Strategic Detection of Escherichia coli in the Poultry Industry: Food Safety Challenges, One Health Approaches, and Advances in Biosensor Technologies
Abstract
1. Introduction
- Sanitation Practices: ensuring clean and dry bedding, proper housing, and transportation conditions to minimize contamination.
- Water and Feed Management: treating drinking water and optimizing feed types and feeding strategies to reduce pathogen load.
- Direct Anti-Pathogen Strategies: utilizing bacteriophages, competitive exclusion products, and vaccines targeting specific E. coli strains.
1.1. Current Issues in Food Safety and Poultry Products
1.2. Where and When Is E. coli Introduced in the Poultry Production Pipeline
1.2.1. Farm Level Introduction
1.2.2. Hatchery and Brooding Stage
1.2.3. Processing Plant Contamination
1.2.4. Transportation and Storage
1.2.5. Importance of Biosecurity in Poultry Production
1.3. Current Methods of E. coli Detection in Poultry Products
1.3.1. Polymerase Chain Reaction (PCR)
1.3.2. Antimicrobial Susceptibility Testing (AST)
1.3.3. Microbiological Culture
2. Biosensor Methods for Detection of E. coli in the Poultry Production Pipeline
2.1. Types of Biosensors
2.1.1. Immunological Biosensors
2.1.2. Nucleic Acid-Biosensors
2.1.3. The Role of Nanomaterials
2.2. Screening for Detection
2.2.1. Life Stages
2.2.2. In Ovo
2.2.3. Hatchlings
2.2.4. Monitoring During Growth and Maturation
2.3. Markers of Interest
2.3.1. Antibiotic Screening and Resistance
2.3.2. Bacterial Toxins
2.3.3. Biomarkers and Immune Responses
2.3.4. Zoonotic Potential
2.4. Strategic Implementation
2.4.1. Slaughterhouse Quality Control
2.4.2. Marketplace Quality Control
2.5. Benefits
2.5.1. Affordability
2.5.2. Time
2.5.3. Ease of Use
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Food & Drug Administration (FDA). E. coli and Foodborne Illness: Information for the Public, FDA Actions, and Recommendations. 2020. Available online: https://www.fda.gov/news-events/public-health-focus/e-coli-and-foodborne-illness (accessed on 2 June 2025).
- World Health Organization (WHO). E. coli, Factsheet. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/e-coli (accessed on 2 June 2025).
- Hasso-Agopsowicz, M.; Lopman, B.A.; Lanata, C.F.; McQuade, E.T.R.; Kang, G.; Prudden, H.J.; Khalil, I.; Platts-Mills, J.A.; Kotloff, K.; Jit, M. World Health Organization Expert Working Group: Recommendations for assessing morbidity associated with enteric pathogens. Vaccine 2021, 39, 7521–7525. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.R. Importance of a One Health approach in advancing global health security and the Sustainable Development Goals. Rev. Sci. Tech. (Int. Off. Epizoot.) 2019, 38, 145–154. [Google Scholar] [CrossRef]
- García, A.; Fox, J.G. A one health perspective for defining and deciphering Escherichia coli pathogenic potential in multiple hosts. Comp. Med. 2021, 71, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, CDC. Estimates of Foodborne Illness in the United States. Available online: https://www.cdc.gov/food-safety/php/data-research/foodborne-illness-burden/index.html (accessed on 2 June 2025).
- United States Department of Agriculture (USDA); Food Safety and Inspection Service (FSIS). Pre-Harvest Management Controls and Intervention Options for Reducing Shiga Toxin-Producing Escherichia coli Shedding in Cattle: An Overview of the Current Research. USDA-FSIS-GD-2014-0012. 2014. Available online: https://www.fsis.usda.gov/sites/default/files/import/Reducing-Ecoli-Shedding-in-Cattle.pdf (accessed on 2 June 2025).
- United States Department of Agriculture (USDA); Food Safety and Inspection Service (FSIS). FSIS Compliance Guideline: Modernization of Poultry Slaughter Inspection, Microbiological Sampling of Raw Poultry. USDA-FSIS-GD-2015-0013. 2015. Available online: https://www.fsis.usda.gov/sites/default/files/import/Microbiological-Testing-Raw-Poultry.pdf (accessed on 2 June 2025).
- Verma, S.; Wadhwa, N.K.; Bajaj, D.; Sharma, R.; Rajni, E.; Priyadarshani, A. A Microbiological Analysis of Egg Shell Bacteria. Vantage J. Themat. Anal 2023, 4, 34–46. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); Foodborne Diseases and Active Surveillance Network. Hemolytic Uremic Syndrome (HS) Surveillance Tool Guide. 2024. Available online: https://www.cdc.gov/foodnet/data/hus-surveillance.html (accessed on 2 June 2025).
- Fuga, B.; Sellera, F.P.; Cerdeira, L.; Esposito, F.; Cardoso, B.; Fontana, H.; Moura, Q.; Cardenas-Arias, A.; Sano, E.; Ribas, R.M.; et al. WHO Critical Priority Escherichia coli as One Health Challenge for a Post-Pandemic Scenario: Genomic Surveillance and Analysis of Current Trends in Brazil. Microbiol. Spectr. 2022, 10, e0125621. [Google Scholar] [CrossRef]
- Zhuang, L.; Gong, J.; Zhao, Y.; Yang, J.; Liu, G.; Zhao, B.; Song, C.; Zhang, Y.; Shen, Q. Progress in methods for the detection of viable Escherichia coli. Analyst 2024, 149, 1022–1049. [Google Scholar] [CrossRef]
- Dlamini, S.B.; Mlambo, V.; Mnisi, C.M.; Ateba, C.N. Virulence, multiple drug resistance, and biofilm-formation in Salmonella species isolated from layer, broiler, and dual-purpose indigenous chickens. PLoS ONE 2024, 19, e0310010. [Google Scholar] [CrossRef]
- Motola, G.; Hafez, H.M.; Brüggemann-Schwarze, S. Efficacy of six disinfection methods against extended-spectrum beta-lactamase (ESBL) producing E. coli on eggshells in vitro. PLoS ONE 2020, 15, e0238860. [Google Scholar] [CrossRef]
- Feng, A.; Akter, S.; Leigh, S.A.; Wang, H.; Pharr, G.T.; Evans, J.; Branton, S.L.; Landinez, M.P.; Pace, L.; Wan, X.F. Genomic diversity, pathogenicity and antimicrobial resistance of Escherichia coli isolated from poultry in the southern United States. BMC Microbiol. 2023, 23, 15. [Google Scholar] [CrossRef]
- Tegegne, H.; Filie, K.; Tolosa, T.; Debelo, M.; Ejigu, E. Isolation, and Identification of Escherichia coli O157:H7 Recovered from Chicken Meat at Addis Ababa Slaughterhouses. Infect. Drug Resist. 2024, 17, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kromann, S.; Jensen, H.E. In vivo models of Escherichia coli infection in poultry. Acta Vet. Scand. 2022, 64, 33. [Google Scholar] [CrossRef] [PubMed]
- Blaak, H.; van Hoek, A.H.A.M.; Hamidjaja, R.A.; van der Plaats, R.Q.J.; Kerkhof-de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Distribution, Numbers, and Diversity of ESBL-Producing E. coli in the Poultry Farm Environment. PLoS ONE 2015, 10, e0135402. [Google Scholar] [CrossRef]
- Shtylla Kika, T.; Cocoli, S.; Ljubojević Pelić, D.; Puvača, N.; Lika, E.; Pelić, M. Colibacillosis in Modern Poultry Production. J. Agron. Technol. Eng. Manag. JATEM 2023, 6, 975–987. [Google Scholar] [CrossRef]
- Nguyen, X.D.; Zhao, Y.; Evans, J.D.; Lin, J.; Purswell, J.L. Survival of Escherichia coli in Airborne and Settled Poultry Litter Particles. Animals 2022, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.; Moganedi, K.; Chitura, T. Presence of Coliforms in Water, Poultry Mouth and Rectal Swabs from Selected Smallholder Poultry Farming Projects of Capricorn District, South Africa. Agric. Sci. Dig. Res. J. 2023, 43, 248–254. [Google Scholar] [CrossRef]
- Waliaula, P.K.; Kiarie, E.G.; Diarra, M.S. Predisposition factors and control strategies of avian pathogenic Escherichia coli in laying hens. Front. Vet. Sci. 2024, 11, 1474549. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA); Food Safety & Inspection Service (FSIS). Guidelines for Escherichia coli Testing for Process Control Verification in Poultry Slaughter Establishments. USDA-FSIS-GD-1996-0002. 2005. Available online: https://www.fsis.usda.gov/sites/default/files/import/Guideline_for_Ecoli_Testing_Slaughter_Estab.pdf (accessed on 2 June 2025).
- United States Department of Agriculture (USDA); Food Safety & Inspection Service (FSIS). PSIT HACCP Overview. FSIS-USDA. 2016. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-03/PSIT_HACCP_Overview.pdf (accessed on 2 June 2025).
- dos Santos, V.M.; Oliveira, G.d.S.; Salgado, C.B.; Pires, P.G.d.S.; Santos, P.H.G.d.S.; McManus, C. Outcomes of Microbiological Challenges in Poultry Transport: A Mini Review of the Reasons for Effective Bacterial Control. Microbiol. Res. 2024, 15, 962–971. [Google Scholar] [CrossRef]
- Coleman, M.E.; Sandberg, S.; Anderson, S.A. Impact of microbial ecology of meat and poultry products on predictions from exposure assessment scenarios for refrigerated storage. Risk Anal. Int. J. 2003, 23, 215–228. [Google Scholar] [CrossRef]
- Koutsianos, D.; Athanasiou, L.; Mossialos, D.; Koutoulis, K. Colibacillosis in poultry: A disease overview and the new perspectives for its control and prevention. J. Hell. Vet. Med. Soc. 2020, 71, 2425–2436. [Google Scholar] [CrossRef]
- Byrd, J.; Sams, A.; Hargis, B.; Caldwell, D. Effect of selected modified atmosphere packaging on Campylobacter survival in raw poultry. Poult. Sci. 2011, 90, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Delpont, M.; Salazar, L.G.; Dewulf, J.; Zbikowski, A.; Szeleszczuk, P.; Dufay-Lefort, A.-C.; Rousset, N.; Spaans, A.; Amalraj, A.; Tilli, G.; et al. Monitoring biosecurity in poultry production: An overview of databases reporting biosecurity compliance from seven European countries. Front. Vet. Sci. 2023, 10, 1231377. [Google Scholar] [CrossRef] [PubMed]
- Woyda, R.; Oladeinde, A.; Abdo, Z. Chicken Production and Human Clinical Escherichia coli Isolates Differ in Their Carriage of Antimicrobial Resistance and Virulence Factors. Appl. Environ. Microbiol. 2023, 89, e01167-22. [Google Scholar] [CrossRef]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Loor-Giler, A.; Castillo-Reyes, S.; Santander-Parra, S.; Caza, M.; Kyriakidis, N.C.; Ferreira, A.J.P.; Nuñez, L. Development of a fast and sensitive RT-qPCR assay based on SYBR® green for diagnostic and quantification of Avian Nephritis Virus (ANV) in chickens affected with enteric disease. BMC Vet. Res. 2024, 20, 33. [Google Scholar] [CrossRef]
- Zimoń, B.; Psujek, M.; Matczak, J.; Guziński, A.; Wójcik, E.; Dastych, J. Novel multiplex-PCR test for Escherichia coli detection. Microbiol. Spectr. 2024, 12, e03773-23. [Google Scholar] [CrossRef]
- Aranda, K.R.; Fagundes-Neto, U.; Scaletsky, I.C. Evaluation of multiplex PCRs for diagnosis of infection with diarrheagenic Escherichia coli and Shigella spp. J. Clin. Microbiol. 2004, 42, 5849–5853. [Google Scholar] [CrossRef]
- Hassanin, F.S.; Salem, A.M.; Shorbagy, E.M.; Kholy, R.L. Traditional and recent techniques for detection of Escherichia coli in fresh chicken cuts and giblets. Benha Vet. Med. J. 2014, 26, 21–29. [Google Scholar]
- United States Department of Agriculture (USDA); Food Safety & Inspection Service (FSIS). FSIS Guidance for Test Kit Manufacturers, Laboratories: Evaluating the Performance of Pathogen Detection Methods. USDA-FSIS-GD-2010-0004. 2010. Available online: https://www.fsis.usda.gov/sites/default/files/import/Validation_Studies_Pathogen_Detection_Methods.pdf (accessed on 2 June 2025).
- Schroeder, C.M.; Zhao, C.; DebRoy, C.; Torcolini, J.; Zhao, S.; White, D.G.; Wagner, D.D.; McDermott, P.F.; Walker, R.D.; Meng, J. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl. Environ. Microbiol. 2002, 68, 576–581. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Dargatz, D.A.; Erdman, M.M.; Harris, B. A survey of methods used for antimicrobial susceptibility testing in veterinary diagnostic laboratories in the United States. J. Vet. Diagn. Investig. 2017, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Aworh, M.K.; Kwaga, J.K.P.; Hendriksen, R.S.; Okolocha, E.C.; Thakur, S. Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob. Resist. Infect. Control. 2021, 10, 58. [Google Scholar] [CrossRef]
- Watts, A.; Wigley, P. Avian Pathogenic Escherichia coli: An Overview of Infection Biology, Antimicrobial Resistance and Vaccination. Antibiotics 2024, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ran, M.; Wang, X.; Chen, Q.; Wang, J.; Ruan, Z.; Wang, J.; Tang, B.; Fang, J. Prevalence and characterization of class I integrons in multidrug-resistant Escherichia coli isolates from humans and food-producing animals in Zhejiang Province, China. BMC Microbiol. 2025, 25, 76. [Google Scholar] [CrossRef]
- Agusi, E.R.; Kabantiyok, D.; Mkpuma, N.; Atai, R.B.; Okongwu-Ejike, C.; Bakare, E.L.; Budaye, J.; Sule, K.G.; Rindaps, R.J.; James, G.K.; et al. Prevalence of multidrug-resistant Escherichia coli isolates and virulence gene expression in poultry farms in Jos, Nigeria. Front. Microbiol. 2024, 15, 1298582. [Google Scholar] [CrossRef] [PubMed]
- Plumblee Lawrence, J.R.; Cudnik, D.; Oladeinde, A. Bacterial Detection and Recovery From Poultry Litter. Front. Microbiol. 2022, 12, 803150. [Google Scholar] [CrossRef]
- Ma, R.-N.; Wang, L.-L.; Zhang, M.; Jia, L.-P.; Zhang, W.; Shang, L.; Jia, W.-L.; Wang, H.-S. A novel one-step triggered “signal-on/off” electrochemical sensing platform for lead based on the dual-signal ratiometric output and electrode-bound DNAzyme assembly. Sens. Actuators B Chem. 2018, 257, 678–684. [Google Scholar] [CrossRef]
- Liu, Y.; Canoura, J.; Alkhamis, O.; Xiao, Y. Immobilization Strategies for Enhancing Sensitivity of Electrochemical Aptamer-Based Sensors. ACS Appl. Mater. Interfaces 2021, 13, 9491–9499. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Assay of plasma insulin in human subjects by immunological methods. Nature 1959, 184 (Suppl. S21), 1648–1649. [Google Scholar] [CrossRef]
- Southern, E.M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 1975, 98, 503–517. [Google Scholar] [CrossRef]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.L.; Roshkolaeva, A.B.; Chapoval, A.I.; Dick, J.E. Recent Advances in Potentiometric Biosensing. Curr. Opin. Electrochem. 2021, 28, 100735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Wu, J.; Eda, S. A rapid, sensitive, and simple-to-use biosensor for on-site detection of attomolar level microRNA biomarkers from serum extracellular vesicles. Sens. Actuators B Chem. 2022, 369, 132314. [Google Scholar] [CrossRef]
- Hatate, K.; Rice, J.H.; Parker, K.; Wu, J.J.; Turner, A.; Stabel, J.R.; Eda, S. Electrochemical Detection of Serum Antibodies Against Mycobacterium avium Subspecies paratuberculosis. Front. Vet. Sci. 2021, 8, 642833. [Google Scholar] [CrossRef]
- Cheng, C.; Cui, H.; Eda, S.; Wu, J. Sensitive and specific point-of-care detection of pathogen infections by an AC electrokinetics-enhanced capacitive immunosensor. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; pp. 176–179. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Xia, X.; Wu, J.; Almeida, R.; Eda, S.; Qi, H. An on-site, highly specific immunosensor for Escherichia coli detection in field milk samples from mastitis-affected dairy cattle. Biosens. Bioelectron. 2020, 165, 112366. [Google Scholar] [CrossRef]
- Zhang, J.; Oueslati, R.; Cheng, C.; Zhao, L.; Chen, J.; Almeida, R.; Wu, J. Rapid, highly sensitive detection of Gram-negative bacteria with lipopolysaccharide based disposable aptasensor. Biosens. Bioelectron. 2018, 112, 48–53. [Google Scholar] [CrossRef]
- Arshad, F.; Abdillah, A.N.; Shivanand, P.; Ahmed, M.U. Dual-Mode RPA/CRISPR-Cas12a Biosensor Based on Silica and Magnetic Hyid Nanobeads for Rapid Detection of Campylobacter jejuni. ACS Appl. Bio Mater. 2025, 8, 2977–2984. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Q.; Brennan, J.D.; Li, Y. Graphene-DNAzyme-based fluorescent biosensor for Escherichia coli detection. MRS Commun. 2018, 8, 687–694. [Google Scholar] [CrossRef]
- Heo, T.; Kang, H.; Choi, S.; Kim, J. Detection of pks Island mRNAs Using Toehold Sensors in Escherichia coli. Life 2021, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alocilja, E.C. Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J. Biol. Eng. 2015, 9, 16. [Google Scholar] [CrossRef]
- Vu, Q.K.; Tran, Q.H.; Vu, N.P.; Anh, T.-L.; Dang, T.T.L.; Matteo, T.; Nguyen, T.H.H. A label-free electrochemical biosensor based on screen-printed electrodes modified with gold nanoparticles for quick detection of bacterial pathogens. Mater. Today Commun. 2021, 26, 101726. [Google Scholar] [CrossRef]
- Yamada, K.; Kim, C.-T.; Kim, J.-H.; Chung, J.-H.; Lee, H.G.; Jun, S. Single Walled Carbon Nanotube-Based Junction Biosensor for Detection of Escherichia coli. PLoS ONE 2014, 9, e105767. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Alocilja, E.C. Portable nuclear magnetic resonance biosensor and assay for a highly sensitive and rapid detection of foodborne bacteria in complex matrices. J. Biol. Eng. 2017, 11, 14. [Google Scholar] [CrossRef]
- Shi, C.; Tang, Y.; Yang, H.; Yang, J.; Wu, Y.; Sun, H.; Yin, S.; Wang, G. Capture and detection of Escherichia coli with graphene aerogels. J. Mater. Chem. B 2022, 10, 8211–8217. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Hou, X.; Ga, L.; Ai, J. Aptamer-carbon quantum dots and silver nanoparticles construct a FRET sensor for sensitive detection of E. coli. Biomed. Anal. 2024, 1, 162–173. [Google Scholar] [CrossRef]
- Ma, H.; O’Kennedy, R. Generation and optimisation of antibodies for biosensor applications. In Nanobiosensors for Personalized and Onsite Biomedical Diagnosis; The Institution of Engineering and Technology: London, UK, 2016; pp. 209–230. [Google Scholar] [CrossRef]
- Cruz, H.J.; Rosa, C.C.; Oliva, A.G. Immunosensors for diagnostic applications. Parasitol. Res. 2002, 88, S4–S7. [Google Scholar] [CrossRef]
- Alamer, S.; Eissa, S.; Chinnappan, R.; Zourob, M. A rapid colorimetric immunoassay for the detection of pathogenic bacteria on poultry processing plants using cotton swabs and nanobeads. Mikrochim. Acta 2018, 185, 164. [Google Scholar] [CrossRef]
- Fronczek, C.F.; You, D.J.; Yoon, J.Y. Single-pipetting microfluidic assay device for rapid detection of Salmonella from poultry package. Biosens. Bioelectron. 2013, 40, 342–349. [Google Scholar] [CrossRef]
- Hong, Y.; Berrang, M.E.; Liu, T.; Hofacre, C.L.; Sanchez, S.; Wang, L.; Maurer, J.J. Rapid detection of Campylobacter coli, C. jejuni, and Salmonella enterica on poultry carcasses by using PCR-enzyme-linked immunosorbent assay. Appl. Environ. Microbiol. 2003, 69, 3492–3499. [Google Scholar] [CrossRef] [PubMed]
- Ledlod, S.; Areekit, S.; Santiwatanakul, S.; Chansiri, K. Colorimetric aptasensor for detecting Salmonella spp., Listeria monocytogenes, and Escherichia coli in meat samples. Food Sci. Technol. Int. 2020, 26, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Almalaysha, M.; Singh, A.; Muhsin, S.A.; Morey, A.; Zhang, S.; Channaiah, L.H.; Almasri, M. Microfluidic biosensor for rapid detection of Salmonella in raw chicken products. In Proceedings of the 2024 IEEE 37th International Conference on Micro Electro Mechanical Systems (MEMS), Austin, TX, USA, 21–25 January 2024; pp. 308–311, ISBN 978-8-3503-5793-6. [Google Scholar]
- Paniel, N.; Noguer, T. Detection of Salmonella in Food Matrices, from Conventional Methods to Recent Aptamer-Sensing Technologies. Foods 2019, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Chan, K.; Weaver, S.C.; Wong, P.-Y.; Lie, S.; Wang, E.; Guerbois, M.; Vayugundla, S.P.; Wong, S. Rapid, affordable and portable medium-throughput molecular device for Zika virus. Sci. Rep. 2016, 6, 38223. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R.; Wang, Y.; Su, X.; Ying, Y.; Wang, J.; Li, Y. Evaluation of different micro/nanobeads used as amplifiers in QCM immunosensor for more sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2011, 29, 23–28. [Google Scholar] [CrossRef]
- Sewid, A.H.; Dylewski, H.C.; Ramos, J.H.; Morgan, B.M.; Gelalcha, B.D.; D’Souza, D.H.; Wu, J.J.; Dego, O.K.; Eda, S. Colorimetric and electrochemical analysis of DNAzyme-LAMP amplicons for the detection of Escherichia coli in food matrices. Sci. Rep. 2024, 14, 28942. [Google Scholar] [CrossRef]
- Sewid, A.H.; Ramos, J.H.; Dylewski, H.C.; Castro, G.I.; D’Souza, D.H.; Eda, S. Colorimetric dual DNAzyme reaction triggered by loop-mediated isothermal amplification for the visual detection of Shiga toxin-producing Escherichia coli in food matrices. PLoS ONE 2025, 20, e0320393. [Google Scholar] [CrossRef]
- Kuralay, F.; Campuzano, S.; Haake, D.A.; Wang, J. Highly sensitive disposable nucleic acid biosensors for direct bioelectronic detection in raw biological samples. Talanta 2011, 85, 1330–1337. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Kumar A, S.; Mathiyarasu, J.; Suthindhiran, K. Detection of Escherichia coli in Food Samples by Magnetosome-based Biosensor. Biotechnol. Bioprocess Eng. 2023, 28, 152–161. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.A.; Ringgit, G.; Sarker, S.; Abu Bakar, A.M.S.; Saallah, S.; Amin, Z.; Shaarani, S.M.; Siddiquee, S. Electrochemical DNA Biosensor for the Detection of Infectious Bronchitis Virus Using a Multi-Walled Carbon Nanotube-Modified Gold Electrode. Poultry 2025, 4, 12. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.J.; Song, Y.; Zhu, M.J.; Lin, Y.H.; Du, D. A portable smart-phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, S.A.; Al-Amidie, M.; Shen, Z.; Mlaji, Z.; Liu, J.; Abdullah, A.; El-Dweik, M.; Zhang, S.; Almasri, M. A microfluidic biosensor for rapid simultaneous detection of waterborne pathogens. Biosens. Bioelectron. 2022, 203, 113993. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Shoja Doost, J.; Sharif, S. Biosensors for Monitoring, Detecting, and Tracking Dissemination of Poultry-Borne Bacterial Pathogens Along the Poultry Value Chain: A Review. Animals 2024, 14, 3138. [Google Scholar] [CrossRef]
- Kumar, J.; Xu, M.; Li, Y.A.; You, S.W.; Doherty, B.M.; Gardiner, W.D.; Cirrito, J.R.; Yuede, C.M.; Benegal, A.; Vahey, M.D.; et al. Capacitive Biosensor for Rapid Detection of Avian (H5N1) Influenza and E. coli in Aerosols. ACS Sens. 2025, 10, 3381–3389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Mitiku, B.A.; Tamrat Admasu, H. Veterinary Drug Residues in Food Products of Animal Origin and Their Public Health Consequences: A Review. Vet. Med. Sci. 2024, 10, e70049. [Google Scholar] [CrossRef]
- Sattar, S.; Hassan, M.M.; Islam, S.; Alam, M.; Chowdhury, S.; Saifuddin, A. Antibiotic residues in broiler and layer meat in Chittagong district of Bangladesh. Vet. World 2014, 7, 738–743. [Google Scholar] [CrossRef]
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef]
- Yang, Y.; Wenqian, Q.; Yuxiang, L.; and Liu, L. Antibiotic residues in poultry food in Fujian Province of China. Food Addit. Contam. Part B 2020, 13, 177–184. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- United States (US) Food and Drug Administration (FDA). 2022 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; US Department of Health and Human Services: Rockville, MD, USA, 2023. Available online: https://www.fda.gov/animal-veterinary/antimicrobial-resistance/2022-summary-report-antimicrobials-sold-or-distributed-use-food-producing-animals (accessed on 2 June 2025).
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16, Erratum in J. Antimicrob. Chemother. 2002, 49, 1049. [Google Scholar] [CrossRef]
- Sun, J.; Warden, A.R.; Huang, J.; Wang, W.; Ding, X. Colorimetric and Electrochemical Detection of Escherichia coli and Antibiotic Resistance Based on a p-Benzoquinone-Mediated Bioassay. Anal. Chem. 2019, 91, 7524–7530. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, F. Graphene-Assisted Sensor for Rapid Detection of Antibiotic Resistance in Escherichia coli. Front. Chem. 2021, 9, 696906. [Google Scholar] [CrossRef]
- Gao, W.; Li, B.; Yao, R.; Li, Z.; Wang, X.; Dong, X.; Qu, H.; Li, Q.; Li, N.; Chi, H.; et al. Intuitive Label-Free SERS Detection of Bacteria Using Aptamer-Based in Situ Silver Nanoparticles Synthesis. Anal. Chem. 2017, 89, 9836–9842. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Wang, A.; Zhou, D. Biosensor Technology: Advances and Applications in Livestock Infectious Disease Diagnosis. Vet. Sci. 2025, 12, 23. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Helali, S.; Sawelem Eid Alatawi, A.; Abdelghani, A. Pathogenic Escherichia coli biosensor detection on chicken food samples. J. Food Saf. 2018, 38, e12510. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; LeJeune, J.T.; Zhao, T.; Doyle, M.P. Enterohemorrhagic Escherichia coli. In Food Microbiology; Doyle, M.P., Buchanan, R.L., Eds.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Wasiewska, L.A.; Diaz, F.G.; Teixeira, S.R.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Amplification-free, highly sensitive electrochemical DNA-based sensor for simultaneous detection of stx1 and stx2 genes of Shiga toxin-producing E. coli (STEC). Electrochim. Acta 2023, 441, 141814. [Google Scholar] [CrossRef]
- Medina, M.B. A Biosensor Method for Detection of Staphylococcal Enterotoxin A in Raw Whole Egg. J. Rapid Methods Autom. Microbiol. 2006, 14, 119–132. [Google Scholar] [CrossRef]
- Koya, S.K.; Yurgelevic, S.; Brusatori, M.; Huang, C.; Diebel, L.N.; Auner, G.W. Rapid Detection of Clostridium difficile Toxins in Stool by Raman Spectroscopy. J. Surg. Res. 2019, 244, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Geleta, G.S. A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: An alternative approach to conventional methods. Anal. Bioanal. Chem. 2022, 414, 7103–7122. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, X.; Li, J.; Lan, Q.; Jing, Q.; Min, L.; Ren, C.; Hu, X.; Lambert, A.; Cheng, Q.; et al. Multiplex immunoassay of chicken cytokines via highly-sensitive chemiluminescent imaging array. Anal. Chim. Acta 2019, 1049, 213–218. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, X.; Ding, M.; Zhang, A. Dual-mode biosensor platform based on synergistic effects of dual-functional hybrid nanomaterials. Talanta 2023, 260, 124584. [Google Scholar] [CrossRef]
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef]
- Abuhelwa, M.; Singh, A.; Liu, J.; Almalaysha, M.; Carlson, A.V.; Trout, K.E.; Morey, A.; Kinzel, E.; Channaiah, L.H.; Almasri, M. Fiber optics-based surface enhanced Raman Spectroscopy sensors for rapid multiplex detection of foodborne pathogens in raw poultry. Microsyst. Nanoeng. 2024, 10, 199. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, Y.; Wang, Z.; Wang, Z.; Xie, J.; Zhai, H.; Huang, Z.; Wang, Y.; Wu, Q.; Ding, Y. The recent advances of high-throughput biosensors for rapid detection of foodborne pathogens. TrAC Trends Anal. Chem. 2024, 176, 117736. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Z.; Song, Y.; Yang, X.; Chen, S.; Fu, S.; Qin, X.; Zhang, W.; Man, C.; Jiang, Y. A novel smartphone-based colorimetric aptasensor for on-site detection of Escherichia coli O157:H7 in milk. J. Dairy Sci. 2021, 104, 8506–8516. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Public health risks associated with Enteroaggregative Escherichia coli (EAEC) as a food-borne pathogen. EFSA J. 2015, 13, 4330. [Google Scholar] [CrossRef]
- Lekowska-Kochaniak, A.; Czajkowska, D.; Popowski, J. Detection of Escherichia coli O157: H7 in raw meat by immunomagnetic separation and multiplex PCR. Acta Microbiol. Pol. 2002, 51, 327–337. [Google Scholar]
- Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Lai, C.W.; Ibrahim, F.; Thong, K.L.; Leo, B.F. Graphene-based label-free electrochemical aptasensor for rapid and sensitive detection of foodborne pathogen. Anal. Bioanal. Chem. 2017, 409, 6893–6905. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, X.; Wang, S.; Liu, Y. A smartphone-integrated paper sensing system for fluorescent and colorimetric dual-channel detection of foodborne pathogenic bacteria. Anal. Bioanal. Chem. 2020, 412, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Kim, B. Lab-on-a-chip pathogen sensors for food safety. Sensors 2012, 12, 10713–10741. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Noll, L.W.; Shridhar, P.B.; Dewsbury, D.M.; Shi, X.; Cernicchiaro, N.; Renter, D.G.; Nagaraja, T.G. A Comparison of Culture- and PCR-Based Methods to Detect Six Major Non-O157 Serogroups of Shiga Toxin-Producing Escherichia coli in Cattle Feces. PLoS ONE 2015, 10, e0135446. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Du, X. Electrochemical biosensors for detection of foodborne pathogens. Micromachines 2019, 10, 222. [Google Scholar] [CrossRef]
- Dong, X.; Huang, A.; He, L.; Cai, C.; You, T. Recent advances in foodborne pathogen detection using photoelectrochemical biosensors: From photoactive material to sensing strategy. Front. Sustain. Food Syst. 2024, 8, 2024. [Google Scholar] [CrossRef]
- Mandal, P.K.; Biswas, A.K.; Choi, K.; Pal, U.K. Methods for Rapid Detection of Foodborne Pathogens: An Overview. Am. J. Food Technol. 2011, 6, 87–102. [Google Scholar] [CrossRef]
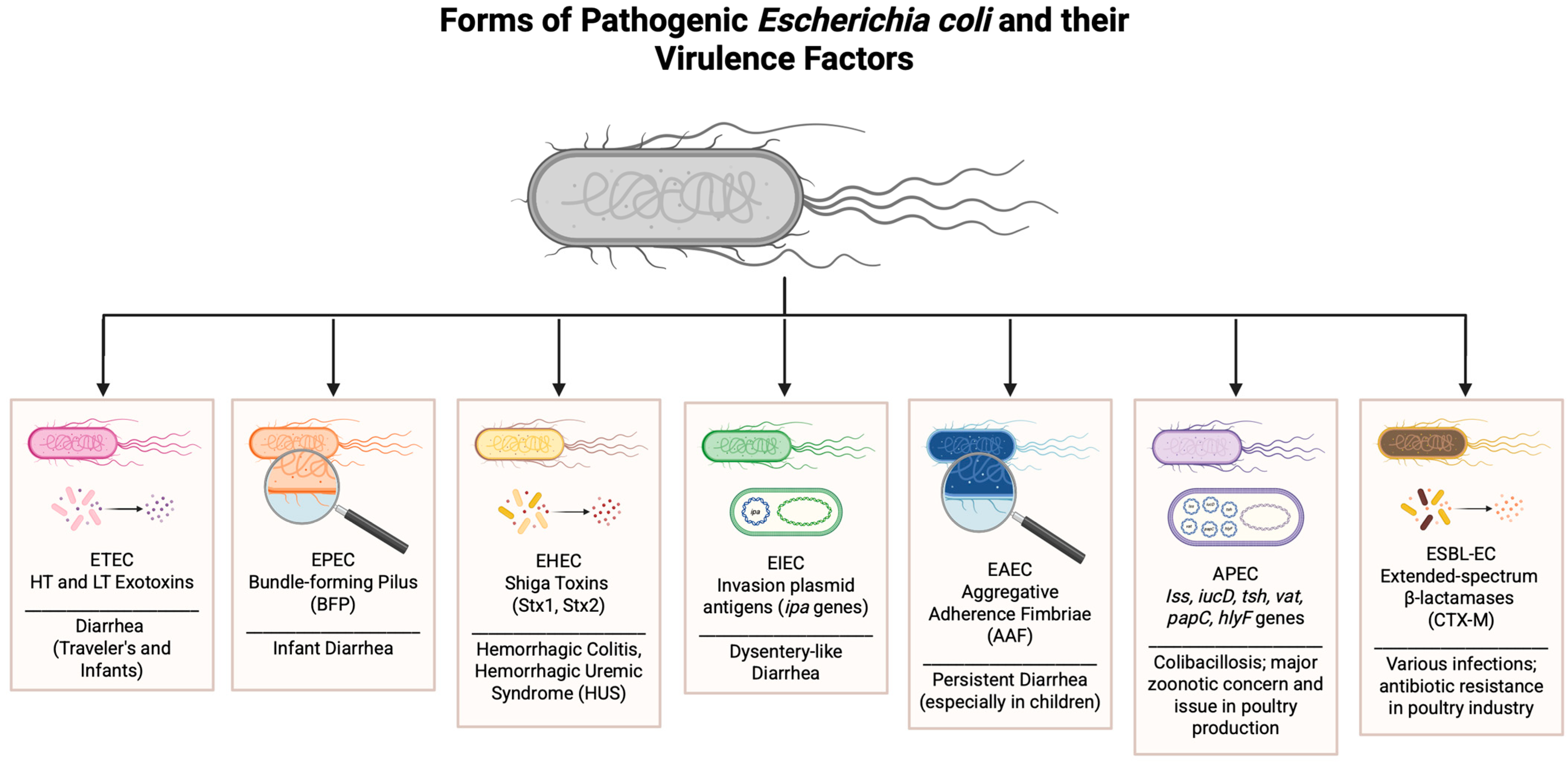


| Strain (Abbreviation) | Full Name | Primary Hosts | Disease(s) Caused | Key Virulence Factors | Notes on Relevance to Poultry/Food Safety |
|---|---|---|---|---|---|
| ETEC | Enterotoxigenic E. coli | Humans, animals | Traveler’s diarrhea, infant diarrhea | Heat-labile (LT) and heat-stable (ST) toxins | Rarely poultry-specific, but possible contamination in products |
| EPEC | Enteropathogenic E. coli | Humans (infants) | Infantile diarrhea | Attaching and effacing lesions, bundle-forming pilus (BFP) | Typically a human concern; not poultry-associated directly |
| EHEC | Enterohemorrhagic E. coli | Humans, cattle | Hemorrhagic colitis, HUS | Shiga toxins (Stx1, Stx2), intimin | Foodborne transmission; serious human pathogen via undercooked meat |
| EIEC | Enteroinvasive E. coli | Humans | Dysentery-like illness | Invasion plasmid antigens (ipa genes) | Human-specific; not associated with poultry |
| EAEC | Enteroaggregative E. coli | Humans | Persistent diarrhea (esp. in children) | Aggregative adherence fimbriae (AAF) | Human concern; not poultry-associated |
| APEC | Avian Pathogenic E. coli | Poultry | Colibacillosis (septicemia, airsacculitis, pericarditis, peritonitis) | Iss, iucD, tsh, vat, papC, hlyF genes | Major concern in poultry production and zoonotic potential |
| ESBL-EC | ESBL-producing E. coli | Poultry, humans | Various infections; antibiotic resistance | Extended-spectrum β-lactamases (e.g., CTX-M) | Emerging food safety threat; linked to antibiotic use in poultry |
| Life Stage | Biosensor Type | Target Biomarker | Sample Type | Linit of Detection (LOD) | Reference |
|---|---|---|---|---|---|
| In Ovo | Electrochemical DNA biosensor | Nucleic acid; E. coli O157:H7 antigen | Allantoic or amniotic fluid | 2 × 10−12 mol/L DNA; 10 CFU/mL | Bhuiyan et al., 2025 [83] Zeinhom et al., 2018 [84] |
| Hatchlings | Microfluidic device with interdigitated electrodes | E. coli O157:H7 antigen | Tap water and waste water | 3 bacterial cells/mL | Muhsin et al., 2022 [85] |
| Growing Birds | Label-free capacitive biosensor with Prussian blue/graphene oxide | E. coli somatic and capsular (O and K) antigen (LPS and bacterial wall) | Aerosol samples | 8 bacterial cells/m3 | Kumar et al., 2025 [87] |
| Grow-Out Phase | CRISPR-Cas-based nucleic acid sensor | Virulence-associated DNA sequences | Water, litter, cloacal swabs | Not provided | Gootenberg et al., 2017 [88] |
| Life Stage | Target | Sample Type | Biosensor Approach | Limit of Detection (LOD) | Reference |
|---|---|---|---|---|---|
| Hatchling (1–7 days of age) | E.coli bacterial load, resistance | Cloacal swabs, environmental swabs | Colorimetric smartphone-based sensor | 103 to 109 CFU/mL of E. coli O157:H7 was tested | Sun et al., 2019 [98] |
| Grow-out (2–6 weeks of age) | E.coli, AMR genes (e.g., bla_TEM) | Feed, feces, drinking water | SERS biosensors | Linear dependence on bacteria ranged from 101 to 107 CFU/mL (R2 = 0.9871); 1.5 CFU/mL (LOD) | Gao et al., 2017 [100] |
| Pre-slaughter (6+ weeks of age) | Antibiotic presence; E. coli antigens | Aerosol | Prussian blue/graphene oxide network on a screen-printed carbon electrode | 5 to 8 bacterial cells/mL (LOD) | Kumar et al., 2025 [87] |
| Post-slaughter (meat products) | E. coli K12 antigen | Frozen chicken meat | Electrochemical impedance spectroscopy with anti-E. coli antibody (immunological) | 103 CFU/mL (LOD) | Helali et al., 2018 [103] |
| Biosensor Type | Target | Sample Type | Limit of Detection | Time | Reference |
|---|---|---|---|---|---|
| Label-free capacitive biosensor with Prussian blue/graphene oxide | E. coli somatic and capsular (O and K) antigen (LPS and bacterial wall) | Aerosol samples | 8 bacterial cells/m3 | Up to 30 min | Kumar et al., 2025 [87] |
| Microfluidic device with interdigitated electrodes | E. coli O157:H7 antigen | Environmental (water) | 3 bacterial cells/mL | <2 h | Muhsin et al., 2022 [85] |
| Surface-Enhanced Raman Spectroscopy (SERS) Aptamer Biosensor | Whole E. coli cells (zoonotic strains) | Carcass rinse, wash water | ~103 CFU/mL | Minutes to ~1 h | Abuhelwa et al., 2024 [113] |
| Smartphone-integrated Colorimetric Biosensor | E. coli O157:H7 antigen | Milk | 5.24 × 103 CFU/mL | ~30–60 min | Yang et al., 2021 [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risalvato, J.; Sewid, A.H.; Eda, S.; Gerhold, R.W.; Wu, J.J. Strategic Detection of Escherichia coli in the Poultry Industry: Food Safety Challenges, One Health Approaches, and Advances in Biosensor Technologies. Biosensors 2025, 15, 419. https://doi.org/10.3390/bios15070419
Risalvato J, Sewid AH, Eda S, Gerhold RW, Wu JJ. Strategic Detection of Escherichia coli in the Poultry Industry: Food Safety Challenges, One Health Approaches, and Advances in Biosensor Technologies. Biosensors. 2025; 15(7):419. https://doi.org/10.3390/bios15070419
Chicago/Turabian StyleRisalvato, Jacquline, Alaa H. Sewid, Shigetoshi Eda, Richard W. Gerhold, and Jie Jayne Wu. 2025. "Strategic Detection of Escherichia coli in the Poultry Industry: Food Safety Challenges, One Health Approaches, and Advances in Biosensor Technologies" Biosensors 15, no. 7: 419. https://doi.org/10.3390/bios15070419
APA StyleRisalvato, J., Sewid, A. H., Eda, S., Gerhold, R. W., & Wu, J. J. (2025). Strategic Detection of Escherichia coli in the Poultry Industry: Food Safety Challenges, One Health Approaches, and Advances in Biosensor Technologies. Biosensors, 15(7), 419. https://doi.org/10.3390/bios15070419







