Synergistic Signal Amplification via Weak Value Amplification Effect and Sandwich Structure for Highly Sensitive and Specific Real-Time Detection of CA125
Abstract
1. Introduction
2. Materials and Methods
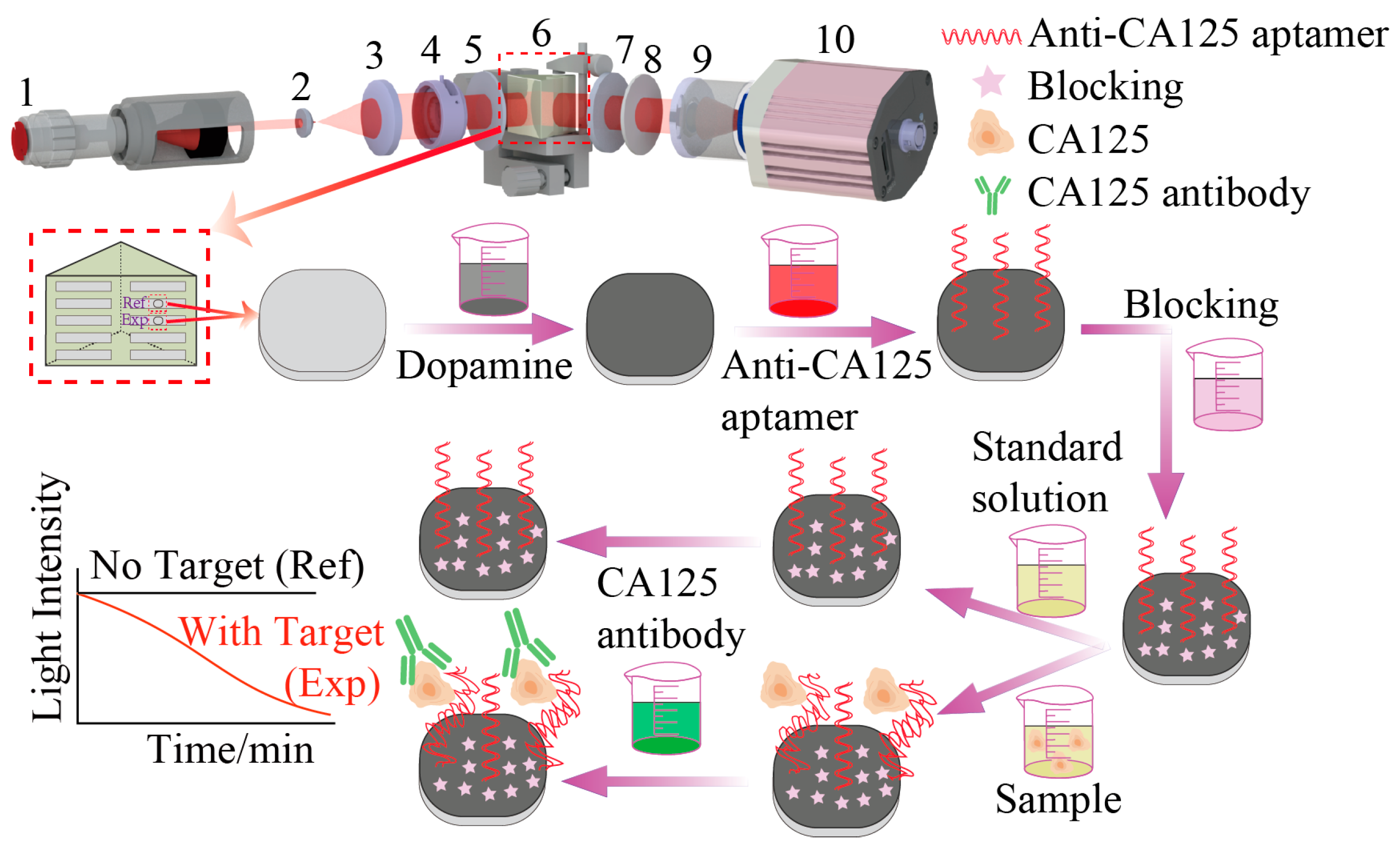
2.1. Imaging Weak Measurement System and Detection Principles
2.2. Experimental Procedures
3. Results and Discussion
3.1. Weak Measurement Characterization
3.2. Optimization of Anti-CA125 Aptamer Concentration
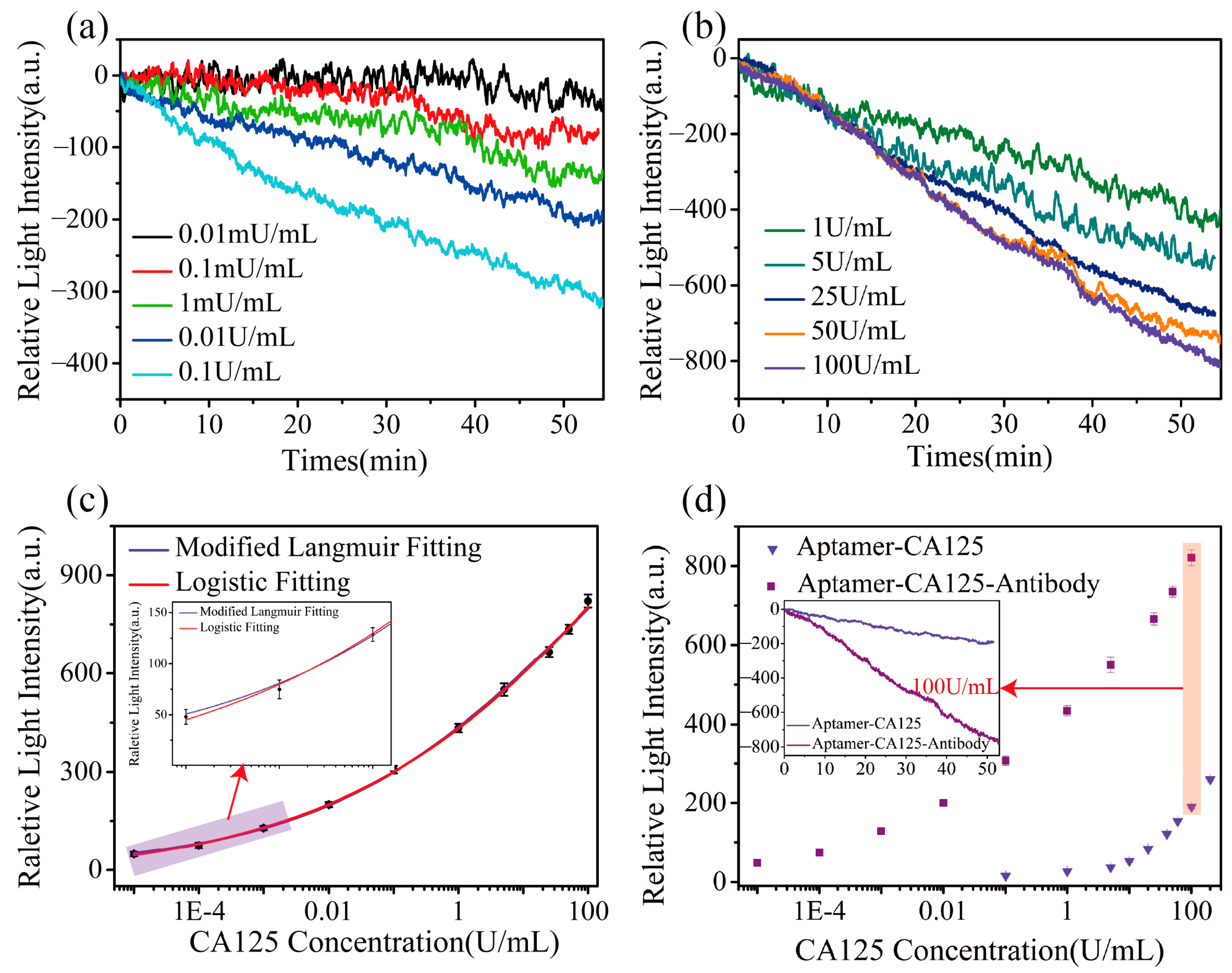
3.3. Specific Detection of CA125 Based on the Sandwich Method
3.4. Gradient Detection of CA125 Using Sandwich Method
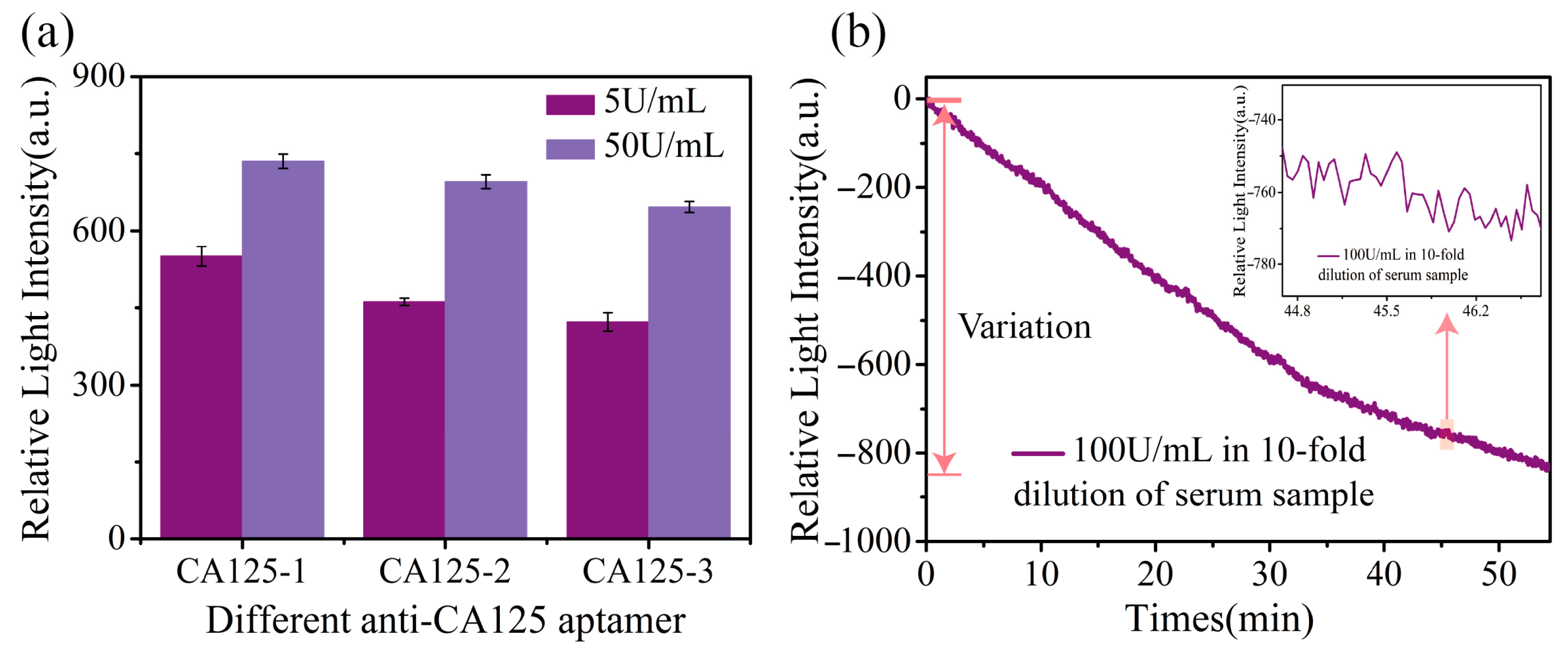
3.5. Detection Experiments of Different Sequence Aptamers
3.6. Detection of CA125 in Complex Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hIgG | Human IgG |
| hIgE | Human IgE |
| AFP | Alpha-fetoprotein |
| PBS | Phosphate-buffered solution |
| ELISA | Enzyme-linked immunosorbent assays |
References
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef]
- Ji, G.; Tian, J.; Xing, F.; Feng, Y. Optical Biosensor Based on Graphene and Its Derivatives for Detecting Biomolecules. Int. J. Mol. Sci. 2022, 23, 10838. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Recent optical sensing technologies for the detection of various biomolecules: Review. Opt. Laser Technol. 2021, 134, 106620. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Yan, H.; Li, X.; Yazd, H.S.; Li, X.; Huang, T.; Cui, C.; Jiang, J.; Tan, W. Nucleic Acid Aptamers for Molecular Diagnostics and Therapeutics: Advances and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Koprowski, H. Molecular recognition and the future of monoclonal antibodies. Nature 1982, 296, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Y.; Zhang, J.; Shi, X.; Ma, L.; Yuan, R. Highly Specific Aptamer–Antibody Birecognized Sandwich Module for Ultrasensitive Detection of a Low Molecular Weight Compound. Anal. Chem. 2024, 96, 11326–11333. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yi, N.; Jiang, D. Advances in single molecule arrays (SIMOA) for ultra-sensitive detection of biomolecules. Talanta 2024, 270, 125529. [Google Scholar] [CrossRef]
- Peng, P.; Liu, C.; Li, Z.; Xue, Z.; Mao, P.; Hu, J.; Xu, F.; Yao, C.; You, M. Emerging ELISA derived technologies for in vitro diagnostics. TrAC Trends Anal. Chem. 2022, 152, 116605. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Zhou, M.; Yao, Y.; Gao, X.; Fan, X.; Wu, G. Advancements in the preparation technology of small molecule artificial antigens and their specific antibodies: A comprehensive review. Analyst 2024, 149, 4583–4599. [Google Scholar] [CrossRef]
- Low, D.; O’Leary, R.; Pujar, N.S. Future of antibody purification. J. Chromatogr. B 2007, 848, 48–63. [Google Scholar] [CrossRef]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Wang, Z.; Cao, H.; Zhang, K.; Li, X.; Yang, Z. Nanomaterial-based aptamer sensors for arsenic detection. Biosens. Bioelectron. 2020, 148, 111785. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.M.; Plaxco, K.W. Real-Time, In Vivo Molecular Monitoring Using Electrochemical Aptamer Based Sensors: Opportunities and Challenges. ACS Sens. 2022, 7, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Meng, L.; Liang, G.; Zhang, L.; Xu, Y.; Zhang, X.; Li, H.; Song, Z.; Guo, C.; Guan, T.; et al. Optical Label-Free Aptasensor Based on Weak Value Amplification for Real-Time and Ultrasensitive Detection of IgE. ACS Sens. 2024, 9, 3625–3632. [Google Scholar] [CrossRef]
- Yang, L.F.; Kacherovsky, N.; Panpradist, N.; Wan, R.; Liang, J.; Zhang, B.; Salipante, S.J.; Lutz, B.R.; Pun, S.H. Aptamer Sandwich Lateral Flow Assay (AptaFlow) for Antibody-Free SARS-CoV-2 Detection. Anal. Chem. 2022, 94, 7278–7285. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Deng, H.; Xiong, X.; Zhang, H.; Liang, T.; Li, C. An aptamer biosensor for CA125 quantification in human serum based on upconversion luminescence resonance energy transfer. Microchem. J. 2021, 161, 105761. [Google Scholar] [CrossRef]
- Valizadeh Shahbazlou, S.; Vandghanooni, S.; Dabirmanesh, B.; Eskandani, M.; Hasannia, S. Biotinylated aptamer-based SPR biosensor for detection of CA125 antigen. Microchem. J. 2023, 194, 109276. [Google Scholar] [CrossRef]
- Kang, S.; Hah, S.S. Improved Ligand Binding by Antibody–Aptamer Pincers. Bioconjugate Chem. 2014, 25, 1421–1427. [Google Scholar] [CrossRef]
- Kim, G.; Yang, H. Electrochemical biosensor using direct electron transfer and an antibody–aptamer hybrid sandwich for target detection in complex biological samples. Biosens. Bioelectron. 2024, 253, 116184. [Google Scholar] [CrossRef]
- Ge, C.; Feng, J.; Zhang, J.; Hu, K.; Wang, D.; Zha, L.; Hu, X.; Li, R. Aptamer/antibody sandwich method for digital detection of SARS-CoV2 nucleocapsid protein. Talanta 2022, 236, 122847. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, J.; Hur, H.; Oh, S.; Lee, H.H. Highly Sensitive Colorimetric Assay of Cortisol Using Cortisol Antibody and Aptamer Sandwich Assay. Biosensors 2021, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Aharonov, Y.; Albert, D.Z.; Vaidman, L. How the result of a measurement of a component of the spin of a spin-1/2 particle can turn out to be 100. Phys. Rev. Lett. 1988, 60, 1351–1354. [Google Scholar] [CrossRef]
- Ritchie, N.W.M.; Story, J.G.; Hulet, R.G. Realization of a measurement of a “weak value”. Phys. Rev. Lett. 1991, 66, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Kofman, A.G.; Ashhab, S.; Nori, F. Nonperturbative theory of weak pre- and post-selected measurements. Phys. Rep. 2012, 520, 43–133. [Google Scholar] [CrossRef]
- Aharonov, Y.; Cohen, E.; Elitzur, A.C. Foundations and applications of weak quantum measurements. Phys. Rev. A 2014, 89, 052105. [Google Scholar] [CrossRef]
- Peres, A. Quantum measurements with postselection. Phys. Rev. Lett. 1989, 62, 2326. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; He, Y.; Shen, Z.; He, Q. Optical weak measurement system with common path implementation for label-free biomolecule sensing. Opt. Lett. 2016, 41, 5409–5412. [Google Scholar] [CrossRef] [PubMed]
- Knee, G.C.; Briggs, G.A.D.; Benjamin, S.C.; Gauger, E.M. Quantum sensors based on weak-value amplification cannot overcome decoherence. Phys. Rev. A 2013, 87, 012115. [Google Scholar] [CrossRef]
- Chen, G.; Yin, P.; Zhang, W.-H.; Li, G.-C.; Li, C.-F.; Guo, G.-C. Beating Standard Quantum Limit with Weak Measurement. Entropy 2021, 23, 354. [Google Scholar] [CrossRef]
- Cong, S.; Tang, Y.; Harraz, S.; Li, K.; Yang, J. On-line quantum state estimation using continuous weak measurement and compressed sensing. Sci. China Inf. Sci. 2020, 64, 189202. [Google Scholar] [CrossRef]
- Strübi, G.; Bruder, C. Measuring Ultrasmall Time Delays of Light by Joint Weak Measurements. Phys. Rev. Lett. 2013, 110, 083605. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, W.-H.; Xu, L.; Liu, Z.-G.; Zhuang, W.-F.; Chen, L.; Gong, M.; Ma, Y.; Peng, X.-X.; Li, G.-C.; et al. Improving the precision of optical metrology by detecting fewer photons with biased weak measurement. Light Sci. Appl. 2021, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, L.; Guan, T.; Guo, C.; Zhong, S.; Zhou, C.; Zhang, H.; Li, Z.; Xing, X.; Ji, Y.; et al. Specific detection of glucose by an optical weak measurement sensor. Biomed. Opt. Express 2021, 12, 5128–5138. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Liang, G.; Meng, L.; Xu, Y.; Li, H.; Song, Z.; Zhang, X.; Li, Z.; Guo, C.; et al. Realization of high-performance biosensor through sandwich analysis utilizing weak value amplification. Talanta 2024, 277, 126302. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, C.; Li, D.; Guo, C.; Li, Z.; Xing, X.; Li, S.; Guan, T.; Liu, L.; He, Y. A stabilized weak measurement sensor for aptamer detection. Sens. Actuators B Chem. 2022, 371, 132509. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Jiang, Y.; Liu, Y.; Wang, A.; Ye, L.; Wu, J.; Ge, R.; Gao, F.; Zhang, Z. Fast-response and stable weak measurement system for protein–antibody specific detection. Sens. Actuators B Chem. 2025, 426, 136828. [Google Scholar] [CrossRef]
- Li, Y.-L.; Sun, F.; Yang, J.; Xiao, X. Enhancing the teleportation of quantum Fisher information by weak measurement and environment-assisted measurement. Quantum Inf. Process. 2021, 20, 55. [Google Scholar] [CrossRef]
- Hu, Q.; Yusufu, T.; Turek, Y. Quantum state engineering using weak measurements. Phys. Rev. A 2022, 105, 022608. [Google Scholar] [CrossRef]
- Song, B.; Chen, B.; Yu, C.; Yang, H. Ultrasensitive Protein Concentration Characterization Based on Weak Measurements. Photonic Sens. 2022, 12, 220301. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, L.; Liang, G.; Wang, B.; Xu, Y.; Li, H.; Song, Z.; Yan, H.; Guo, C.; Guan, T.; et al. Highly sensitive antibiotic sensing based on optical weak value amplification: A case study of chloramphenicol. Food Chem. 2024, 458, 140184. [Google Scholar] [CrossRef]
- Li, D.; Weng, C.; Li, K.; Lin, Q.; Zhang, J.; Wang, S.; Ruan, Y.; Yao, J. Quantification of HER2 in COS7 cells using quantum weak measurement. Opt. Express 2021, 29, 30337–30347. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, H.; Xu, Y.; Meng, L.; Liang, G.; Wang, B.; Liu, L.; Guan, T.; Guo, C.; et al. Weak Value Amplification Based Optical Sensor for High Throughput Real-Time Immunoassay of SARS-CoV-2 Spike Protein. Biosensors 2024, 14, 332. [Google Scholar] [CrossRef]
- Hu, C.; Qin, Z.; Fu, J.; Gao, Q.; Chen, C.; Tan, C.S.; Li, S. Aptamer-based carbohydrate antigen 125 sensor with molybdenum disulfide functional hybrid materials. Anal. Biochem. 2023, 675, 115213. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A biosensor for determination of the circulating biomarker CA125/MUC16 by Surface Plasmon Resonance Imaging. Talanta 2020, 206, 120187. [Google Scholar] [CrossRef] [PubMed]
- Bahari, D.; Babamiri, B.; Salimi, A. Ultrasensitive molecularly imprinted fluorescence sensor for simultaneous determination of CA125 and CA15–3 in human serum and OVCAR-3 and MCF-7 cells lines using Cd and Ni nanoclusters as new emitters. Anal. Bioanal. Chem. 2021, 413, 4049–4061. [Google Scholar] [CrossRef]
- Sadasivam, M.; Sakthivel, A.; Nagesh, N.; Hansda, S.; Veerapandian, M.; Alwarappan, S.; Manickam, P. Magnetic bead-amplified voltammetric detection for carbohydrate antigen 125 with enzyme labels using aptamer-antigen-antibody sandwiched assay. Sens. Actuators B Chem. 2020, 312, 127985. [Google Scholar] [CrossRef]
- Hamd-Ghadareh, S.; Salimi, A.; Fathi, F.; Bahrami, S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelectron. 2017, 96, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Gedi, V.; Song, C.K.; Kim, G.B.; Lee, J.O.; Oh, E.; Shin, B.S.; Jung, M.; Shim, J.; Lee, H.; Kim, Y.-P. Sensitive on-chip detection of cancer antigen 125 using a DNA aptamer/carbon nanotube network platform. Sens. Actuators B Chem. 2018, 256, 89–97. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ponche, A.; Ringwald, C.; Hemmerlé, J.; Raya, J.; Bechinger, B.; Voegel, J.-C.; Schaaf, P.; Ball, V. Characterization of Dopamine−Melanin Growth on Silicon Oxide. J. Phys. Chem. C 2009, 113, 8234–8242. [Google Scholar] [CrossRef]
- Tripathi, P.; Kumar, A.; Sachan, M.; Gupta, S.; Nara, S. Aptamer-gold nanozyme based competitive lateral flow assay for rapid detection of CA125 in human serum. Biosens. Bioelectron. 2020, 165, 112368. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Nunna, B.B.; Zhuang, S.; Rakshit, S.; Lee, E.S. Carbon nanotubes based biosensor for detection of cancer antigens (CA-125) under shear flow condition. Nano-Struct. Nano-Objects 2018, 15, 180–185. [Google Scholar] [CrossRef]
- Baradoke, A.; Jose, B.; Pauliukaite, R.; Forster, R.J. Properties of Anti-CA125 antibody layers on screen-printed carbon electrodes modified by gold and platinum nanostructures. Electrochim. Acta 2019, 306, 299–306. [Google Scholar] [CrossRef]


| Detection Method | Receptor | Measurement Range | Resolution | Ref. |
|---|---|---|---|---|
| Surface plasmon resonance | Aptamer | 10–100 U/mL | 0.01 U/mL | [17] |
| Electrochemical | Aptamer | 0.0001–500 U/mL | 0.1 mU/mL | [43] |
| Surface plasmon Resonance imaging | Antibody | 2.2–150 U/mL | 0.66 U/mL | [44] |
| Fluorescent and magnetic MIP | Antibody | 0.0005–40 U/mL | 50 μU/mL | [45] |
| Electrochemical affinity sensor | Aptamer, antibody | 2–100 U/mL | 0.08 U/mL | [46] |
| Fluorescence resonance Energy transfer | Aptamer, antibody | 1.0 μU/mL–1.0 U/mL | 0.5 μU/mL | [47] |
| 3D carbon nanotube network biochip | Aptamer, antibody | 10 mU/mL–1000 U/mL | 10 mU/mL | [48] |
| WVA-based sandwich method | Aptamer, antibody | 0.01 mU/mL–100 U/mL | 3.98 μU/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Xu, Y.; Li, H.; Song, Z.; Guan, T.; He, Y. Synergistic Signal Amplification via Weak Value Amplification Effect and Sandwich Structure for Highly Sensitive and Specific Real-Time Detection of CA125. Biosensors 2025, 15, 268. https://doi.org/10.3390/bios15050268
Wang B, Xu Y, Li H, Song Z, Guan T, He Y. Synergistic Signal Amplification via Weak Value Amplification Effect and Sandwich Structure for Highly Sensitive and Specific Real-Time Detection of CA125. Biosensors. 2025; 15(5):268. https://doi.org/10.3390/bios15050268
Chicago/Turabian StyleWang, Bei, Yang Xu, Han Li, Zishuo Song, Tian Guan, and Yonghong He. 2025. "Synergistic Signal Amplification via Weak Value Amplification Effect and Sandwich Structure for Highly Sensitive and Specific Real-Time Detection of CA125" Biosensors 15, no. 5: 268. https://doi.org/10.3390/bios15050268
APA StyleWang, B., Xu, Y., Li, H., Song, Z., Guan, T., & He, Y. (2025). Synergistic Signal Amplification via Weak Value Amplification Effect and Sandwich Structure for Highly Sensitive and Specific Real-Time Detection of CA125. Biosensors, 15(5), 268. https://doi.org/10.3390/bios15050268





