AI-Driven Wearable Bioelectronics in Digital Healthcare
Abstract
1. Introduction
2. Wearable Bioelectronics
2.1. Overview of Wearable Sensors and Bioelectronics
2.1.1. Biosensors
| Generation | Response Time | Sensitivity | Stability | Selectivity |
|---|---|---|---|---|
| 2000–2010 | >30 s | μM–mM | Hours/days | Antibody dependent |
| 2010–2020 | 2–10 s | nM–μM | Weeks | Aptamer/MIP-based |
| 2020-present | <1 s | pM–nM | Months | AI-enhanced multimodal |
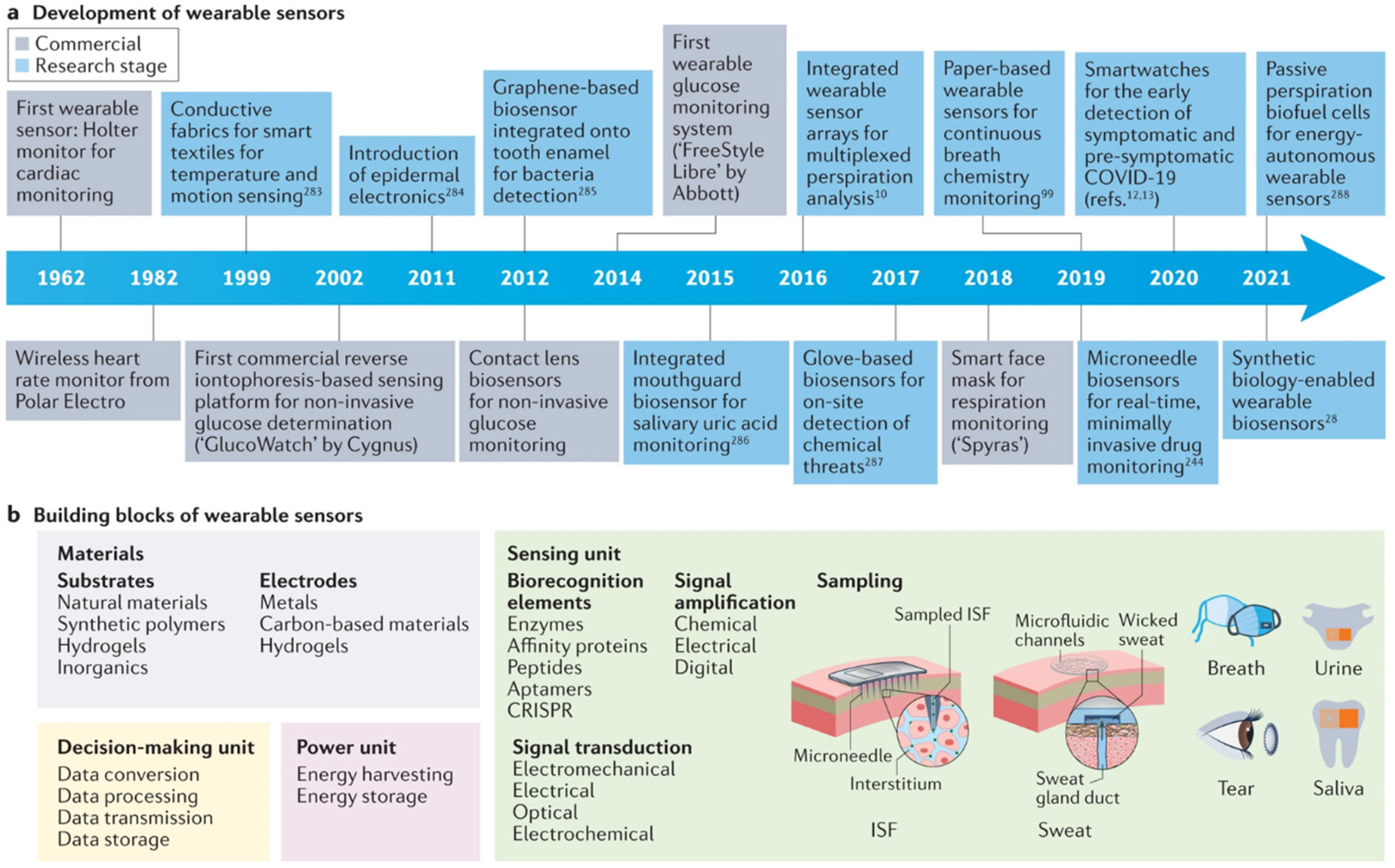
2.1.2. Smartwatches and Fitness Trackers
2.1.3. Wearable Patches
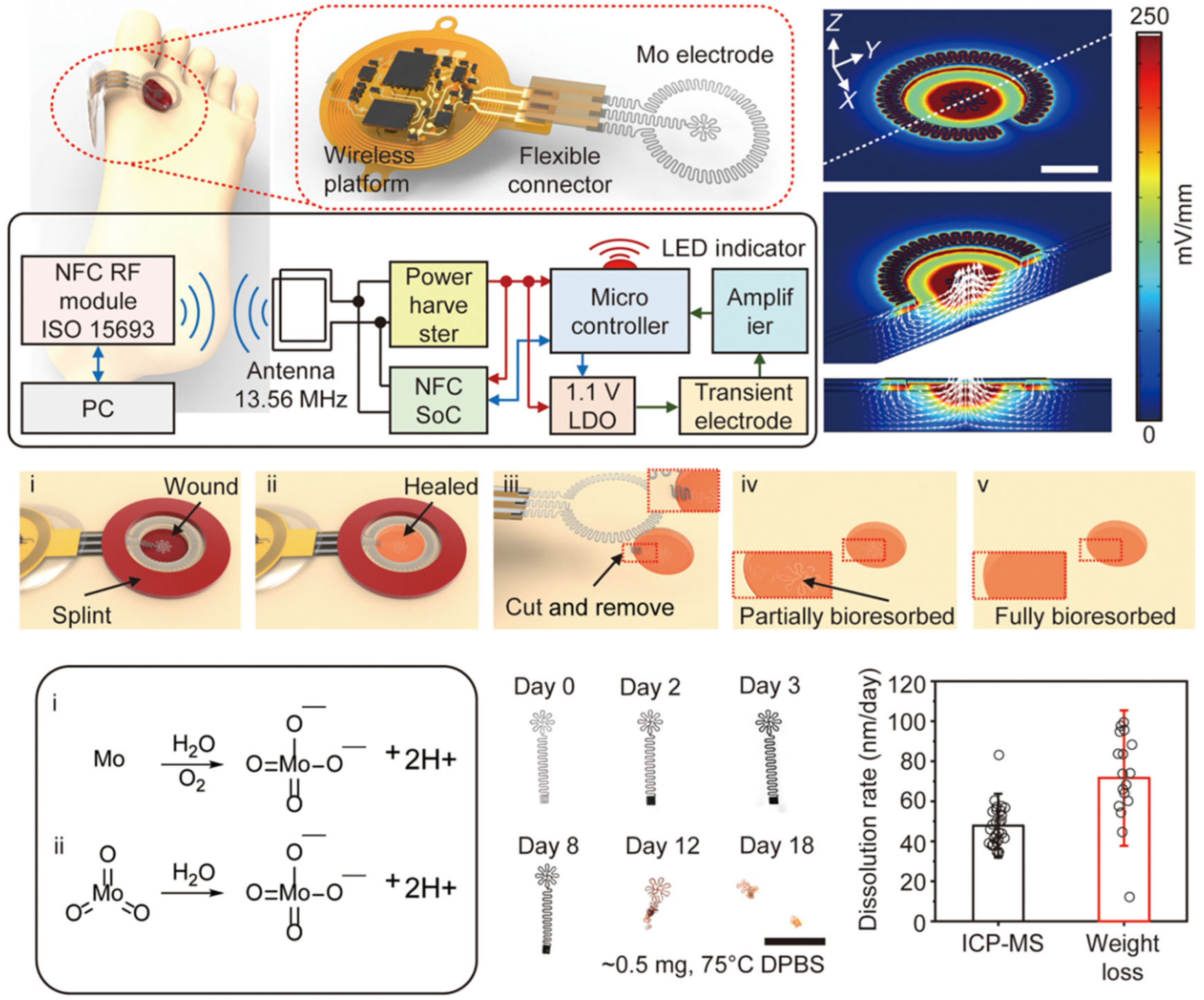
2.1.4. Implantable Devices

2.1.5. Smart Textiles

2.2. Materials Properties
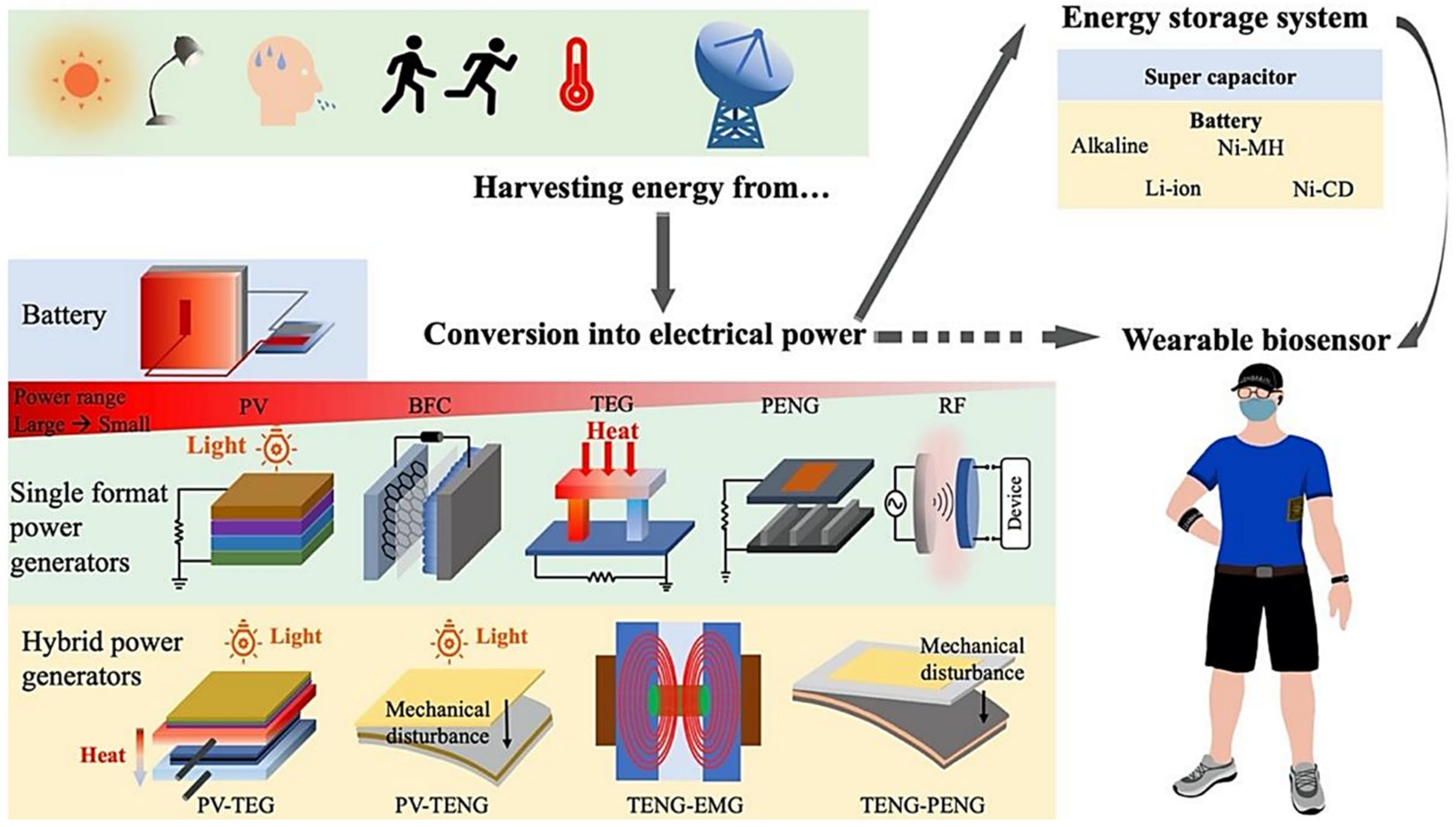
2.3. Power Sources and Energy Harvesting Methods
2.4. Signal Processing and Data Acquisition
3. Artificial Intelligence
3.1. Overview of AI Algorithms in Healthcare
3.2. Data Processing and Analysis Techniques
3.3. Integration of AI with Edge Computing
3.4. AI–Biosensor Fusion Framework: Methodological Pillars
3.4.1. AI-Enhanced Sensing Principles
3.4.2. Closed-Loop Optimization Methods
3.4.3. Intelligent Diagnostic Paradigms
3.4.4. Implementation Roadmap
4. Applications in Digital Healthcare
4.1. Health Monitoring
4.1.1. Continuous Monitoring of Vital Signs

4.1.2. Detection of Anomalies and Early Warning Systems for Chronic Diseases
4.1.3. Wearable Devices for Mental Health Monitoring
4.2. Diagnosis and Prognosis

4.2.1. AI-Driven Diagnostic Tools for Early Detection of Diseases
4.2.2. Predictive Analytics for Disease Progression and Patient Outcomes
4.2.3. Integration of Wearable Bioelectronics with EHRs
5. Challenges for AI-Driven Wearable Bioelectronics
5.1. Ethical and Privacy Concerns in the Integration of Wearable Data
5.1.1. Data Security: Protecting Sensitive Health Information
5.1.2. Ethical Implications of AI Decision-Making in Healthcare
5.1.3. Informed Consent and Patient Autonomy in Health Data Management
5.2. Regulatory and Legal Issues in AI and Wearable Technologies in Healthcare
5.2.1. Compliance with Healthcare Regulations to Safeguard Patient Safety and Data Privacy
5.2.2. Standardization of AI Algorithms and Wearable Technologies: Ensuring Consistency and Interoperability
5.2.3. Liability and Accountability in AI-Driven Healthcare Decisions: Navigating Complex Legal Landscapes
5.3. Cost-Reduction for AI-Driven Wearable Bioelectronics
6. Future Directions
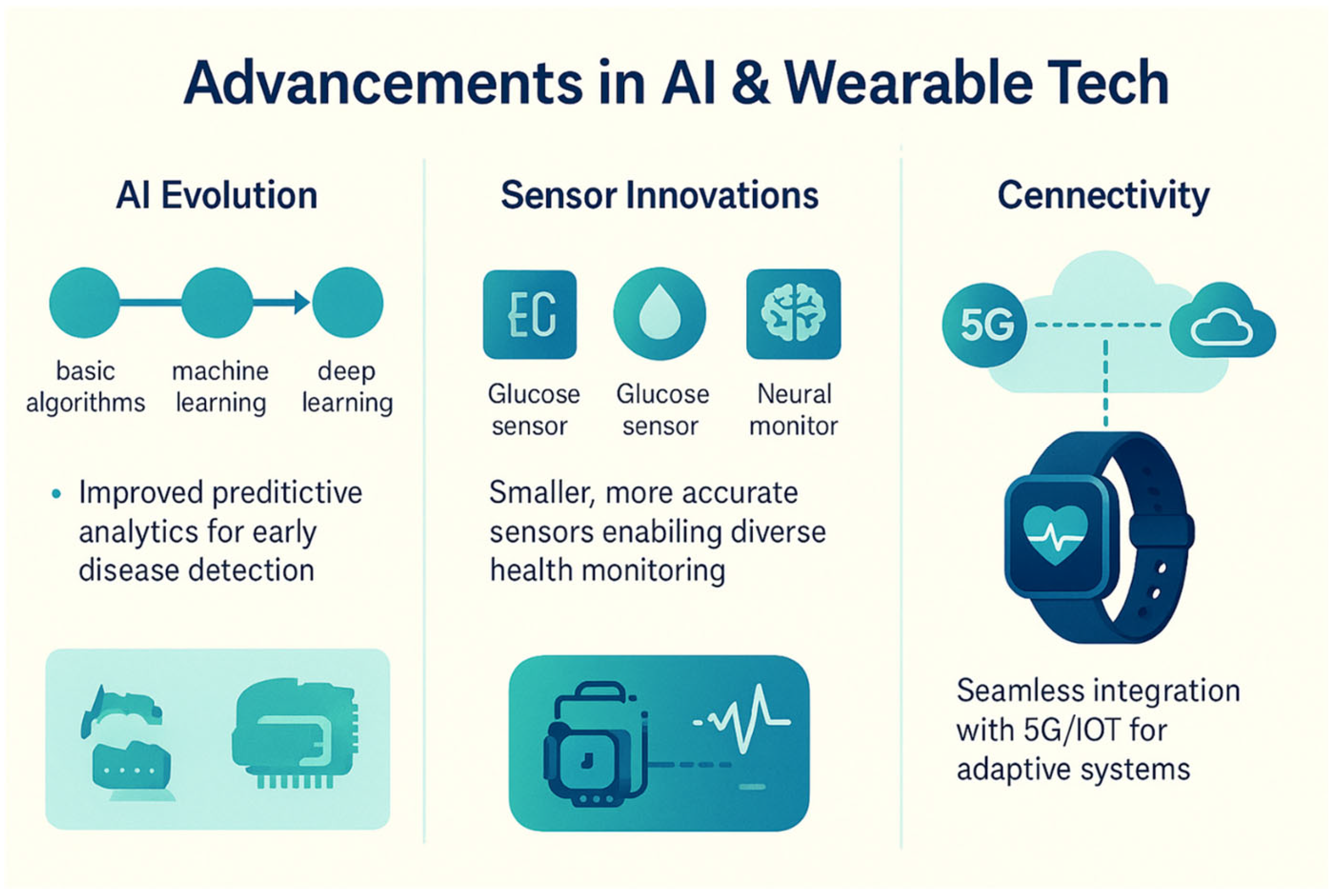
6.1. Advancements in AI and Wearable Technologies
6.2. Personalized and Preventive Healthcare
6.3. Enhancing Affordability and Accessibility
6.3.1. Scalable Manufacturing Innovations
6.3.2. Open-Source Ecosystem Development
6.3.3. Policy-Driven Accessibility Frameworks
6.4. Global Impact
7. Policy Implementation Framework
7.1. Phased Implementation Roadmap
7.2. Equity Assurance Mechanisms
7.3. Stakeholder Engagement Protocol
7.4. Monitoring and Evaluation Framework
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.; Tang, N.; Omar, R.; Hu, Z.; Duong, T.; Wang, J.; Wu, W.; Haick, H. Smart Materials Enabled with Artificial Intelligence for Healthcare Wearables. Adv. Funct. Mater. 2021, 31, 2105482. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, J.; Gao, S. Advanced Electronics and Artificial Intelligence: Must-Have Technologies Toward Human Body Digital Twins. Adv. Intell. Syst. 2022, 4, 2100263. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gebicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Song, Y. Wearable healthcare monitoring and therapeutic bioelectronics. Wearable Electron. 2025, 2, 18–22. [Google Scholar] [CrossRef]
- Liao, C.; Xiong, Y.; Fu, Y.; Chen, X.; Occhipinti, L.G. Organic semiconductors based wearable bioelectronics. Wearable Electron. 2025, 2, 23–39. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.; Sluyter, R.; Konstantinov, K.; Kim, J.H.; Kim, S.; Kim, Y.H. Wearable and implantable bioelectronics as eco-friendly and patient-friendly integrated nanoarchitectonics for next-generation smart healthcare technology. EcoMat 2023, 5, e12356. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; Zhou, Y.; Du, W.; Liang, F.; Yu, H.D.; Li, L. Recent Progress in Semi-Implantable Bioelectronics for Precision Health Monitoring. Adv. Funct. Mater. 2025, 2424463. [Google Scholar] [CrossRef]
- Wang, B.; Lv, A.; Wu, H.; Guo, B.; Lu, Y.; Chang, Z.; Wu, Y.; Li, X.; Yang, Q.; Nie, J.; et al. Antifreezing Ultrathin Bioionic Gel-Based Wearable System for Artificial Intelligence-Assisted Arrhythmia Diagnosis in Hypothermia. ACS Nano 2025, 19, 8176–8188. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Chen, X. Autonomous Bioelectronic Devices Based on Silk Fibroin. Adv. Mater. 2025, 37, e2500073. [Google Scholar] [CrossRef]
- Rivnay, J.; Raman, R.; Robinson, J.T.; Schreib, C.; Cohen-Karni, T.; Galloway, K.E.; Veiseh, O. Integrating bioelectronics with cell-based synthetic biology. Nat. Rev. Bioeng. 2025, 3, 317–332. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Z.; Liu, J.; Hu, C.; Du, Y.; Li, J.; Pan, Z.; Ding, K. Advancing Sports Cardiology: Integrating Artificial Intelligence with Wearable Devices for Cardiovascular Health Management. ACS Appl. Mater. Interfaces. 2025, 17, 17895–17920. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, J.; Jeong, E.Y.; Jang, Y.; Kim, H.; Choi, B.; Han, D.; Oh, Y.; Jeong, J.W. Wireless Technologies for Wearable Electronics: A Review. Adv. Electron. Mater. 2025, 2400884. [Google Scholar] [CrossRef]
- Ko, A.; Liao, C. Paper-based colorimetric sensors for point-of-care testing. Anal. Methods 2023, 15, 4377–4404. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef]
- Finster, R.; Sankaran, P.; Bihar, E. Computational and AI-Driven Design of Hydrogels for Bioelectronic Applications. Adv. Electron. Mater. 2025, 2400763. [Google Scholar] [CrossRef]
- Liao, C.; Wu, H.; Occhipinti, L.G. Machine Learning-Assisted 3D Flexible Organic Transistor for High-Accuracy Metabolites Analysis and Other Clinical Applications. Chemosensors 2024, 12, 174. [Google Scholar] [CrossRef]
- Ma, X.; Guo, G.; Wu, X.; Wu, Q.; Liu, F.; Zhang, H.; Shi, N.; Guan, Y. Advances in Integration, Wearable Applications, and Artificial Intelligence of Biomedical Microfluidics Systems. Micromachines 2023, 14, 972. [Google Scholar] [CrossRef]
- Shan, G.; Li, X.; Huang, W. AI-Enabled Wearable and Flexible Electronics for Assessing Full Personal Exposures. Innovation 2020, 1, 100031. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Wang, K.; Cao, Z.; Ren, F.; Zhou, G.; Chen, Y.; Sun, B. Silk Fibroin-Based Biomemristors for Bionic Artificial Intelligence Robot Applications. ACS Nano 2025, 19, 17173–17198. [Google Scholar] [CrossRef]
- Yin, J.; Wang, S.; Xiao, X.; Manshaii, F.; Scott, K.; Chen, J. Leveraging biomimetic materials for bioelectronics. Matter 2025, 8, 101961. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, F.; Yan, Z.; Chen, P.Y. Wearable bioelectronics based on emerging nanomaterials for telehealth applications. Device 2025, 3, 100676. [Google Scholar] [CrossRef] [PubMed]
- Katsoulakis, A.; Nakyazze, F.; McHugh, M.; Morris, S.; Bhavsar, M.; Tank, O.; Kireev, D. Squishy bioelectronic circuits. Device 2025, 3, 100553. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, M.Y.; Kim, D.H.; Koo, J.H. Recent Advances in Hydrogel-Based Soft Bioelectronics and its Convergence with Machine Learning. Adv. Eng. Mater. 2024, 26, 2401432. [Google Scholar] [CrossRef]
- Wang, C.; Sani, E.S.; Gao, W. Wearable Bioelectronics for Chronic Wound Management. Adv. Funct. Mater. 2022, 32, 2111022. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Sui, X.; Shao, X.; Li, K.; Zhang, H.; Xu, Z.; Zhang, D. An artificial intelligence-assisted microfluidic colorimetric wearable sensor system for monitoring of key tear biomarkers. npj Flex. Electron. 2024, 8, 35. [Google Scholar] [CrossRef]
- Gong, S.; Lu, Y.; Yin, J.; Levin, A.; Cheng, W. Materials-Driven Soft Wearable Bioelectronics for Connected Healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef]
- Chen, S.; Fan, S.; Qiao, Z.; Wu, Z.; Lin, B.; Li, Z.; Riegler, M.A.; Wong, M.Y.H.; Opheim, A.; Korostynska, O.; et al. Transforming Healthcare: Intelligent Wearable Sensors Empowered by Smart Materials and Artificial Intelligence. Adv. Mater. 2025, 37, e2500412. [Google Scholar] [CrossRef]
- Lin, S.; Hu, S.; Song, W.; Gu, M.; Liu, J.; Song, J.; Liu, Z.; Li, Z.; Huang, K.; Wu, Y.; et al. An ultralight, flexible, and biocompatible all-fiber motion sensor for artificial intelligence wearable electronics. npj Flex. Electron. 2022, 6, 27. [Google Scholar] [CrossRef]
- Dastgeer, G.; Nisar, S.; Shahzad, Z.M.; Rasheed, A.; Kim, D.K.; Jaffery, S.H.A.; Wang, L.; Usman, M.; Eom, J. Low-Power Negative-Differential-Resistance Device for Sensing the Selective Protein via Supporter Molecule Engineering. Adv. Sci. 2022, 10, e2204779. [Google Scholar] [CrossRef]
- Nisar, S.; Dastgeer, G.; Shazad, Z.M.; Zulfiqar, M.W.; Rasheed, A.; Iqbal, M.Z.; Hussain, K.; Rabani, I.; Kim, D.K.; Irfan, A.; et al. 2D Materials in Advanced Electronic Biosensors for Point-of-Care Devices. Adv. Sci. 2024, 11, e2401386. [Google Scholar] [CrossRef]
- Kassanos, P.; Rosa, B.G.; Keshavarz, M.; Yang, G.-Z. Power and data communication in wearable and implantable devices. In Wearable Sensors; Academic Press: San Diego, CA, USA, 2021; pp. 279–309. [Google Scholar] [CrossRef]
- Sani, E.S.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G.; et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023, 9, eadf7388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Haick, H.; Guo, S.; Wang, C.; Lee, S.; Yokota, T.; Someya, T. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 2022, 51, 3759–3793. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jekal, J.; Liu, J.; Kim, J.; Park, J.U.; Lee, T.; Jang, K.I. Bioelectronic Implantable Devices for Physiological Signal Recording and Closed-Loop Neuromodulation. Adv. Funct. Mater. 2024, 34, 2403562. [Google Scholar] [CrossRef]
- Chen, L.; Chen, S.; Sun, B.; Chen, J.; Zhang, Y. Wireless bioelectronic devices for next-generation electrotherapy. Cell Biomater. 2025, 1, 100054. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, Y.; Song, L.; Luo, L.; Qin, Y.; Han, N.; Yang, Z.; Liu, L.; Ho, J.C.; Wang, F. High-performance enhancement-mode thin-film transistors based on Mg-doped In2O3 nanofiber networks. Nano Research 2018, 11, 1227–1237. [Google Scholar] [CrossRef]
- Backiyalakshmi, G.; Snekhalatha, U.; Salvador, A.L. Recent advancements in non-invasive wearable electrochemical biosensors for biomarker analysis—A review. Anal. Biochem. 2024, 692, 115578. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Z.; Zhang, X.; Wang, W.; Li, Z. Recent progress on PEDOT-based wearable bioelectronics. View 2022, 3, 20220030. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Liao, C.; Chen, X.; Fu, Y. Salivary analysis: An emerging paradigm for non-invasive healthcare diagnosis and monitoring. Interdiscip. Med. 2023, 1, e20230009. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.; Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.; Orozco, J. Wearable electrochemical biosensors to measure biomarkers with complex blood-to-sweat partition such as proteins and hormones. Microchimica Acta. 2022, 189, 127. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.; Crewther, B.; Cook, C.; Punyadeera, C.; Pandey, A.K. Sensing methods for stress biomarker detection in human saliva: A new frontier for wearable electronics and biosensing. Mater. Adv. 2024, 5, 5339–5350. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Patle, S.; Rotake, D. Recent advances, technological challenges and requirements to predict the future treads in wearable sweat sensors: A critical review. Microchem. J. 2024, 200, 110457. [Google Scholar] [CrossRef]
- Cho, E.H.; Choi, H.R.; Park, Y.; Jeong, S.Y.; Song, Y.J.; Hwang, Y.H.; Lee, J.; Chi, Y.; Wang, S.F.; Jeon, Y.; et al. Wearable and Wavelength-Tunable Near-Infrared Organic Light-Emitting Diodes for Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 57415–57426. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramirez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Organic electrochemical transistors with graphene-modified gate electrodes for highly sensitive and selective dopamine sensors. J. Mater. Chem B 2014, 2, 191–200. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Wu, J.; Li, H.; Peng, H.; Zhang, J.; Ding, K.; Wang, X.; Hou, C.; Zhang, H.; et al. Smart Wearable Sensor Fuels Noninvasive Body Fluid Analysis. ACS Appl. Mater. Interfaces 2025, 17, 13279–13301. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.C.; Soltis, I.; Lee, S.H.; Yeo, W.H. Advances in Electrochemical Sensors for Detecting Analytes in Biofluids. Adv. Sens. Res. 2023, 2, 2200088. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry. Anal. Chem. 2020, 92, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Antaw, F.; Wuethrich, A.; Anderson, W.; Trau, M. Configurable Miniaturized 3D Pores for Robust Single-Nanoparticle Analysis. Small Struct. 2020, 1, 2000011. [Google Scholar] [CrossRef]
- Ravariu, C.; Parvulescu, C.C.; Manea, E.; Tucureanu, V. Optimized Technologies for Cointegration of MOS Transistor and Glucose Oxidase Enzyme on a Si-Wafer. Biosensors 2021, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Kalai, M.; Becha, H.; Helali, K. Effect of artificial intelligence on economic growth in European countries: A symmetric and asymmetric cointegration based on linear and non-linear ARDL approach. J. Econ. Struct. 2024, 13, 22. [Google Scholar] [CrossRef]
- Hosseinzadeh Fakhr, M.; Lopez Carrasco, I.; Belyaev, D.; Kang, J.; Shin, Y.; Yeo, J.-S.; Koh, W.-G.; Ham, J.; Michaelis, A.; Opitz, J.; et al. Recent advances in wearable electrochemical biosensors towards technological and material aspects. Biosens. Bioelectron. X 2024, 19, 100503. [Google Scholar] [CrossRef]
- Duan, H.; Peng, S.; He, S.; Tang, S.Y.; Goda, K.; Wang, C.H.; Li, M. Wearable Electrochemical Biosensors for Advanced Healthcare Monitoring. Adv. Sci. 2025, 12, e2411433. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, H.; Li, J.; Bandodkar, A.J.; Rogers, J.A. Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids. Trends Chem. 2019, 1, 559–571. [Google Scholar] [CrossRef]
- Apoorva, S.; Nguyen, N.T.; Sreejith, K.R. Recent developments and future perspectives of microfluidics and smart technologies in wearable devices. Lab Chip 2024, 24, 1833–1866. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narvaez, E.; Guder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Shu, L.; Yu, Y.; Chen, W.; Hua, H.; Li, Q.; Jin, J.; Xu, X. Wearable Emotion Recognition Using Heart Rate Data from a Smart Bracelet. Sensors 2020, 20, 718. [Google Scholar] [CrossRef]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. npj Digit Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.W.; Low, C.A.; Jacobson, N.; Arean, P.; Torous, J.; Allen, N.B. Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. npj Digit. Med. 2020, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.Y.; Ryan, N.P.; Chen, D.; McNeil, J.; Hopper, I. Novel wearable and contactless heart rate, respiratory rate, and oxygen saturation monitoring devices: A systematic review and meta-analysis. Anaesthesia 2022, 77, 1268–1280. [Google Scholar] [CrossRef]
- Gill, S.K.; Barsky, A.; Guan, X.; Bunting, K.V.; Karwath, A.; Tica, O.; Stanbury, M.; Haynes, S.; Folarin, A.; Dobson, R.; et al. Consumer wearable devices for evaluation of heart rate control using digoxin versus beta-blockers: The RATE-AF randomized trial. Nat. Med. 2024, 30, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Strain, T.; Wijndaele, K.; Dempsey, P.C.; Sharp, S.J.; Pearce, M.; Jeon, J.; Lindsay, T.; Wareham, N.; Brage, S. Wearable-device-measured physical activity and future health risk. Nat. Med. 2020, 26, 1385–1391. [Google Scholar] [CrossRef]
- Ferguson, T.; Olds, T.; Curtis, R.; Blake, H.; Crozier, A.J.; Dankiw, K.; Dumuid, D.; Kasai, D.; O’Connor, E.; Virgara, R.; et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: A systematic review of systematic reviews and meta-analyses. Lancet Digit. Health 2022, 4, e615–e626. [Google Scholar] [CrossRef]
- Dcosta, J.V.; Ochoa, D.; Sanaur, S. Recent Progress in Flexible and Wearable All Organic Photoplethysmography Sensors for SpO(2) Monitoring. Adv. Sci. 2023, 10, e2302752. [Google Scholar] [CrossRef]
- Rincon, F.; Pidoux, J.; Murali, S.; Goy, J.J. Performance of the new SmartCardia wireless, wearable oximeter: A comparison with arterial SaO2 in healthy volunteers. BMC Anesthesiol. 2022, 22, 77. [Google Scholar] [CrossRef]
- Pericleous, P.; van Staa, T.P. The use of wearable technology to monitor physical activity in patients with COPD: A literature review. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1317–1322. [Google Scholar] [CrossRef]
- Hannan, A.L.; Harders, M.P.; Hing, W.; Climstein, M.; Coombes, J.S.; Furness, J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: Systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2019, 11, 14. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent advances in wearable sensors and portable electronics for sleep monitoring. iScience 2021, 24, 102461. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.S.; Annis, J.; Master, H.; Han, L.; Gleichauf, K.; Ching, J.H.; Nasser, M.; Coleman, P.; Desine, S.; Ruderfer, D.M.; et al. Sleep patterns and risk of chronic disease as measured by long-term monitoring with commercial wearable devices in the All of Us Research Program. Nat. Med. 2024, 30, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhang, X.; Li, M.; Yu, J.; Zhang, Z.; He, Q.; Chen, S.; Zhu, L.; Jiang, T. Wearable wireless real-time cerebral oximeter for measuring regional cerebral oxygen saturation. Sci. China Inf. Sci. 2020, 64, 112203. [Google Scholar] [CrossRef]
- Ong, J.L.; Tandi, J.; Patanaik, A.; Lo, J.C.; Chee, M.W.L. Large-scale data from wearables reveal regional disparities in sleep patterns that persist across age and sex. Sci. Rep. 2019, 9, 3415. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Fu, Y.; Liao, C. Colorimetric sweat analysis using wearable hydrogel patch sensors for detection of chloride and glucose. Anal Methods 2023, 15, 43, 5855–5866. [Google Scholar]
- Geng, Y.; Cao, R.; Innocent, M.T.; Hu, Z.; Zhu, L.; Wang, L.; Xiang, H.; Zhu, M. A high-sensitive wearable sensor based on conductive polymer composites for body temperature monitoring. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107269. [Google Scholar] [CrossRef]
- Dahiya, E.S.; Kalra, A.M.; Lowe, A.; Anand, G. Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review. Sensors 2024, 24, 1318. [Google Scholar] [CrossRef]
- Kumar, A.; Komaragiri, R.; Kumar, M. From Pacemaker to Wearable: Techniques for ECG Detection Systems. J. Med. Syst. 2018, 42, 2, 34. [Google Scholar]
- Ahn, J.W.; Ku, Y.; Kim, H.C. A Novel Wearable EEG and ECG Recording System for Stress Assessment. Sensors 2019, 19, 1991. [Google Scholar] [CrossRef]
- Shao, M.; Zhou, Z.; Bin, G.; Bai, Y.; Wu, S. A Wearable Electrocardiogram Telemonitoring System for Atrial Fibrillation Detection. Sensors 2020, 20, 606. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Q.; Lei, L.; Zheng, K.; Xiang, W. An IoT-cloud Based Wearable ECG Monitoring System for Smart Healthcare. J. Med. Syst. 2016, 40, 286. [Google Scholar] [CrossRef] [PubMed]
- Nozariasbmarz, A.; Collins, H.; Dsouza, K.; Polash, M.H.; Hosseini, M.; Hyland, M.; Liu, J.; Malhotra, A.; Ortiz, F.M.; Mohaddes, F.; et al. Review of wearable thermoelectric energy harvesting: From body temperature to electronic systems. Appl. Energy 2020, 258, 114069. [Google Scholar] [CrossRef]
- Alam, M.S.; Kim, J.K.; Choi, J. Multifunctional Wearable System for Mapping Body Temperature and Analyzing Sweat. ACS Sens. 2023, 8, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Li, J.; Kim, Y.; Van Os, J.M.C.; Brounts, S.H.; Choi, C.Y. Using implantable biosensors and wearable scanners to monitor dairy cattle’s core body temperature in real-time. Comput. Electron. Agric. 2020, 174, 105453. [Google Scholar] [CrossRef]
- Liaqat, S.; Dashtipour, K.; Rizwan, A.; Usman, M.; Shah, S.A.; Arshad, K.; Assaleh, K.; Ramzan, N. Personalized wearable electrodermal sensing-based human skin hydration level detection for sports, health and wellbeing. Sci. Rep. 2022, 12, 3715. [Google Scholar] [CrossRef]
- Yao, S.; Myers, A.; Malhotra, A.; Lin, F.; Bozkurt, A.; Muth, J.F.; Zhu, Y. A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater. 2017, 6, 1601159. [Google Scholar] [CrossRef]
- Volkova, E.; Perchik, A.; Pavlov, K.; Nikolaev, E.; Ayuev, A.; Park, J.; Chang, N.; Lee, W.; Kim, J.Y.; Doronin, A.; et al. Multispectral sensor fusion in SmartWatch for in situ continuous monitoring of human skin hydration and body sweat loss. Sci. Rep. 2023, 13, 13371. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. npj Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Cho, S.; Han, H.; Park, H.; Lee, S.-U.; Kim, J.-H.; Jeon, S.W.; Wang, M.; Avila, R.; Xi, Z.; Ko, K.; et al. Wireless, multimodal sensors for continuous measurement of pressure, temperature, and hydration of patients in wheelchair. npj Flex. Electron. 2023, 7, 8. [Google Scholar] [CrossRef]
- Ota, H.; Chao, M.; Gao, Y.; Wu, E.; Tai, L.C.; Chen, K.; Matsuoka, Y.; Iwai, K.; Fahad, H.M.; Gao, W.; et al. 3D Printed “Earable” Smart Devices for Real-Time Detection of Core Body Temperature. ACS Sens. 2017, 2, 990–997. [Google Scholar] [CrossRef]
- Song, J.W.; Ryu, H.; Bai, W.; Xie, Z.; Guardado, A.V.; Nandoliya, K.; Avila, R.; Lee, G.; Song, Z.; Kim, J.; et al. Bioresorbable, wireless, and battery-free system for electrotherapy and impedance sensing at wound sites. Sci. Adv. 2023, 9, eade4687. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Park, H.M.; Kim, M.K.; Kim, B.; Myoung, H.S.; Kim, T.Y.; Yoon, H.J.; Kwak, S.S.; Kim, J.; Hwang, T.H.; et al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat. Commun. 2021, 12, 4374. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- Cutrone, A.; Micera, S. Implantable Neural Interfaces and Wearable Tactile Systems for Bidirectional Neuroprosthetics Systems. Adv. Healthc. Mater. 2019, 8, e1801345. [Google Scholar] [CrossRef]
- Topalovic, U.; Barclay, S.; Ling, C.; Alzuhair, A.; Yu, W.; Hokhikyan, V.; Chandrakumar, H.; Rozgic, D.; Jiang, W.; Basir-Kazeruni, S.; et al. A wearable platform for closed-loop stimulation and recording of single-neuron and local field potential activity in freely moving humans. Nat. Neurosci. 2023, 26, 517–527. [Google Scholar] [CrossRef]
- Saha, T.; Khan, M.I.; Sandhu, S.S.; Yin, L.; Earney, S.; Zhang, C.; Djassemi, O.; Wang, Z.; Han, J.; Abdal, A.; et al. A Passive Perspiration Inspired Wearable Platform for Continuous Glucose Monitoring. Adv. Sci. 2024, 11, e2405518. [Google Scholar] [CrossRef]
- van Enter, B.J.; von Hauff, E. Challenges and perspectives in continuous glucose monitoring. Chem. Commun. 2018, 54, 5032–5045. [Google Scholar] [CrossRef]
- Hanna, J.; Bteich, M.; Tawk, Y.; Ramadan, A.H.; Dia, B.; Asadallah, F.A.; Eid, A.; Kanj, R.; Costantine, J.; Eid, A.A. Noninvasive, wearable, and tunable electromagnetic multisensing system for continuous glucose monitoring, mimicking vasculature anatomy. Sci. Adv. 2020, 6, eaba5320. [Google Scholar] [CrossRef]
- Cernera, S.; Alcantara, J.D.; Opri, E.; Cagle, J.N.; Eisinger, R.S.; Boogaart, Z.; Pramanik, L.; Kelberman, M.; Patel, B.; Foote, K.D.; et al. Wearable sensor-driven responsive deep brain stimulation for essential tremor. Brain Stimul. 2021, 14, 1434–1443. [Google Scholar] [CrossRef]
- Habibagahi, I.; Omidbeigi, M.; Hadaya, J.; Lyu, H.; Jang, J.; Ardell, J.L.; Bari, A.A.; Babakhani, A. Vagus nerve stimulation using a miniaturized wirelessly powered stimulator in pigs. Sci. Rep. 2022, 12, 8184. [Google Scholar] [CrossRef]
- Qian, X.; Ko, A.; Li, H.; Liao, C. Flexible non-enzymatic glucose strip for direct non-invasive diabetic management. Microchemical J. 2024, 197, 109818. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, G.; Wang, S.; Zhang, Y.; Jian, Y.; He, L.; Yu, T.; Luo, H.; Kong, D.; Xianyu, Y.; et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2023, 7, 51–65. [Google Scholar] [CrossRef]
- Di, J.; Zhang, X.; Yong, Z.; Zhang, Y.; Li, D.; Li, R.; Li, Q. Carbon-Nanotube Fibers for Wearable Devices and Smart Textiles. Adv. Mater. 2016, 28, 10529–10538. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, S.; Zhang, L.; Yang, B.; Shu, L.; Yang, Y.; Ren, M.; Wang, Y.; Chen, J.; Chen, W.; et al. Smart Textile-Integrated Microelectronic Systems for Wearable Applications. Adv. Mater. 2020, 32, e1901958. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Xiao, Y.; Dong, Y.; Wang, X.; Lin, L. A Naturally Integrated Smart Textile for Wearable Electronics Applications. Adv. Mater. Technol. 2019, 5, 1900781. [Google Scholar] [CrossRef]
- Ma, X.; Wang, P.; Huang, L.; Ding, R.; Zhou, K.; Shi, Y.; Chen, F.; Zhuang, Q.; Huang, Q.; Lin, Y.; et al. A monolithically integrated in-textile wristband for wireless epidermal biosensing. Sci. Adv. 2023, 9, eadj2763. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Rao, R.P.; Reddy, M.V.; Chellappan, V.; Ramakrishna, S. Novel low-carbon energy solutions for powering emerging wearables, smart textiles, and medical devices. Energy Environ. Sci. 2022, 15, 4928–4981. [Google Scholar] [CrossRef]
- Leal-Junior, A.; Avellar, L.; Frizera, A.; Marques, C. Smart textiles for multimodal wearable sensing using highly stretchable multiplexed optical fiber system. Sci. Rep. 2020, 10, 13867. [Google Scholar] [CrossRef]
- Islam, M.R.; Afroj, S.; Novoselov, K.S.; Karim, N. Smart Electronic Textile-Based Wearable Supercapacitors. Adv. Sci. 2022, 9, e2203856. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, X.; Qu, L.; Miao, J. Smart Fibers and Textiles for Personal Thermal Management in Emerging Wearable Applications. Adv. Mater. Technol. 2022, 8, 2201137. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Huang, Y. Material innovation and mechanics design for substrates and encapsulation of flexible electronics: A review. Mater. Horiz. 2021, 8, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible Electrochemical Bioelectronics: The Rise of In Situ Bioanalysis. Adv. Mater. 2020, 32, e1902083. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liang, Z.; Cao, Y.; Han, Z.; Feng, X. Flexible inorganic bioelectronics. npj Flex. Electron. 2020, 4, 2. [Google Scholar] [CrossRef]
- Choi, C.; Lee, Y.; Cho, K.W.; Koo, J.H.; Kim, D.H. Wearable and Implantable Soft Bioelectronics Using Two-Dimensional Materials. Acc. Chem. Res. 2019, 52, 73–81. [Google Scholar] [CrossRef]
- Ko, A.; Liao, C. Hydrogel wound dressings for diabetic foot ulcer treatment: Status-quo, challenges, and future perspectives. BMEMat 2023, 1, e12037. [Google Scholar] [CrossRef]
- Liao, C.; Yan, F. Organic Semiconductors in Organic Thin-Film Transistor-Based Chemical and Biological Sensors. Polym. Rev. 2013, 53, 352–406. [Google Scholar] [CrossRef]
- Liao, C.; Wuethrich, A.; Trau, M. A material odyssey for 3D nano/microstructures: Two photon polymerization based nanolithography in bioapplications. Appl. Mater. Today 2020, 19, 100635. [Google Scholar] [CrossRef]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.Z.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S.T. Biocompatible Material-Based Flexible Biosensors: From Materials Design to Wearable/Implantable Devices and Integrated Sensing Systems. Small 2023, 19, e2207879. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, X.; Liang, J.; Shao, M.; Xie, E.; Yu, C.; Lan, W. Recent Advances of Energy Solutions for Implantable Bioelectronics. Adv. Healthc. Mater. 2021, 10, e2100199. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; De Oliveira, N.; Wang, S. Implantable bioelectronics toward long-term stability and sustainability. Matter 2021, 4, 1125–1141. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Veena, E.; Kumar, N.S.; Naidu, K.C.B.; Mallikarjuna, A.; Basha, D.B. A Review on Piezoelectric Materials and Their Applications. Cryst. Res. Technol. 2022, 58, 2200130. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Ding, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Flexible and Wearable Power Sources for Next-Generation Wearable Electronics. Batter. Supercaps 2020, 3, 1262–1274. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Wang, J. On-Body Bioelectronics: Wearable Biofuel Cells for Bioenergy Harvesting and Self-Powered Biosensing. Adv. Funct. Mater. 2020, 30, 1906243. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kramer, B.; Kroposki, B. A review of power electronics interfaces for distributed energy systems towards achieving low-cost modular design. Renew. Sustain. Energy Rev. 2009, 13, 2323–2335. [Google Scholar] [CrossRef]
- Chandrakasan, A.P.; Verma, N.; Daly, D.C. Ultralow-power electronics for biomedical applications. Annu. Rev. Biomed. Eng. 2008, 10, 247–274. [Google Scholar] [CrossRef]
- Dewan, A.; Ay, S.U.; Karim, M.N.; Beyenal, H. Alternative power sources for remote sensors: A review. J. Power Sources 2014, 245, 129–143. [Google Scholar] [CrossRef]
- Rong, G.; Zheng, Y.; Sawan, M. Energy Solutions for Wearable Sensors: A Review. Sensors 2021, 21, 3806. [Google Scholar] [CrossRef]
- Martinek, R.; Nedoma, J.; Fajkus, M.; Kahankova, R.; Konecny, J.; Janku, P.; Kepak, S.; Bilik, P.; Nazeran, H. A Phonocardiographic-Based Fiber-Optic Sensor and Adaptive Filtering System for Noninvasive Continuous Fetal Heart Rate Monitoring. Sensors 2017, 17, 890. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Seok, H.S.; Kim, S.S.; Shin, H. Photoplethysmogram Analysis and Applications: An Integrative Review. Front. Physiol. 2021, 12, 808451. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.L.; Rocha, M.X.; Vasconcelos, G.G.; Vasconcelos Filho, J.E.; de Albuquerque, V.H.C.; Alexandria, A.R. Advances in Photopletysmography Signal Analysis for Biomedical Applications. Sensors 2018, 18, 1894. [Google Scholar] [CrossRef] [PubMed]
- Mahato, K.; Saha, T.; Ding, S.; Sandhu, S.S.; Chang, A.-Y.; Wang, J. Hybrid multimodal wearable sensors for comprehensive health monitoring. Nat. Electron. 2024, 7, 735–750. [Google Scholar] [CrossRef]
- Ma, C.B.; Shang, X.; Sun, M.; Bo, X.; Bai, J.; Du, Y.; Zhou, M. Emerging Multifunctional Wearable Sensors: Integrating Multimodal Sweat Analysis and Advanced Material Technologies for Next-Generation Health Monitoring. ACS Sens. 2025, 10, 2388–2408. [Google Scholar] [CrossRef]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Panigrahi, D.; Rewatkar, P.; Haick, H. Role of Machine Learning Assisted Biosensors in Point-of-Care-Testing For Clinical Decisions. ACS Sens. 2024, 9, 4495–4519. [Google Scholar] [CrossRef]
- Olyanasab, A.; Annabestani, M. Leveraging Machine Learning for Personalized Wearable Biomedical Devices: A Review. J. Pers. Med. 2024, 14, 203. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors—Enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- van Engelen, J.E.; Hoos, H.H. A survey on semi-supervised learning. Mach. Learn. 2019, 109, 373–440. [Google Scholar] [CrossRef]
- Nettleton, D.F.; Orriols-Puig, A.; Fornells, A. A study of the effect of different types of noise on the precision of supervised learning techniques. Artif. Intell. Rev. 2010, 33, 275–306. [Google Scholar] [CrossRef]
- Jiang, T.; Gradus, J.L.; Rosellini, A.J. Supervised Machine Learning: A Brief Primer. Behav. Ther. 2020, 51, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Mehedi Hassan, M.; Mollick, S.; Yasmin, F. An unsupervised cluster-based feature grouping model for early diabetes detection. Healthc. Anal. 2022, 2, 100112. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Therneau, T.M.; Atkinson, E.J.; Tafti, A.P.; Zhang, N.; Amin, S.; Limper, A.H.; Khosla, S.; Liu, H. Unsupervised machine learning for the discovery of latent disease clusters and patient subgroups using electronic health records. J. Biomed. Inform. 2020, 102, 103364. [Google Scholar] [CrossRef]
- Lopez, C.; Tucker, S.; Salameh, T.; Tucker, C. An unsupervised machine learning method for discovering patient clusters based on genetic signatures. J. Biomed. Inform. 2018, 85, 30–39. [Google Scholar] [CrossRef]
- Ying, L.P.; Yin, O.X.; Quan, O.W.; Jain, N.; Mayuren, J.; Pandey, M.; Gorain, B.; Candasamy, M. Continuous glucose monitoring data for artificial intelligence-based predictive glycemic event: A potential aspect for diabetic care. Int. J. Diabetes Dev. Ctries. 2024, 45, 272–287. [Google Scholar] [CrossRef]
- Guan, Z.; Li, H.; Liu, R.; Cai, C.; Liu, Y.; Li, J.; Wang, X.; Huang, S.; Wu, L.; Liu, D.; et al. Artificial intelligence in diabetes management: Advancements, opportunities, and challenges. Cell Rep. Med. 2023, 4, 101213. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Coelho, L.; Carvalho, E.; Ferreira-Pinto, M.J.; Vaz, R.; Aguiar, P. Machine learning for adaptive deep brain stimulation in Parkinson’s disease: Closing the loop. J. Neurol. 2023, 270, 5313–5326. [Google Scholar] [CrossRef]
- He, G.Q.; Li, H.; Liu, J.; Hu, Y.L.; Liu, Y.; Wang, Z.L.; Jiang, P. Recent Progress in Implantable Drug Delivery Systems. Adv. Mater. 2024, 36, e2312530. [Google Scholar] [CrossRef]
- Kang, S.; Paul, A.; Jeon, G. Reduction of mixed noise from wearable sensors in human-motion estimation. Comput. Electr. Eng. 2017, 61, 287–296. [Google Scholar] [CrossRef]
- Rodeheaver, N.; Kim, H.; Herbert, R.; Seo, H.; Yeo, W.-H. Breathable, Wireless, Thin-Film Wearable Biopatch Using Noise-Reduction Mechanisms. ACS Appl. Electron. Mater. 2022, 4, 503–512. [Google Scholar] [CrossRef]
- Matthews, J.; Kim, J.; Yeo, W.H. Advances in Biosignal Sensing and Signal Processing Methods with Wearable Devices. Anal. Sens. 2022, 3, e202200062. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Rajabi, N.; Taleb, F.; Yang, Q.; Kragic, D.; Li, Z. Shaping high-performance wearable robots for human motor and sensory reconstruction and enhancement. Nat. Commun. 2024, 15, 1760. [Google Scholar] [CrossRef] [PubMed]
- Benson, L.C.; Clermont, C.A.; Osis, S.T.; Kobsar, D.; Ferber, R. Classifying running speed conditions using a single wearable sensor: Optimal segmentation and feature extraction methods. J. Biomech. 2018, 71, 94–99. [Google Scholar] [CrossRef]
- Phinyomark, A.; Khushaba, R.N.; Scheme, E. Feature Extraction and Selection for Myoelectric Control Based on Wearable EMG Sensors. Sensors 2018, 18, 1615. [Google Scholar] [CrossRef]
- Quaid, M.A.K.; Jalal, A. Wearable sensors based human behavioral pattern recognition using statistical features and reweighted genetic algorithm. Multimed. Tools Appl. 2019, 79, 6061–6083. [Google Scholar] [CrossRef]
- Piciucco, E.; Di Lascio, E.; Maiorana, E.; Santini, S.; Campisi, P. Biometric recognition using wearable devices in real-life settings. Pattern Recognit. Lett. 2021, 146, 260–266. [Google Scholar] [CrossRef]
- Ganesan, A.; Paul, A.; Nagabushnam, G.; Gul, M.J.J. Human-in-the-Loop Predictive Analytics Using Statistical Learning. J. Healthc. Eng. 2021, 2021, 9955635. [Google Scholar] [CrossRef]
- Witt, D.; Kellogg, R.; Snyder, M.; Dunn, J. Windows Into Human Health Through Wearables Data Analytics. Curr. Opin. Biomed. Eng. 2019, 9, 28–46. [Google Scholar] [CrossRef]
- Dargazany, A.R.; Stegagno, P.; Mankodiya, K. WearableDL: Wearable Internet-of-Things and Deep Learning for Big Data Analytics—Concept, Literature, and Future. Mob. Inf. Syst. 2018, 2018, 1–20. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, P.K.; Chauhan, N.; Roy, S.; Tiwari, A.; Gupta, S.; Tiwari, A.; Patra, S.; Das, T.R.; Mishra, P.; et al. Emergence of integrated biosensing-enabled digital healthcare devices. Sens. Diagn. 2024, 3, 718–744. [Google Scholar] [CrossRef]
- Sachdeva, S.; Bhatia, S.; Al Harrasi, A.; Shah, Y.A.; Anwer, K.; Philip, A.K.; Shah, S.F.A.; Khan, A.; Ahsan Halim, S. Unraveling the role of cloud computing in health care system and biomedical sciences. Heliyon 2024, 10, e29044. [Google Scholar] [CrossRef] [PubMed]
- Quy, N.M.; Ngoc, L.A.; Ban, N.T.; Hau, N.V.; Quy, V.K. Edge Computing for Real-Time Internet of Things Applications: Future Internet Revolution. Wirel. Pers. Commun. 2023, 132, 1423–1452. [Google Scholar] [CrossRef]
- Nain, G.; Pattanaik, K.K.; Sharma, G.K. Towards edge computing in intelligent manufacturing: Past, present and future. J. Manuf. Syst. 2022, 62, 588–611. [Google Scholar] [CrossRef]
- Singh, A.; Chatterjee, K. Securing smart healthcare system with edge computing. Comput. Secur. 2021, 108, 102353. [Google Scholar] [CrossRef]
- Almalawi, A.; Zafar, A.; Unhelkar, B.; Hassan, S.; Alqurashi, F.; Khan, A.I.; Fahad, A.; Alam, M.M. Enhancing security in smart healthcare systems: Using intelligent edge computing with a novel Salp Swarm Optimization and radial basis neural network algorithm. Heliyon 2024, 10, e33792. [Google Scholar] [CrossRef]
- Muhoza, A.C.; Bergeret, E.; Brdys, C.; Gary, F. Power consumption reduction for IoT devices thanks to Edge-AI: Application to human activity recognition. Internet Things 2023, 24, 100930. [Google Scholar] [CrossRef]
- Shi, Q.; Dong, B.; He, T.; Sun, Z.; Zhu, J.; Zhang, Z.; Lee, C. Progress in wearable electronics/photonics—Moving toward the era of artificial intelligence and internet of things. InfoMat 2020, 2, 1131–1162. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, X. Utilizing AI and IoT technologies for identifying risk factors in sports. Heliyon 2024, 10, e32477. [Google Scholar] [CrossRef]
- Sreedevi, A.G.; Nitya Harshitha, T.; Sugumaran, V.; Shankar, P. Application of cognitive computing in healthcare, cybersecurity, big data and IoT: A literature review. Inf. Process. Manag. 2022, 59, 102888. [Google Scholar] [CrossRef]
- Liao, M.T.; Yu, C.C.; Lin, L.Y.; Pan, K.H.; Tsai, T.H.; Wu, Y.C.; Liu, Y.B. Impact of recording length and other arrhythmias on atrial fibrillation detection from wrist photoplethysmogram using smartwatches. Sci. Rep. 2022, 12, 5364. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, e2109357. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.Y.; et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 2023, 7, 1293–1306. [Google Scholar] [CrossRef]
- Luo, J.; Sun, C.; Chang, B.; Zhang, B.; Li, K.; Li, Y.; Zhang, Q.; Wang, H.; Hou, C. On-Skin Paintable Water-Resistant Biohydrogel for Wearable Bioelectronics. Adv. Funct. Mater. 2024, 34, 2400884. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.; Chicas, R.; Xiuhtecutli, N.; Matthews, J.; Zavanelli, N.; Kwon, S.; Lee, S.H.; Hertzberg, V.S.; Yeo, W.H. Soft Wireless Bioelectronics Designed for Real-Time, Continuous Health Monitoring of Farmworkers. Adv. Healthc. Mater. 2022, 11, e2200170. [Google Scholar] [CrossRef]
- Shah, A.J.; Althobiani, M.A.; Saigal, A.; Ogbonnaya, C.E.; Hurst, J.R.; Mandal, S. Wearable technology interventions in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. npj Digit. Med. 2023, 6, 222. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, J.; Zhao, L.; Rhoades, R.D.; Kim, D.Y.; Wu, N.; Liang, J.; Chae, J. Machine-learning enabled wireless wearable sensors to study individuality of respiratory behaviors. Biosens. Bioelectron. 2021, 173, 112799. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Xie, Y.; Gao, F.; Xu, S.; Wu, X.; Ye, Z. Wearable Health Devices in Health Care: Narrative Systematic Review. JMIR Mhealth Uhealth 2020, 8, e18907. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.Z.; Odetayo, A.; David-Olawade, A.C.; Asaolu, F.; Eberhardt, J. Enhancing mental health with Artificial Intelligence: Current trends and future prospects. J. Public Health Med. 2024, 3, 100099. [Google Scholar] [CrossRef]
- Samson, C.; Koh, A. Stress Monitoring and Recent Advancements in Wearable Biosensors. Front. Bioeng. Biotechnol. 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.S.; Mahmood, M.; Kwon, S.; Zavanelli, N.; Kim, H.S.; Rim, Y.S.; Epps, F.; Yeo, W.H. Fully Integrated, Stretchable, Wireless Skin-Conformal Bioelectronics for Continuous Stress Monitoring in Daily Life. Adv. Sci. 2020, 7, 2000810. [Google Scholar] [CrossRef]
- Yen, H.Y. Smart wearable devices as a psychological intervention for healthy lifestyle and quality of life: A randomized controlled trial. Qual. Life Res. 2021, 30, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Conley, C.S.; Raposa, E.B.; Bartolotta, K.; Broner, S.E.; Hareli, M.; Forbes, N.; Christensen, K.M.; Assink, M. The Impact of Mobile Technology-Delivered Interventions on Youth Well-being: Systematic Review and 3-Level Meta-analysis. JMIR Ment. Health 2022, 9, e34254. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.S.; Mahmood, M.; Kwon, S.; Epps, F.; Rim, Y.S.; Yeo, W.H. Wireless, continuous monitoring of daily stress and management practice via soft bioelectronics. Biosens. Bioelectron. 2021, 173, 112764. [Google Scholar] [CrossRef]
- Wang, C.; Fan, K.; Sani, E.S.; Lasalde-Ramírez, J.A.; Heng, W.; Min, J.; Solomon, S.A.; Wang, M.; Li, J.; Han, H.; et al. A microfluidic wearable device for wound exudate management and analysis in human chronic wounds. Sci. Transl. Med. 2025, 17, eadt0882. [Google Scholar] [CrossRef]
- Wang, Y.C.; Xu, X.; Hajra, A.; Apple, S.; Kharawala, A.; Duarte, G.; Liaqat, W.; Fu, Y.; Li, W.; Chen, Y.; et al. Current Advancement in Diagnosing Atrial Fibrillation by Utilizing Wearable Devices and Artificial Intelligence: A Review Study. Diagnostics 2022, 12, 689. [Google Scholar] [CrossRef]
- Pereira, T.; Tran, N.; Gadhoumi, K.; Pelter, M.M.; Do, D.H.; Lee, R.J.; Colorado, R.; Meisel, K.; Hu, X. Photoplethysmography based atrial fibrillation detection: A review. npj Digit. Med. 2020, 3, 3. [Google Scholar] [CrossRef]
- Khan, B.; Riaz, Z.; Ahmad, R.u.S.; Khoo, B.L. Advancements in wearable sensors for cardiovascular disease detection for health monitoring. Mater. Sci. Eng. R Rep. 2024, 159, 100804. [Google Scholar] [CrossRef]
- Channa, A.; Popescu, N.; Ciobanu, V. Wearable Solutions for Patients with Parkinson’s Disease and Neurocognitive Disorder: A Systematic Review. Sensors 2020, 20, 2713. [Google Scholar] [CrossRef]
- Moore, K.; O’Shea, E.; Kenny, L.; Barton, J.; Tedesco, S.; Sica, M.; Crowe, C.; Alamaki, A.; Condell, J.; Nordstrom, A.; et al. Older Adults’ Experiences With Using Wearable Devices: Qualitative Systematic Review and Meta-synthesis. JMIR mHealth uHealth 2021, 9, e23832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Zhou, P.; Zou, Y.; Yang, J.; Haick, H.; Wang, Y. Soft Bioelectronics for Therapeutics. ACS Nano 2023, 17, 17634–17667. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Hanna, J.; Gutruf, P. Wearable devices for continuous monitoring of biosignals: Challenges and opportunities. APL Bioeng. 2022, 6, 021502. [Google Scholar] [CrossRef]
- Jain, P.; Gupta, R.; Joshi, A.; Kuzmin, A. Enhanced cardiovascular diagnostics using wearable ECG and bioimpedance monitoring with LightGBM classifier. Biosens. Bioelectron. X 2025, 24, 100617. [Google Scholar] [CrossRef]
- Chen, S.; Ouyang, Q.; Meng, X.; Yang, Y.; Li, C.; Miao, X.; Chen, Z.; Zhao, G.; Lei, Y.; Ghanem, B.; et al. Starfish-inspired wearable bioelectronic systems for physiological signal monitoring during motion and real-time heart disease diagnosis. Sci. Adv. 2025, 11, eadv2406. [Google Scholar] [CrossRef]
- Dinh-Le, C.; Chuang, R.; Chokshi, S.; Mann, D. Wearable Health Technology and Electronic Health Record Integration: Scoping Review and Future Directions. JMIR Mhealth Uhealth 2019, 7, e12861. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, B.; Cho, S.; Heo, E.; Kim, Y.; Lee, J.; Jung, S.Y.; Yoo, S. Impact of Personal Health Records and Wearables on Health Outcomes and Patient Response: Three-Arm Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e12070. [Google Scholar] [CrossRef]
- Akbar, F.; Mark, G.; Prausnitz, S.; Warton, E.M.; East, J.A.; Moeller, M.F.; Reed, M.E.; Lieu, T.A. Physician Stress During Electronic Health Record Inbox Work: In Situ Measurement With Wearable Sensors. JMIR Med. Inform. 2021, 9, e24014. [Google Scholar] [CrossRef]
- Ayyaswami, V.; Subramanian, J.; Nickerson, J.; Erban, S.; Rosano, N.; McManus, D.D.; Gerber, B.S.; Faro, J.M. A Clinician and Electronic Health Record Wearable Device Intervention to Increase Physical Activity in Patients With Obesity: Formative Qualitative Study. JMIR Form Res. 2024, 8, e56962. [Google Scholar] [CrossRef]
- Pawelek, J.; Baca-Motes, K.; Pandit, J.A.; Berk, B.B.; Ramos, E. The Power of Patient Engagement With Electronic Health Records as Research Participants. JMIR Med. Inform. 2022, 10, e39145. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, N.; Heo, E.; Yoo, S.; Lee, K.; Hwang, H.; Kim, J.W.; Kim, Y.; Lee, J.; Jung, S.Y. Impact of an Electronic Health Record-Integrated Personal Health Record on Patient Participation in Health Care: Development and Randomized Controlled Trial of MyHealthKeeper. J. Med. Internet Res. 2017, 19, e401. [Google Scholar] [CrossRef]
- Shull, J.G. Digital Health and the State of Interoperable Electronic Health Records. JMIR Med. Inform. 2019, 7, e12712. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Stroo, M.; Fiander, C.; McMillan, K. Selecting Mobile Health Technologies for Electronic Health Record Integration: Case Study. J. Med. Internet Res. 2020, 22, e23314. [Google Scholar] [CrossRef] [PubMed]
- Thomason, J. Big tech, big data and the new world of digital health. Glob. Public Health 2021, 5, 165–168. [Google Scholar] [CrossRef]
- Chen, J.; Tao, X.; Xu, X.; Sun, L.; Huang, R.; Nilghaz, A.; Tian, J. Making commercial bracelet smarter with a biochemical button module. Biosens. Bioelectron. 2024, 253, 116163. [Google Scholar] [CrossRef]
- Abouelmehdi, K.; Beni-Hessane, A.; Khaloufi, H. Big healthcare data: Preserving security and privacy. J. Big Data 2018, 5, 1. [Google Scholar] [CrossRef]
- Templ, M.; Sariyar, M. A systematic overview on methods to protect sensitive data provided for various analyses. Int. J. Inf. Secur. 2022, 21, 1233–1246. [Google Scholar] [CrossRef]
- Cartolovni, A.; Tomicic, A.; Lazic Mosler, E. Ethical, legal, and social considerations of AI-based medical decision-support tools: A scoping review. Int. J. Med. Inform. 2022, 161, 104738. [Google Scholar] [CrossRef]
- Karimian, G.; Petelos, E.; Evers, S.M.A.A. The ethical issues of the application of artificial intelligence in healthcare: A systematic scoping review. AI Ethics 2022, 2, 539–551. [Google Scholar] [CrossRef]
- Vedder, A.; Spajić, D. Moral autonomy of patients and legal barriers to a possible duty of health related data sharing. Ethics Inf. Technol. 2023, 25, 23. [Google Scholar] [CrossRef]
- Woodward, B. Confidentiality, Consent and Autonomy in the Physician-Patient Relationship. Health Care Anal. 2001, 9, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.; Fulford, K.W.M.; Herring, J.; Handa, A. Between the Reasonable and the Particular: Deflating Autonomy in the Legal Regulation of Informed Consent to Medical Treatment. Health Care Anal. 2019, 27, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Bani Issa, W.; Al Akour, I.; Ibrahim, A.; Almarzouqi, A.; Abbas, S.; Hisham, F.; Griffiths, J. Privacy, confidentiality, security and patient safety concerns about electronic health records. Int. Nurs. Rev. 2020, 67, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, U.; Song, J.; Han, B.T.; Dietzman, D. Do data security measures, privacy regulations, and communication standards impact the interoperability of patient health information? A cross-country investigation. Int. J. Med. Inform. 2021, 148, 104401. [Google Scholar] [CrossRef]
- Ahmad, A.; Cuomo, S.; Wu, W.; Jeon, G. Intelligent algorithms and standards for interoperability in Internet of Things. Future Gener. Comput. Syst. 2019, 92, 1187–1191. [Google Scholar] [CrossRef]
- Zafar, A. Balancing the scale: Navigating ethical and practical challenges of artificial intelligence (AI) integration in legal practices. Discov. Artif. Intell. 2024, 4, 27. [Google Scholar] [CrossRef]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef]
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and recommendations for wearable devices in digital health: Data quality, interoperability, health equity, fairness. PLOS Digit. Health 2022, 1, e0000104. [Google Scholar] [CrossRef]
- LaBoone, P.A.; Marques, O. Overview of the future impact of wearables and artificial intelligence in healthcare workflows and technology. Int. J. Inf. Manag. Data Insights 2024, 4, 100294. [Google Scholar] [CrossRef]
- Dao, N.-N. Internet of wearable things: Advancements and benefits from 6G technologies. Future Gener. Comput. Syst. 2023, 138, 172–184. [Google Scholar] [CrossRef]
- Ali, A.; Shaukat, H.; Bibi, S.; Altabey, W.A.; Noori, M.; Kouritem, S.A. Recent progress in energy harvesting systems for wearable technology. Energy Strat. Rev. 2023, 49, 101124. [Google Scholar] [CrossRef]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Feron, K.; Lim, R.; Sherwood, C.; Keynes, A.; Brichta, A.; Dastoor, P.C. Organic Bioelectronics: Materials and Biocompatibility. Int. J. Mol. Sci. 2018, 19, 2382. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wen, F.; Yang, Y.; Le, X.; Liu, W.; Lee, C. Emerging Wearable Chemical Sensors Enabling Advanced Integrated Systems toward Personalized and Preventive Medicine. Anal. Chem. 2023, 95, 490–514. [Google Scholar] [CrossRef]


| Material | Key Properties | Performance Metrics | Applications | Advantages | Limitations |
|---|---|---|---|---|---|
| Graphene | High conductivity, flexibility, biocompatibility | Sensitivity: 0.1–10 µM (glucose), Response time: <1 s | ECG electrodes, sweat sensors, strain sensors | Ultra-thin, high electron mobility | Expensive, complex fabrication |
| PDMS (Polydimethylsiloxane) | Stretchable (up to 300%), biocompatible | Elastic modulus: 0.1–3 MPa, Durability: >10 k cycles | Flexible substrates, epidermal patches | Conformal skin adhesion, inert | Low conductivity (requires composites) |
| PEDOT:PSS (Conductive Polymer) | High conductivity (100–1000 S/cm), tunable flexibility | Sheet resistance: 50–300 Ω/sq, Stability: >1 month | Organic electrochemical transistors (OECTs), neural interfaces | Printable, lightweight | Hygroscopic (sensitive to humidity) |
| Hydrogels | Soft (Young’s modulus ~kPa), ionically conductive | Swelling ratio: 200–500%, Adhesion: 10–50 kPa | Wound monitoring, drug delivery, electrophysiology | Tissue-like mechanics, self-healing | Poor long-term stability (dehydration) |
| Silver Nanowires (AgNWs) | High conductivity (~106 S/m), bendable | Transparency: >90%, Flexibility: <5 mm bending radius | Transparent electrodes, pressure sensors | Solution-processable, low cost | Oxidation risk, cytotoxicity concerns |
| Ecoflex (Silicone Elastomer) | Ultra-stretchable (up to 900%), soft | Tensile strength: ~1 MPa, High Biocompatibility | Wearable motion sensors, soft robotics | Extreme stretchability, durable | Low intrinsic conductivity |
| MXenes (Ti3C2Tₓ) | Metallic conductivity, hydrophilic surface | Capacitance: 500–1500 F/cm3, Sensitivity: 0.1–5 kPa−1 | Energy storage, multimodal sensors | High surface area, customizable | Susceptible to oxidation, scalability challenges |
| Cellulose Nanofibers | Biodegradable, flexible, low-cost | Tensile strength: 2 |
| AI Algorithm | Healthcare Applications | Advantages | Limitations |
|---|---|---|---|
| Logistic Regression | Disease prediction (e.g., diabetes, cancer risk); binary classification tasks | Simple, interpretable, fast training | Limited to linear relationships; less accurate on complex data |
| Decision Trees | Diagnostic support, triage systems, patient outcome prediction | Easy to interpret, handles categorical data well | Prone to overfitting, less stable |
| Random Forest | Predictive modeling (e.g., ICU mortality, sepsis detection) | Robust, handles high-dimensional data, reduces overfitting | Less interpretable than single trees |
| Support Vector Machine (SVM) | Image classification (e.g., tumor detection), genomics | Effective in high-dimensional spaces, good for classification | Computationally intensive, less interpretable |
| K-Nearest Neighbors (KNN) | Disease classification, patient similarity search | Simple, non-parametric, intuitive | Slow with large datasets, sensitive to noise |
| Naïve Bayes | Medical text classification (e.g., clinical notes), disease diagnosis | Fast, handles missing data well, works with small datasets | Assumes feature independence (often unrealistic) |
| Neural Networks (MLP) | Medical diagnosis, electronic health record (EHR) modeling | Learns complex patterns, flexible | Requires large data, hard to interpret |
| Convolutional Neural Networks (CNN) | Medical imaging (e.g., radiology, pathology), dermatology | High accuracy for image data, automatic feature extraction | Needs large, labeled datasets, less interpretable |
| Recurrent Neural Networks (RNN), LSTM | Sequence data (e.g., EHR time-series, patient monitoring) | Captures temporal dependencies, useful for time-series | Difficult to train, vanishing gradient issues |
| Transformers (e.g., BERT, GPT) | Clinical NLP, medical coding, chatbot assistants, summarizing medical records | State-of-the-art in language understanding, pre-trained models available | High computational cost, requires fine-tuning for domain-specific tasks |
| Reinforcement Learning | Treatment recommendation, personalized medicine, drug dosing optimization | Learns optimal policy, adapts to dynamic environments | Complex to design, needs reward modeling, safety concerns |
| Clustering (e.g., K-means) | Patient stratification, phenotype discovery, cohort analysis | Unsupervised learning, finds hidden structures | Sensitive to initialization, assumes spherical clusters |
| Dimensionality Reduction (e.g., PCA, t-SNE) | Genomic data analysis, visualization, feature selection | Reduces noise, aids visualization | May lose interpretability, not always preserves global structure |
| Metric | Traditional Methods | AI-Enhanced Approach | Improvement | Clinical Impact |
|---|---|---|---|---|
| Diagnostic Accuracy | 72.3% (±5.1%) | 89.7% (±2.3%) | +24% | 38% reduction in false negatives |
| Prediction Latency | 2.1 s (±0.3 s) | 0.4 s (±0.1 s) | 5.2× faster | Enables real-time intervention |
| Multi-analyte Resolution | 3–5 biomarkers | 9–12 biomarkers | 3× capacity | Comprehensive profiling |
| Long-term Stability | 15% signal drift/week | 4% drift/week (with self-calibration) | 73% reduction | Fewer relapse |
| AI Model/Algorithm | Medical Application | Data Source/Modality | Sensitivity | Specificity | Accuracy | AUC | Clinical Setting |
|---|---|---|---|---|---|---|---|
| Deep Learning CNN | Diabetic Retinopathy Screening | Retinal Fundus Images | 90.5% | 91.6% | 91.3% | 0.963 | Primary Care Clinics |
| AI-Rad Companion (Siemens) | Lung Nodule Detection | Chest CT Scans | 92.0% | 86.5% | 89.3% | 0.94 | Radiology Departments |
| Aidoc (AI Triage Tool) | Intracranial Hemorrhage Detection | Non-contrast Head CT | 89.4% | 93.6% | 91.0% | 0.91 | Emergency Departments |
| Google Health AI | Breast Cancer Detection | Mammography | 89.0% | 94.5% | — | 0.945 | Retrospective Multicenter Studies |
| PathAI | Histopathology (Breast cancer) | H&E-Stained Slides | 94.6% | 93.8% | — | 0.98 | Pathology Labs |
| Tempus xT AI | Predictive Genomics for Oncology | NGS + Clinical Data | 88.0% | 85.0% | — | 0.87 | Clinical Decision Support |
| SkinVision | Skin Cancer Risk Assessment (Melanoma) | Smartphone Images | 95.1% | 78.3% | — | 0.89 | Patient Self-Screening/Teledermatology |
| Eko AI (Heart Murmur) | Atrial/Valve Murmur Classification | Digital Stethoscope Audio | 87.6% | 91.1% | — | 0.90 | Point-of-Care, Cardiology Clinics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Chen, X.; Liao, C. AI-Driven Wearable Bioelectronics in Digital Healthcare. Biosensors 2025, 15, 410. https://doi.org/10.3390/bios15070410
Huang G, Chen X, Liao C. AI-Driven Wearable Bioelectronics in Digital Healthcare. Biosensors. 2025; 15(7):410. https://doi.org/10.3390/bios15070410
Chicago/Turabian StyleHuang, Guangqi, Xiaofeng Chen, and Caizhi Liao. 2025. "AI-Driven Wearable Bioelectronics in Digital Healthcare" Biosensors 15, no. 7: 410. https://doi.org/10.3390/bios15070410
APA StyleHuang, G., Chen, X., & Liao, C. (2025). AI-Driven Wearable Bioelectronics in Digital Healthcare. Biosensors, 15(7), 410. https://doi.org/10.3390/bios15070410





