Impedimetric DNA Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and Polyproflavine Electropolymerized from Natural Deep Eutectic Solvent for Anthracycline Medications Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. SPCE Surface Modification
2.4. Anthracycline Medication Determination in Standard Solutions and Real Samples
3. Results
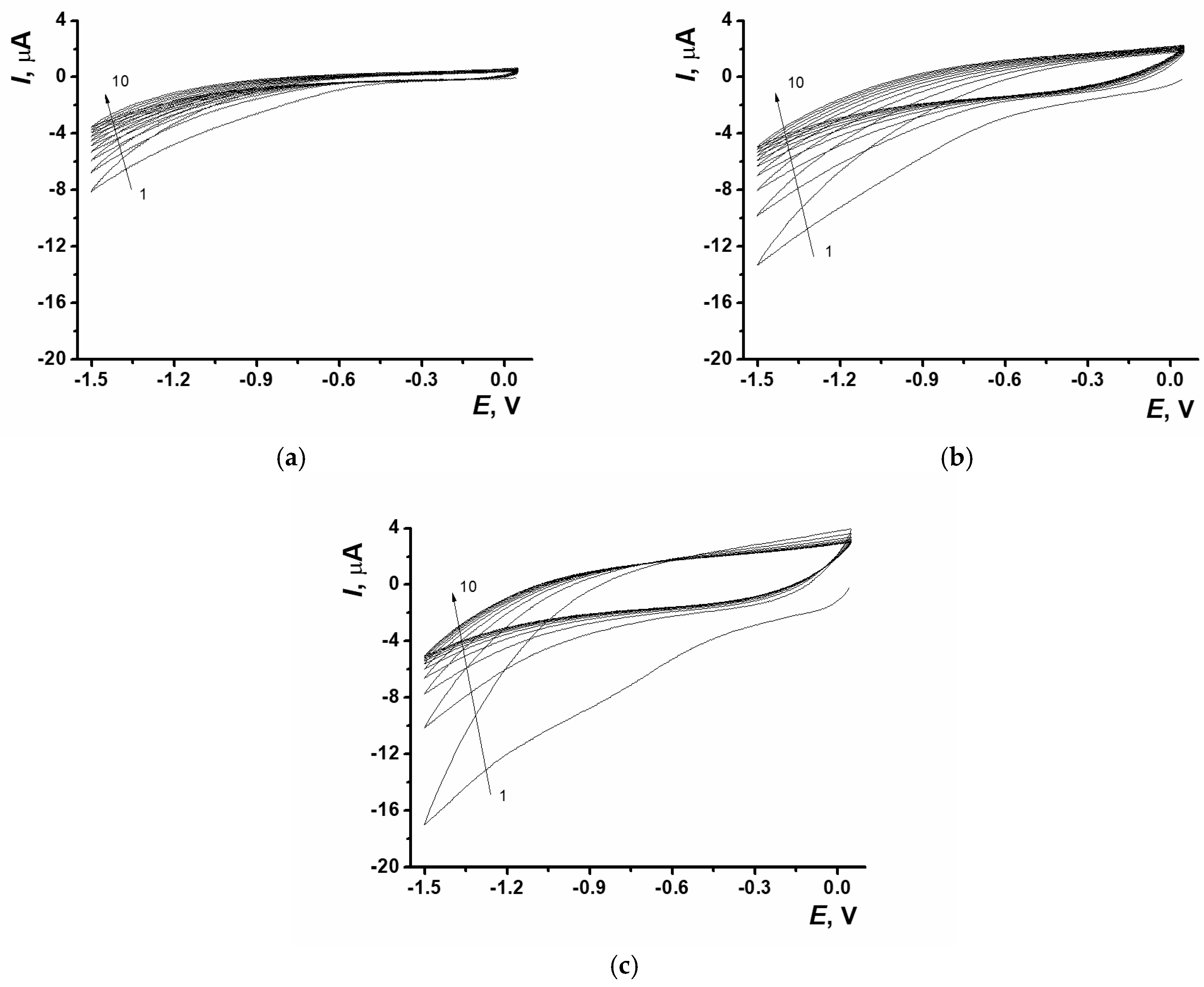
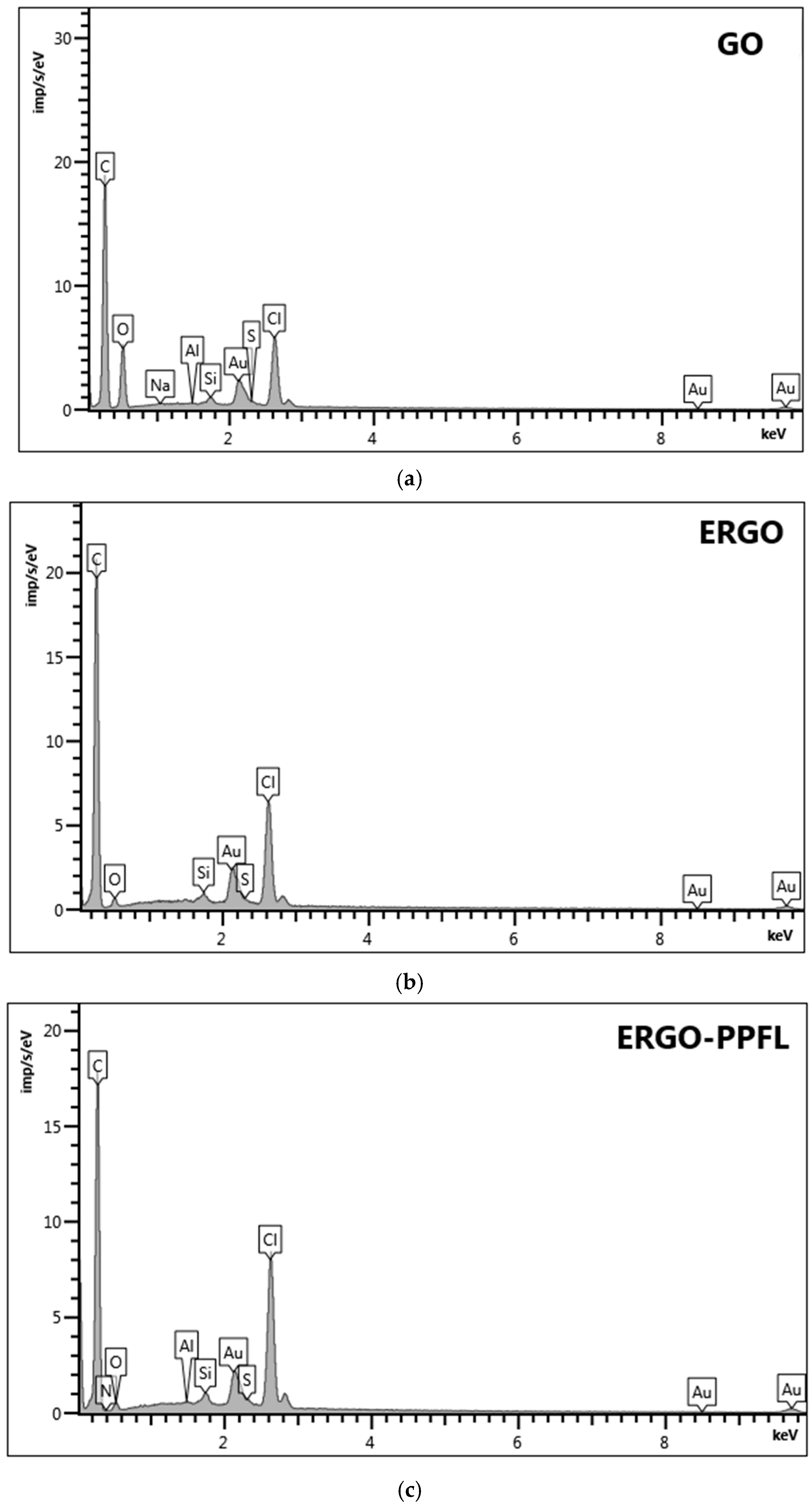
3.1. Electrochemically Reduced Graphene Oxide Production
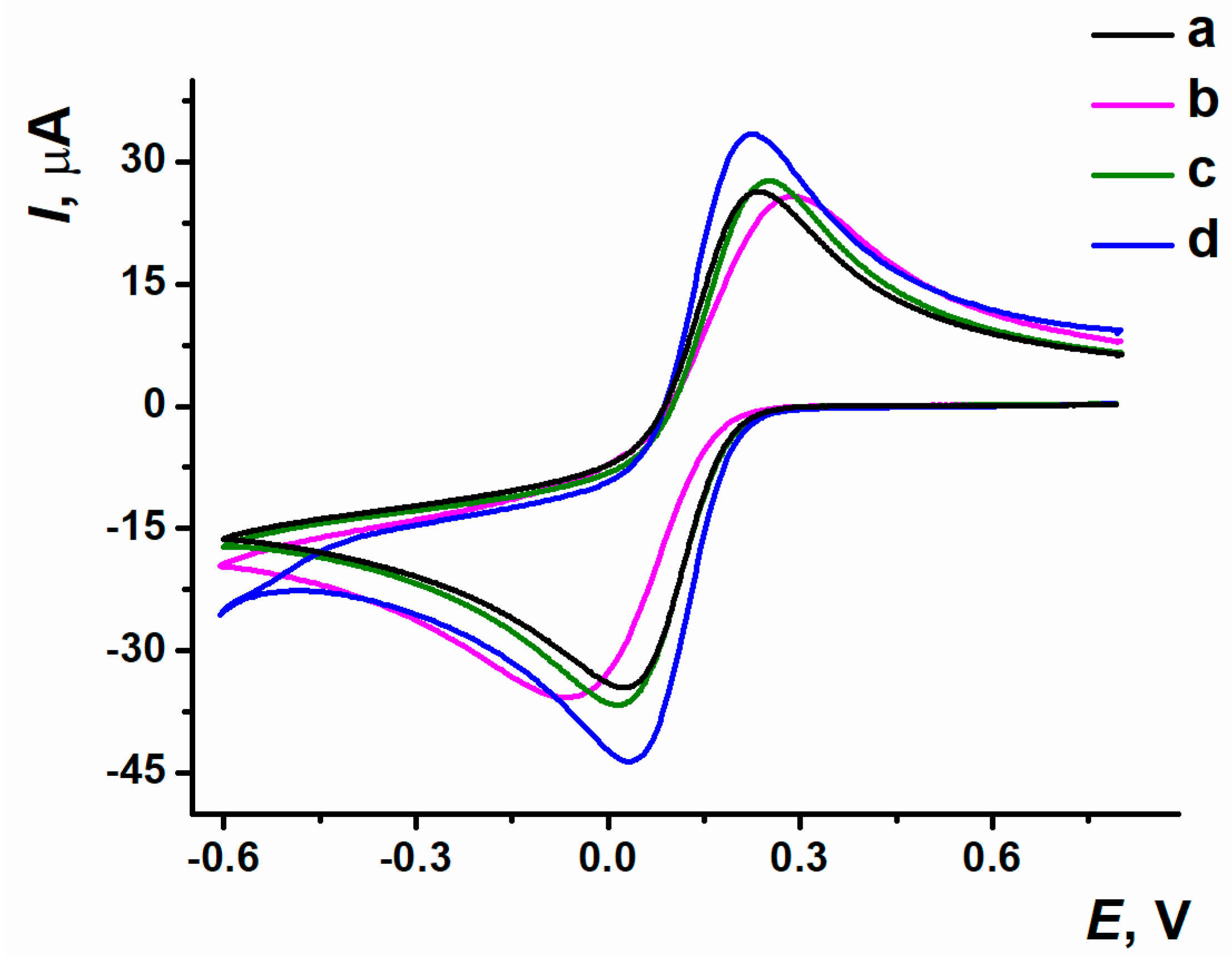
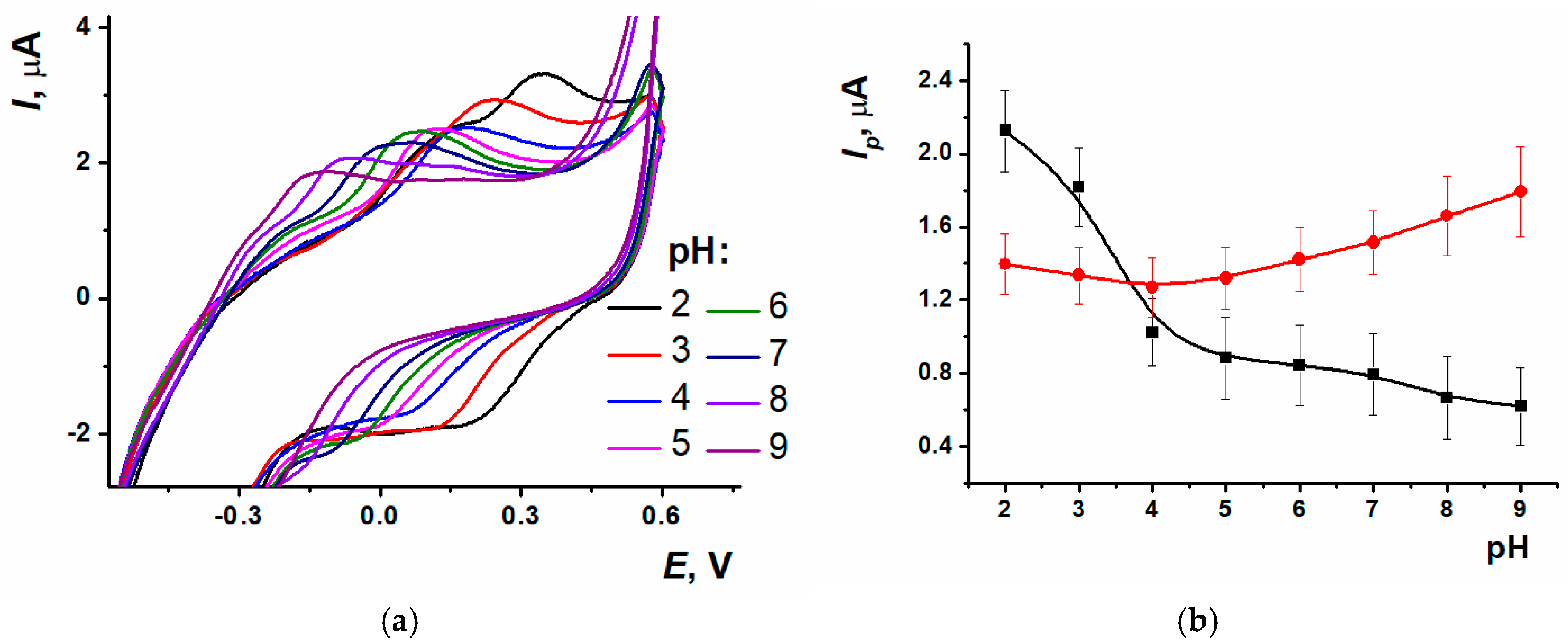
3.2. Electrochemical Polymerization of Proflavine and the Estimation of ERGO-PPFLNADES Composite Electrochemical Characteristics
3.3. ERGONADES and ERGO-PPFLNADES Coatings Characterization with SEM Images
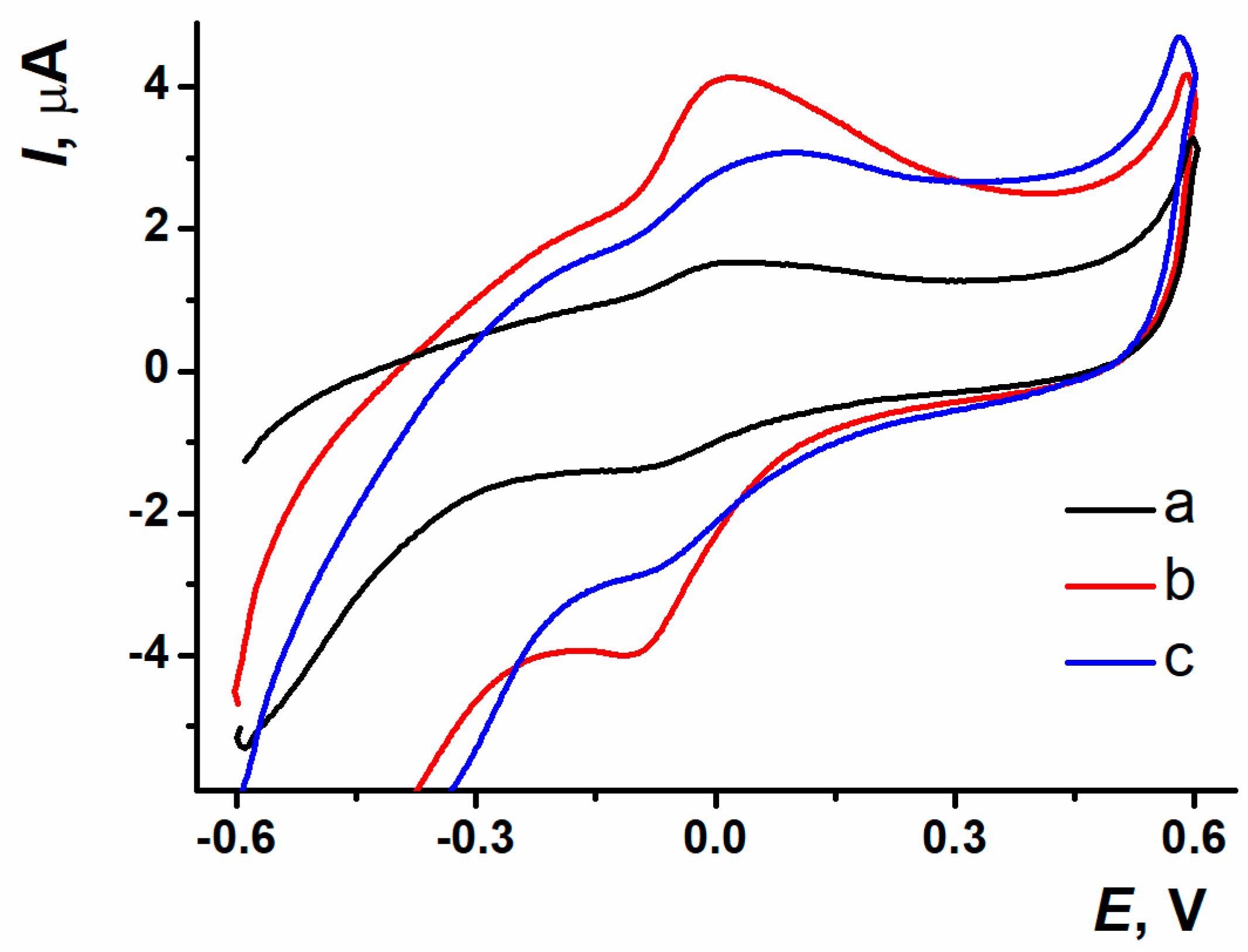
3.4. DNA Sensor Assembling
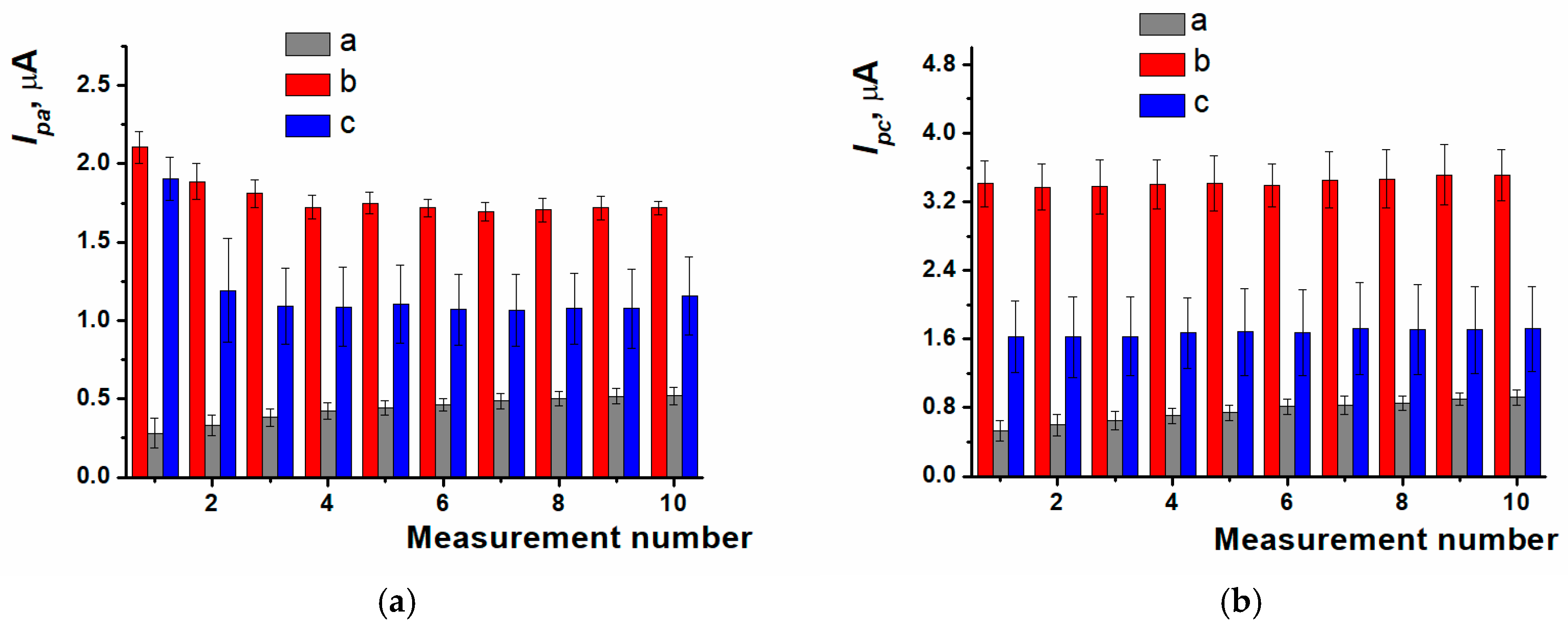
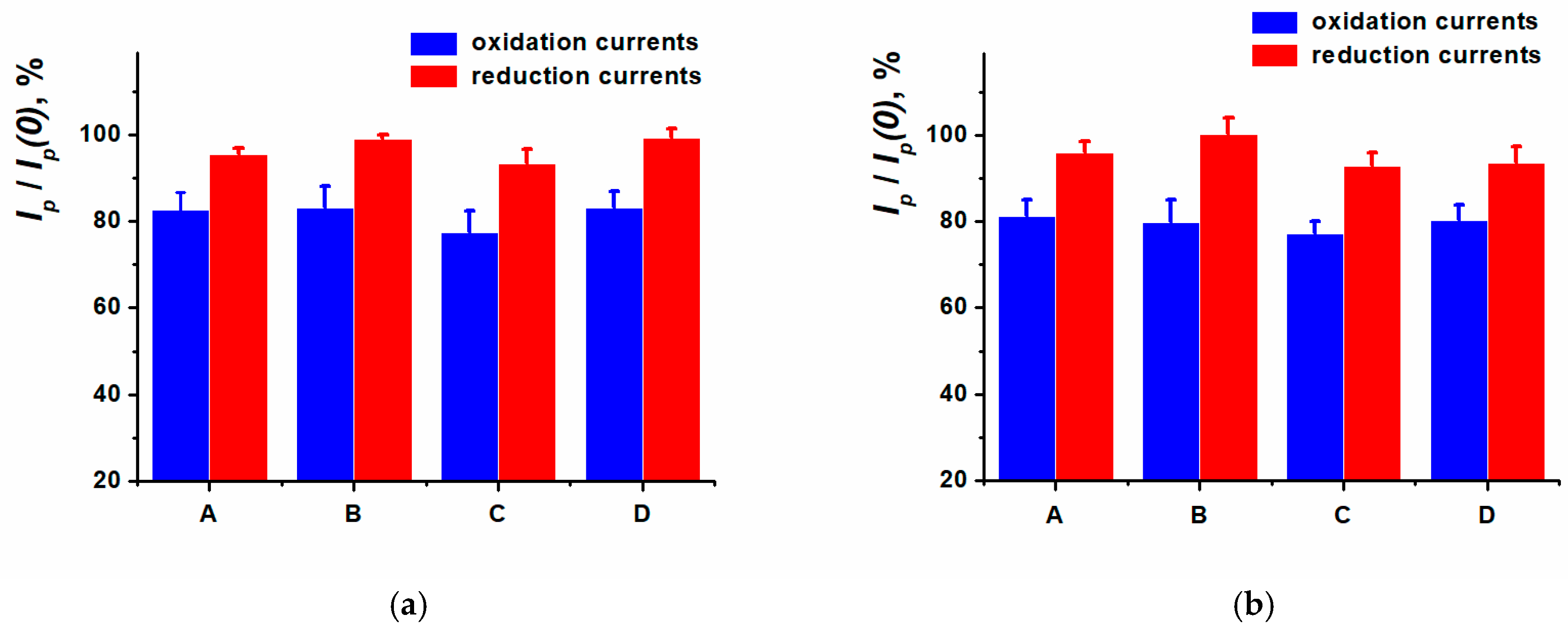
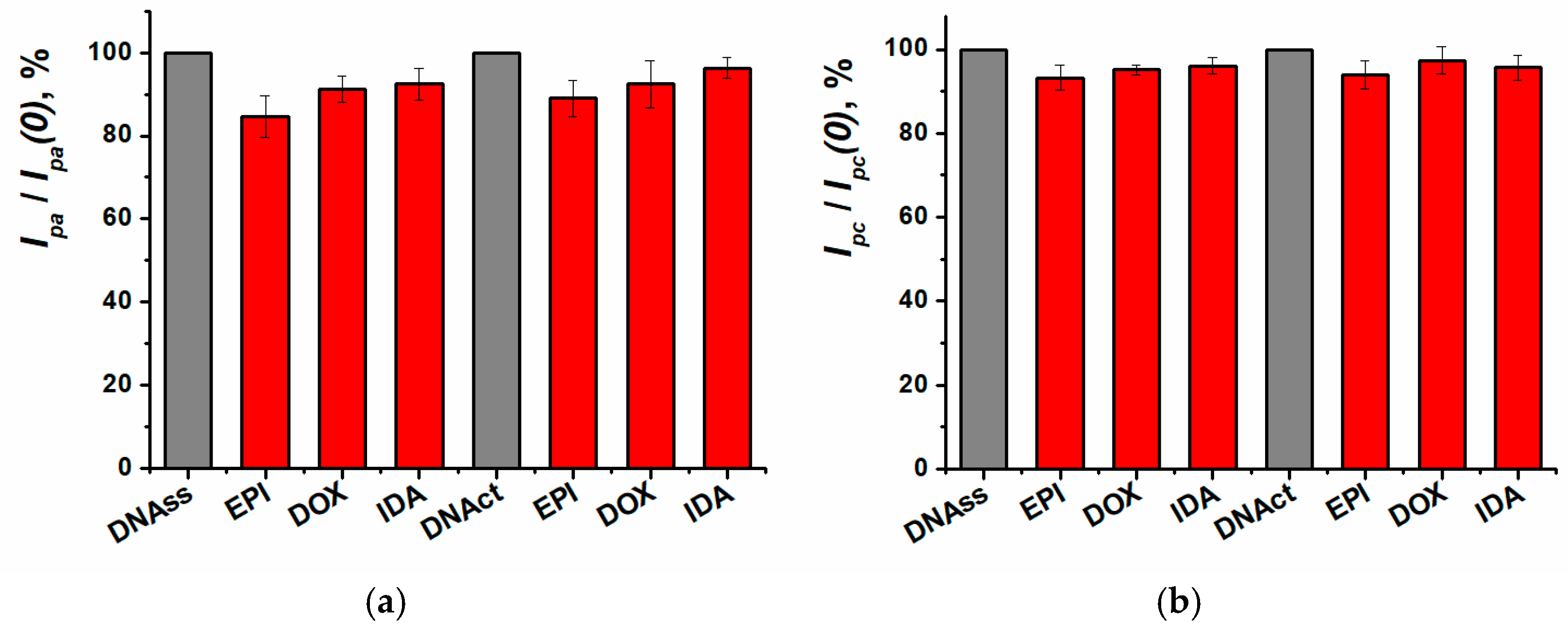
3.5. Anthracycline Medications Determination
3.6. Impedimetric Determination of Anthracycline Medications in Pharmaceutical Forms and Real Sample Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabiani, I.; Chianca, M.; Cipolla, C.M.; Cardinale, D.M. Anthracycline-induced cardiomyopathy: Risk prediction, prevention and treatment. Nat. Rev. Cardiol. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Madaudo, C.; Macaione, F.; Galassi, A.R.; Novo, G. Cancer survivors and cardiovascular diseases: From preventive strategies to treatment. J. Cardiovasc. Med. 2025, 26, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, Y.; Mehrabi, M.; Shekarchi, M. A review on various analytical methods for determination of anthracyclines and their metabolites as anti–cancer chemotherapy drugs in different matrices over the last four decades. TrAC Trends Anal. Chem. 2020, 130, 115991. [Google Scholar] [CrossRef]
- Rezaei, Z.; Wang, N.; Rodriguez, A.D.J.A.; Higashi, S.; Shin, S.R. Biosensors in biomedical research: Bridging cell and tissue engineering and real-time monitoring. Curr. Opin. Biomed. Eng. 2025, 34, 100582. [Google Scholar] [CrossRef]
- Ozbey, S.; Keles, G.; Kurbanoglu, S. Innovations in graphene-based electrochemical biosensors in healthcare applications. Microchim. Acta 2025, 192, 290. [Google Scholar] [CrossRef]
- Truong, P.L.; Yin, Y.; Lee, D.; Ko, S.H. Advancement in COVID-19 detection using nanomaterial-based biosensors. Exploration 2023, 3, 20210232. [Google Scholar] [CrossRef] [PubMed]
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (bio) sensors go green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef]
- Porfireva, A.; Goida, A.; Evtugyn, V.; Mozgovaya, M.; Krasnova, T.; Evtugyn, G. Electrochemical DNA Sensor Based on Poly(proflavine) Deposited from Natural Deep Eutectic Solvents for DNA Damage Detection and Antioxidant Influence Assessment. Chemosensors 2024, 12, 215. [Google Scholar] [CrossRef]
- Plaza-Mayoral, E.; Dalby, K.N.; Falsig, H.; Chorkendorff, I.; Sebastián-Pascual, P.; Escudero-Escribano, M. Preparation of Tunable Cu Ag Nanostructures by Electrodeposition in a Deep Eutectic Solvent. ChemElectroChem 2024, 11, e202400094. [Google Scholar] [CrossRef]
- Sugiarto, S.; Weerasinghe, U.A.; Muiruri, J.K.; Chai, A.Y.Q.; Yeo, J.C.C.; Wang, G.; Zhu, Q.; Loh, X.J.; Li, Z.; Kai, D. Nanomaterial synthesis in deep eutectic solvents. Chem. Eng. J. 2024, 499, 156177. [Google Scholar] [CrossRef]
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of acidic deep eutectic solvents on synthesis, properties and applications. Green. Energy Environ. 2020, 5, 8–21. [Google Scholar] [CrossRef]
- Li, M.; Yin, B.; Gao, C.; Guo, J.; Zhao, C.; Jia, C.; Guo, X. Graphene: Preparation, tailoring, and modification. Exploration 2023, 3, 20210233. [Google Scholar] [CrossRef]
- Zhou, A.; Bai, J.; Hong, W.; Bai, H. Electrochemically reduced graphene oxide: Preparation, composites, and applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Gao, M.; Xu, Y.; Wang, X.; Sang, Y.; Wang, S. Analysis of Electrochemical Reduction Process of Graphene Oxide and its Electrochemical Behavior. Electroanalysis 2016, 28, 1377–1382. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Qian, L.; Xiao, J.; Wang, S.; Liu, Y. Facile Synthesis of 3D MnO2–Graphene and Carbon Nanotube–Graphene Composite Networks for High-Performance, Flexible, All-Solid-State Asymmetric Supercapacitors. Adv. Energy Mater. 2014, 4, 1400064. [Google Scholar] [CrossRef]
- Sheng, K.; Sun, Y.; Li, C.; Yuan, W.; Shi, G. Ultrahigh-rate supercapacitors based on eletrochemically reduced graphene oxide for ac line-filtering. Sci. Rep. 2012, 2, 247. [Google Scholar] [CrossRef]
- Tite, T.; Chiticaru, E.A.; Burns, J.S.; Ioniţă, M. Impact of nano-morphology, lattice defects and conductivity on the performance of graphene based electrochemical biosensors. J. Nanobiotechnol. 2019, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Svitková, V.; Hanzelyová, M.; Macková, H.; Blaškovičová, J.; Vyskočil, V.; Farkašová, D.; Labuda, J. Behaviour and detection of acridine-type DNA intercalators in urine using an electrochemical DNA-based biosensor with the protective polyvinyl alcohol membrane. J. Electroanal. Chem. 2018, 821, 87–91. [Google Scholar] [CrossRef]
- Porfireva, A.V.; Goida, A.I.; Rogov, A.M.; Evtugyn, G.A. Impedimetric DNA Sensor Based on Poly(Proflavine) for Determination of Anthracycline Drugs. Electroanalysis 2020, 32, 827–834. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical DNA sensors and aptasensors based on electropolymerized materials and polyelectrolyte complexes. TrAC Trends Anal. Chem. 2016, 79, 168–178. [Google Scholar] [CrossRef]
- Beretta, G.L.; Zunino, F. Molecular Mechanisms of Anthracycline Activity. Top. Curr. Chem. 2008, 283, 1–19. [Google Scholar]
- Pérez-Arnaiz, C.; Busto, N.; Leal, J.M.; García, B. New Insights into the Mechanism of the DNA/Doxorubicin Interaction. J. Phys. Chem. B 2014, 118, 1288–1295. [Google Scholar] [CrossRef]
- Malanina, A.N.; Kuzin, Y.I.; Padnya, P.L.; Ivanov, A.N.; Stoikov, I.I.; Evtugyn, G.A. Cationic and anionic phenothiazine derivatives: Electrochemical behavior and application in DNA sensor development. Analyst 2025, 150, 2087–2100. [Google Scholar] [CrossRef] [PubMed]
- Charak, S.; Jangir, D.K.; Tyagi, G.; Mehrotra, R. Interaction studies of Epirubicin with DNA using spectroscopic techniques. J. Mol. Struct. 2011, 1000, 150–154. [Google Scholar] [CrossRef]
- Hajian, R.; Ekhlasi, E.; Daneshvar, R. Spectroscopic and Electrochemical Studies on the Interaction of Epirubicin with Fish Sperm DNA. J. Chem. 2012, 9, 738678. [Google Scholar] [CrossRef]
- Charak, S.; Mehrotra, R. Structural investigation of idarubicin–DNA interaction: Spectroscopic and molecular docking study. Int. J. Biol. Macromol. 2013, 60, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Goida, A.; Kuzin, Y.; Evtugyn, V.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical Sensing of Idarubicin—DNA Interaction Using Electropolymerized Azure B and Methylene Blue Mediation. Chemosensors 2022, 10, 33. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Garkani Nejad, F.; Dourandish, Z. Electrochemical Determination of Doxorubicin in the Presence of Dacarbazine Using MWCNTs/ZnO Nanocomposite Modified Disposable Screen-Printed Electrode. Biosensors 2025, 15, 60. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Hou, A.; Fang, R.; Shi, L. Bimetallic Pd-Au nanoparticles decorated MoS2/GO nanoflowers for sensitive detection of doxorubicin. Int. J. Electrochem. Sci. 2025, 20, 100914. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Cao, M.; Liu, Y. Preparation of electrochemical sensor for detection of cancer medicine doxorubicin based on multi-walled carbon nanotube composites modified electrode. Alex. Eng. J. 2024, 93, 51–58. [Google Scholar] [CrossRef]
- Ehsani, M.; Soleymani, J.; Hasanzadeh, M.; Vaez-Gharamaleki, Y.; Khoubnasabjafari, M.; Jouyban, A. Sensitive monitoring of doxorubicin in plasma of patients, MDA-MB-231 and 4T1 cell lysates using electroanalysis method. J. Pharm. Biomed. Anal. 2021, 192, 113701. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 291, 132928. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, S.; Hashemi, B.; Chegeni, M.; Aeenmehr, A. The Application of Perlite/Cobalt Oxide/Reduced Graphene Oxide (PC-rGO)/Metal Organic Framework (MOF) Composite as Electrode Modifier for Direct Sensing of Anticancer Drug Idarubicin. IEEE Sens. J. 2019, 19, 11739–11745. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Behbahanian, N.; Taherzadeh, K.M.; Hashemi, B. Development of electrochemical sensor based on multiwall carbon nanotube for determination of anticancer drug idarubicin in biological samples. Adv. Nanochem. 2019, 1, 22–28. [Google Scholar]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Budnikov, G. Electrochemical Biosensors Based on Native DNA and Nanosized Mediator for the Detection of Anthracycline Preparations. Electroanalysis 2015, 27, 629–637. [Google Scholar] [CrossRef]
- Bavandpour, R.; Rajabi, M.; Asghari, A. Electrochemical determination of epirubicin in the presence of topotecan as essential anti-cancer compounds using paste electrode amplified with Pt/SWCNT nanocomposite and a deep eutectic solvent. Chemosphere 2022, 289, 133060. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, A.; Faghih-Mirzaei, E.; Karimi-Maleh, H.; Abbaspourrad, A.; Agarwal, S.; Gupta, V.K. A new epirubicin biosensor based on amplifying DNA interactions with polypyrrole and nitrogen-doped reduced graphene: Experimental and docking theoretical investigations. Sens. Actuators B 2019, 284, 568–574. [Google Scholar] [CrossRef]
- Shams, A.; Yari, A. A new sensor consisting of Ag-MWCNT nanocomposite as the sensing element for electrochemical determination of Epirubicin. Sens. Actuators B 2019, 286, 131–138. [Google Scholar] [CrossRef]


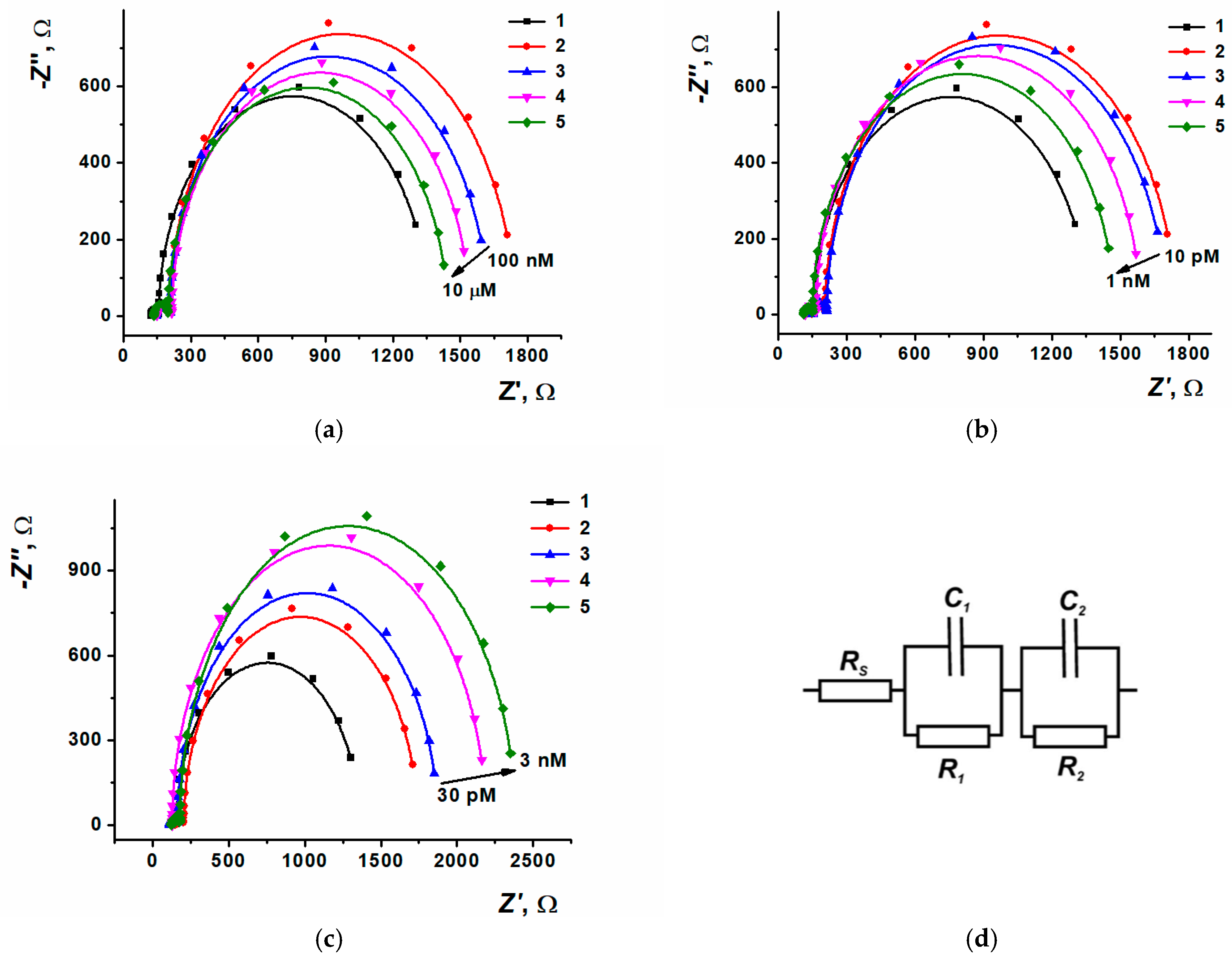
| Analyte | Sensor Content | Method | Concentration Range, LOD | Selectivity Assessment | Real Samples | Refs |
|---|---|---|---|---|---|---|
| DOX | SPCE/ZnO/ MWCNT | DPV | 0.007–150.0 µM, 0.002 µM | in 200-folds of K+, Na+, Mg2+, Ca2+, NH4+, Cl−, CO32−, SO42− | doxorubicin injection solution | [29] |
| GCE/GO/MoS2/ Pd(4)-Au(3 | DPV | 0.11–127.5 μM, 0.042 μM | in 100-folds of Ni2+, Zn2+, Mg2+, Cl−, NO3−; 10-folds of Ca2+, Cu2+, glucose, uric acid, dopamine and ascorbic acid | human urine samples | [30] | |
| GCE/ mSiO2@ MWCNT | DPV | 30–750 μM, 14 nM | in 3-folds of ascorbic acid, uric acid, acetaminophen, sulfamethoxazole, glucose, mannose, lactose | human urine samples | [31] | |
| GCE/poly(Toluidine Blue | DPV | 17 nM–8.6 μM, 17 nM | acetate, serine | treated whole plasma, untreated whole plasma, various cell lysates, plasma samples of patients | [32] | |
| SPCE/ERGO-PPFLNADES | EIS | 10 nM–0.1 mM, 3 nM | in 10-folds of glucose, ascorbic acid, uric acid | artificial urine, human urine samples, medical preparations: Doxorubicin-TEVA®, Doxorubicin-LANS® | [This work] | |
| IDA | GCE/SWCNTs/Pt-Pd-ZnO/ds-DNA | DPV | 1 nM–65 μM, 0.8 nM | in 1000-folds of K+, Na+, Li+, F−, Ca2+, 800-folds of alanine and glycine, valine, 500-folds of glucose, 4-folds of doxorubicin and daunorubicin | human urine samples | [33] |
| CPE/ MOF-199/PC-rGO | DPV | 5–1000 nM, 1.5 nM | in 500-fold Mg2+, Na+, Ca2+, K+, Cl−, Li+, Al3+, NH4+, dopamine and ascorbic acid | human urine samples, serum samples | [34] | |
| GPE/MWCNT | DPV | 1–1000 nM, 0.2 nM | in 700-fold Mg2+, Li+, Al3+, Na+, K+, NH4+, and dopamine | human urine samples, serum samples | [35] | |
| GCE/PNR/ TKA/ NR/DNA | DPV, EIS | 1 nM–100 μM, 1 nM | in binary solutions of 1000-fold excess of anthracyclines or sulfonylamide preparations (sulfomethoxazole, sulfadiazine, sulfamethazine, and sulfaguanine) | medical preparations: Doxorubicin- LANS® and “Rastocin”® | [36] | |
| SPCE/ERGO-PPFLNADES | EIS | 10 pM–10 nM, 5 pM | in 10-folds of glucose, ascorbic acid, uric acid | artificial urine, human urine samples, medical preparation Idarubicin-Zavedos® | [This work] | |
| EPI | CPE/DES/Pt-SWCNT | DPV | 0.001–500 μM, 0.8 nM | in 1000-folds of Li+, K+, Cl−, Br−, Mg2+, 600-folds of methionine, tryptophan, phenylalanine, valine, 100-folds of ascorbic acid and vitamin B6 | epirubicin injection solution and dextrose saline samples | [37] |
| PGE/ds-DNA/ds-DNA/PP | DPV | 0.004–55.0 μM, 1.0 nM | in 1000-folds of K+, Cl−, Na+, Br− and Mg2+ | epirubicin injection solution, human urine samples | [38] | |
| GCE/Ag-MWCNT | SWV | 0.003–0.25 μM, 1 nM | in 5000-folds of ascorbic acid, glucose; 2000-folds of uric acid, caffeine, vitamin A, vitamin E; 1000-folds of dopamine; 2000-folds of Na+, K+, Fe2+, Fe3+, Cu2+, Hg2+, Pb2+, Ca2+, Zn2+, Cl−, SO42−, NO3− | blood, pharmaceuticals and urine samples | [39] | |
| SPCE/ERGO-PPFLNADES | EIS | 1 pM–10 nM, 1 pM | in 10-folds of glucose, ascorbic acid, uric acid | artificial urine, human urine samples, medical preparation Idarubicin-Zavedos® | [This work] |
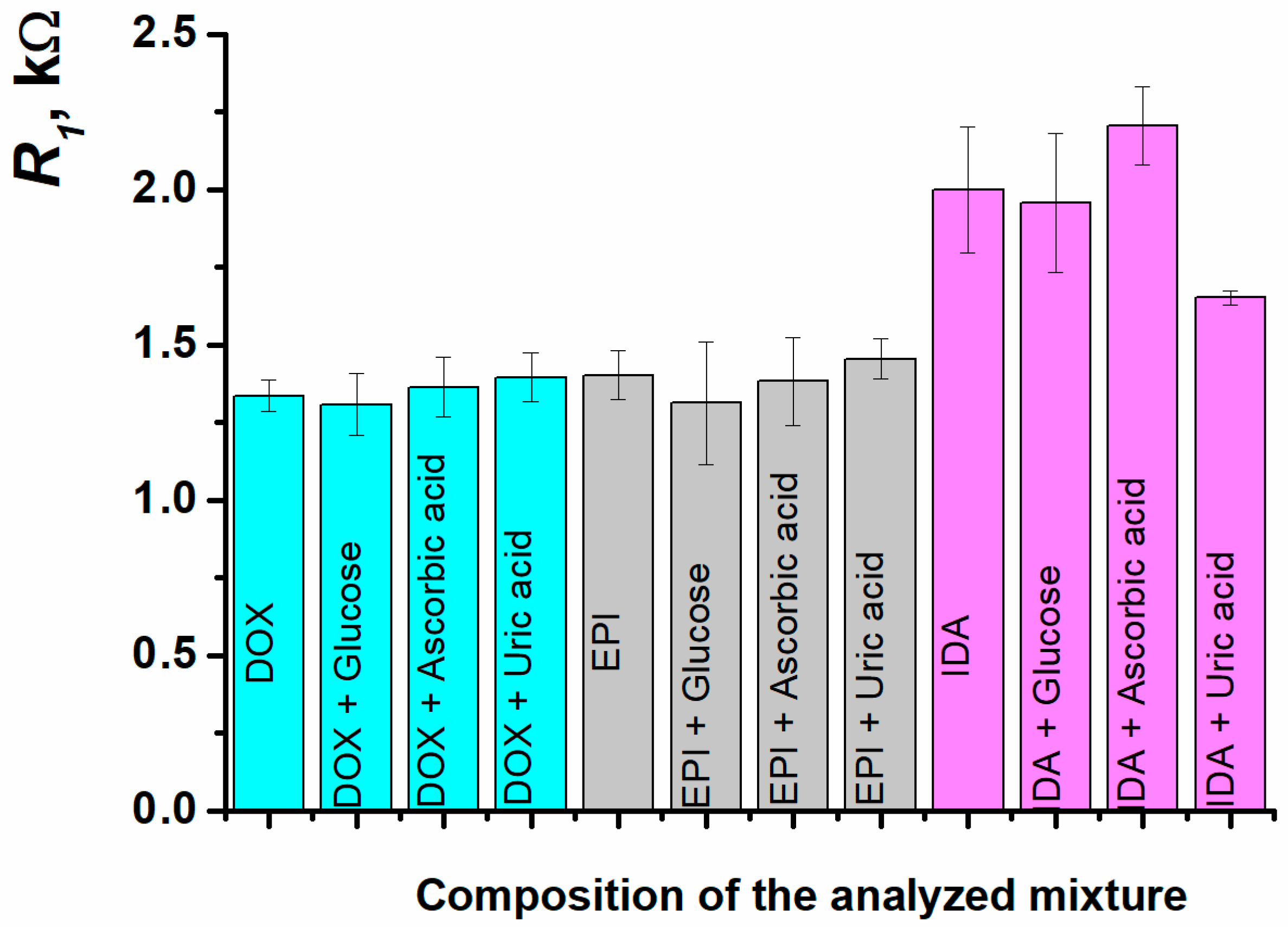
| Sample | R1, kΩ | Recovery, % |
|---|---|---|
| 1 µM doxorubicin (standard solution, R1 = 1.34 ± 0.05 kΩ) | ||
| doxorubicin + glucose | 1.31 ± 0.09 | 98 ± 7 |
| doxorubicin + ascorbic acid | 1.36 ± 0.09 | 102 ± 7 |
| doxorubicin + uric acid | 1.39 ± 0.08 | 104 ± 6 |
| 0.3 nM idarubicin (standard solution, R1 = 1.99 ± 0.20 kΩ) | ||
| idarubicin + glucose | 1.96 ± 0.22 | 98 ± 11 |
| idarubicin + ascorbic acid | 2.20 ± 0.13 | 110 ± 6 |
| idarubicin + uric acid | 1.65 ± 0.02 | 83 ± 1 |
| 0.1 nM epirubicin (standard solution, R1 = 1.40 ± 0.08 kΩ) | ||
| epirubicin + glucose | 1.31 ± 0.19 | 94 ± 14 |
| epirubicin + ascorbic acid | 1.38 ± 0.14 | 99 ± 10 |
| epirubicin + uric acid | 1.46 ± 0.07 | 104 ± 5 |
| Sample | R1, kΩ | Recovery, % |
|---|---|---|
| 1 µM doxorubicin (standard solution, R1 = 1.34 ± 0.05 kΩ) | ||
| Doxorubicin-TEVA® | 1.28 ± 0.09 | 96 ± 7 |
| Doxorubicin-LANS® | 1.70 ± 0.09 | 127 ± 7 |
| Doxorubicin with mannitol addition | 1.53 ± 0.13 | 114 ± 10 |
| 0.3 nM idarubicin (standard solution, R1 = 1.99 ± 0.20 kΩ) | ||
| Idarubicin-Zavedos® | 1.60 ± 0.12 | 80 ± 6 |
| Idarubicin with lactose addition | 1.54 ± 0.13 | 77 ± 6 |
| Sample | R1, kΩ | Recovery, % |
|---|---|---|
| 1 µM doxorubicin (standard solution, R1 = 1.34 ± 0.05 kΩ) | ||
| Human urine, non-diluted | 1.88 ± 0.08 | 140 ± 6 |
| Human urine, 1:1 diluted | 1.44 ± 0.11 | 107 ± 8 |
| Artificial urine, non-diluted | 1.63 ± 0.07 | 122 ± 5 |
| Artificial urine, 1:1 diluted | 1.33 ± 0.11 | 99 ± 8 |
| 0.3 nM idarubicin (standard solution, R1 = 1.99 ± 0.20 kΩ) | ||
| Human urine, non-diluted | 1.97 ± 0.14 | 98 ± 7 |
| Artificial urine, non-diluted | 1.83 ± 0.14 | 93 ± 6 |
| 0.1 nM epirubicin (standard solution, R1 = 1.40 ± 0.08 kΩ) | ||
| Human urine, non-diluted | 1.89 ± 0.15 | 134 ± 6 |
| Human urine, 1:1 diluted | 1.36 ± 0.09 | 97 ± 10 |
| Artificial urine, non-diluted | 1.77 ± 0.09 | 126 ± 6 |
| Artificial urine, 1:1 diluted | 1.47 ± 0.06 | 104 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goida, A.; Krasnova, T.; Shamagsumova, R.; Evtugyn, V.; Saveliev, A.; Porfireva, A. Impedimetric DNA Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and Polyproflavine Electropolymerized from Natural Deep Eutectic Solvent for Anthracycline Medications Determination. Biosensors 2025, 15, 385. https://doi.org/10.3390/bios15060385
Goida A, Krasnova T, Shamagsumova R, Evtugyn V, Saveliev A, Porfireva A. Impedimetric DNA Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and Polyproflavine Electropolymerized from Natural Deep Eutectic Solvent for Anthracycline Medications Determination. Biosensors. 2025; 15(6):385. https://doi.org/10.3390/bios15060385
Chicago/Turabian StyleGoida, Anastasia, Tatiana Krasnova, Rezeda Shamagsumova, Vladimir Evtugyn, Anatoly Saveliev, and Anna Porfireva. 2025. "Impedimetric DNA Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and Polyproflavine Electropolymerized from Natural Deep Eutectic Solvent for Anthracycline Medications Determination" Biosensors 15, no. 6: 385. https://doi.org/10.3390/bios15060385
APA StyleGoida, A., Krasnova, T., Shamagsumova, R., Evtugyn, V., Saveliev, A., & Porfireva, A. (2025). Impedimetric DNA Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and Polyproflavine Electropolymerized from Natural Deep Eutectic Solvent for Anthracycline Medications Determination. Biosensors, 15(6), 385. https://doi.org/10.3390/bios15060385





