Individually Modified Microneedle Array for Minimal Invasive Multi-Electrolyte Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ion-Selective Electrodes Based on Microneedles
2.2. Finite Element Modeling and Simulation and Experimental Validation
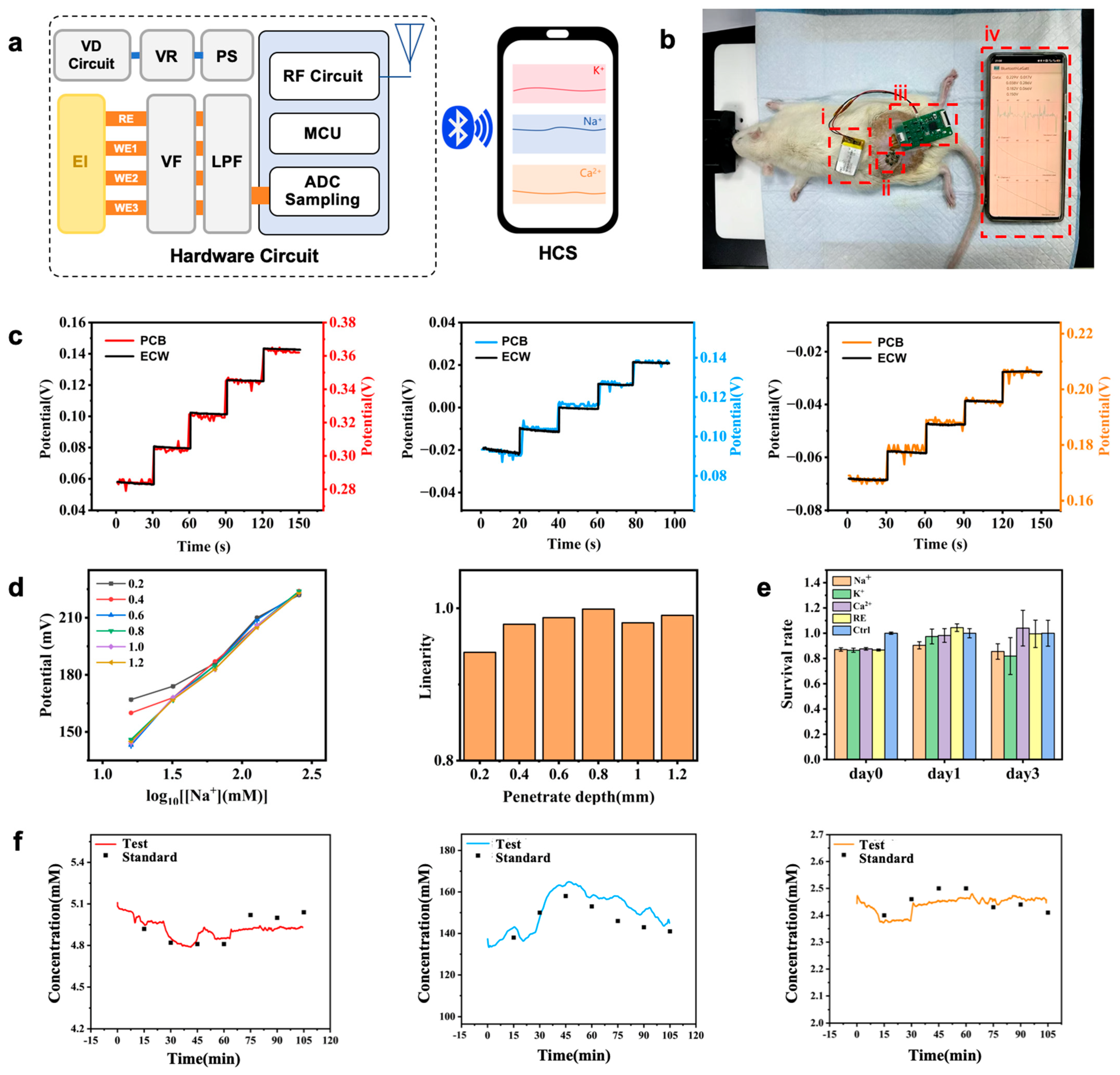
2.3. Integration of Microneedle Arrays and Testing of the Monitoring System
2.4. In Vitro Simulation Experiments
2.5. In Vivo Animal Experiments
3. Results and Discussion
3.1. Performance Characterization of Ion-Selective Electrodes Based on Microneedles
3.2. Optimization of Array Design Using Finite Element Analysis
3.3. Design of Monitoring System Based on Microneedle Arrays
3.4. In Vitro Simulation Experiments
3.5. In Vivo Animal Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadifard, N.; Gotay, C.; Humphries, K.H.; Ignaszewski, A.; Esmaillzadeh, A.; Sarrafzadegan, N. Electrolyte minerals intake and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2019, 59, 2375–2385. [Google Scholar] [CrossRef]
- Liamis, G.; Rodenburg, E.M.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Electrolyte disorders in community subjects: Prevalence and risk factors. Am. J. Med. 2013, 126, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; De Wael, K. Wearable self-powered electrochemical devices for continuous health management. Adv. Funct. Mater. 2021, 31, 2107042. [Google Scholar] [CrossRef]
- Haghayegh, F.; Norouziazad, A.; Haghani, E.; Feygin, A.A.; Rahimi, R.H.; Ghavamabadi, H.A.; Sadighbayan, D.; Madhoun, F.; Papagelis, M.; Felfeli, T. Revolutionary Point-of-Care Wearable Diagnostics for Early Disease Detection and Biomarker Discovery through Intelligent Technologies. Adv. Sci. 2024, 11, 2400595. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Ortiz-Go, I.; Ca, R.; Salinas-Castillo, A.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion patch for on-body electrolyte monitoring in sweat: Toward a validation strategy to ensure physiological relevance. Anal. Chem. 2019, 91, 8644–8651. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Dong, Y.; Mao, S.; Chen, S.; Ma, J.; Jaffrezic-Renault, N.; Guo, Z. Opportunities and challenges of microneedle electrochemical sensors for interstitial fluid detection. TrAC Trends Anal. Chem. 2024, 180, 117891. [Google Scholar] [CrossRef]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: Toward Parkinson management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Bakhshandeh, F.; Zheng, H.; Barra, N.G.; Sadeghzadeh, S.; Ausri, I.; Sen, P.; Keyvani, F.; Rahman, F.; Quadrilatero, J.; Liu, J. Wearable aptalyzer integrates microneedle and electrochemical sensing for in vivo monitoring of glucose and lactate in live animals. Adv. Mater. 2024, 36, 2313743. [Google Scholar] [CrossRef]
- Liu, H.; Gu, Z.; Zhao, Q.; Li, S.; Ding, X.; Xiao, X.; Xiu, G. Printed circuit board integrated wearable ion-selective electrode with potential treatment for highly repeatable sweat monitoring. Sens. Actuators B Chem. 2022, 355, 131102. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Z.; Zhong, G.; Xu, T.; Zhang, X. Self-Sterilizing Microneedle Sensing Patches for Machine Learning-Enabled Wound pH Visual Monitoring. Adv. Funct. Mater. 2024, 34, 2315067. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Freeman, D.M.E.; Rawson, T.M.; Rogers, M.L.; Wilson, R.C.; Holmes, A.H.; Cass, A.E.; O’Hare, D. Development of a minimally invasive microneedle-based sensor for continuous monitoring of β-lactam antibiotic concentrations in vivo. ACS Sens. 2019, 4, 1072–1080. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, Q.; Wang, Q.; Qiu, H.; Li, H.; Xu, T. Fully integrated microneedle biosensor array for wearable multiplexed fitness biomarkers monitoring. Biosens. Bioelectron. 2024, 265, 116697. [Google Scholar] [CrossRef]
- Teymourian, H.; Tehrani, F.; Mahato, K.; Wang, J. Lab under the skin: Microneedle based wearable devices. Adv. Healthc. Mater. 2021, 10, 2002255. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Sabri, A.H.; McKenna, P.E.; Himawan, A.; Hutton, A.R.J.; Detamornrat, U.; Paredes, A.J.; Larrañeta, E.; Donnelly, R.F. Microneedle-based biosensing. Nat. Rev. Bioeng. 2024, 2, 64–81. [Google Scholar] [CrossRef]
- Hu, Y.; Chatzilakou, E.; Pan, Z.; Traverso, G.; Yetisen, A.K. Microneedle sensors for point-of-care diagnostics. Adv. Sci. 2024, 11, 2306560. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Weng, Z.; Sun, H.; Nistala, R.; Zhang, Y. Microneedle-based potentiometric sensing system for continuous monitoring of multiple electrolytes in skin interstitial fluids. ACS Sens. 2021, 6, 2181–2190. [Google Scholar] [CrossRef]
- Abdullah, H.; Phairatana, T.; Jeerapan, I. Tackling the challenges of developing microneedle-based electrochemical sensors. Microchim. Acta 2022, 189, 440. [Google Scholar] [CrossRef]
- Erdem, Ö.; Eş, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent advances in microneedle-based sensors for sampling, diagnosis and monitoring of chronic diseases. Biosensors 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Poudineh, M. Microneedle assays for continuous health monitoring: Challenges and solutions. ACS Sens. 2024, 9, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zheng, S.; Liang, B.; He, M.; Wu, F.; Yang, J.; Chen, H.; Xie, X. 3D-assembled microneedle ion sensor-based wearable system for the transdermal monitoring of physiological ion fluctuations. Microsystems Nanoeng. 2023, 9, 25. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Casanova, A.; Wang, Q.; Cuartero, M.; Crespo, G.A. In vivo transdermal multi-ion monitoring with a potentiometric microneedle-based sensor patch. ACS Sens. 2022, 8, 158–166. [Google Scholar] [CrossRef]
- Kang, J.; Kim, K.Y.; Kim, S.; Hong, H.; Bae, B.-S.; Kang, S.-K.; Lee, W. A conformable microneedle sensor with photopatternable skin adhesive and gel electrolyte for continuous glucose monitoring. Device 2023, 1, 100112. [Google Scholar] [CrossRef]
- LeSueur, J.; Hampton, C.; Kleinberger, M.; Dzwierzynski, W.; Pintar, F.A. In vitro skin puncture methodology for material characterization. Med. Eng. Phys. 2024, 130, 104199. [Google Scholar] [CrossRef]
- Su, R.; Zhang, R.; Wang, Y.; Li, Z.; Zhang, L.; Ma, S.; Li, X.; Ma, F.; Fu, H. Simulated skin model for in vitro evaluation of insertion performance of microneedles: Design, development, and application verification. Comput. Methods Biomech. Biomed. Engin. 2024, 30, 1–10. [Google Scholar] [CrossRef]
- Pereda, E.D.C.; Rojas, H.A.G.; Egea, A.J.S. Dynamic modelling of needle-tissue interaction applied to soft tissue damage during needle extraction. IEEE Access 2025, 13, 25457–25464. [Google Scholar] [CrossRef]
- Ebrahiminejad, V.; Prewett, P.D.; Davies, G.J.; Rad, Z.F. Microneedle arrays for drug delivery and diagnostics: Toward an optimized design, reliable insertion, and penetration. Adv. Mater. Interfaces 2022, 9, 2101856. [Google Scholar] [CrossRef]
- Weber, B.; Scheibert, J.; de Boer, M.P.; Dhinojwala, A. Experimental insights into adhesion and friction between nominally dry rough surfaces. MRS Bull. 2022, 47, 1237–1246. [Google Scholar] [CrossRef]
- Wei, B.; Liu, J.; Ouyang, L.; Kuo, C.-C.; Martin, D.C. Significant enhancement of PEDOT thin film adhesion to inorganic solid substrates with EDOT-acid. ACS Appl. Mater. Interfaces 2015, 7, 15388–15394. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.W.; Lim, S.; Yi, H.; Wu, Z.; Kwon, I.G.; Yeo, W.; Song, E.; Yu, K.J. Conformal fixation strategies and bioadhesives for soft bioelectronics. Adv. Funct. Mater. 2024, 34, 2313728. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, X.; Fan, J.A.; Xu, S.; Song, Y.M.; Choi, K.; Yeo, W.; Lee, W.; Nazaar, S.N. Experimental and theoretical studies of serpentine microstructures bonded to prestrained elastomers for stretchable electronics. Adv. Funct. Mater. 2014, 24, 2028–2037. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Gao, B. A review of recent advances in microneedle-based sensing within the dermal ISF that could transform medical testing. ACS Sens. 2024, 9, 1149–1161. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, H.; Chaj, J.; Wei, U. Microneedle sensors for dermal interstitial fluid analysis. Med-X 2024, 2, 15. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Ma, Y.; Wei, Y.; Chen, W.; Dai, Z.; Cai, Y.; Ye, X.; Liang, B. Individually Modified Microneedle Array for Minimal Invasive Multi-Electrolyte Monitoring. Biosensors 2025, 15, 310. https://doi.org/10.3390/bios15050310
Yu K, Ma Y, Wei Y, Chen W, Dai Z, Cai Y, Ye X, Liang B. Individually Modified Microneedle Array for Minimal Invasive Multi-Electrolyte Monitoring. Biosensors. 2025; 15(5):310. https://doi.org/10.3390/bios15050310
Chicago/Turabian StyleYu, Ketian, Yukun Ma, Yiming Wei, Wanying Chen, Zhen Dai, Yu Cai, Xuesong Ye, and Bo Liang. 2025. "Individually Modified Microneedle Array for Minimal Invasive Multi-Electrolyte Monitoring" Biosensors 15, no. 5: 310. https://doi.org/10.3390/bios15050310
APA StyleYu, K., Ma, Y., Wei, Y., Chen, W., Dai, Z., Cai, Y., Ye, X., & Liang, B. (2025). Individually Modified Microneedle Array for Minimal Invasive Multi-Electrolyte Monitoring. Biosensors, 15(5), 310. https://doi.org/10.3390/bios15050310






