Abstract
Innovative biosensor technologies are revolutionizing cancer detection by offering non-invasive, sensitive, and rapid diagnostic tools, addressing the limitations of conventional screening. Non-invasive samples like breath, saliva, urine, and sweat, analyzed using advanced technologies like electronic nose systems and AI, show promise for early detection and frequent monitoring, though validation is needed. AI integration enhances data analysis and personalization. While blood-based methods remain the gold standard, combining them with less invasive sample types like saliva or sweat, and using sensitive techniques, is a promising direction. Conventional methods (mammography, MRI, etc.) offer proven efficacy, but are costly and invasive. Innovative methods using biosensors offer reduced infrastructure needs, lower costs, and patient-friendly sampling. However, challenges remain in validation, standardization, and low biomarker concentrations. Integrating both methodologies could create a comprehensive framework, combining reliability with accessibility. Future research should focus on robust biosensor development, standardization, expanding application to other cancers, exploring less-studied samples like sweat, and improving affordability for wider adoption, especially in resource-limited settings. The future lies in integrating diverse approaches for more sensitive, specific, and patient-friendly screening, improving early detection and outcomes.
1. Introduction
This introduction is divided into two sections: current epidemiological perspectives and state-of-the-art diagnostic methods.
1.1. Epidiemology and Current Challenges
Breast cancer is the most frequently diagnosed cancer among women globally and a leading cause of cancer-related mortality. In 2022, approximately 2.3 million new cases (Figure 1) and 685,000 deaths were reported worldwide, accounting for 23.8% of all cancer cases and 15.4% of cancer deaths among women; moreover, an update from 2024 on the IARC site discusses the evidence suggesting that female breast cancer has surpassed lung cancer as the most diagnosed cancer worldwide. This is the reason why the early and timely diagnosis of breast cancer, along with comprehensive cancer treatment, is essential in many countries experiencing social and economic transition, where late-stage presentation remains widespread [1].
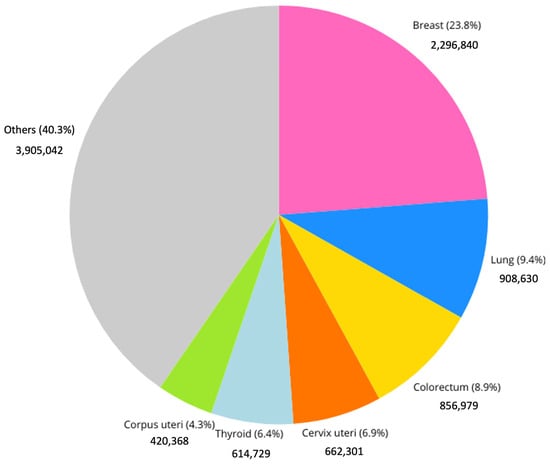
Figure 1.
Incidence of different cancer types among females in 2022. “IARC, 2022” [1].
In Europe, around one in eight women will develop breast cancer during their lifetime, with a mortality rate of approximately 15% [2].
The incidence and mortality of breast cancer vary significantly across different regions, influenced by factors such as genetic predisposition, lifestyle, healthcare infrastructure, and public health policies.
In particular, as foreseen in the report of 2022 [2] and confirmed by the recent update in February 2025 [3], to effectively address disparities and track progress toward cancer control goals, countries with a low or medium Human Development Index (HDI) require improved cancer treatment and vital statistics, along with advancements in early detection and treatment access. Although 29 countries with a very high HDI have seen reductions in mortality rates, and 7 are already achieving the Global Breast Cancer Initiative target of an annual 2.5% decrease, the global burden remains uneven. By 2050, new breast cancer cases are projected to rise by 38% and related deaths by 68%, with the most significant impact present in low-HDI nations [3,4].
The incidence and mortality rates of breast cancer are closely linked to age and stage at diagnosis (Figure 2). The risk of developing breast cancer increases with age, particularly after 50 years. The highest incidence rates are observed in the 70–79 age group. Although younger women (under 40 years of age) constitute a smaller proportion of breast cancer cases, they often present with more aggressive cancer subtypes [5]. The stage at which breast cancer is diagnosed significantly influences patient prognosis. Early-stage cancers (Stages I and II) generally have higher survival rates, while advanced stages (Stages III and IV) require more intensive treatments and have lower survival rates [6].
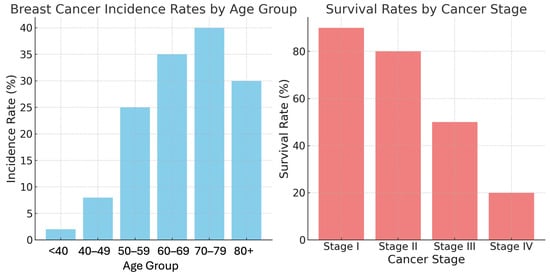
Figure 2.
Breast cancer incidence rates by age group and survival rates by cancer stage [1].
1.2. Conventional Methods for Breast Cancer Detection
Mammography, ultrasound, MRI, and PET scans are the current standard for breast cancer detection [7]. While crucial for early diagnosis, these methods suffer from significant drawbacks [8], including high costs, limited availability, and inconsistent accuracy, especially in younger women with dense breasts. Furthermore, the invasive nature of some procedures can discourage regular screening, hindering early detection and potentially impacting patient outcomes.
Mammography, the standard for breast cancer screening, is especially effective for women over 50. Using low-dose X-rays, it produces detailed breast tissue images and has proven to decrease breast cancer mortality. However, its effectiveness is reduced in women with dense breasts. Additionally, it involves low-level radiation exposure and carries a risk of false positives, leading to unnecessary biopsies [9].
Magnetic Resonance Imaging (MRI) uses magnetic fields and radio waves to create detailed images of breast tissue. It is especially useful for high-risk individuals, and can detect cancers in dense breasts [10], thus resolving some of the issues raised by mammography. However, MRI is more expensive, less accessible, and prone to more false positives, resulting in additional tests and biopsies [11], as already evidenced for mammography.
Ultrasound uses high-frequency sound waves to image breast tissue, and is a valuable complement to mammography, particularly for women under 50 and those with dense breasts [12]. While it avoids radiation and is generally well tolerated, thus overcoming MRI’s drawbacks, its accuracy is dependent on the operator’s skill, and it produces more false positives than mammography [13].
Positron Emission Tomography (PET) scans detect gamma rays from radioactive tracers that collect in areas of high metabolic activity [14]. Combined with CT scans, PET provides both functional and anatomical information, improving the accuracy of cancer staging. However, PET suffers from lower spatial resolution, complex image interpretation, and the limited availability of certain tracers [15].
Table 1 summarizes the pros and cons of the methods described above, highlighting the challenges that emerging technologies should address.

Table 1.
Pros and cons of conventional methods for breast cancer detection.
2. Emerging Diagnostic Methods
Given that early detection is strongly associated with improved survival rates and treatment outcomes in breast cancer, the development and implementation of effective diagnostic methodologies for early-stage identification are of paramount importance [16].
Early breast cancer detection significantly improves treatment success and long-term survival. These early-stage cancers are often less aggressive and more responsive to treatment, minimizing the need for extensive surgery and less toxic therapies, ultimately enhancing both survival rates and quality of life [17].
An extensive literature review was conducted to explore the various innovative methodologies developed for early breast cancer detection. This review categorizes the studies based on differences in sample types, techniques, technologies, and research objectives. The findings suggest a wide range of potential approaches and applications in the field. For each sample type, different technologies and methods can be used. In the following two sections, the sample types will be briefly presented and the different methodologies summarized, together with the different research objectives faced by the current literature. Then, the rest of the review will be organized into a series of paragraphs, each devoted to a specific sample type, reporting all the methods/technologies used for its analysis.
Innovative breast cancer detection employs diverse sample types (see Figure 3 for the percentages of distribution). Blood-based samples are the most prevalent (48.1%) due to their accessibility and the wealth of systemic information they provide. These samples are frequently used in studies targeting specific biomarkers. In situ methods (18.5%), encompassing direct imaging and tissue sampling, offer detailed visualization of breast tissue alterations via techniques based on different sensors’ working principles, like piezoelectric analysis, ultrasound, tissue elasticity assessment, imaging, and thermal sensing. Exhaled breath analysis (7.4%) detects cancer-related metabolic shifts by analyzing volatile organic compounds (VOCs). Though less explored, non-invasive samples such as saliva (11.1%), urine (3.7%), and sweat (3.7%) hold promise and offer potential advantages for various diagnostic applications.
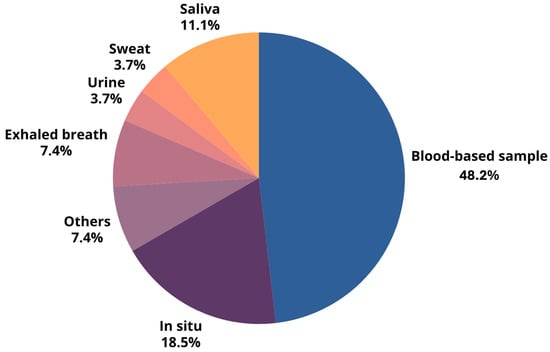
Figure 3.
Percentage distribution of different sample types used in breast cancer detection, highlighting the predominance of blood-based samples and in situ methods.
A variety of analytical techniques have been used across different sample types, with some methods suitable for multiple sample types. The chosen method depends on the specific research goals and the unique characteristics of each sample. This diverse approach is illustrated in Figure 4, which shows the range of analytical techniques applied to the various samples.
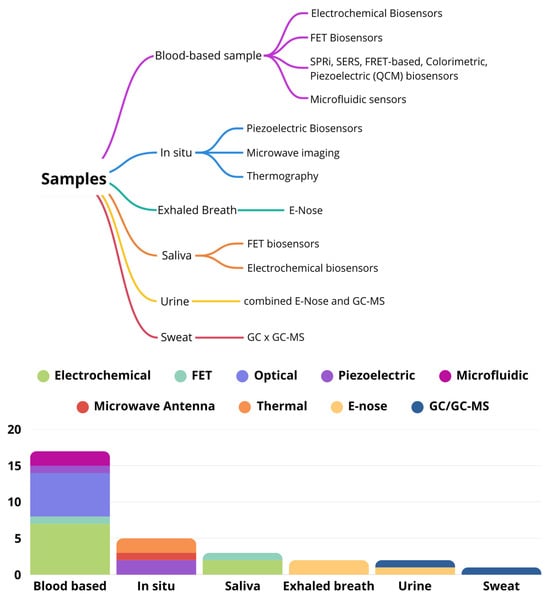
Figure 4.
Different analytical techniques applied to different samples.
This literature review reveals several key research objectives driving the development of these innovative technologies. One major focus is identifying specific biological markers indicative of breast cancer, such as genetic biomarkers (genes and mRNA), protein biomarkers, cell lines, and cancer stem cell-associated biomarkers. Another objective is creating predictive models that analyze patterns and diagnose breast cancer based on diverse data inputs. These models can process large datasets, uncovering subtle patterns that improve early detection and diagnostic accuracy. Finally, some methods aim to detect and monitor structural and physiological changes in breast tissue. Techniques like piezoelectric sensors and microwave imaging offer non-invasive ways to identify abnormal tissue properties.
3. Analysis of Blood, Serum, and Plasma
Blood tests are a common tool in cancer research because they can detect a wide range of biomarkers. These biomarkers include genetic markers (like BRCA1 and miRNA-155), protein markers (such as HER2, MUC1, and CEA), and cell surface markers (like CD44 and ERα). Scientists use various techniques like electrochemical biosensors, optical and acoustic sensors, and microfluidic devices to study these biomarkers. These methods help to detect and measure the amount of biomarkers with different levels of accuracy.
3.1. Electrochemical Biosensors
Electrochemical biosensors stand out for their ability to accurately and sensitively detect biomarkers in blood. These devices utilize various signal transduction techniques, including impedimetric, voltammetric, and field-effect transistor (FET) methods, each offering unique advantages for biomarker detection.
Shahrokhian et al. created a highly sensitive electrochemical DNA biosensor for BRCA1 detection, as detailed in their study [18]. The biosensor utilizes electrochemically reduced graphene oxide and conducting polymers, and employs both voltammetric and impedimetric detection methods. This approach yielded a remarkable detection limit of 3 fM, crucial for identifying low-concentration genetic markers. While this design offers exceptional sensitivity, it also presents challenges, including complex electrode fabrication and the potential for non-specific binding. More recently, Mohammadpour-Haratbar et al. reported a series of electrochemical biosensors in which the use of graphene derivates (graphene oxide, reduced graphene oxide, and graphene quantum dots) increased the diagnostic performances in terms of LOD, lowering the concentration of detectable biomarkers and related compounds to challenging levels [19].
Voltammetric biosensors offer quantitative analysis by measuring the current changes resulting from redox reactions. For example, Hakimian et al. used cyclic voltammetry (CV) and a thiolated DNA probe-modified gold electrodes to detect miRNA-155. This method achieved a high sensitivity, with a detection range of 2.0 × 10−20 to 2.0 × 10−12 M. However, the stability of the DNA probes, which last about 1.5 months when stored at 4 °C, can be a limiting factor for long-term use [20]. In another study, Hu et al. developed a highly sensitive MUC1 aptasensor using differential pulse voltammetry (DPV). This aptasensor incorporated gold nanoparticles and horseradish peroxidase for signal amplification, achieving a low detection limit and a wide linear range. Testing in human serum samples demonstrated high sensitivity and specificity, with recoveries between 101.2% and 108.9%. Despite these promising results, aptamer synthesis and nanoparticle stability pose ongoing challenges [21]. Zhao et al. developed a novel folding-based electrochemical aptasensor for vascular endothelial growth factor (VEGF) detection using alternating current voltammetry (ACV). This sensor achieved a 5 pM (190 pg mL−1) detection limit in complex biological samples, even whole blood. Its high sensitivity, ease of preparation, and ability to operate in complex media make it a promising candidate for clinical use. However, aptamer degradation in serum could affect its long-term reliability [22]. For HER2 detection, Marques et al. used linear sweep voltammetry (LSV) with a gold nanoparticle-modified electrode in a sandwich assay. This method achieved a 4.4 ng/mL detection limit. While sensitive, it suffers from potential enzyme instability and a long assay time of approximately 2 h and 50 min [23]. Zhu et al. also focused on HER2 detection, creating a square wave voltammetry (SWV) biosensor with a nanocomposite-modified gold nanoparticle electrode. Their sensor achieved a significantly lower detection limit of 0.037 ± 0.002 pg/mL, though the complex surface modification process presents a challenge [24]. Ribeiro et al. developed a novel electrochemical biosensor for detecting CA 15-3, employing poly(Toluidine Blue) as a molecularly imprinted polymer (MIP) receptor. This sensor achieved a detection limit below 0.10 U mL−1 in diluted artificial serum and demonstrated a linear response across a broad concentration range (0.10 to 100 U mL−1). This work highlights the potential of combining MIPs with electrochemical detection for improved cancer biomarker detection, especially for point-of-care diagnostics [25]. Field-effect transistor (FET) biosensors hold great promise for cancer biomarker detection. Majd et al. designed an FET biosensor for miRNA-155 detection using MoS₂ as the conductive channel. Their design achieved an exceptionally low detection limit of 0.03 fM and exhibited high specificity, successfully differentiating between perfectly matched and single-base mismatched miRNA-155 sequences. While the biosensor showed good reproducibility, the stability of both the MoS₂ and the DNA probes requires further improvement for robust and reliable clinical application [26]. A recent comprehensive review [27,28] summarized the evidence and advancements in electrochemical biosensors that utilize advanced nanotechnologies for detecting breast cancer genes. These technologies include gold nanoparticle-reduced graphene oxide (AuNPs-GO), carbon nanotube-modified glassy carbon electrodes (CNT/GCE), zinc oxide nanowires (ZnONWs), carbon nanotube-modified screen-printed electrodes (SPEs), and reduced graphene oxide–yttrium nanocomposites (Y2O3–rGO/Apt/BSA). Specifically, regarding the detection of the HER2 protein, a more specialized review [29] highlighted recent advancements in the field. This review focused on the importance of detecting cancer early—whether at its onset, during recurrence, or while monitoring therapeutic interventions.
A detailed analysis of HER2 protein detection in serum was presented in another study, which examined two sensors utilizing gold sensor chips combined with amperometric detection of the enzyme label horseradish peroxidase (HRP). These biosensors/immunosensors were based on indirect sandwich enzyme-linked immunosorbent assays (ELISAs), where monoclonal antibodies (Abs) against HER-1 and HER-2 were attached to the sensors to capture the biomarkers [30]. Their performance was comparable to current standard techniques, although the overall process duration could and should be reduced.
To conclude the discussion on electrochemical biosensors, we find it useful to list their limitations and the challenges associated with them: complex fabrication; sensitivity to environmental factors; potential for non-specific binding; limited multiplexing capabilities; calibration and standardization issues; dynamic range limitations; regulatory hurdles; and stability issues.
3.2. Optical and Acoustic Biosensors
Optical and acoustic biosensors exploit changes in optical properties or acoustic waves upon target binding. These methods include Surface Plasmon Resonance Imaging (SPRi), Surface-Enhanced Raman Scattering (SERS), fluorescence, and colorimetric and piezoelectric sensors. Surface Plasmon Resonance (SPR) sensors are highly sensitive, label-free detectors that measure changes in the refractive index, making them ideal for identifying biomarkers in complex samples like blood. SPR’s adaptability allows for the tailoring of the sensor for specific biomarkers and detection needs by modifying the surface chemistry, functionalization, and detection environment. This versatility makes SPR effective for detecting a wide range of cancer biomarkers, as demonstrated by the studies presented below.
Szymańska et al. proposed that CEA could be a valuable biomarker for breast cancer detection, although their study was not specifically focused on this application. They developed an SPRi sensor utilizing cysteamine-modified gold chips for CEA detection, achieving a highly sensitive linear calibration curve with a detection limit of 0.1 ng/mL. However, the careful control of non-specific binding was required to ensure accuracy [31]. Erol et al. demonstrated the potential of molecularly imprinted polymers in SPR-based biosensors by developing a nanoMIP–SPR sensor for HER2 detection in human serum. Their approach exhibited a high affinity with and selectivity for HER2, while enabling rapid and cost-effective analysis without requiring signal amplification. This innovative design advanced SPR technology, achieving a detection limit of 11.6 pg/mL [32]. Verma et al. enhanced SPR technology by integrating a supervised machine learning approach (MLP regressor) to optimize sensor performance. They developed a gold/TiO₂-coated photonic crystal fiber SPR sensor, which exhibited exceptional sensitivity for MCF-7 breast cancer cells, achieving a maximum wavelength sensitivity of 11,034 nm/RIU. The addition of the gold/TiO₂ layer significantly improved the sensor’s detection capabilities, demonstrating the effectiveness of this novel approach [33]. Han et al. developed a SERS-based sensor for miR-K12-5-5p detection using Au/Ag hybrid porous GaN substrates, highlighting its potential for clinical diagnostics. Their approach demonstrated high sensitivity, with a detection limit of 884 pM, while the substrates exhibited promising uniformity and reproducibility. However, they emphasized the need for further improvements in stability to enhance long-term reliability [34]. Wang’s FRET-based aptasensor demonstrated high sensitivity for CEA detection, with limits of 7.9 pg/mL in aqueous solution and 10.7 pg/mL in human serum. However, the inherent complexities of the FRET system pose challenges for practical implementation [35]. Bai et al. developed a highly sensitive colorimetric sensor for BRCA1 detection, achieving an impressive detection limit of 10−1⁸ M. Their multiple-signal amplification strategy enhanced sensitivity, yet obtaining sufficient direct signals at low concentrations remains a challenge, indicating the need for further optimization [36]. Yang et al. developed a quartz crystal microbalance (QCM) biosensor targeting CD44 to assess the metastatic potential of breast cancer cells. The biosensor effectively distinguished between cells with different CD44 expression levels, achieving a detection limit of 300 cells mL−1 for MDA-MB-231 cells (high CD44 expression) and 1000 cells mL−1 for MCF-7 cells (lower CD44 expression). This differential sensitivity highlights its potential for evaluating metastatic potential. However, further optimization is necessary to improve its specificity and clinical applicability, as stability and reproducibility remain critical factors for its practical use [37]. The detection of CD44 expressing cancer cells was also recently performed using a fiber-optic ball resonator sensor via the measurement of the reflected light. The limit of detection obtained was 335 cells/mL [38]. The next challenge of this technique will be its application in vitro.
To conclude the discussion on optical and acoustic biosensors, we find it useful to list their limitations and the challenges associated with them: sensitivity to environmental factors; non-specific interactions; multiplexing limitations; stability concerns; sample interference; dynamic range limitations; and regulatory hurdles.
3.3. Microfluidic Devices
Microfluidic technologies offer a compact and efficient platform for biomarker detection by integrating multiple processes—sampling, dilution, reaction, separation, and detection—into a single chip.
Uliana et al. developed a fully disposable microfluidic electrochemical device (μFED) for ERα detection in calf serum, designed from low-cost materials for easy manufacturing. This innovative system achieved a remarkable detection limit of 10.0 fg mL−1, with high reproducibility, making it a promising candidate for point-of-care applications. In addition to improving reaction kinetics and minimizing sample and reagent consumption, the μFED offers significant advantages. However, the researchers noted that fabrication complexities could present challenges for large-scale adoption [39]. Gao et al. introduced a high-throughput microfluidic platform for the simultaneous detection of multiple miRNA biomarkers associated with breast cancer. Their system utilizes a self-assembled Poly-L-Lysine (PLL) substrate combined with microfluidic chips, enabling efficient multi-target analysis. By employing a three-segment hybridization approach, the platform allows for concurrent miRNA detection across various samples, achieving a detection limit of 1 pM with an impressive 30 min turnaround time [40]. Gao et al.’s microfluidic chip presents a more versatile and cost-effective alternative to single-target devices, like Uliana et al.’s ERα detector. By leveraging the charge polarity interactions of PLL for DNA probe immobilization, this design improves practicality for point-of-care applications. Its adaptability suggests a broader potential for on-site cancer diagnostics, making it a promising tool in the field.
To conclude the discussion on optical and acoustic biosensors, we find it useful to list their limitations and the challenges associated with them: fabrication complexity; sensitivity to environmental factors; and potential for the clogging of the channels.
3.4. Pros and Cons of Blood, Serum, and Plasma Samples
To conclude the illustration of the methods used for the analysis of blood, serum and plasma, general pros and cons are listed.
- Pros: High Biomarker Abundance: Blood, serum, and plasma contain rich sources of biomarkers essential for diagnosing and monitoring disease progression [22,34]. Early Detection: These samples allow for the detection of biomarkers at early stages, crucial for timely intervention and improved survival rates [20]. Variety of Detection Methods: Techniques such as electrochemical biosensors, SPR sensors, and microfluidic devices can be employed, offering flexibility and sensitivity in biomarker detection [19,32]. Stability of Samples: Serum and plasma can be stored for extended periods, maintaining the stability of many biomarkers, which is advantageous for longitudinal studies [34].
- Cons: Invasiveness: The collection of these samples can be uncomfortable for patients [33]. Handling and Storage: Proper handling and storage are required to prevent the degradation of the samples, which can affect the accuracy of the test results [34]. Potential for False Positives: There is a risk of cross-reactivity with non-target molecules, which can lead to false-positive results and unnecessary follow-up procedures [31]. Complex Fabrication and Maintenance: Advanced sensor technologies often require intricate preparation and maintenance, which can be resource-intensive and challenging to implement consistently across different settings [19,38]. Variability in Biomarker Levels: Biomarker levels can vary significantly between individuals and due to external factors, complicating result interpretation [38]. Waste Management: The disposal of biological waste must adhere to strict regulations, adding to operational complexity and costs [38]. Cost and Resource-intensive Nature: The collection and preparation of samples require resources and personnel, increasing operational costs, especially in mass screening contexts [34].
4. Analysis In Situ
In situ analyses are performed directly on tissues and include methods such as piezoelectric sensors, microwave imaging, and thermography.
4.1. Piezoelectric Sensors and Continuous Ultrasound Breast Monitor (cUSBr)
Piezoelectric sensors work by generating an electric charge in response to mechanical stress. When used for breast tissue analysis, they detect structural changes by translating pressure variations into electrical signals. This mechanism is extensively utilized in medical diagnostics and imaging to identify tissue abnormalities, facilitating the early detection and evaluation of potential health concerns.
The cUSBr, developed by Wenya Du et al. [41], enhances real-time breast tissue monitoring with its advanced imaging capabilities. This technology features a conformable ultrasound patch that employs a one-dimensional phased array based on piezoelectric principles, ensuring standardized and reproducible imaging. It offers a contrast sensitivity of approximately 3 dB, with axial and lateral resolutions of 0.25 mm and 1.0 mm at a depth of 30 mm, enabling the detection of cysts as small as 0.3 cm. Additionally, the cUSBr achieves a maximum imaging depth of 80 mm and provides a broader field of view than conventional handheld probes. Xu et al. developed a high-performance piezoelectric sensor array designed for breast cancer detection, achieving an accuracy rate of 88% [42]. Their research focused on assessing how factors such as DC voltage duration, application depth, and breast density influence detection sensitivity. By applying a direct-current (DC) voltage, the sensor detects tissue deformation, enabling the evaluation of tissue stiffness—an important indicator of potential tumors.
To conclude the discussion on piezoelectric sensors and cUSBr, we find it useful to list their limitations and the challenges associated with them: operator dependency, potential skin contact issues, long-term stability of materials, and time interval issues dependent on scanning operations.
4.2. Microvawe Imaging
By detecting differences in dielectric properties, microwave imaging can distinguish between normal and cancerous tissues. This cutting-edge technology offers a promising alternative for detecting breast cancer.
Elsheakh et al. investigated the use of machine learning techniques to enhance the performance of a microwave imaging system for breast cancer detection [43]. Support vector machines (SVM) and logistic regression classifiers were trained on datasets derived from microwave signals of 3D tumor models, enabling the effective classification of malignant tumors. Their study also introduced a smart bra embedded with microwave textile-based antenna sensors, achieving an impressive detection sensitivity of 85%. By integrating wearable technology with advanced imaging, this approach has the potential to facilitate more frequent and comfortable monitoring.
To conclude the discussion on microwave imaging, we find it useful to list its limitations and the challenges associated with it: this technology relies on advanced equipment, which may limit its availability in less-equipped clinical settings; the accuracy of detection is influenced by tumor size, with smaller tumors being more challenging to detect; additionally, patient compliance is crucial, otherwise, the effectiveness of monitoring can be compromised.
4.3. Thermography
Thermography detects cancerous tissues by measuring the higher temperatures they exhibit compared to normal tissues due to increased metabolic activity.
Elouerghi et al. designed a flexible wearable thermography system for early breast cancer detection, utilizing a network of bioheat microsensors [44]. The system features 28 miniaturized sensors arranged on a flexible star-shaped surface to cover the most sensitive breast areas. To validate the concept, the researchers conducted computer simulations and experimental tests on a breast phantom. The system successfully detected simulated tumors at different depths, recording temperature variations of up to 0.6 °C for tumors at 15 mm, 0.5 °C at 20 mm, and 0.45 °C at 30 mm. While the study does not report specific sensitivity and specificity values for this system, the authors reference previous studies indicating that breast thermography can achieve sensitivity and specificity rates of 95%. Future clinical evaluations are planned to assess the system’s actual diagnostic performance. Sree et al. investigated the Cyrcadia Breast Monitor (CBM), a thermal sensing device designed for breast cancer detection [45]. By continuously monitoring circadian temperature variations in breast tissue, the CBM provides significant advantages in identifying malignant tissues. Its non-invasive nature and ability to collect data over time make it a valuable tool for early detection. The recorded thermal data are analyzed using machine learning models, improving the accuracy of distinguishing between benign and malignant lesions. The study highlights the growing role of thermography in breast cancer detection, emphasizing the CBM’s potential in enhancing diagnostic capabilities.
To conclude the discussion on thermography, we find it useful to list its limitations and the challenges associated with it: the need for the robust calibration for reliable results; influence of environmental confounding factors; individual variability; limited data on tissue structure; and clinical acceptance and patient compliance.
4.4. Pros and Cons of In Situ Sample Analyses
To conclude the illustration of the methods used for the in situ analysis, general pros and cons are listed.
- Pros:
- Real-time Monitoring: Technologies such as thermography systems allow for continuous monitoring, which is useful for the early detection of breast cancer [46]. Potential for Clinical Use: These systems can be integrated into clinical settings, enhancing diagnostic capabilities and the timeliness of interventions [42]. Detection of Anomalies: Technologies like ultrasound can provide significant imaging, facilitating the identification of tissue abnormalities [36]. Non-invasive Approach: The proposed technologies are designed to be non-invasive, reducing patient discomfort and improving the acceptance of procedures [44].
- Cons:
- Stringent Measurement Conditions: Patients are required to comply with specific conditions, which may restrict the practical applicability of these technologies [42]. Mechanical Challenges: Geometric variability and the deformability of breast tissue can complicate the accuracy of analyses and measurements [44]. Technical Limitations: these two technologies have technical gaps, such as dependence on technician experience and issues with skin contact [44]. Sensor Limitations: The sensors used may have limited bandwidths, affecting the effectiveness of analyses and the capability for self-screening [45].
5. Exhaled Breath
Analyzing exhaled breath is becoming a promising non-invasive approach for detecting volatile organic compounds (VOCs) linked to breast cancer. By collecting and examining breath samples, this method can identify patterns that may indicate the presence of cancer.
5.1. Electronic Nose Technology
Breath analysis using electronic nose (E-Nose) technology has shown great potential in cancer detection. Yang et al. conducted a study utilizing an E-Nose with 32 carbon nanotube sensors to analyze the exhaled breath of 899 individuals, including 351 breast cancer patients [46]. Predictive classification models were developed using machine learning algorithms, specifically Random Forest, which achieved an impressive 91% detection accuracy, with 86% sensitivity and 97% specificity. These results highlight the promise of E-Nose technology for early breast cancer detection. However, certain limitations must be considered, such as the potential influence of anesthetics on VOC composition in intraoperative settings and the exclusion of smokers, which may affect sample diversity. Further research is needed to validate breath-based tests for breast cancer diagnosis in more diverse populations. Díaz de León-Martínez et al. (2020) conducted a study using an E-Nose for exhaled breath analysis, achieving an impressive 98% classification accuracy [47]. Despite these promising results, the study faced limitations, including a small sample size and potential environmental factors that could influence breath composition. Ref. [46] in the discussion and ref. [47] in the introduction propose two different lists of possible VOC biomarkers. This apparent contradiction accounts for extreme difficulties in identifying specific biomarkers, which opens the way for a VOC profile alteration study as an alternative strategy proposed by 46 and 47, which is based on exhaled breath fingerprinting via E-Nose-like instruments.
To conclude the discussion on E-Nose, we find it useful to list its limitations and the challenges associated with it: the environmental sensitivity of sensors, variability in breath composition due to personal factors, and the need for validation in larger, diverse populations
5.2. Pros and Cons of VOC Analysis in Breath Samples
- Pros:
- Non-Invasive: Breath analysis is a comfortable, unobtrusive method [48]; this characteristic makes it particularly suitable for frequent screening and monitoring. Rapid Results: E-Nose technology allows for quick analysis, providing timely diagnostic information [46].
- Cons:
- Environmental Sensitivity: Accuracy can be affected by environmental factors like temperature and humidity [46]. Standardization Challenges: Variability in breath composition due to personal factors complicates standardization [46]. Factors such as diet, medication, and smoking habits can influence VOC profiles, requiring robust normalization methods. Need for Validation: Further validation in larger, diverse populations is necessary to confirm the results [46,47].
6. Saliva
Saliva offers a non-invasive and convenient method for cancer detection, as its biomarkers can be correlated with those found in blood. Its easy collection process makes saliva an ideal option for frequent monitoring.
6.1. Field-Effect Transistor (FET) Biosensors
Field-effect transistor (FET) biosensors have emerged as a promising technology for detecting cancer biomarkers in saliva. Wan et al. [48] developed a highly sensitive saliva-based biosensor capable of detecting HER2 and CA15-3, with an impressive detection limit of 1 fg/mL. This innovative device integrates FET technology with disposable test strips, similar to those used for glucose monitoring, which were functionalized to selectively target these breast cancer biomarkers. By employing a synchronized double-pulse method, the biosensor achieved remarkable sensitivity levels of approximately 70/dec for HER2 and 30/dec for CA15-3. It also demonstrated a rapid testing time of under 15 ms and required only 3 μL of saliva, highlighting its potential for early breast cancer detection. Despite its operational simplicity and high sensitivity, challenges remain, such as ensuring specificity, standardizing sample collection, and assessing long-term stability. Further clinical validation with diverse patient cohorts is necessary to confirm its diagnostic accuracy and enhance its applicability in clinical settings. Wei et al. designed integrated electrochemical sensors capable of detecting both IL-8 mRNA and protein simultaneously, highlighting their potential for oral cancer diagnosis [49]. Similarly, Torrente-Rodríguez et al. developed an amperometric platform for IL-8 detection, demonstrating the feasibility of using saliva-based diagnostics for other cancers, including lung and pancreatic tumors [50].
A novel technique has demonstrated remarkable efficiency in detecting breast cancer biomarkers HER2 and CA15-3, utilizing commercially available disposable strips similar to common glucose detection strips [50]. Notably, this method achieves an exceptionally low detection limit of just 1 fg/mL, significantly surpassing the sensitivity of conventional enzyme-linked immunosorbent assays. Its advantages are further highlighted by a rapid testing time of under 15 ms and a minimal sample requirement of only 3 μL of saliva.
6.2. Pros and Cons of Saliva Analysis
- Pros:
- Non-Invasive: Saliva collection is simple, non-invasive, and painless, making it suitable for frequent monitoring and reducing patient discomfort [48]. Cost-Effective: The use of saliva-based tests can reduce the costs associated with more invasive procedures, making them more accessible for patients [49]. Potential for Multiple Biomarkers: Saliva can contain a variety of biomarkers, allowing for the simultaneous detection of multiple conditions [49,50]. Lower Interference: Saliva generally has lower levels of interfering substances compared to blood, which can simplify the analysis and improve the accuracy of the results.
- Cons:
- Lower Biomarker Concentration: Biomarker concentrations in saliva may be lower than those in blood, potentially requiring more sensitive detection methods to achieve reliable results [51]. Variability: Factors such as hydration, food intake, and oral health can influence saliva composition, leading to variability in the results and potentially affecting diagnostic accuracy [49]. Standardization: Standardized protocols for saliva collection and processing are essential to ensure reliable and reproducible results, which can be challenging to implement across different settings [50]. Limited Research for Some Biomarkers: While there is growing interest in saliva-based diagnostics, some biomarkers may not yet have established correlations with disease states, necessitating further research [48,49,50].
7. Urine
Urine contains various cancer-related metabolites and biomarkers, making it a simple and non-invasive diagnostic tool. However, its easy collection is offset by the challenge of detecting biomarkers present in low concentrations.
Electronic nose (E-Nose) technology has also demonstrated significant potential in urine analysis for cancer detection. Benet et al. developed an E-Nose system modeled on a dog’s olfactory capabilities, designed to detect volatile organic compounds (VOCs) in human urine. The study analyzed 90 urine samples from both breast cancer patients and control subjects, with the E-Nose prototype achieving a 75% classification rate, including 100% sensitivity, but only 50% specificity. To enhance accuracy, gas chromatography-mass spectrometry (GC-MS) data were used to train a convolutional neural network (CNN) algorithm, which improved the classification rate, taking it to 92.31%. The E-Nose utilized a sensor array to identify VOC patterns in urine, effectively distinguishing between cancerous and non-cancerous samples in a clinical setting [51].
Pros and Cons of Urine Analysis
- Pros:
- Non-Invasive: Urine collection is simple, non-invasive, and painless, making it suitable for frequent monitoring. Ease of Collection: Urine samples can be collected easily without the need for specialized medical personnel or equipment. Cost-Effective: The use of urine-based tests can reduce the costs related to more invasive procedures.
- Cons:
- Lower Biomarker Concentration: Biomarker concentrations in urine may be lower than those in blood, potentially requiring more sensitive detection methods. Variability: Factors such as diet, hydration, and lifestyle can influence urine composition and affect the consistency of results. Standardization Challenges: Standardized protocols for urine collection and processing are essential to ensure reliable and reproducible results.
8. Sweat
Sweat analysis offers a non-invasive method for real-time biomarker monitoring, enabling continuous tracking without the need for invasive procedures. Although it is less commonly used for cancer detection than breath or saliva analysis, recent studies have started investigating the presence of volatile organic compounds (VOCs) in sweat. This emerging approach has the potential to expand non-invasive cancer screening options.
The study conducted by Leemans et al. focused on identifying breast cancer-specific VOCs in the sweat of patients [52]. To achieve this, the researchers collected sweat samples from the breast and hand areas of 21 breast cancer patients before and after tumor ablation. These samples were then analyzed using thermal desorption coupled with two-dimensional gas chromatography and mass spectrometry (GC × GC − MS), which allowed for the analysis of a total of 761 VOCs.
The study successfully detected at least 77 VOCs, revealing significant differences between the pre- and post-surgery states of the patients. By utilizing machine learning models, particularly logistic regression, the researchers identified specific VOCs that could distinguish between these states, achieving high sensitivity rates. However, the study faced limitations, including a small sample size and variability in VOC profiles due to individual differences and external factors.
Pros and Cons of Sweat Analysis
- Pros:
- Non-Invasive Collection: Sweat sampling is a non-invasive method, making it more patient-friendly compared to blood or tissue samples. Cost-Effective: If validated, sweat analysis could serve as a low-cost alternative to traditional screening methods, particularly beneficial in resource-limited settings.
- Cons:
- Limited Sample Size: The small number of participants in the study may limit the applicability of the findings to the broader population. Variability in VOC Composition: Factors such as diet, hydration, and individual metabolism can influence sweat composition, leading to variability in VOC profiles. Standardization Challenges: There is a need for standardized protocols for sweat collection and analysis to ensure consistent and reliable results across different studies and populations.
9. Conclusions
Biosensor technologies are transforming cancer detection by providing rapid, sensitive, and non-invasive diagnostic tools. While these innovations enhance early detection and patient outcomes, they must also overcome the challenges of traditional screening methods. The use of diverse sample types—including blood, saliva, breath, urine, and sweat—offers flexibility in application, though each comes with its own advantages and limitations. As a summary to aid the discussion and conclusion, Table 2 presents a comparative overview of all the cited experiments, highlighting the key factors involved.

Table 2.
Comparative overview of recent studies (2009–2024) on various innovative methodologies for early detection of breast cancer in different biological sample types. Review papers cited in the text have not been included in this table.
The shift toward non-invasive cancer detection is evident, with breath, saliva, urine, and sweat analyses emerging as promising options for patient-friendly and frequent monitoring. Although blood-based methods remain the gold standard due to their high biomarker abundance, research is increasingly focused on integrating multiple approaches. Applying highly sensitive detection techniques from blood analysis to less invasive samples like saliva or sweat could enhance diagnostic accuracy while minimizing limitations.
Advanced technologies, such as electronic nose systems and machine learning algorithms, are further supporting early detection, though standardization and validation remain necessary. Additionally, the integration of artificial intelligence across various detection methods is improving data analysis and interpretation, paving the way for more precise and personalized diagnostics. This trend is expected to continue and strengthen the overall effectiveness of breast cancer screening.
Traditional imaging techniques such as mammography, MRI, ultrasound, and PET remain the gold standard for breast cancer detection, providing direct tissue visualization and clinically validated accuracy. Their widespread adoption is supported by established protocols and integration within healthcare systems. However, these methods require substantial infrastructure, specialized facilities, and trained personnel, leading to high costs and limited accessibility. Additionally, patients often face challenges such as procedure invasiveness, radiation exposure, and long wait times for appointments and results.
In contrast, innovative approaches leverage non-invasive sampling—such as breath, saliva, sweat, and urine—alongside advanced biosensor technologies. These methods offer key benefits, including lower infrastructure requirements, reduced operational costs, rapid result delivery, and the potential for point-of-care or home-based testing. Their non-invasive nature enhances patient comfort and allows for more frequent monitoring.
Despite these advantages, novel methodologies still face hurdles in clinical validation, protocol standardization, and result consistency. Technical challenges, such as low biomarker concentrations and environmental influences, must also be addressed. Nevertheless, these innovations hold significant promise, particularly for early screening and continuous monitoring [54].
Recent advancements in biosensors and sensing technologies that utilize nanostructured materials are significantly transforming the landscape of traditional diagnostic tools. These innovations are driving the development of more practical, accurate, and efficient diagnostic platforms, making them increasingly suitable for real-world clinical applications. Nanostructured materials, in particular, have emerged as a cornerstone in this evolution due to their exceptional attributes—such as cost-effectiveness, superior sensitivity, versatility in detecting multiple targets (multimodal detection), and the ability to be easily miniaturized into compact devices.
Their unique physical and chemical properties make them especially promising for the detection of a wide range of analytes, including critical clinical targets like cancer biomarkers [55]. However, for these advanced diagnostic systems to deliver reliable and reproducible results, it is crucial that the nanomaterials employed exhibit a high degree of selectivity and binding efficiency. This means they must be engineered to specifically interact with target molecules while minimizing non-specific interactions that could interfere with the accuracy of the test. Achieving this level of specificity is essential to fully harness the potential of nanostructured materials in next-generation diagnostic solutions [56]. In the context of breast cancer diagnosis using nano-enabled biosensors, it is critically important to ensure that the nanomaterials employed are capable of binding to their target molecules with high specificity and efficiency. This selective binding is essential to accurately detect cancer-related biomarkers amidst the complex biochemical environment of biological samples. Minimizing non-specific interactions with unrelated molecules not only enhances the precision of the diagnostic process, but also significantly reduces the likelihood of false positives or negatives. Therefore, achieving a high degree of molecular recognition and binding fidelity is fundamental to the development of reliable, sensitive, and clinically viable diagnostic tools for early breast cancer detection [57].
With reference to SPR, which has been discussed in this review [31,32,33], a derivation of this method is the application of the Localized Surface Plasmon Resonance (LSPR) sensors, extensively explored for breast cancer diagnostics, and several studies demonstrate the effectiveness of LSPR-based technologies in detecting breast cancer biomarkers [53,58,59,60]. By enlarging the target to cancer biomarkers in general, excellent lower detection limits can be obtained, together with affordability and durability, by using 2D nano-engineered sensing materials [61,62].
A hybrid diagnostic model that integrates both conventional and innovative methods could create a more effective and accessible screening framework. By combining the proven reliability of traditional imaging with the convenience and affordability of emerging technologies, early detection rates could improve, making screening more widely available across diverse populations
Future research should prioritize overcoming the current limitations of biosensor technologies, particularly by improving their reproducibility and ensuring high specificity and sensitivity across diverse populations. Standardizing testing conditions—especially for breath- and sweat-based biosensors—is essential for reducing environmental and physiological variability and enhancing result reliability.
Expanding biosensor applications beyond breast cancer detection presents another promising avenue. Developing multiplexed biosensors capable of detecting multiple biomarkers simultaneously could provide a more comprehensive diagnostic profile and improve early cancer detection across various types. Additionally, applying detection techniques that have proven to be effective in traditional samples, such as blood, to less studied sample types like sweat, could enable non-invasive and continuous monitoring, combining high sensitivity with patient-friendly sampling methods.
Enhancing the affordability and accessibility of these technologies is also critical, particularly for resource-limited regions. The development of point-of-care devices that integrate multiple detection methods could significantly expand the reach of breast cancer screening programs, making early diagnosis more widely available.
While significant progress has been made, continued research and innovation remain crucial to refining these diagnostic tools. The future of breast cancer detection lies in integrating diverse approaches to create more sensitive, specific, and patient-friendly screening methods, ultimately improving early detection rates and patient outcomes.
Roadmap
By concluding with a roadmap for a future outlook in the field, some points can be assessed:
- -
- Traditional imaging—such as mammography, MRI, ultrasound, and PET—remains essential for breast cancer detection, offering validated accuracy, but requiring costly infrastructure and trained personnel [63]. These methods also come with drawbacks such as invasiveness, radiation, and long wait times;
- -
- In contrast, non-invasive approaches using biosensors and bodily fluids offer lower costs, faster results, and better accessibility, including potential home testing. Despite challenges like low biomarker levels and inconsistent results, these innovations show strong promise for early detection and continuous monitoring [64];
- -
- The shift toward non-invasive cancer detection is gaining momentum, with breath, saliva, urine, and sweat offering patient-friendly options for frequent monitoring. While blood tests remain the gold standard due to high biomarker levels, research is increasingly exploring multi-sample integration. Applying sensitive blood detection methods to non-invasive samples could boost accuracy, with fewer drawbacks;
- -
- Technologies like electronic noses and machine learning support early detection, though standardization and validation are still needed. AI integration is improving analysis and interpretation, moving diagnostics toward greater precision and personalization;
- -
- The integration of unobtrusive sensor technology for monitoring and frequent examination could favor early detection and early intervention in a collaborative synergy between robotics and sensors [65].
Author Contributions
Conceptualization, A.G., V.A. and G.P.; methodology, M.S. and A.Z.; investigation, G.F. and A.G.; data curation, A.Z. and G.F.; writing—original draft preparation, G.F. and A.G.; writing—review and editing, G.P. and M.S.; supervision, A.G. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IARC. International Agency for Research on Cancer. 2024. Available online: https://www.iarc.who.int/ (accessed on 14 April 2025).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer Facts & Figures 2021–2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Zheng, D.; He, X.; Jing, J. Overview of Artificial Intelligence in Breast Cancer Medical Imaging. J. Clin. Med. 2023, 12, 419. [Google Scholar] [CrossRef]
- Gegios, A.R.; Peterson, M.S.; Fowler, A.M. Breast Cancer Screening and Diagnosis: Recent Advances in Imaging and Current Limitations. Pet Clin. 2023, 18, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Monticciolo, D.L.; Newell, M.S.; Hendrick, R.E.; Helvie, M.A.; Moy, L.; Monsees, B.; Kopans, D.B.; Eby, P.R.; Sickles, E.A. Breast Cancer Screening for Average-Risk Women: Recommendations from the ACR Commission on Breast Imaging. J. Am. Coll. Radiol. 2017, 14, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.L.; Moy, L. Breast MRI Screening: Benefits and Limitations. Curr. Breast Cancer Rep. 2016, 8, 248–257. [Google Scholar] [CrossRef]
- Emaus, M.; Bakker, M.; Peeters, H.; Loo, C.E.; Mann, R.M.; de Jong, M.D.F.; Bisschops, R.H.C.; Veltman, J.; Duvivier, K.M.; Lobbes, M.B.I.; et al. MR Imaging as an Additional Screening Modality for the Detection of Breast Cancer in Women Aged 50–75 Years with Extremely Dense Breasts: The DENSE Trial Study Design. Radiology 2015, 277, 527–537. [Google Scholar] [CrossRef]
- Berg, W.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Böhm-Vélez, M.; Mahoney, M.C.; Evans, W.P.; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef]
- Mahoney, M.C.; Newell, M.S. Screening MR Imaging Versus Screening Ultrasound: Pros and Cons. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.M. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 55–76. [Google Scholar] [PubMed]
- Heusner, T.A.; Kuemmel, S.; Umutlu, L.; Koeninger, A.; Freudenberg, L.S.; Hauth, E.A.; Kimmig, K.R.; Forsting, M.; Bockisch, A.; Antoch, G. Breast Cancer Staging in a Single Session: Whole-Body PET/CT Mammography. J. Nucl. Med. 2008, 49, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Bhatia, S.; Brindle, K.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, 6586. [Google Scholar] [CrossRef]
- Ginsburg, O.; Yip, C.; Brooks, A.; Cabanes, A.; Caleffi, M.; Yataco, J.A.D.; Gyawali, B.; McCormack, V.; de Anderson, M.M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Salimian, R. Ultrasensitive Detection of Cancer Biomarkers Using Conducting Polymer/Electrochemically Reduced Graphene Oxide-Based Biosensor: Application toward BRCA1 Sensing. Sens. Actuators B Chem. 2018, 266, 160–169. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Boraei, S.B.A.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Graphene-based electrochemical biosensors for breast cancer detection. Biosensors 2023, 13, 80. [Google Scholar] [CrossRef]
- Hakimian, F.; Ghourchian, H. Ultrasensitive electrochemical biosensor for detection of microRNA-155 as a breast cancer risk factor. Anal. Chim. Acta 2020, 1136, 1–8. [Google Scholar] [CrossRef]
- Hu, R.; Wen, W.; Wang, Q.; Xiong, H.; Zhang, X.; Gu, H.; Wang, S. Novel electrochemical aptamer biosensor based on an enzyme–gold nanoparticle dual label for the ultrasensitive detection of epithelial tumor marker MUC1. Biosens. Bioelectron. 2014, 53, 384–389. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, W.; Lai, R.Y. A folding-based electrochemical aptasensor for detection of vascular endothelial growth factor in human whole blood. Biosens. Bioelectron. 2011, 26, 2442–2447. [Google Scholar] [CrossRef]
- Marques, R.C.B.; Viswanathan, S.; Nouws, H.P.A.; Delerue-Matos, C.; Gonzales-Garcia, M.B. Electrochemical immunosensor for the analysis of the breast cancer biomarker HER2 ECD. Talanta 2014, 129, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chandra, P.; Shim, Y.B. Ultrasensitive and Selective Electrochemical Diagnosis of Breast Cancer Based on a Hydrazine–Au Nanoparticle–Aptamer Bioconjugate. Anal. Chem. 2013, 85, 1058–1064. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Sales, G.M.F. Disposable electrochemical detection of breast cancer tumour marker CA 15-3 using poly(Toluidine Blue) as imprinted polymer receptor. Biosens. Bioelectron. 2018, 109, 246–254. [Google Scholar] [CrossRef]
- Majd, S.M.; Salimi, A.; Ghasemi, F. An ultrasensitive detection of miRNA-155 in breast cancer via direct hybridization assay using two-dimensional molybdenum disulfide field-effect transistor biosensor. Biosens. Bioelectron. 2018, 105, 6–13. [Google Scholar] [CrossRef]
- Yazdani, Y.; Jalali, F.; Tahmasbi, H.; Akbari, M.; Talebi, N.; Shahrtash, S.A.; Mobed, A.; Alem, M.; Ghazi, F.; Dadashpour, M. Recent advancements in nanomaterial-based biosensors for diagnosis of breast cancer: A comprehensive review. Cancer Cell Int. 2025, 25, 50. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sadeghi, S.; Naghib, S.M.; Garshasbi, H.R. A comprehensive review on electrochemical nano biosensors for precise detection of blood-based oncomarkers in breast cancer. Biosensors 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Turk, Z.; Armani, A.; Jafari-Gharabaghlou, D.; Madakbas, S.; Bonabi, E.; Zarghami, N. A new insight into the early detection of HER2 protein in breast cancer patients with a focus on electrochemical biosensors approaches: A review. Int. J. Biol. Macromol. 2024, 272, 132710. [Google Scholar] [CrossRef]
- Wignarajah, S.; Chianella, I.; Tothill, I.E. Development of electrochemical immunosensors for HER-1 and HER-2 analysis in serum for breast cancer patients. Biosensors 2023, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An immunosensor for the determination of carcinoembryonic antigen by Surface Plasmon Resonance imaging. Anal. Biochem. 2020, 609, 113964. [Google Scholar] [CrossRef]
- Erol, K.; Hasabnis, G.; Altintas, Z. A Novel NanoMIP–SPR Sensor for the Point-of-Care Diagnosis of Breast Cancer. Micromachines 2023, 14, 1086. [Google Scholar] [CrossRef]
- Verma, P.; Kumar, A.; Jindal, P. Machine Learning Approach for SPR-based Photonic Crystal Fiber Sensor for Breast Cancer Cells Detection. In Proceedings of the 2022 IEEE 7th Forum on Research and Technologies for Society and Industry Innovation (RTSI), Paris, France, 24–26 August 2022. [Google Scholar]
- Han, Y.; Qiang, L.; Gao, Y.; Gao, J.; He, Q.; Liu, H.; Han, L.; Zhang, Y. Large-area surface-enhanced Raman spectroscopy substrate by hybrid porous GaN with Au/Ag for breast cancer miRNA detection. Appl. Surf. Sci. 2020, 541, 148456. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Luo, X.; Wan, Q.; Qiu, R.; Wang, S. An ultrasensitive homogeneous aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Talanta 2018, 195, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, H.; Xu, J.; Huang, Y.; Zhang, X.; Weng, J.; Li, Z.; Sun, L. Ultrasensitive colorimetric biosensor for BRCA1 mutation based on multiple signal amplification strategy. Biosens. Bioelectron. 2020, 166, 112424. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, R.; Hao, Y.; Yang, P. A CD44-biosensor for evaluating metastatic potential of breast cancer cells based on quartz crystal microbalance. Sci. Bull. 2017, 62, 923–930. [Google Scholar] [CrossRef]
- Uliana, C.V.; Peverari, C.R.; Afonso, A.S.; Cominetti, M.R.; Faria, R.C. Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosens. Bioelectron. 2017, 99, 156–162. [Google Scholar] [CrossRef]
- Nurlankyzy, M.; Kantoreyeva, K.; Myrkhiyeva, Z.; Ashikbayeva, Z.; Baiken, Y.; Kanayeva, D.; Tosi, D.; Bekmurzayeva, A. Label-free optical fiber biosensor for the detection of CD44-expressing breast cancer cells. Sens. Bio-Sens. Res. 2024, 44, 100661. [Google Scholar] [CrossRef]
- Gao, Y.; Qiang, L.; Chu, Y.; Han, Y.; Zhang, Y.; Han, L. Microfluidic chip for multiple detection of miRNA biomarkers in breast cancer based on three-segment hybridization. AIP Adv. 2020, 10, 045022. [Google Scholar] [CrossRef]
- Du, W.; Zhang, L.; Suh, E.; Lin, D.; Marcus, C.; Ozkan, L.; Ahuja, A.; Fernandez, S.; Shuvo, I.I.; Sadat, D.; et al. Conformable ultrasound breast patch for deep tissue scanning and imaging. Sci. Adv. Eng. 2023, 9, eadh5325. [Google Scholar] [CrossRef]
- Xu, X.; Chung, Y.; Brooks, A.D.; Shih, W.-H.; Shih, W.Y. Development of array piezoelectric fingers towards in vivo breast tumor detection. Rev. Sci. Instrum. 2016, 87, 124301. [Google Scholar] [CrossRef]
- Elsheakh, D.N.; Mohamed, R.A.; Fahmy, O.M.; Ezzat, K.; Eldamak, A.R. Complete Breast Cancer Detection and Monitoring System by Using Microwave Textile-Based Antenna Sensors. Biosensors 2023, 13, 87. [Google Scholar] [CrossRef]
- Elouerghi, A.; Bellarbi, L.; Khomsi, Z.; Jbari, A.; Errachid, A.; Yaakoubi, N. A Flexible Wearable Thermography System Based on Bioheat Microsensors Network for Early Breast Cancer Detection: IoT Technology. J. Electr. Comput. Eng. 2022, 2022, 5921691. [Google Scholar] [CrossRef]
- Sree, V.S.; Royea, R.; Buckman, K.J.; Benardis, M.; Holmes, J.; Fletcher, R.L.; EYK, N.; Acharya, R.; Ellenhorn, J.D.I. An introduction to the Cyrcadia Breast Monitor: A wearable breast health monitoring device. Comput. Methods Programs Biomed. 2020, 197, 105758. [Google Scholar]
- Yang, H.-Y.; Wang, Y.-C.; Peng, H.-Y.; Huang, C.-H. Breath biopsy of breast cancer using sensor array signals and machine learning analysis. Sci. Rep. 2022, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Díaz de León-Martínez, L.; Rodríguez-Aguilar, M.; Gorocica-Rosete, P.; Domínguez-Reyes, C.A.; Martínez-Bustos, V.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Cruz-Ramos, J.A.; Balderas-Segura, B.; Flores-Ramírez, R. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: A case-control study. J. Breath Res. 2020, 14, 046009. [Google Scholar] [CrossRef]
- Wan, H.-H.; Zhu, H.; Chiang, C.-C.; Li, J.-S.; Ren, F.; Tsai, C.-T.; Liao, Y.-T.; Neal, D.; Esquivel-Upshaw, J.F.; Pearton, S.J. High sensitivity saliva-based biosensor in detection of breast cancer biomarkers: HER2 and CA15-3. J. Vac. Sci. Technol. B 2024, 42, 023202. [Google Scholar] [CrossRef]
- Wei, F.; Patel, P.; Liao, W.; Chaudhry, K.; Zhang, L.; Arellano-Garcia, M.; Hu, S.; Elashoff, D.; Zhou, H.; Shukla, S.; et al. Electrochemical Sensor for Multiplex Biomarkers Detection. Imaging Diagn. Progn. 2009, 15, 13. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Gamella, M.; Pingarrón, J.M. Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 mRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosens. Bioelectron. 2016, 77, 538–548. [Google Scholar] [CrossRef]
- Benet, J.G.; Seo, M.; Khine, M.; Padró, J.G.; Martínez, A.P.; Kurdahi, F. Breast cancer detection by analyzing the volatile organic compound (VOC) signature in human urine. Sci. Rep. 2022, 12, 14873. [Google Scholar]
- Leemans, M.; Cuzuel, V.; Bauër, P.; Aissa, H.B.; Cournelle, G.; Baelde, A.; Thuleau, A.; Cognon, G.; Pouget, N.; Guillot, E.; et al. Screening of Breast Cancer from Sweat Samples Analyzed by 2D-GC-MS: A Preliminary Study. Cancers 2023, 15, 2939. [Google Scholar] [CrossRef]
- Elsheakh, D.N.; Fahmy, O.M.; Farouk, M.; Ezzat, K.; Eldamak, A.R. An early breast cancer detection by using wearable flexible sensors and artificial intelligent. IEEE Access 2024, 12, 48511–48529. [Google Scholar] [CrossRef]
- Katsika, L.; Boureka, E.; Kalogiannidis, I.; Tsakiridis, I.; Tirodimos, I.; Lallas, K.; Tsimtsiou, Z.; Dagklis, T. Screening for Breast Cancer: A Comparative Review of Guidelines. Life 2024, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.R.A.; Palaniyandi, T.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Sugumaran, A.; Sivaji, A.; Kaliamoorthy, S.; Kumarasamy, S. Biomarker-specific biosensors revolutionise breast cancer diagnosis. Clin. Chim. Acta 2024, 555, 117792. [Google Scholar] [CrossRef]
- Chugh, V.; Basu, A.; Kaushik, A.; Bhansali, S.; Basu, A.K. Employing nano-enabled artificial intelligence (AI)-based smart technologies for prediction, screening, and detection of cancer. Nanoscale 2024, 16, 5458–5486. [Google Scholar] [CrossRef]
- Joshi, A.; GK, A.V.; Sakorikar, T.; Kamal, A.M.; Vaidya, J.S.; Pandya, H.J. Recent advances in biosensing approaches for point-of-care breast cancer diagnostics: Challenges and future prospects. Nanoscale Adv. 2021, 3, 5542–5564. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Loke, S.Y.; Lim, H.Q.; Balasundaram, G.; Chan, P.; Chong, B.K.; Tan, E.Y.; Lee, A.S.G.; Olivo, M. Circulating microRNA breast cancer biomarker detection in patient sera with surface plasmon resonance imaging biosensor. J. Biophotonics 2021, 14, e202100153. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Lobry, M.; Hassan, E.M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 breast cancer biomarker detection using a sandwich optical fiber assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef]
- Yildizhan, Y.; Driessens, K.; Tsao, H.S.K.; Boiy, R.; Thomas, D.; Geukens, N.; Hendrix, A.; Lammertyn, J.; Spasic, D. Detection of breast cancer-specific extracellular vesicles with fiber-optic SPR biosensor. Int. J. Mol. Sci. 2023, 24, 3764. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Solanki, P.R. Enhanced Electrochemical Biosensing of the Sp17 Cancer Biomarker in Serum Samples via Engineered Two-Dimensional MoS2 Nanosheets on the Reduced Graphene Oxide Interface. ACS Appl. Biol. Mater. 2023, 6, 4250–4268. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Kumar, A.; Bhatt, A.N.; Solanki, P.R. Biocompatible epoxysilane substituted polymer-based nano biosensing platform for label-free detection of cancer biomarker SP17 in patient serum samples. Int. J. Biol. Macromol. 2023, 239, 124325. [Google Scholar] [CrossRef]
- Katal, S.; McKay, M.J.; Taubman, K. PET molecular imaging in breast cancer: Current applications and future perspectives. J. Clin. Med. 2024, 13, 3459. [Google Scholar] [CrossRef]
- Ghosh, S.; Rajendran, R.L.; Mahajan, A.A.; Chowdhury, A.; Bera, A.; Guha, S.; Chakraborty, K.; Chowdhury, R.; Paul, A.; Jha, S.; et al. Harnessing exosomes as cancer biomarkers in clinical oncology. Cancer Cell Int. 2024, 24, 278. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Mughees, M.; Shaikh, S.; Choudhary, F.; Nizam, A.; Rizwan, A.; Narang, J. From Biosensors to Robotics: Pioneering Advances in Breast Cancer Management. Sensors 2024, 24, 6149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).