Improving the Accuracy of a Wearable Uroflowmeter for Incontinence Monitoring Under Dynamic Conditions: Leveraging Machine Learning Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Approach
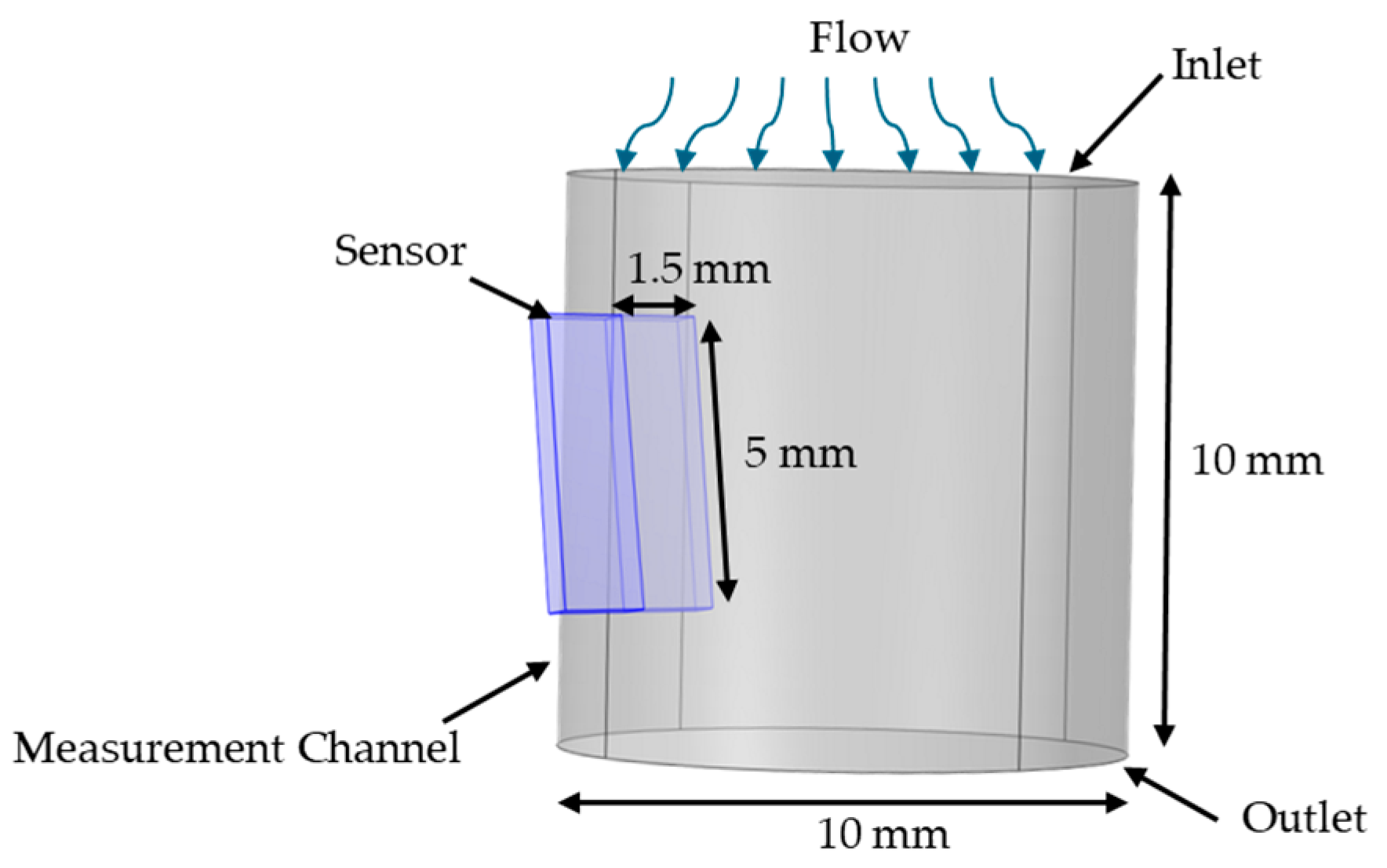
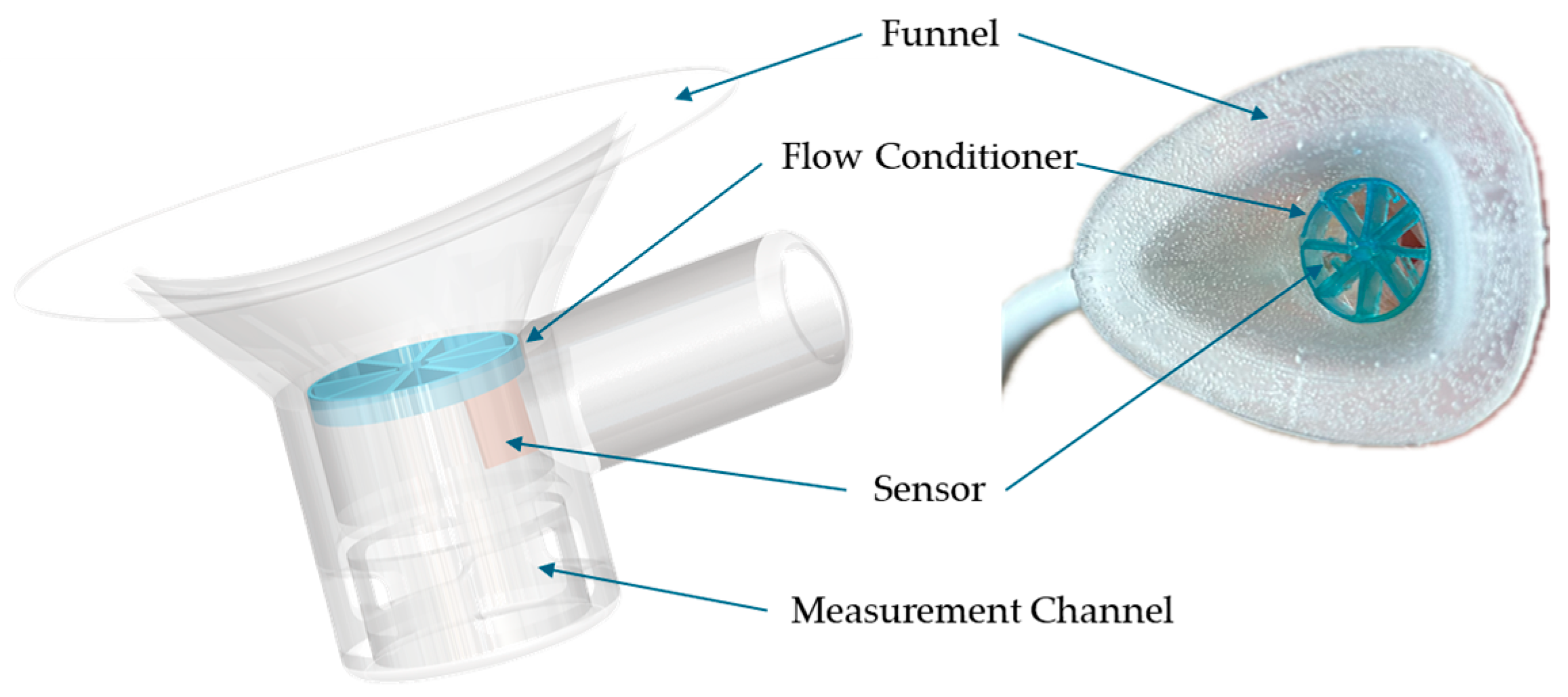
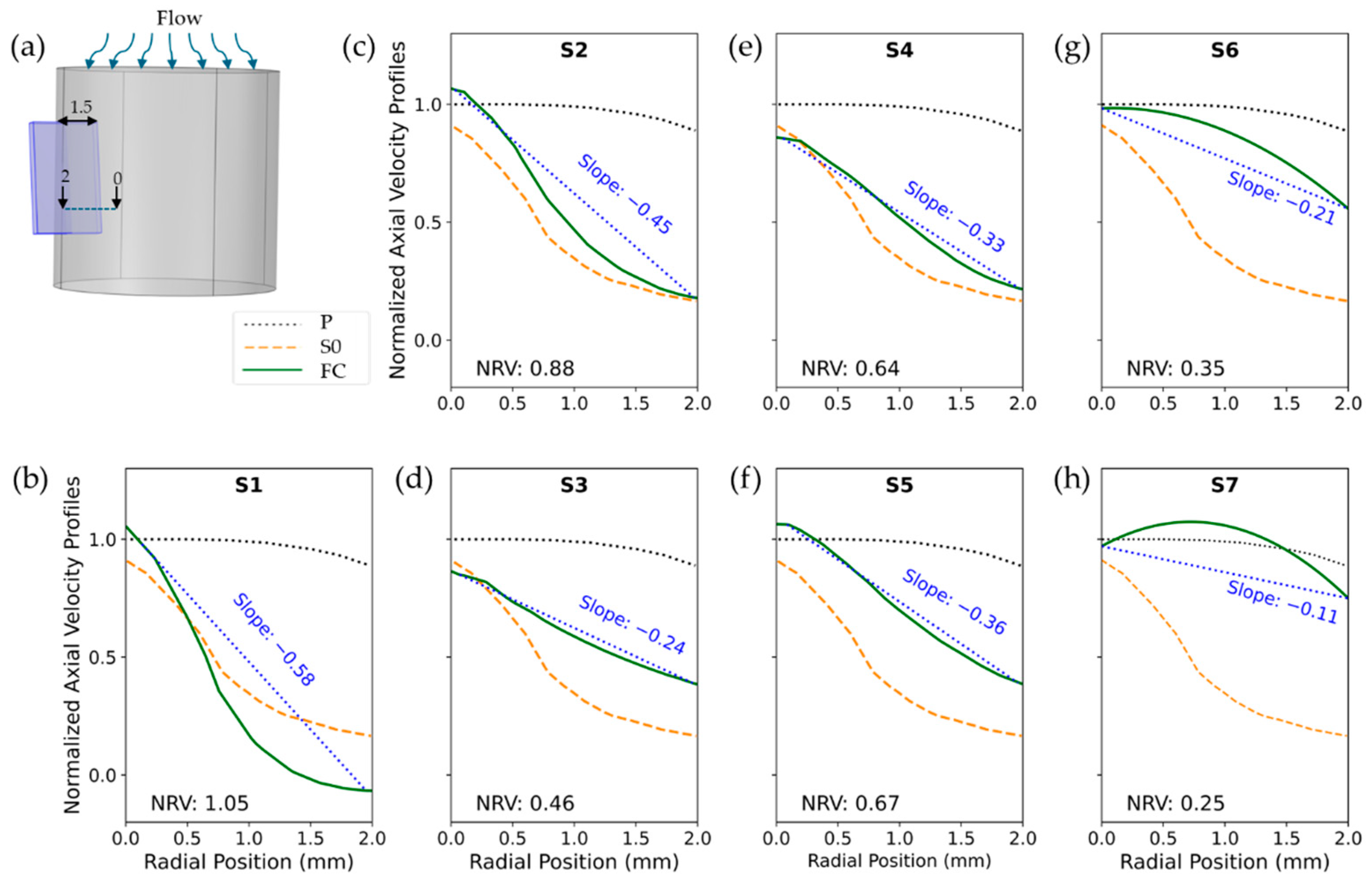
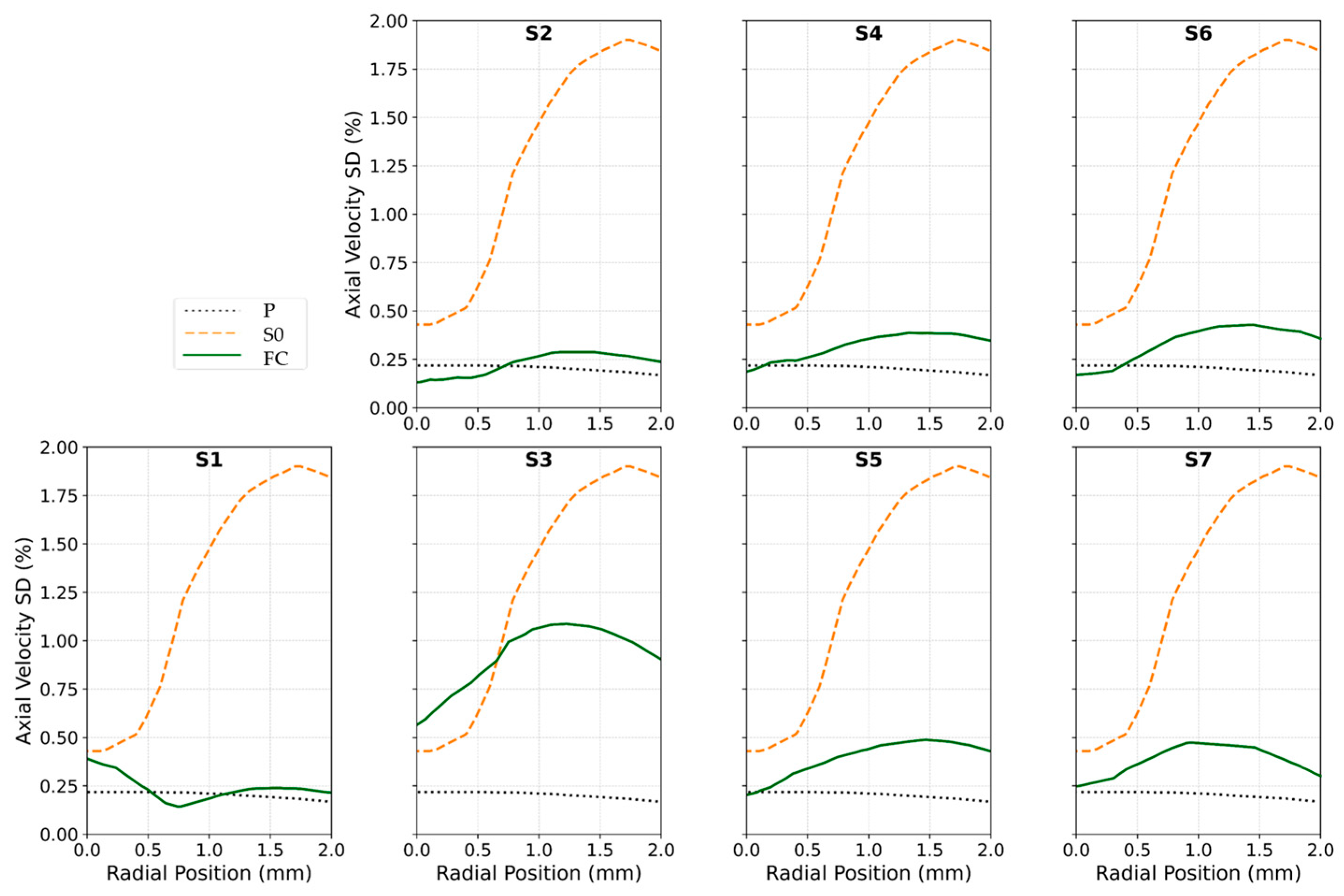
2.3. Flow Conditioner Design and Optimization
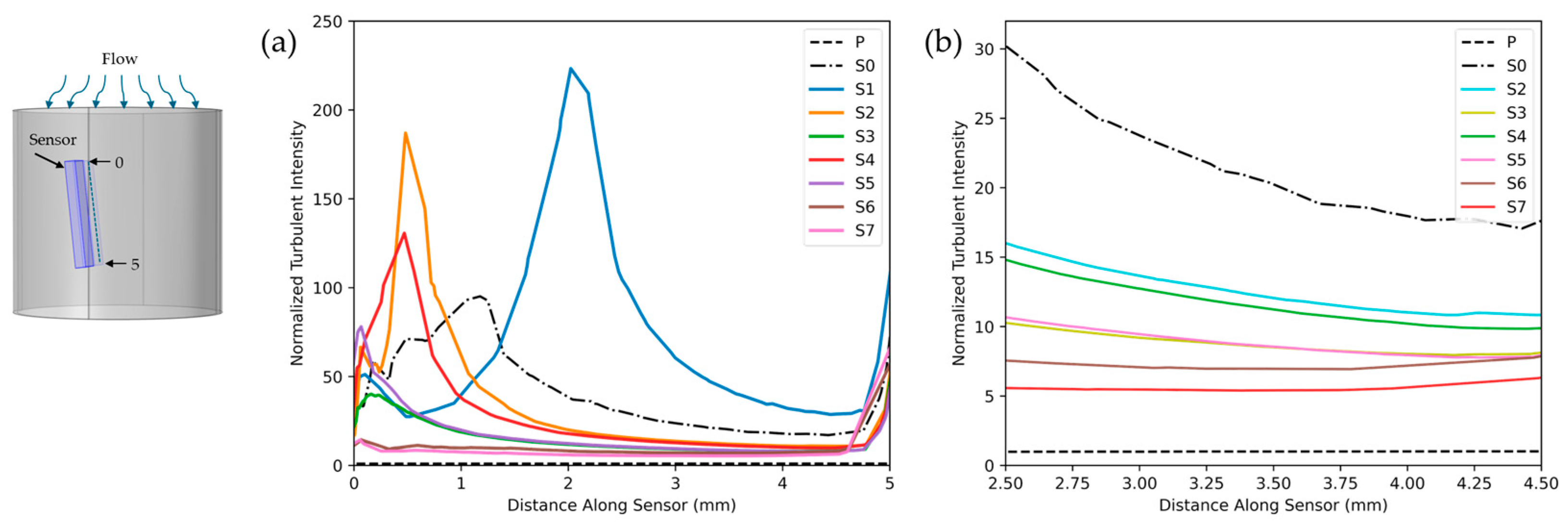
2.4. Data Collection and Processing
2.4.1. Dataset and Preprocessing
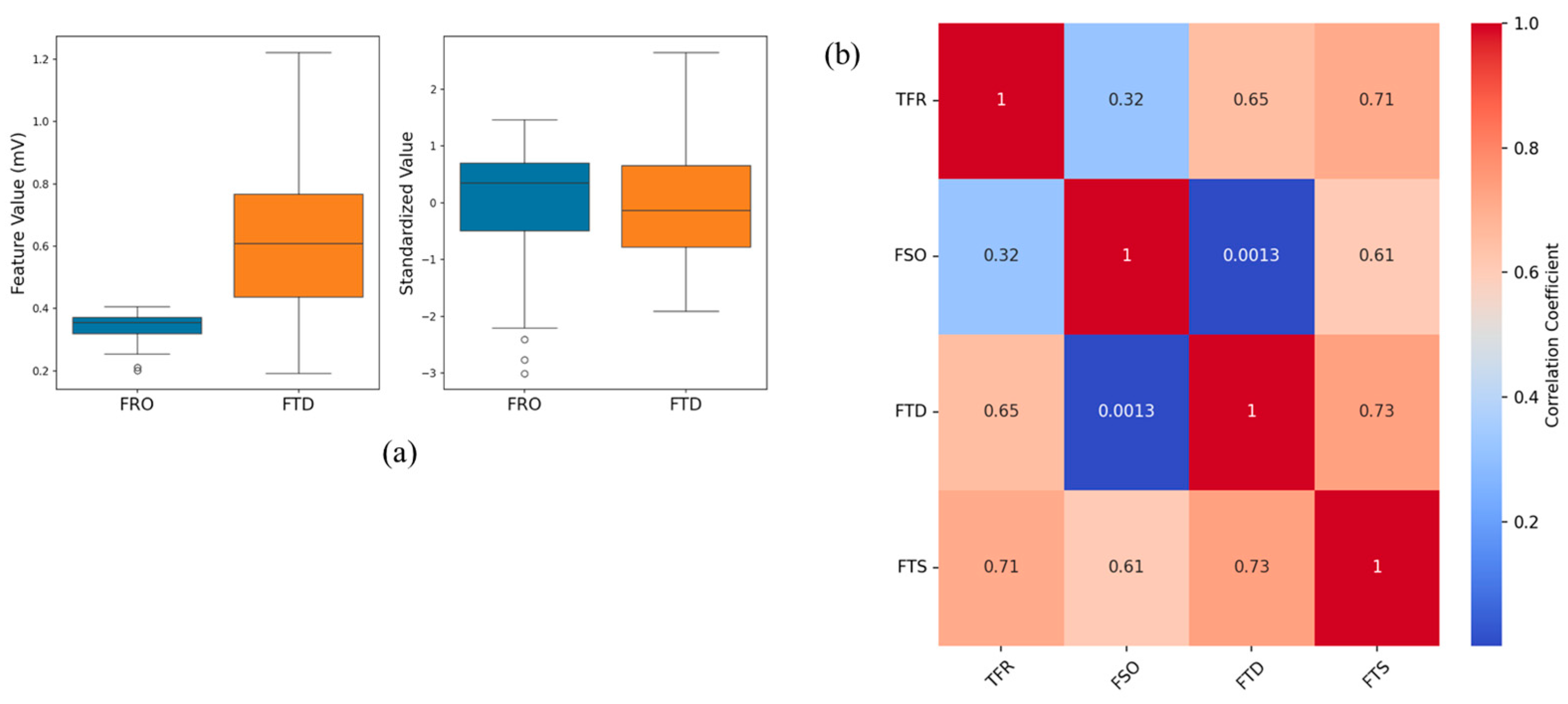
2.4.2. Feature Selection and Transformation
2.4.3. Machine Learning Models
2.4.4. Hyperparameter Optimization
2.4.5. Evaluation Matrices
3. Results
| Model | Hyperparameter | Values | Best Parameters | Time Cost | ||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | ||||
| RF | n_estimators | 30, 40, 50, 100 | 30 | 40 | 30 | 50 | 50 | 88.8 s |
| max_depth | 10, 20, 30, 40 | 30 | 40 | 30 | 30 | 30 | ||
| min_samples_split | 2, 5, 10 | 2 | 10 | 2 | 5 | 2 | ||
| min_samples_leaf | 1, 2, 4 | 4 | 4 | 2 | 1 | 1 | ||
| bootstrap | True, False | True | True | True | True | True | ||
| XGBoost | n_estimators | 40, 50, 100, 200 | 50 | 40 | 40 | 50 | 50 | 16 s |
| learning_rate | 0.01, 0.05, 0.1, 0.2 | 0.05 | 0.05 | 0.1 | 0.1 | 0.1 | ||
| max_depth | 3, 5, 7, 10 | 3 | 3 | 10 | 3 | 3 | ||
| subsample | 0.6, 0.8, 1.0 | 1.0 | 0.8 | 1.0 | 0.6 | 0.6 | ||
| colsample_bytree | 0.6, 0.8, 1.0 | 0.6 | 0.6 | 0.8 | 1.0 | 1 | ||
| SVM | C | 0.1, 1, 10, 100 | 10 | 1 | 100 | 2 s | ||
| Gamma | ‘scale’, ‘auto’, 0.1, 0.01, 0.001 | Scale | Scale | Scale | ||||
| kernel | ‘linear’, ‘rbf’, ‘poly’, ‘sigmoid’ | rbf | rbf | rbf | ||||
| NN in AutoGloun | num_layers | 1, 2, 3, 4 | 9.1 s * | |||||
| dropout_prob | 0.1, 0.2, 0.3, 0.4 | |||||||
4. Discussion
4.1. Sensor Performance and Flow Dynamics
4.2. Experimental Integration and Calibration
4.3. Machine Learning Model Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| CFD | Computational fluid dynamics |
| FC | Flow conditioner |
| FSO | Flow-rate sensor output |
| FTD | Differential of flow-rate and temperature sensors |
| FTS | Composite metric of FSO and FTD |
| MAE | Mean absolute error |
| MSE | Mean squared error |
| NN | Neural network |
| NRV | Normalized range of velocity |
| PCA | Principal component analysis |
| PUF | Personal uroflowmeter |
| RF | Random forest |
| RMSE | Root mean square error |
| SCU | Signal conditioner unit |
| SVM | Support vector machine |
| TFR | True flow rate |
| TI | Turbulence intensity |
| TT | True temperature |
| UI | Urinary incontinence |
| XGBoost | Extreme gradient boosting |
References
- Wood, L.N.; Anger, J.T. Urinary Incontinence in Women. BMJ 2014, 349, g4531. [Google Scholar] [CrossRef] [PubMed]
- Lukacz, E.S.; Santiago-Lastra, Y.; Albo, M.E.; Brubaker, L. Urinary Incontinence in Women: A Review. JAMA 2017, 318, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Wadensten, T.; Nyström, E.; Franzén, K.; Lindam, A.; Wasteson, E.; Samuelsson, E. A Mobile App for Self-Management of Urgency and Mixed Urinary Incontinence in Women: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e19439. [Google Scholar] [CrossRef]
- Vaughan, C.P.; Markland, A.D. Urinary Incontinence in Women. Ann. Intern. Med. 2020, 172, ITC17–ITC32. [Google Scholar] [CrossRef]
- Batmani, S.; Jalali, R.; Mohammadi, M.; Bokaee, S. Prevalence and Factors Related to Urinary Incontinence in Older Adults Women Worldwide: A Comprehensive Systematic Review and Meta-Analysis of Observational Studies. BMC Geriatr. 2021, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Lok, M.K.; Pang, S.M.; Wun, Y.T. Stress Urinary Incontinence in Younger Women in Primary Care: Prevalence and Opportunistic Intervention. J. Women’s Health 2014, 23, 65–68. [Google Scholar] [CrossRef]
- Young, C.; Cooper, D.; Mostafa, A.; Abdel-Fattah, M. The “Aberdeen Home Continence Stress Test”: A Novel Objective Assessment Tool for Female Stress Urinary Incontinence. Int. Urogynecol. J. 2023, 34, 1961–1969. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Shi, H.; Xue, K.; Zhou, F. Advances in the Natural History of Urinary Incontinence in Adult Females. J. Obstet. Gynaecol. 2023, 43, 2171774. [Google Scholar] [CrossRef]
- Hafid, A.; Difallah, S.; Alves, C.; Abdullah, S.; Folke, M.; Lindén, M.; Kristoffersson, A. State of the Art of Non-Invasive Technologies for Bladder Monitoring: A Scoping Review. Sensors 2023, 23, 2758. [Google Scholar] [CrossRef]
- Ben Arous, M.; Haddar, I.; Truong, A.; Ayena, J.C.; Ouakrim, Y.; El Kamel, L.; Chikhaoui, B.; Mezghani, N. Non-Invasive Wearable Devices for Urinary Incontinence Detection—A Mini Review. Front. Sens. 2023, 4, 1279158. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Palmieri, S.; Cola, A.; Barba, M.; Manodoro, S.; Frigerio, M. Correlation between Urinary Symptoms and Urodynamic Findings: Is the Bladder an Unreliable Witness? Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Block, V.J.; Mclntyre, L.; Sisodia, N.; Van Kuiken, M.E.; Suskind, A.M.; Bove, R. Wearables for the Bladder: Stakeholder Perspectives on Moving Multiple Sclerosis Bladder Dysfunction Interventions Into the 21st Century. Int. J. MS Care 2024, 26, 290–301. [Google Scholar] [CrossRef]
- Medeiros Araujo, C.; de Morais, N.R.; Sacomori, C.; de Sousa Dantas, D. Pad Test for Urinary Incontinence Diagnosis in Adults: Systematic Review of Diagnostic Test Accuracy. Neurourol. Urodyn. 2022, 41, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, I.; Holcomb, R. Reproducibility of the Seven-Day Voiding Diary in Women with Stress Urinary Incontinence. Int. Urogynecol. J. 2000, 11, 15–17. [Google Scholar] [CrossRef]
- Tincello, D.G.; Williams, K.S.; Joshi, M.; Assassa, R.P.; Abrams, K.R. Urinary Diaries: A Comparison of Data Collected for Three Days versus Seven Days. Obstet. Gynecol. 2007, 109, 277–280. [Google Scholar] [CrossRef]
- Rabin, J.M.; McNett, J.; Badlani, G.H. Computerized Voiding Diary. Neurourol. Urodyn. 1993, 12, 541–552. [Google Scholar] [CrossRef]
- Ryhammer, A.M.; Djurhuus, J.C.; Laurberg, S. Pad Testing in Incontinent Women: A Review. Int. Urogynecol. J. 1999, 10, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Curran, P.; Barda, D.A.; Bradley, D.B.; Phillips, J.; Kotlarski, P.; Olkkonen, J.T.; Mattila, T.J.P. Capacitive Wetness Sensor and Method for Manufacturing the Same. WO 2013/016765 A1, 13 January 2013. [Google Scholar]
- Lewis, M.P.; Carey, K.M.; Templestowe, L.; Cottenden, M.A.; Barda, D.A.; Bay, R.; Bergman, F. Incontinence Monitoring and Assessment. US 2012/0268278 A1, 25 October 2012. [Google Scholar]
- Ang, L.M.; Ow, S.H.; Seng, K.P.; Tee, Z.H.; Lee, B.W.; Thong, M.K.; Poi, P.J.H.; Kunanayagam, S. Wireless Intelligent Incontinence Management System Using Smart Diapers. In Proceedings of the 2008 5th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Krabi, Thailand, 14–17 May 2008; IEEE: New Yok, NY, USA, 2008; Volume 1, pp. 69–72. [Google Scholar] [CrossRef]
- Miller, J.M.; Ashton-Miller, J.A.; Delancey, J.O.L. Quantification of Cough-Related Urine Loss Using the Paper Towel Test. Obstet. Gynecol. 1998, 91, 705–709. [Google Scholar] [CrossRef]
- DiMino, A.; Drummer, M.E.; Campbell, J.M.; Malik, A.; Curameng, L. Apparatus and Method for Uroflowmetry. US20120226196A1, 6 September 2012. [Google Scholar]
- Beckwith, R.; Drinnan, M.; Bray, A.; Griffiths, C.J.; Whitaker, M. Urine Flow Measuring Apparatus. GB 2508551A, 4 January 2014. [Google Scholar]
- Gammie, A.; Wachter, S. De Research Priorities for Diagnostic Instrumentation in Urinary Incontinence. Proc. Inst. Mech. Eng. H 2024, 238, 682–687. [Google Scholar] [CrossRef]
- Dawidek, M.T.; Singla, R.; Spooner, L.; Ho, L.; Nguan, C. Clinical Validation of an Audio-Based Uroflowmetry Application in Adult Males. Can. Urol. Assoc. J. 2021, 16, E120–E125. [Google Scholar] [CrossRef]
- Chun, K.; Kim, S.J.; Cho, S.T. Noninvasive Medical Tools for Evaluating Voiding Pattern in Real Life. Int. Neurourol. J. 2017, 21, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Attari, A. Design and Testing Novel Wearable Instrumentation for Assessing Pelvic Floor Function and Exploring Continence Mechanisms. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2020. [Google Scholar]
- Attari, A.; Ashton-Miller, J.A.; DeLancey, J.O.; Burns, M.A.; Kirkbride, T.M.; Carlin, E.P.; Ramachandran, A.J.; Day, C.A. Uroflowmetry Systems Having Wearable Uroflowmeters, and Methods of Operating the Same. US 2021/0121112 A1, 29 April 2021. [Google Scholar]
- Lin, W.C.; Burns, M.A. Low-Power Micro-Fabricated Liquid Flow-Rate Sensor. Anal. Methods 2015, 7, 3981–3987. [Google Scholar] [CrossRef]
- Umek, W.H.; Obermair, A.; Stutterecker, D.; Häusler, G.; Leodolter, S.; Hanzal, E. Three-Dimensional Ultrasound of the Female Urethra: Comparing Transvaginal and Transrectal Scanning. Ultrasound Obstet. Gynecol. 2001, 17, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.M.; Ouazanne, A.K. Compact Installations for Differential Flowmeters. Flow. Meas. Instrum. 1994, 5, 79–85. [Google Scholar] [CrossRef]
- Laws, E.M.; Harris, R. Evaluation of a Swirl-Vor-Tab Flow Conditioner. Flow. Meas. Instrum. 1993, 4, 101–108. [Google Scholar] [CrossRef]
- Laws, E.M.; Ouazzane, A.K. A Preliminary Study into the Effect of Length on the Performance of the Etoile Flow Straightener. Flow. Meas. Instrum. 1995, 6, 225–233. [Google Scholar] [CrossRef]
- Chen, G.; Liu, G. Performance Evaluation and Analysis of a New Flow Conditioner Based on CFD. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 032049. [Google Scholar] [CrossRef]
- Askari, V.; Nicolas, D.; Edralin, M.; Jang, C. Computational Fluid Dynamics Model for Sensitivity Analysis and Design of Flow Conditioners. In Proceedings of the 9th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH 2019), Prague, Czech Republic, 29–31 July 2019; SciTePress: Setúbal, Portugal, 2019; pp. 129–140. [Google Scholar]
- ISO 5167-1:2022; Tracked Changes. Measurement of Fluid Flow by Means of Pressure Differential Devices Inserted in Circular Cross-Section Conduits Running Full. Part 1, General Principles and Requirements. British Standards Institution: London, UK, 2022; ISBN 9780539230604.
- COMSOL. CFD Module User’s Guide; COMSOL, Inc.: Stockholm, Sweden, 2020. [Google Scholar]
- Savcı, İ.H.; Şener, R.; Duman, İ. A Study of Signal Noise Reduction of the Mass Air Flow Sensor Using the Flow Conditioner on the Air Induction System of Heavy-Duty Truck. Flow Meas. Instrum. 2022, 83, 102121. [Google Scholar] [CrossRef]
- Lambert, T.P.; Chan, M.; Sanchez-Perez, J.A.; Nikbakht, M.; Lin, D.J.; Nawar, A.; Bashar, S.K.; Kimball, J.P.; Zia, J.S.; Gazi, A.H.; et al. A Comparison of Normalization Techniques for Individual Baseline-Free Estimation of Absolute Hypovolemic Status Using a Porcine Model. Biosensors 2024, 14, 61. [Google Scholar] [CrossRef]
- Manju, N.; Samiha, C.M.; Kumar, S.P.P.; Gururaj, H.L.; Flammini, F. Prediction of Aptamer Protein Interaction Using Random Forest Algorithm. IEEE Access 2022, 10, 49677–49687. [Google Scholar] [CrossRef]
- Wekalao, J.; Patel, S.K.; U, A.K.; Armghan, A.; Kraiem, H.; Said, Y. Detection of Proteins in a Surface Plasmon Resonance Biosensor Based on Hybrid Metasurface Architecture and Behaviour Prediction Using Random Forest Regression. Plasmonics 2024, 123456789, 1–21. [Google Scholar] [CrossRef]
- Kyriazos, T.; Poga, M. Dealing with Multicollinearity in Factor Analysis: The Problem, Detections, and Solutions. Open J. Stat. 2023, 13, 404–424. [Google Scholar] [CrossRef]
- Kutateladze, V. The Kernel Trick for Nonlinear Factor Modeling. Int. J. Forecast. 2022, 38, 165–177. [Google Scholar] [CrossRef]
- Rumbut, J.; Fang, H.; Carreiro, S.; Smelson, D.; Boyer, E. An Overview of Wearable Biosensor Systems for Real-Time Substance Use Detection. IEEE Internet Things J. 2022, 9, 23405–23415. [Google Scholar] [CrossRef]
- Wekalao, J.; Mandela, N. Terahertz Metasurface Biosensor for High-Sensitivity Salinity Detection and Data Encoding with Machine Learning Optimization Based on Random Forest Regression. Opt. Quant. Electron. 2024, 56, 1826. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Yan, B.; Zhang, L.; Gu, Y. Bionic Electronic Nose Based on MOS Sensors Array and Machine Learning Algorithms Used for Wine Properties Detection. Sensors 2019, 19, 45. [Google Scholar] [CrossRef]
- Kokabi, M.; Tahir, M.N.; Singh, D.; Javanmard, M. Advancing Healthcare: Synergizing Biosensors and Machine Learning for Early Cancer Diagnosis. Biosensors 2023, 13, 884. [Google Scholar] [CrossRef]
- Sharma, H.; Kalita, D.; Naskar, U.; Mishra, B.K.; Kumar, P.; Mirza, K.B. Prediction of Glucose Sensor Sensitivity in the Presence of Biofouling Using Machine Learning and Electrochemical Impedance Spectroscopy. IEEE Sens. J. 2023, 23, 18785–18797. [Google Scholar] [CrossRef]
- Rahman, M.J.; Morshed, B.I. Improving Accuracy of Inkjet Printed Core Body WRAP Temperature Sensor Using Random Forest Regression Implemented with an Android App. In Proceedings of the 2019 United States National Committee of URSI National Radio Science Meeting (USNC-URSI NRSM), Boulder, CO, USA, 9–12 January 2019. [Google Scholar] [CrossRef]
- Boubin, M.; Shrestha, S. Microcontroller Implementation of Support Vector Machine for Detecting Blood Glucose Levels Using Breath Volatile Organic Compounds. Sensors 2019, 19, 2283. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: San Francisco, CA, USA, 2016. [Google Scholar]
- Noh, B.; Youm, C.; Goh, E.; Lee, M.; Park, H.; Jeon, H.; Kim, O.Y. XGBoost Based Machine Learning Approach to Predict the Risk of Fall in Older Adults Using Gait Outcomes. Sci. Rep. 2021, 11, 12183. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Chen, M.; Yang, W.; Fu, R. Deep Regression Network with Sequential Constraint for Wearable ECG Characteristic Point Location. IEEE Access 2023, 11, 63487–63495. [Google Scholar] [CrossRef]
- Althnian, A.; AlSaeed, D.; Al-Baity, H.; Samha, A.; Dris, A.B.; Alzakari, N.; Abou Elwafa, A.; Kurdi, H. Impact of Dataset Size on Classification Performance: An Empirical Evaluation in the Medical Domain. Appl. Sci. 2021, 11, 796. [Google Scholar] [CrossRef]
- Shaikhina, T.; Khovanova, N.A. Handling Limited Datasets with Neural Networks in Medical Applications: A Small-Data Approach. Artif. Intell. Med. 2017, 75, 51–63. [Google Scholar] [CrossRef]
- Erickson, N.; Mueller, J.; Shirkov, A.; Zhang, H.; Larroy, P.; Li, M.; Smola, A. AutoGluon-Tabular: Robust and Accurate AutoML for Structured Data. arXiv 2020, arXiv:2003.06505. [Google Scholar]




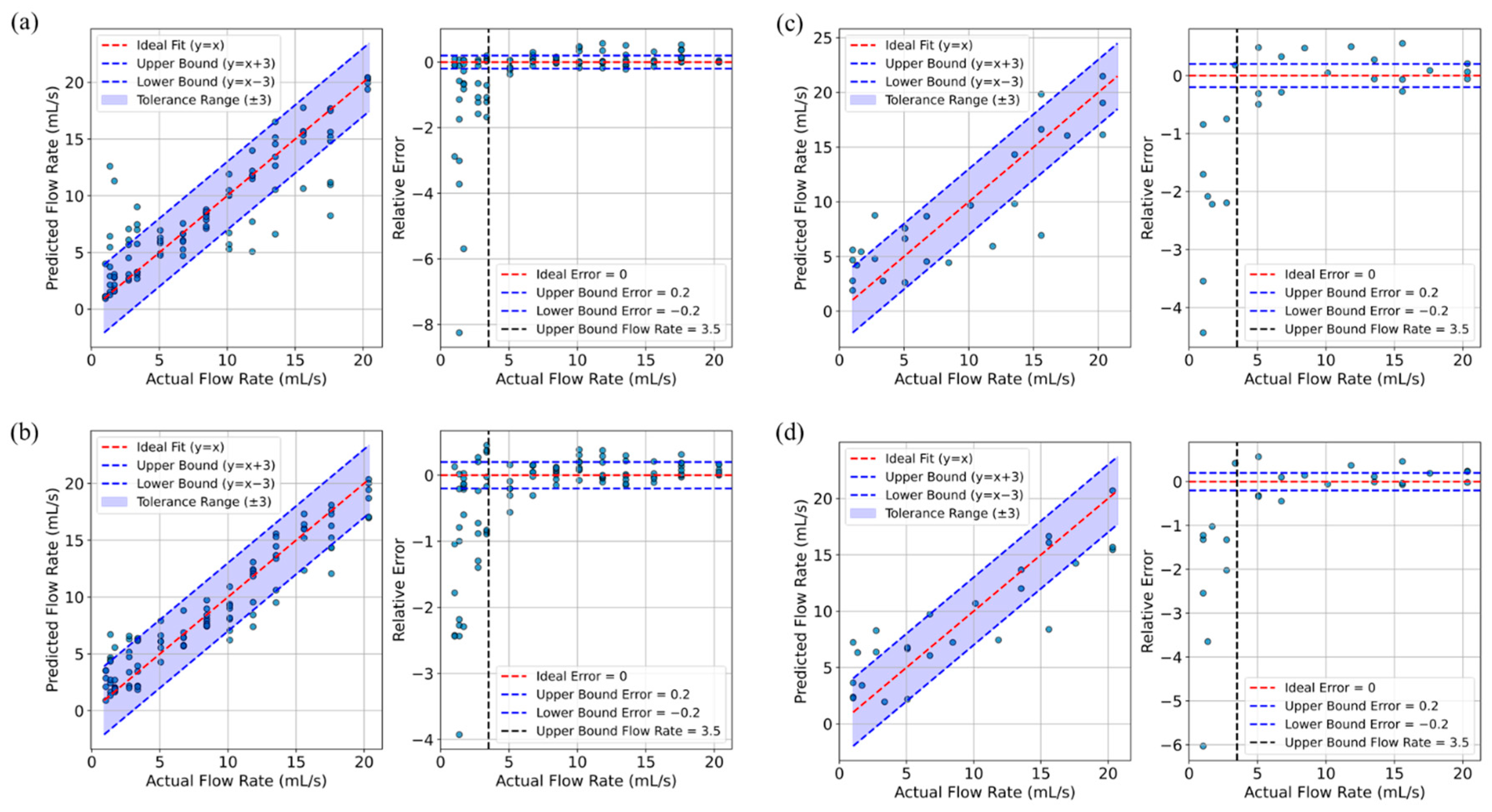
| Training Data | Test Data | |||||||
|---|---|---|---|---|---|---|---|---|
| Input | PCA | ML Model | Corr | R2 | Corr | R2 | MAE | RMSE |
| T1: FTS | - | RF | 0.84 | 0.7 | 0.8 | 0.63 | 3.3 | 4.1 |
| SVM | 0.75 | 0.54 | 0.77 | 0.58 | 3.5 | 4.3 | ||
| XGBoost | 0.85 | 0.7 | 0.77 | 0.55 | 3.7 | 4.5 | ||
| AutoGluon | 0.85 | 0.69 | 0.8 | 0.57 | 3.8 | 4.4 | ||
| T2: FTD | - | RF | 0.74 | 0.54 | 0.84 | 0.65 | 3.2 | 4 |
| SVM | 0.67 | 0.44 | 0.84 | 0.69 | 3 | 4 | ||
| XGBoost | 0.81 | 0.63 | 0.83 | 0.63 | 3.5 | 4.1 | ||
| AutoGluon | 0.66 | 0.35 | 0.87 | 0.75 | 2.5 | 3.4 | ||
| T3: FSO FTS FTD | - | RF | 0.96 | 0.91 | 0.86 | 0.73 | 2.7 | 3.5 |
| SVM | 0.88 | 0.78 | 0.86 | 0.74 | 2.8 | 3.4 | ||
| XGBoost | 1 | 1 | 0.84 | 0.68 | 3 | 3.8 | ||
| AutoGluon | 0.88 | 0.76 | 0.84 | 0.7 | 2.9 | 3.7 | ||
| T4: FSO FTS FTD | Linear | RF | 0.97 | 0.94 | 0.87 | 0.75 | 2.8 | 3.4 |
| XGBoost | 0.95 | 0.9 | 0.88 | 0.76 | 2.6 | 3.3 | ||
| T5: FSO FTS FTD | rbf | RF | 0.93 | 0.85 | 0.78 | 0.6 | 3.3 | 4.2 |
| XGBoost | 0.94 | 0.87 | 0.81 | 0.65 | 3.2 | 4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanehsazzadeh, F.; DeLancey, J.O.L.; Ashton-Miller, J.A. Improving the Accuracy of a Wearable Uroflowmeter for Incontinence Monitoring Under Dynamic Conditions: Leveraging Machine Learning Methods. Biosensors 2025, 15, 306. https://doi.org/10.3390/bios15050306
Shanehsazzadeh F, DeLancey JOL, Ashton-Miller JA. Improving the Accuracy of a Wearable Uroflowmeter for Incontinence Monitoring Under Dynamic Conditions: Leveraging Machine Learning Methods. Biosensors. 2025; 15(5):306. https://doi.org/10.3390/bios15050306
Chicago/Turabian StyleShanehsazzadeh, Faezeh, John O. L. DeLancey, and James A. Ashton-Miller. 2025. "Improving the Accuracy of a Wearable Uroflowmeter for Incontinence Monitoring Under Dynamic Conditions: Leveraging Machine Learning Methods" Biosensors 15, no. 5: 306. https://doi.org/10.3390/bios15050306
APA StyleShanehsazzadeh, F., DeLancey, J. O. L., & Ashton-Miller, J. A. (2025). Improving the Accuracy of a Wearable Uroflowmeter for Incontinence Monitoring Under Dynamic Conditions: Leveraging Machine Learning Methods. Biosensors, 15(5), 306. https://doi.org/10.3390/bios15050306





