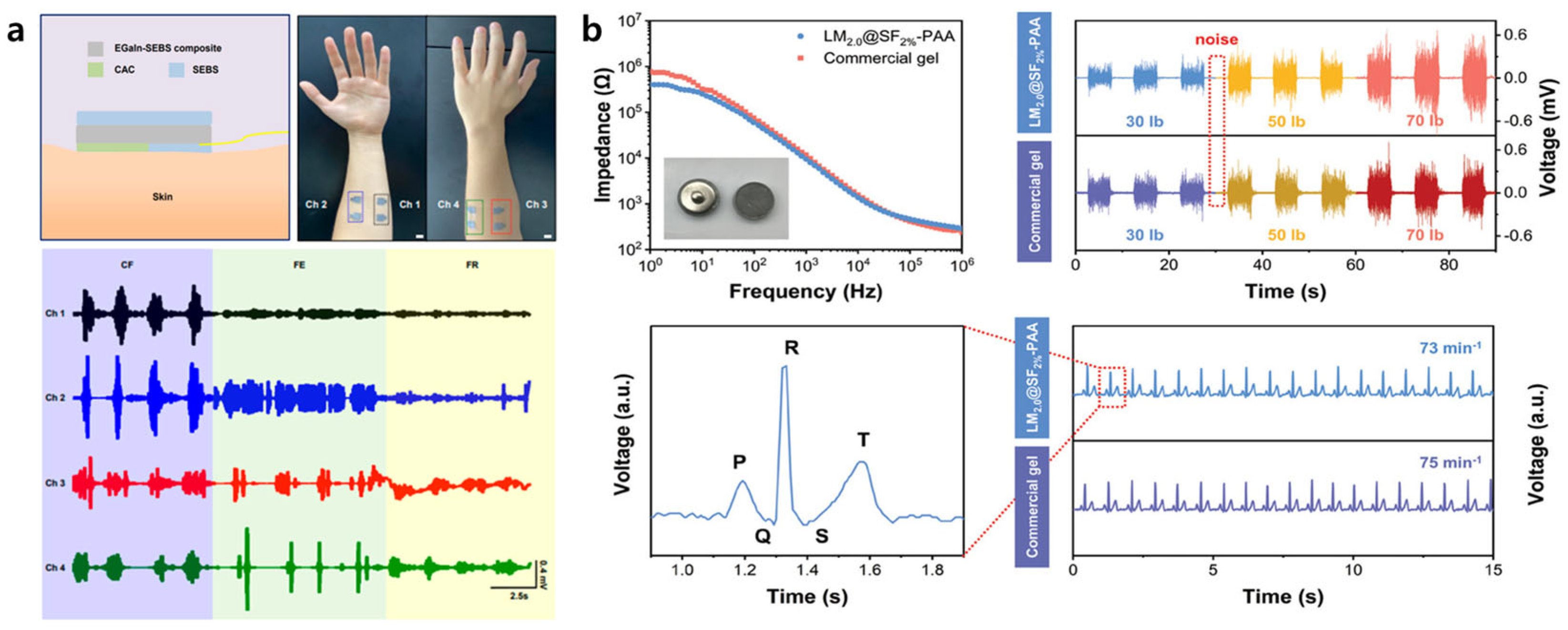
4.2. Electrophysiological Signal Recording
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic skin: Recent progress and future prospects for skin-attachable devices for health monitoring, robotics, and prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1543. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.-H.; Kim, D.-H.; Xiao, J.; Kim, B.H.; Park, S.-I.; Panilaitis, B.; Ghaffari, R.; Yao, J.; Li, M.; Liu, Z. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater. 2010, 9, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoringand personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Dickey, M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Noguchi, Y.; Hata, K.; Fukushima, T.; Aida, T.; Someya, T. A rubberlike stretchable active matrix using elastic conductors. Science 2008, 321, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Krisnadi, F.; Vong, M.H.; Kong, M.; Awartani, O.M.; Dickey, M.D. Shaping a soft future: Patterning liquid metals. Adv. Mater. 2023, 35, 2205196. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, fabrication, and applications of gallium-based liquid metal particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef] [PubMed]
- Kazem, N.; Hellebrekers, T.; Majidi, C. Soft multifunctional composites and emulsions with liquid metals. Adv. Mater. 2017, 29, 1605985. [Google Scholar] [CrossRef] [PubMed]
- Eristoff, S.; Nasab, A.M.; Huang, X.; Kramer-Bottiglio, R. Liquid Metal+ x: A Review of Multiphase Composites Containing Liquid Metal and Other (x) Fillers. Adv. Funct. Mater. 2024, 34, 2309529. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Dickey, M.D. Emerging applications of liquid metals featuring surface oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Ghaffari, R.; Kim, D.-H. Stretchable Bioelectronics for Medical Devices and Systems; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Prokhorenko, V.Y.; Roshchupkin, V.V.; Pokrasin, M.A.; Prokhorenko, S.; Kotov, V. Liquid gallium: Potential uses as a heat-transfer agent. High Temp. 2000, 38, 954–968. [Google Scholar] [CrossRef]
- Liu, T.; Sen, P.; Kim, C.-J. Characterization of nontoxic liquid-metal alloy galinstan for applications in microdevices. J. Microelectromech. Syst. 2011, 21, 443–450. [Google Scholar] [CrossRef]
- Liu, S.; Sweatman, K.; McDonald, S.; Nogita, K. Ga-based alloys in microelectronic interconnects: A review. Materials 2018, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanesh, K.; Tang, S.-Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-Zadeh, K.; Dickey, M.D. Liquid metal enabled microfluidics. Lab Chip 2017, 17, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Ansara, I. The Ga-In (Gallium-Indium) System. J. Phase Equilibria 1991, 12, 64–72. [Google Scholar] [CrossRef]
- Evans, D.; Prince, A. Thermal analysis of ga-in-sn system. Met. Sci. 1978, 12, 411–414. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.-Z.; Zhao, R.-Q.; Rao, W.; Liu, J. Liquid metal composites. Matter 2020, 2, 1446–1480. [Google Scholar] [CrossRef]
- Park, G.; Lee, G.H.; Lee, W.; Kang, J.; Park, S.; Park, S. Divide and Conquer: Design of Gallium-Based Liquid Metal Particles for Soft and Stretchable Electronics. Adv. Funct. Mater. 2024, 34, 2309660. [Google Scholar] [CrossRef]
- Lee, G.-H.; Lee, Y.R.; Kim, H.; Kwon, D.A.; Kim, H.; Yang, C.; Choi, S.Q.; Park, S.; Jeong, J.-W.; Park, S. Rapid meniscus-guided printing of stable semi-solid-state liquid metal microgranular-particle for soft electronics. Nat. Commun. 2022, 13, 2643. [Google Scholar] [CrossRef] [PubMed]
- Boley, J.W.; White, E.L.; Kramer, R.K. Mechanically sintered gallium–indium nanoparticles. Adv. Mater. 2015, 27, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Lear, T.R.; Hyun, S.-H.; Boley, J.W.; White, E.L.; Thompson, D.H.; Kramer, R.K. Liquid metal particle popping: Macroscale to nanoscale. Extrem. Mech. Lett. 2017, 13, 126–134. [Google Scholar] [CrossRef]
- Chen, S.; Fan, S.; Chan, H.; Qiao, Z.; Qi, J.; Wu, Z.; Yeo, J.C.; Lim, C.T. Liquid metal functionalization innovations in wearables and soft robotics for smart healthcare applications. Adv. Funct. Mater. 2024, 34, 2309989. [Google Scholar] [CrossRef]
- Song, H.; Kim, T.; Kang, S.; Jin, H.; Lee, K.; Yoon, H.J. Ga-Based liquid metal micro/nanoparticles: Recent advances and applications. Small 2020, 16, 1903391. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, R.; Sun, X.; Wang, H.; Li, L.; Liu, J. Toxicity and biocompatibility of liquid metals. Adv. Healthc. Mater. 2023, 12, 2201924. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Stavrinidou, E.; Proctor, C.M. Introduction to Bioelectronics; AIP Publishing: Melville, NY, USA, 2022. [Google Scholar]
- Kim, D.C.; Shim, H.J.; Lee, W.; Koo, J.H.; Kim, D.H. Material-based approaches for the fabrication of stretchable electronics. Adv. Mater. 2020, 32, 1902743. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.C.; Tee, B.C. Functional Liquid Metal Polymeric Composites: Fundamentals and Applications in Soft Wearable Electronics. Adv. Funct. Mater. 2024, 34, 2400284. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Mashima, Y.; Iyoda, T. Reversible size control of liquid-metal nanoparticles under ultrasonication. Angew. Chem. Int. Ed. 2015, 54, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- Babatain, W.; Kim, M.S.; Hussain, M.M. From droplets to devices: Recent advances in liquid metal droplet enabled electronics. Adv. Funct. Mater. 2024, 34, 2308116. [Google Scholar] [CrossRef]
- Jeong, S.H.; Chen, S.; Huo, J.; Gamstedt, E.K.; Liu, J.; Zhang, S.-L.; Zhang, Z.-B.; Hjort, K.; Wu, Z. Mechanically stretchable and electrically insulating thermal elastomer composite by liquid alloy droplet embedment. Sci. Rep. 2015, 5, 18257. [Google Scholar] [CrossRef] [PubMed]
- Tutika, R.; Kmiec, S.; Haque, A.T.; Martin, S.W.; Bartlett, M.D. Liquid metal–elastomer soft composites with independently controllable and highly tunable droplet size and volume loading. ACS Appl. Mater. Interfaces 2019, 11, 17873–17883. [Google Scholar] [CrossRef] [PubMed]
- Fassler, A.; Majidi, C. Liquid-phase metal inclusions for a conductive polymer composite. Adv. Mater 2015, 27, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Markvicka, E.J.; Bartlett, M.D.; Huang, X.; Majidi, C. An autonomously electrically self-healing liquid metal–elastomer composite for robust soft-matter robotics and electronics. Nat. Mater. 2018, 17, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Tutika, R.; Haque, A.T.; Bartlett, M.D. Self-healing liquid metal composite for reconfigurable and recyclable soft electronics. Commun. Mater. 2021, 2, 64. [Google Scholar] [CrossRef]
- Liu, S.; Yuen, M.C.; White, E.L.; Boley, J.W.; Deng, B.; Cheng, G.J.; Kramer-Bottiglio, R. Laser sintering of liquid metal nanoparticles for scalable manufacturing of soft and flexible electronics. ACS Appl. Mater. Interfaces 2018, 10, 28232–28241. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, Y.; He, Z.; Rao, W.; Hu, L.; Chen, S.; Lin, J.; Gao, J.; Zhang, P.; Sun, X. A highly stretchable liquid metal polymer as reversible transitional insulator and conductor. Adv. Mater. 2019, 31, 1901337. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.; Kang, I.; Park, H.; Jung, J.; Lee, H.; Park, H.; Park, J.S.; Yuk, J.M.; Ryu, S. Universal assembly of liquid metal particles in polymers enables elastic printed circuit board. Science 2022, 378, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, Z.; Li, G.; Li, Z.; Ye, Z.; Xu, Z.; Chen, W.; Jin, D.; Ma, X. Ultrasonic-Enabled Nondestructive and Substrate-Independent Liquid Metal Ink Sintering. Adv. Sci. 2023, 10, 2301292. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.; Tang, S.-Y.; Lu, H.; Zhang, S.; Dickey, M.D.; Li, W. Hybrid-filler stretchable conductive composites: From fabrication to application. Small Sci. 2021, 1, 2000080. [Google Scholar] [CrossRef]
- Ford, M.J.; Patel, D.K.; Pan, C.; Bergbreiter, S.; Majidi, C. Controlled assembly of liquid metal inclusions as a general approach for multifunctional composites. Adv. Mater. 2020, 32, 2002929. [Google Scholar] [CrossRef] [PubMed]
- Tutika, R.; Zhou, S.H.; Napolitano, R.E.; Bartlett, M.D. Mechanical and functional tradeoffs in multiphase liquid metal, solid particle soft composites. Adv. Funct. Mater. 2018, 28, 1804336. [Google Scholar] [CrossRef]
- Wang, J.; Cai, G.; Li, S.; Gao, D.; Xiong, J.; Lee, P.S. Printable superelastic conductors with extreme stretchability and robust cycling endurance enabled by liquid-metal particles. Adv. Mater. 2018, 30, 1706157. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhang, D.; Wu, Y.; Xing, R.; Li, H.; Yu, T.; Bai, B.; Tao, Y.; Dickey, M.D.; Yang, J. Segregated and non-settling liquid metal elastomer via jamming of elastomeric particles. Adv. Funct. Mater. 2023, 33, 2210553. [Google Scholar] [CrossRef]
- Krings, E.J.; Zhang, H.; Sarin, S.; Shield, J.E.; Ryu, S.; Markvicka, E.J. Lightweight, thermally conductive liquid metal elastomer composite with independently controllable thermal conductivity and density. Small 2021, 17, 2104762. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Ma, B.; Li, G.; Liu, Y.; Zhang, Y.; Ma, X.; Yan, S. A highly stretchable and sintering-free liquid metal composite conductor enabled by ferrofluid. Soft Sci. 2023, 3, 36. [Google Scholar] [CrossRef]
- Zhong, D.; Shi, S.; Yang, X.; Handschuh-Wang, S.; Zhang, Y.; Gan, T.; Zhou, X. Highly stretchable yet degradable and recyclable conductive composites with liquid metal nanodroplets as physical crosslinks. Adv. Funct. Mater. 2024, 34, 2308032. [Google Scholar] [CrossRef]
- Kim, H.; Kim, G.; Kang, J.H.; Oh, M.J.; Qaiser, N.; Hwang, B. Intrinsically conductive and highly stretchable liquid metal/carbon nanotube/elastomer composites for strain sensing and electromagnetic wave absorption. Adv. Compos. Hybrid Mater. 2025, 8, 14. [Google Scholar] [CrossRef]
- Li, S.; Guo, X.; Bai, Z.; Guo, M.; Ren, Y.; Niu, H.; Zhang, H.; Deng, J. Self-Healing Liquid Metal Magnetic Composite Films for Wearable Sensors and Electromagnetic Shielding. ACS Appl. Eng. Mater. 2024, 2, 2899–2909. [Google Scholar] [CrossRef]
- Wang, M.; Tang, X.-H.; Cai, J.-H.; Wu, H.; Shen, J.-B.; Guo, S.-Y. Construction, mechanism and prospective of conductive polymer composites with multiple interfaces for electromagnetic interference shielding: A review. Carbon 2021, 177, 377–402. [Google Scholar] [CrossRef]
- Cao, M.; Han, C.; Wang, X.; Zhang, M.; Zhang, Y.; Shu, J.; Yang, H.; Fang, X.; Yuan, J. Graphene nanohybrids: Excellent electromagnetic properties for the absorbing and shielding of electromagnetic waves. J. Mater. Chem. C 2018, 6, 4586–4602. [Google Scholar] [CrossRef]
- Huang, M.-L.; Luo, C.-L.; Sun, C.; Zhao, K.-Y.; Wang, M. In-situ microfibrilization of liquid metal droplets in polymer matrix for enhancing electromagnetic interference shielding and thermal conductivity. Compos. Sci. Technol. 2024, 255, 110724. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Wu, Y.; Xiong, X.; Yang, J.; Dickey, M.D. Liquid metal interdigitated capacitive strain sensor with normal stress insensitivity. Adv. Intell. Syst. 2022, 4, 2100201. [Google Scholar] [CrossRef]
- Choi, H.; Luo, Y.; Olson, G.; Won, P.; Shin, J.H.; Ok, J.; Yang, Y.J.; Kim, T.i.; Majidi, C. Highly stretchable and strain-insensitive liquid metal based elastic kirigami electrodes (LM-eKE). Adv. Funct. Mater. 2023, 33, 2301388. [Google Scholar] [CrossRef]
- Gul, O.; Kim, K.; Gu, J.; Choi, J.; Del Orbe Henriquez, D.; Ahn, J.; Park, I. Sensitivity-controllable liquid-metal-based pressure sensor for wearable applications. ACS Appl. Electron. Mater. 2021, 3, 4027–4036. [Google Scholar] [CrossRef]
- Mou, L.; Xia, Y.; Jiang, X. Liquid metal-polymer conductor-based wireless, battery-free epidermal patch. Biosens. Bioelectron. 2022, 197, 113765. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Lee, G.Y.; Jang, J.; Yun, S.M.; Kim, E.; Park, J.U. Liquid metal-based soft electronics for wearable healthcare. Adv. Healthc. Mater. 2021, 10, 2002280. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Han, Q.; Chen, A. A liquid metal/polypyrrole electrospun TPU composite conductive network for highly sensitive strain sensing in human motion monitoring. J. Mater. Chem. B 2024, 12, 4655–4665. [Google Scholar] [CrossRef] [PubMed]
- Kouediatouka, A.N.; Wang, J.; Mawignon, F.J.; Wang, W.; Liu, Q.; Meng, Z.; Makanda, I.L.D.; Djandja, O.S.; Dong, G. Carbon nanotube/liquid metal hybrid coating-based flexible pressure piezoresistive sensors. Chem. Eng. J. 2024, 481, 148637. [Google Scholar] [CrossRef]
- Stevens, M.; Yun, G.; Hasan, T. Porous conductive hybrid composite with superior pressure sensitivity and dynamic range. Adv. Funct. Mater. 2024, 34, 2309347. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Lee, J.; Shin, M.; Son, D. Wearable Liquid Metal Composite with Skin-Adhesive Chitosan–Alginate–Chitosan Hydrogel for Stable Electromyogram Signal Monitoring. Polymers 2023, 15, 3692. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Fu, Y.; Chen, X.; Gan, S.; Yang, W.; Chen, S.; Liu, L. Liquid Metal@ Silk Fibroin Peptide Particles Initiated Hydrogels with High Toughness, Adhesion, and Conductivity for Portable and Continuous Electrophysiological Monitoring. Adv. Funct. Mater. 2025, 35, 2420240. [Google Scholar] [CrossRef]
- Wei, P.; Guo, X.; Qiu, X.; Yu, D. Flexible capacitive pressure sensor with sensitivity and linear measuring range enhanced based on porous composite of carbon conductive paste and polydimethylsiloxane. Nanotechnology 2019, 30, 455501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, H.; Li, S.; Liang, X.; Zhang, M.; Dai, X.; Zhang, Y. Flexible electrodes for in vivo and in vitro electrophysiological signal recording. Adv. Healthc. Mater. 2021, 10, 2100646. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Afshari, R.; Jain, S.; Zheng, Y.; Lin, M.-H.; Zenkar, S.; Yin, J.; Chen, J.; Peppas, N.A.; Annabi, N. Advances in conducting nanocomposite hydrogels for wearable biomonitoring. Chem. Soc. Rev. 2025, 54, 2595–2652. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, H.; Wang, L.; Huang, Z.; Haq, F.; Teng, L.; Jin, M.; Ding, B. Recent advances on designs and applications of hydrogel adhesives. Adv. Mater. Interfaces 2022, 9, 2101038. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Martinez, E.E.; Zhu, J.; Wang, T.; Shi, S.; Shin, S.R.; Hassan, S.; Guo, C. Emerging biopolymer-based bioadhesives. Macromol. Biosci. 2022, 22, 2100340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jiang, C.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Skin-inspired double-hydrophobic-coating encapsulated hydrogels with enhanced water retention capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Cai, C.; Li, Q.; Wen, C.; Zhang, X.; Wang, X.; Yang, J.; Zhang, L. Ionic conductive hydrogels with long-lasting antifreezing, water retention and self-regeneration abilities. Chem. Eng. J. 2021, 419, 129478. [Google Scholar] [CrossRef]


| Processing Technique (Droplet Size) | Principle | Advantages | Disadvantages | |
|---|---|---|---|---|
| TOP-DOWN | Drop-on-demand (Tens of µm to a few mm) | Extruding LMs from a syringe or nozzle | 3D structure formation | Limited by the minimum size. Precise pressure control required |
| Molding (Tens of µm to a few mm) | Pressing into pre-patterned mold | Monodispersity. Large area patterning | Complete filling required. Difficulty in detaching from mold | |
| Microfluidics (50–200 µm) | Balance between interfacial tension and viscous force | Reliable and repeatable method | Complex system design. Limited by the minimum size | |
| Sonication (Tens of nm to a few µm) | Fragmentation by sonication | Easy formation of NPs | Polydispersity. Heat generation | |
| Shearing (A few nm to a few µm) | Application of shear stress | Easy formation of NPs | Polydispersity | |
| BOTTOM-UP | Thermal evaporation (5–150 nm) | Condensation after evaporation of Ga | Size controllability | Ultra-high vacuum required |
| Hot injection (12–46 nm) | Growth of Ga precursor | Monodispersity | Low reproducibility | |
| LM Composite | Fabrication Method | Deformation Range | Application | Ref. |
|---|---|---|---|---|
| LM/PPy/TPU | Electrospinning and polymerization | Up to 135.5% stain | Strain sensing (GF = 4.36 at 0–12.5% strain) | [67] |
| LM/CNT/PDMS | Blending | Up to 144.33% strain | Strain sensing (GF = 5.35 at 50–100% strain) | [56] |
| PE/CNT/LM/PE | Laser-assisted coating | Up to 30% compressive strain | Pressure sensing (GF = 57 at 30% strain) | [68] |
| Porous LM/Ni/PDMS | Blending | Up to 8.9 MPa pressure | Pressure sensing (0.306 kPa–1 at 50 kPa) | [69] |
| LM-SEBS/CAC hydrogel | Drop casting | Up to 520% strain | EMG recording | [70] |
| LM@SF-PAA hydrogel | Polymerization | Up to 1050% strain | EMG/ECG recording | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.Y.; Yusoff, W.Y.W.; Matteini, P.; Baumli, P.; Hwang, B. Recent Advances in Liquid Metal-Based Stretchable and Conductive Composites for Wearable Sensor Applications. Biosensors 2025, 15, 466. https://doi.org/10.3390/bios15070466
Kim BY, Yusoff WYW, Matteini P, Baumli P, Hwang B. Recent Advances in Liquid Metal-Based Stretchable and Conductive Composites for Wearable Sensor Applications. Biosensors. 2025; 15(7):466. https://doi.org/10.3390/bios15070466
Chicago/Turabian StyleKim, Boo Young, Wan Yusmawati Wan Yusoff, Paolo Matteini, Peter Baumli, and Byungil Hwang. 2025. "Recent Advances in Liquid Metal-Based Stretchable and Conductive Composites for Wearable Sensor Applications" Biosensors 15, no. 7: 466. https://doi.org/10.3390/bios15070466
APA StyleKim, B. Y., Yusoff, W. Y. W., Matteini, P., Baumli, P., & Hwang, B. (2025). Recent Advances in Liquid Metal-Based Stretchable and Conductive Composites for Wearable Sensor Applications. Biosensors, 15(7), 466. https://doi.org/10.3390/bios15070466






