1. Introduction
The main characteristic of diabetes is that it is typically brought on by extremely high or low blood glucose levels for an extended length of time [
1,
2]. Millions of people worldwide suffer from diabetes, a chronic illness. Over 60% of the global diabetes population lives in a “risk zone”, accounting for 463 million cases of the disease, according to the World Health Organization (WHO) and the International Diabetes Federation (IDF) [
2]. To increase the accuracy of these findings, it is advised that this identification and monitoring can be done frequently. Finger prick tests and glucose monitoring are two methods that can be used to identify diabetics [
3,
4], albeit they are seen as intrusive and harmful to the patient. Blood glucose levels can rise or fall as a result of diabetes, which is characterized by poor glucose utilization. It is well known to be a long-term metabolic disease that impacts important body organs. Diabetes can cause potentially dangerous side effects, such as peripheral neuropathy, heart disease, renal failure, and visual loss if it is not well monitored [
5]. An individual in good health should have blood glucose levels that fall within the normal range of 70–100 mg/dL during fasting and 70–140 mg/dL after meals. Hyperglycemia, or elevated blood sugar, and hypoglycemia, or decreased blood sugar, are both harmful. Hypoglycemia can cause weakness or even unconsciousness. Conversely, blood hyperglycemia may potentially be an emergency or result in diabetic complications [
6]. Diabetes is a chronic illness that arises from either insufficient insulin production or inefficient insulin utilization by the body. As a result, regular blood glucose testing is essential in managing diabetes. Since diabetes cannot be permanently cured, the only treatments are lifestyle modifications and a nutritious diabetic diet [
7].
The patient has discomfort from frequent blood glucose monitoring based on this unpleasant intrusive procedure, and invasive methods like laboratory and one-touch glucometers raise the risk of blood-related infections. Furthermore, diabetics have to pay for recordings and endure the monotony of doing the same tests repeatedly [
8,
9]. The description of conventional glucometers that need finger pricking has prompted research on non-invasive or minimally invasive methods. The accuracy and user discomfort of the current options, such as wrist monitors and forearm glucometers, are criticized. This raises the prospect of using microwave glucose testing methods for a genuinely non-invasive method. For non-invasive glucose monitoring, several technologies have been proposed, some of which have even advanced to the commercialization stage. These technologies include optical [
10], transdermal techniques [
11], thermal spectrum measurement [
12], breath acetone analysis [
13], impedance spectroscopy [
13], graphene-based nano-sensors [
14], electrochemical methods that measure the glucose level in urine [
15,
16,
17], contact lenses [
18], acoustic spectroscopy [
19], Raman spectroscopy [
20], or a combination of these techniques [
21]. However, a drawback of several of these commercial technologies is that they are not entirely non-invasive; contact lenses and implantable devices are two examples, while a few others are launched as semi-invasive devices. They are also costly and place limitations on the user [
22].
Because electromagnetic (EM) microwaves can pass through bodily tissues, they are also used in continuous non-invasive blood glucose measurement and management [
23]. These kinds of systems primarily depend on variations in the permittivity (ε) of real and imaginary parts of tissues or target cells because variations in glucose concentration cause variations in the EM parameters of ISF beneath the skin, tears, sweat, blood in the arteries, and so forth [
24]. Additionally, these devices enable users to check their blood sugar levels without having to prick their fingertips every day [
25]. There are several approaches to detecting electromagnetic properties based on microwave waves that fall into two categories: resonant (narrow band) [
26,
27] and non-resonant (broadband) [
28,
29,
30]. Resonant techniques have grown in popularity among these, particularly for applications requiring high detection speed, precision, and sensitivity [
31,
32,
33]. Because a greater range of frequencies can be covered to measure the microwave parameters of the materials and obtain more detailed information about the electromagnetic properties of the sample under test (SUT), increasing the number of resonances of sensor performance is one method to improve the accuracy of detecting electrical parameters of materials by microwave resonant sensors [
33,
34,
35,
36,
37]. A band-stop filter with a defective ground structure (DGS) sensor is another technique that uses scattering parameters to monitor intravenous glucose levels, as reported in [
38]. Moreover, it is less sensitive than previously reported to variations in blood sample glucose levels and penetration depth. However, there are several options presented that relate to checking blood glucose levels [
39,
40] using near-infrared and electrochemical sensors. In [
41], a regular-shaped SRR sensor was used to monitor the glucose level. Moreover, Refs. [
42,
43] proposed a different pressure sensor, and Ref. [
44] provided several creative microwave sensors, while Deshmukh and Ghongade devised a method for measuring glucose levels by estimating the glucose levels and computing the dielectric characteristics using a ring resonator [
45]. The detection of glucose in the presence of animal tissues was addressed by the authors of [
46] by using mm wave techniques. Furthermore, a variety of novel techniques for more precisely determining the glucose content in blood samples are described in [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40]. The most popular non-invasive glucose monitoring technology is the antenna system because of its great sensitivity to even small fluctuations in blood glucose levels, ease of deployment, resistance to noise, temperature, and other disturbances, and deep penetration.
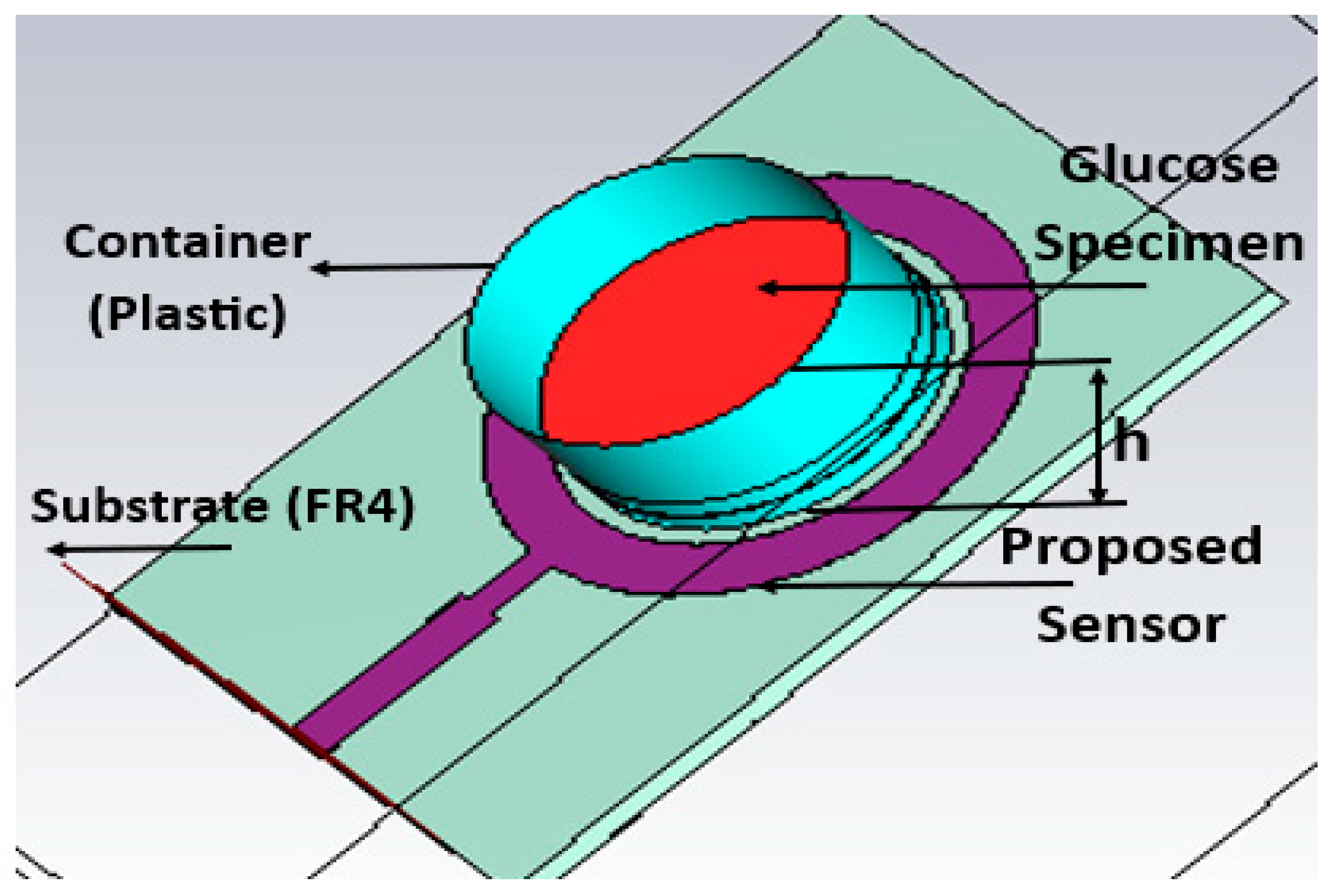
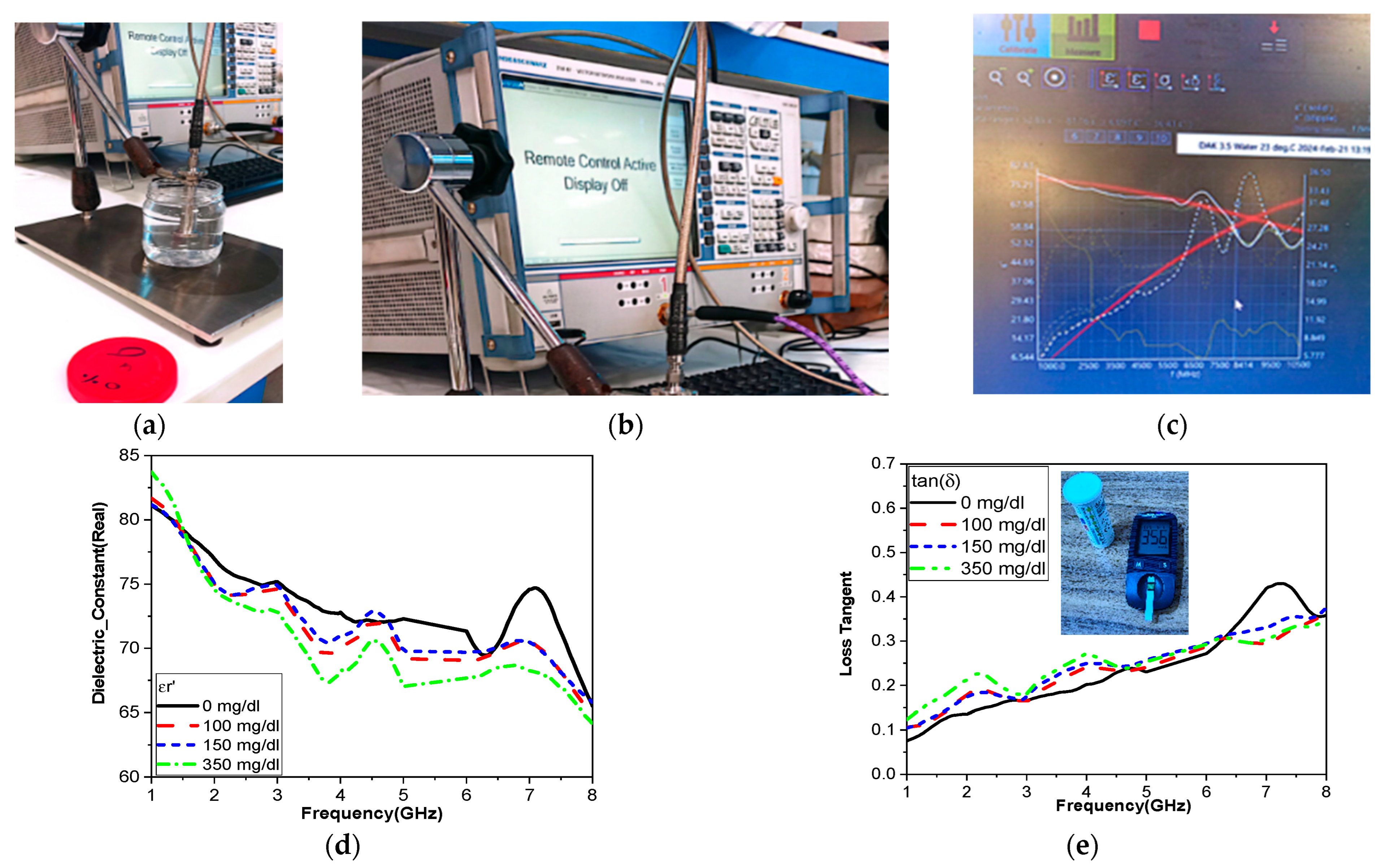
A variety of liquid phantoms, such as physiological solutions and pig blood, were assessed; the samples under analysis had glucose concentrations ranging from 150 mg/dL to 550 mg/dL. It was found that the solution caused a linear shift in frequency of 5 MHz, with no correlation between the readings and the samples’ varying volumes and temperatures [
47]. In [
48], metamaterials can also be used to identify glucose. In [
48], the simulated result sensitivity for differences in a small range of glucose changes from 0 mg/dL to 200 mg/dL is very small, 0.017 dB/mg/dL at three operating bands.
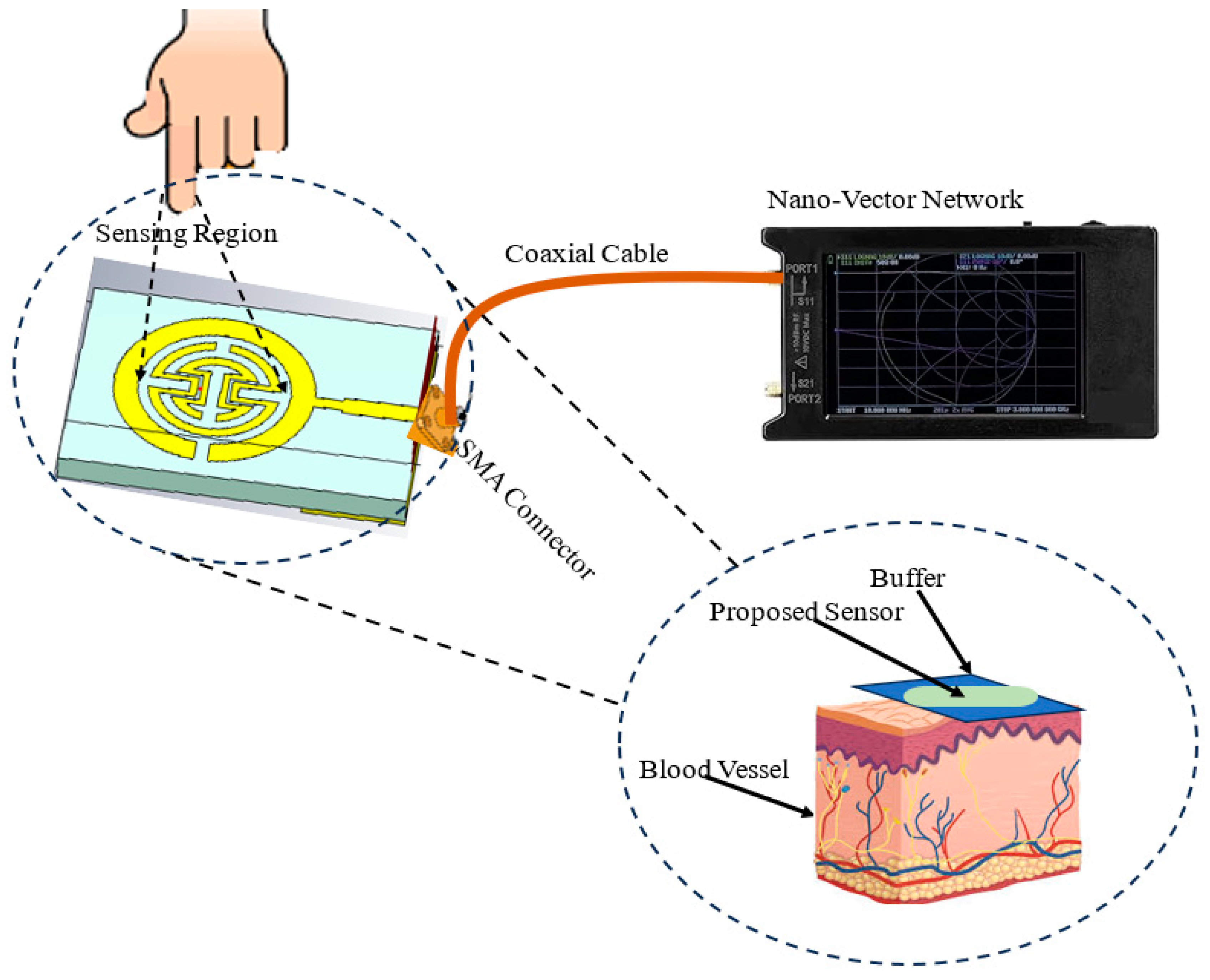
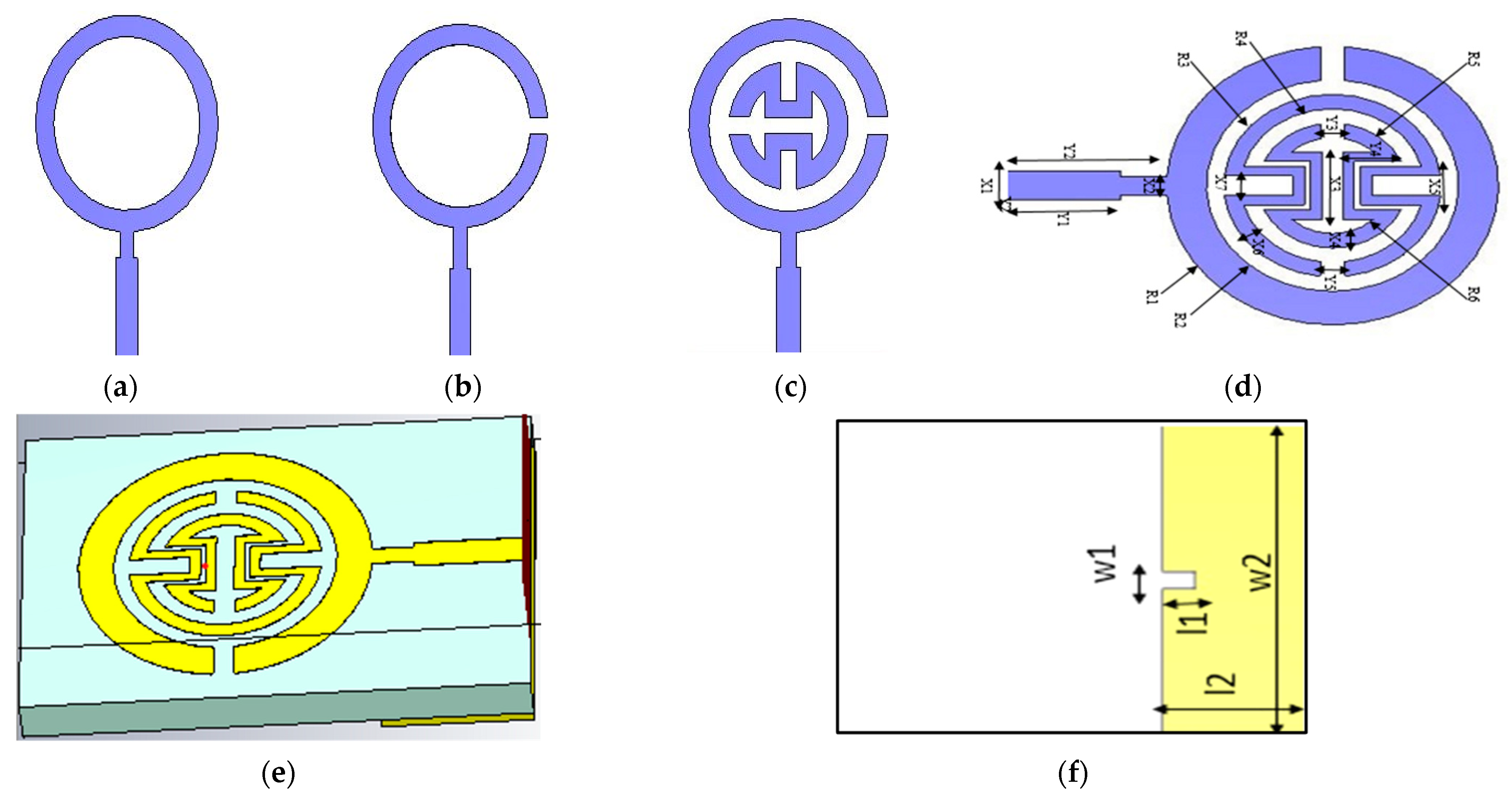
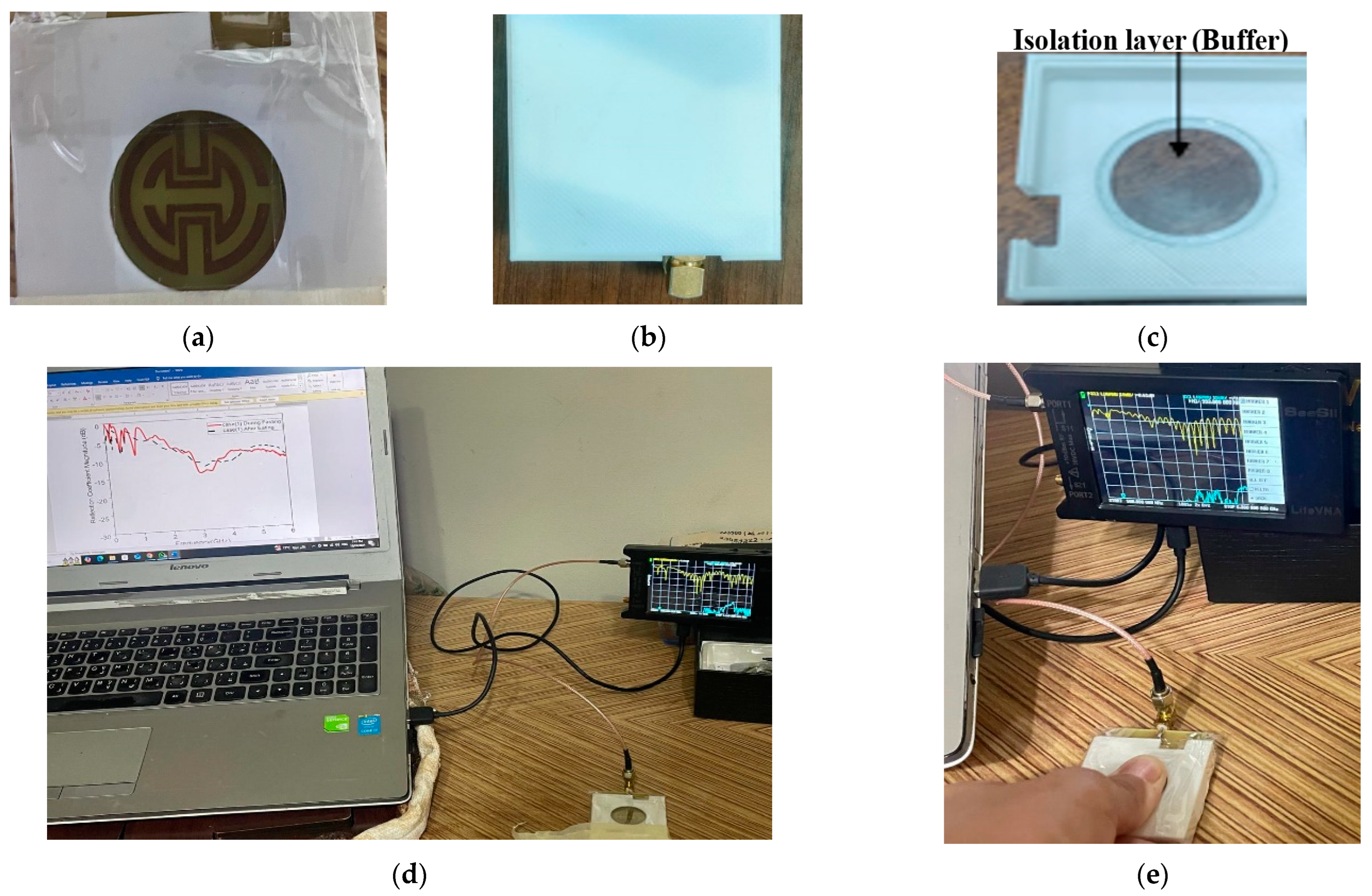
As shown in
Figure 1, this paper introduces a proposed biosensor system using a compact monopole antenna based on an irregularly shaped split-ring resonator (MSRRMA) for monitoring blood glucose levels. This design enables multiple resonant frequencies at 0.94, 1.5, 3, 4.6, 6.3, and 7.2 GHz, which is beneficial for monitoring blood glucose levels across a range of applications.
The proposed sensor allows for continuous monitoring of blood glucose levels without requiring invasive blood sampling. This represents a significant improvement over traditional glucose monitoring methods that require finger pricks or other invasive techniques. The incorporation of two SRR metamaterial cells to create specific notches at 1.7 GHz and 4.4 GHz is innovative. It enhances the antenna’s ability to respond to changes in blood glucose levels selectively. The proof of concept is carried out by using two technologies: containers having solutions with different glucose concentrations and the other one by using a human fingertip.
The proposed design could improve the sensitivity and specificity of the monitoring system by 24 MHz/mg/dL. Constructed on a commercial FR4 substrate with compact dimensions (35 × 50 × 1.6 mm3), the design is practical for real-world applications. The compactness of the antenna makes it suitable for wearable or portable devices. Moreover, the sensor is tested on real human fingers, measuring resonant frequency, magnitude, and phase changes. This method of validation provides strong evidence of the sensor’s effectiveness, enhancing the credibility of the research findings.
The paper is organized as follows: The material and methods used in the design process, parametric analysis, and microstrip antenna structure are all covered in
Section 2. Results and comments are presented in
Section 3, along with modeling of a study on frequency changes related to a finger phantom. Discussion and comments are presented in
Section 4, along with modeling of a finger phantom-related investigation on frequency shifts for various finger phantom positions and glucose levels.
Section 5 concludes the paper’s results.
4. Discussion
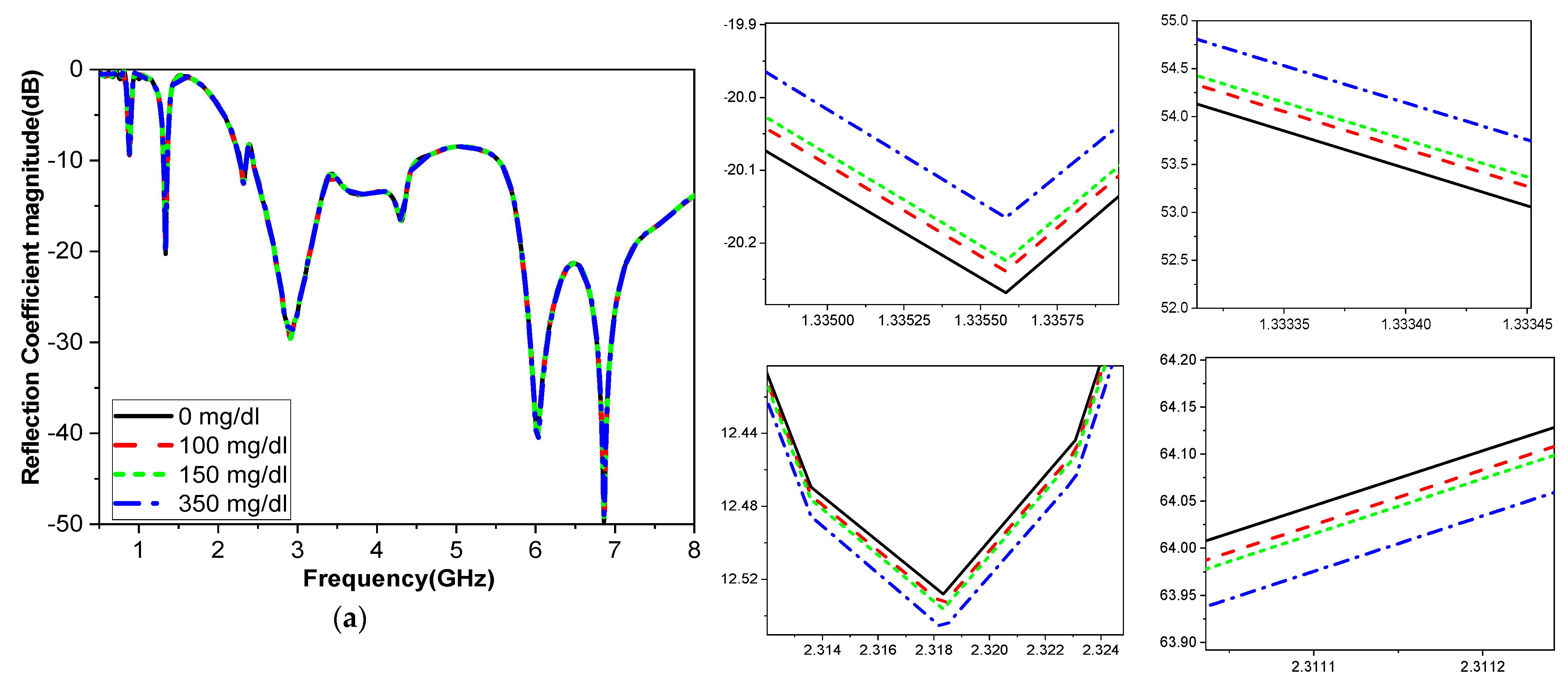
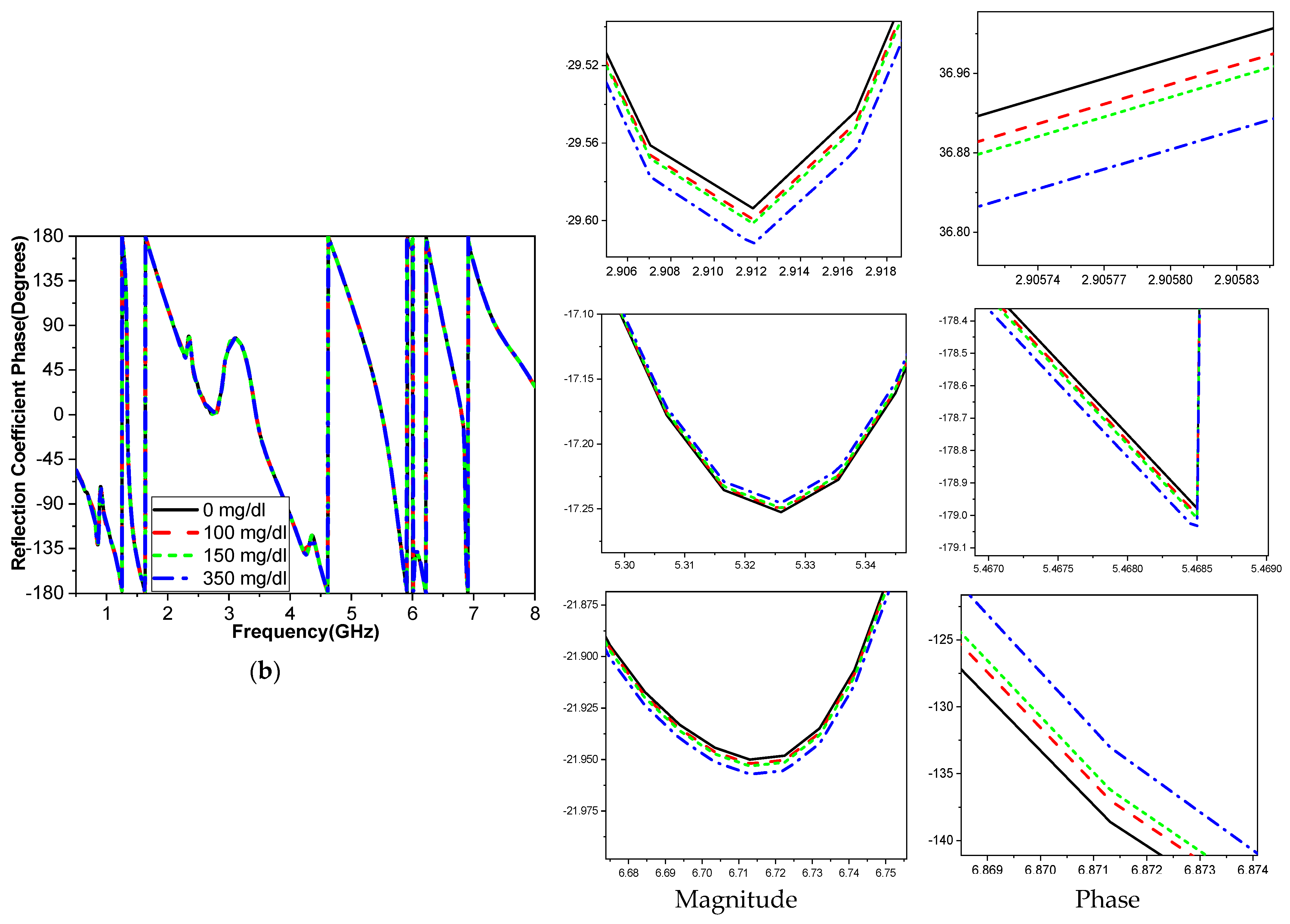
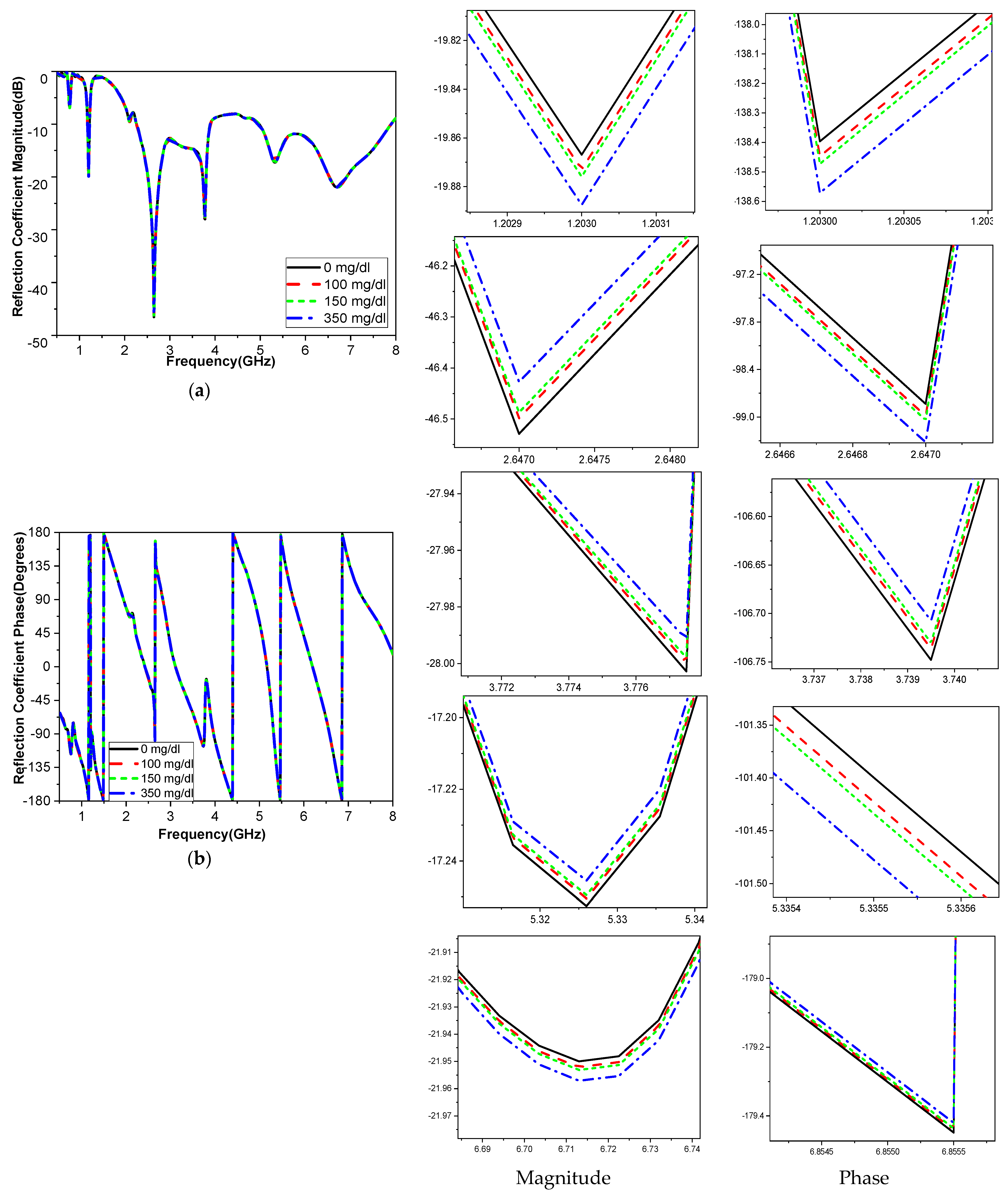
The frequency band is varied and tested throughout a range to determine how variations in glucose concentration affect the reflection coefficient, which was utilized to compute the sensor’s sensitivity. When blood is added, the interaction between the blood glucose samples and the fields modifies the resonant frequency for the relative permittivity εr and the sensitivity of the sensor. The variation in the glucose concentration changed according to the resonant frequency of operation, as shown in
Figure 14. The operating bands 3.1 GHz and 5.95 GHz are more sensitive frequency bands to the variation in the blood glucose concentration. This corroborates with the results shown in
Figure 10. Equation (2) is used to analyze the sensitivity; it shows that any change in the sensor’s resonant frequency (Δf) or magnitude (ΔS
11) depends on a change in the glucose concentration (ΔC). One may extract Equations (4)–(6), as in [
5].
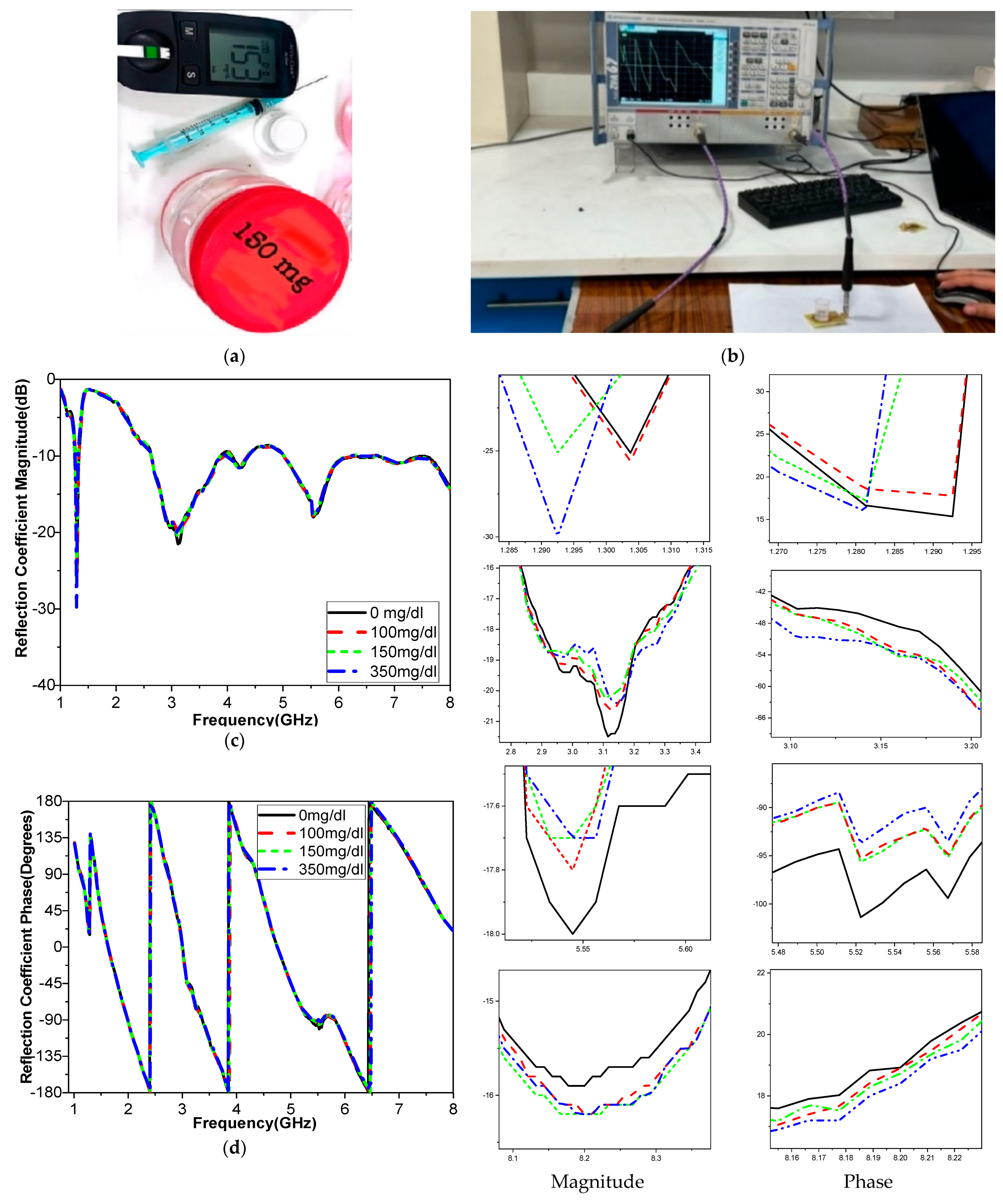
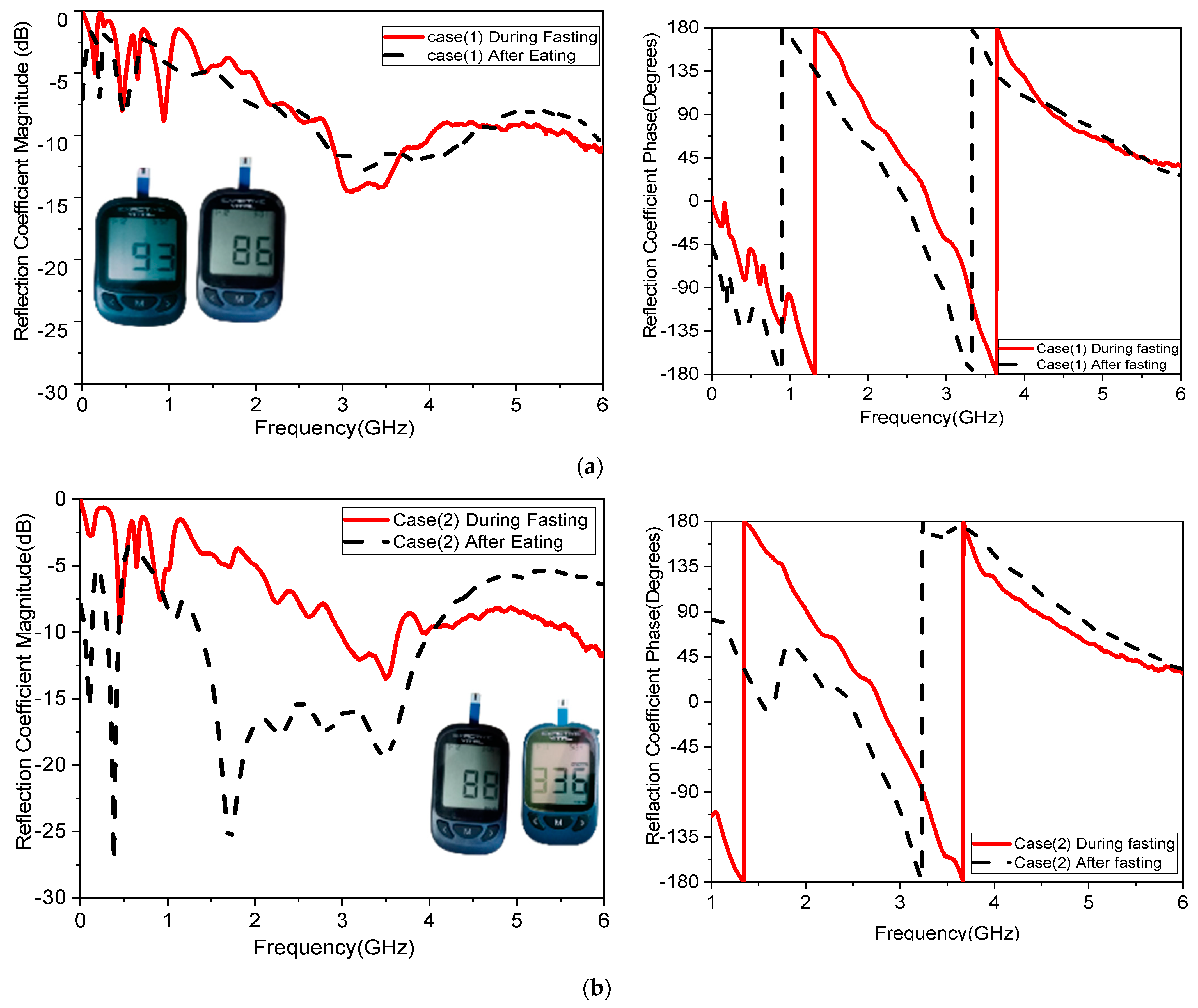
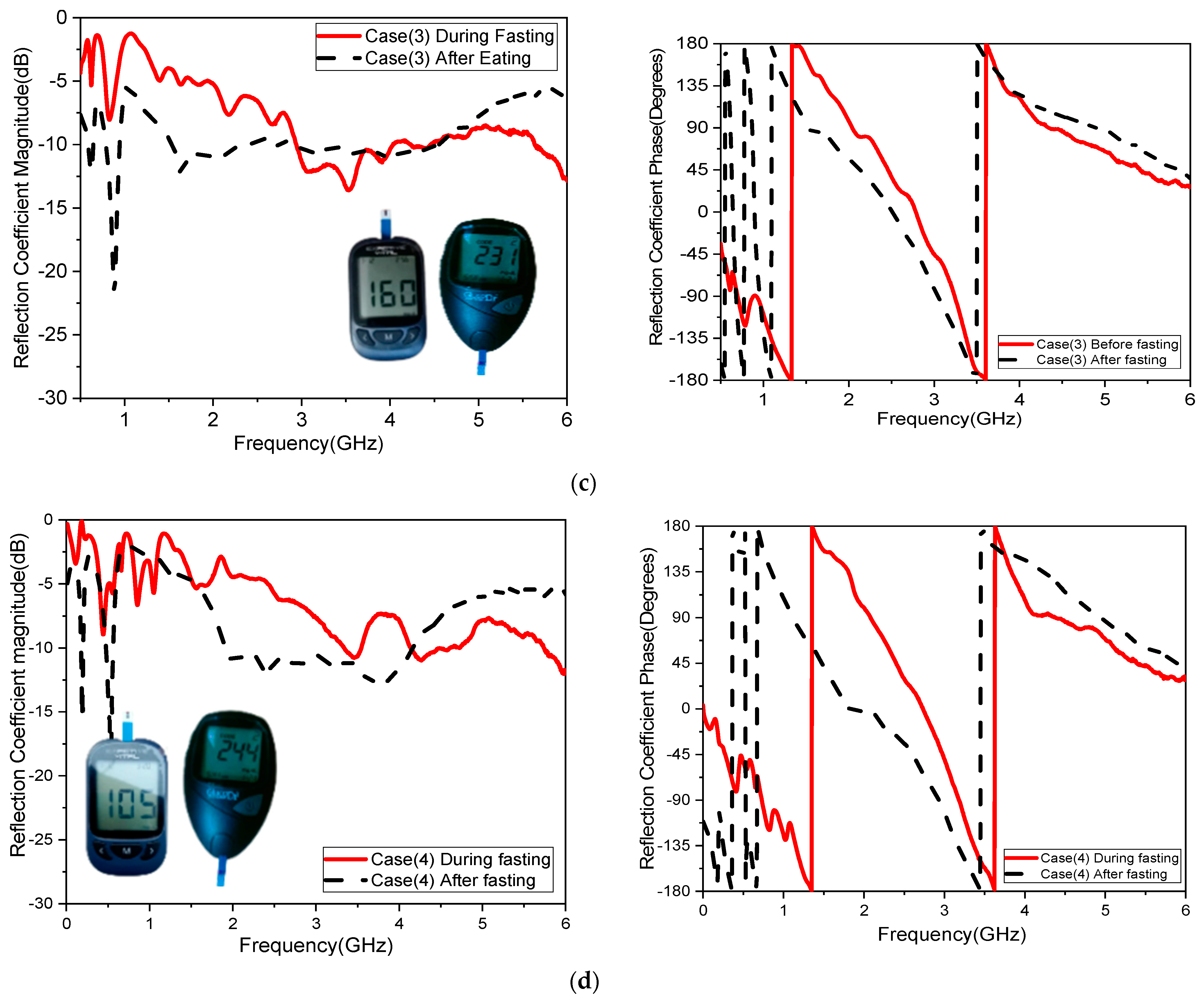
The sensitivity of the measurement by using a container is around 0.015 MHz/mg/dL, 0.003 dB/mg/dL, and 0.003 degree/mg/dL at resonant 3.1 GHz 4.624. When at resonant frequency 5.9 GHz, the sensitivity is 0.5 MHz/mg/dL, 0.005 dB/mg/dL, and 0.05 degree/mg/dL. The measurement sensitivity using a human finger is around 0.299 dB/mg/dL for case 1 and 1.859 dB/mg/dL for case 2, and the sensitivity is around 0.05951 degree/mg/dL for case 1 and 0.510473 degree/mg/dl for case 2.
Moreover, the sensitivity is around 0.2427 MHz/mg/dL for case 1 and 24.624 MHz/mg/dl for case 2, while the value of sensitivity is around 0.299 dB/mg/dL for case 1 and 1.859 dB/mg/dL for case 2, and the sensitivity is around 0.05951 degree/mg/dL for case 1 and 0.510473 degree/mg/dL for case 2. The resonant frequency at 172.37 MHz decreases with increasing glucose concentrations between 3.280 and 3.1080 GHz. The frequency moves somewhat downward at lower levels and drastically lowers at higher quantities, as shown in
Table 8. This alteration causes relative permittivity to drop and sensor sensitivity to increase with glucose concentrations. Our measurement system is designed to minimize the influence of external factors, including perspiration. Measurements are conducted in a controlled environment where factors such as humidity and temperature are regulated to reduce the likelihood of excessive perspiration. Participants are instructed to clean and dry their fingers before measurements to ensure consistent conditions. This step helps remove any existing moisture or contaminants that could affect the results. The sensor interface buffer is designed to maintain consistent contact with the skin, even in the presence of minor moisture. The materials and design of the sensor ensure that perspiration does not significantly alter the electrical or dielectric properties being measured.
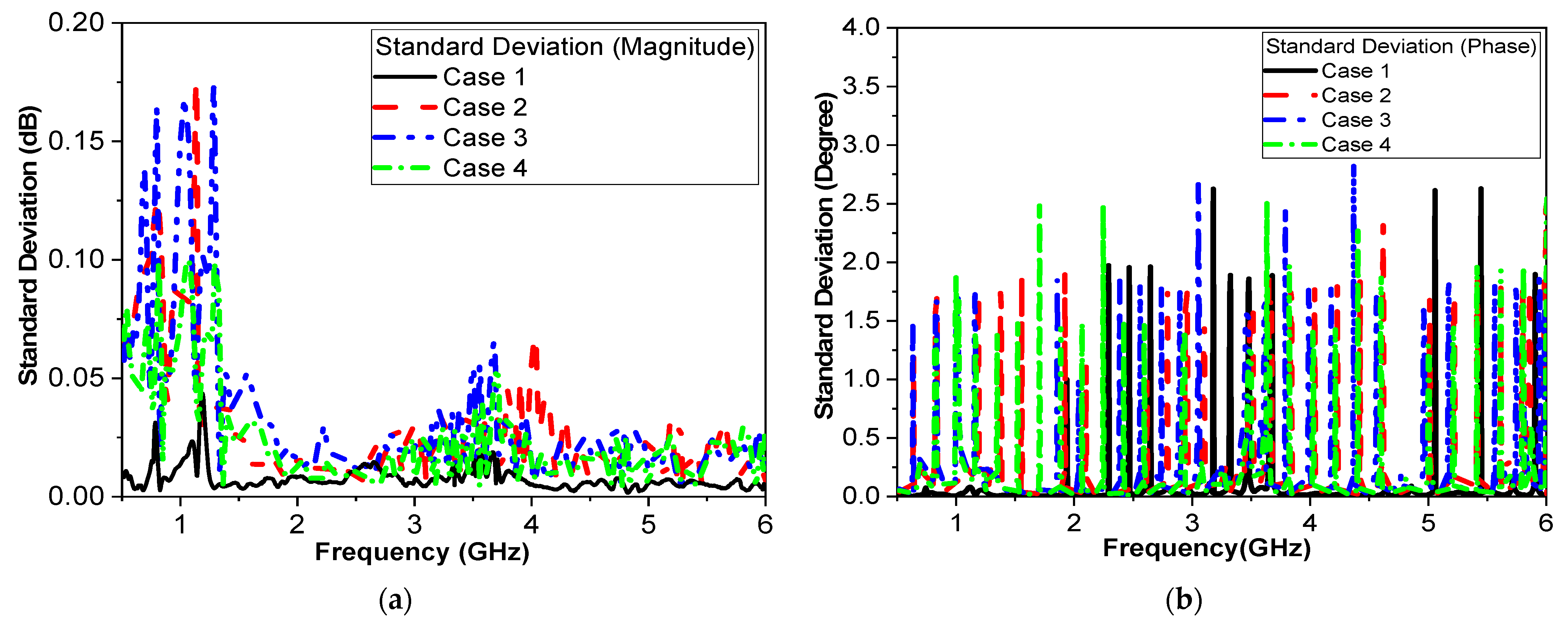
One of the objectives in the proposed study was to record sensor response while testing with humans. Four volunteers were involved in testing as a first round of measurements. These measurements provide a proof-of-concept for the sensor’s sensitivity to glucose variations within a human finger. While the small sample size limits the generalizability of the findings, consistent and significant frequency shifts were observed in the volunteers.
Figure 14 and
Table 8 provide strong preliminary evidence of the sensor’s functionality. In future studies, the influence of finger size, skin thickness, and other physiological factors on the sensor’s performance is planned. Having ethical approval, more measurements could be collected from larger number of volunteers.
Table 9 compares the performance of the proposed sensor relative to that reported in the literature, in terms of sensitivity and size. The vision for the proposed sensor is to replace the traditional glucometer without the invasive Pickering operation at each measurement. The proposed sensor fits better into the portable category than the wearable one. In terms of cost, the proposed sensor costs 10 USD. With potential additional complementary circuit and display, 10 USD more, for an overall cost of 20 USD, compared to a 70 USD traditional glucometer.
Table 9 presents an evaluation of the data collected from the newly developed microwave antenna sensor compared with different configurations that have been previously published. To compare the results of this research with those of previous studies,
Table 9 shows that the proposed sensor is more compact and has better sensitivity than the others. The proposed design, MSRRMA, demonstrates several advantages over other configurations in terms of practical applicability, sensitivity, safety, and versatility. The MSRRMA has a size of 35 × 50 mm
2, which is relatively compact compared to most alternatives. For instance, the DR sensor and Microstrip Patch Antenna have larger areas ([
5] 40 × 40 × 7 mm
2 and [
59] 43 × 43.6 mm
2, respectively), making them bulkier and less practical for wearable or portable applications. The MSRRMA’s size allows for better integration into modern mobile devices. Unlike several configurations, such as the DR sensor [
5], coplanar waveguide [
56], and cylindrical CPW [
57], the MSRRMA has been tested with humans. This practical validation ensures its reliability in real-world conditions and gives it an advantage over designs that are tested only in simulated or non-biological environments. Moreover, the proposed sensor design effectively measures glucose concentrations in the range of 0–350 mg/dL, which covers a substantial spectrum of clinically relevant glucose levels. While some designs like the coplanar waveguide [
56] support a wider range (0–4800 mg/dL), the MSRRMA’s range is sufficient for monitoring hyperglycemia and hypoglycemia. The proposed sensor operates across a wide resonant frequency range of 0.5–7 GHz, enabling greater flexibility and adaptability. This range outperforms configurations like that reported in [
54], which is limited to 1.45–1.55 GHz and [
53] fixed at 6.1 GHz. Furthermore, the proposed sensor has a specific absorption rate (SAR) of 0.687 W/Kg at dBm for 10 g, which aligns with safety standards for electromagnetic exposure. The configuration reported in [
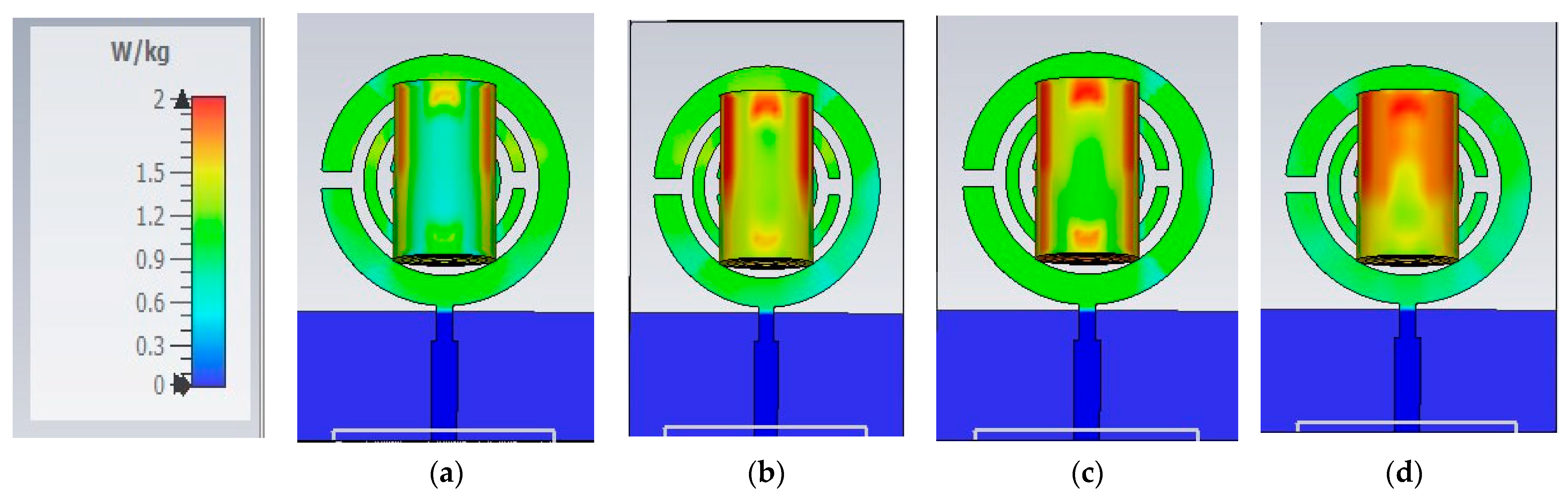
58] has an SAR of 0.05 W/Kg, and the author did not mention at which power level.
The MSRRMA slightly exceeds but still complies with acceptable limits while offering better functionality at less than 2 W/Kg, and the others did not measure or calculate the SAR; designs without SAR evaluation cannot guarantee the same safety level. Compared to other sensors, the MSRRMA combines essential features such as compactness, human trial validation, broad frequency range, and safety. While some designs may excel in specific metrics (e.g., [
5] in sensitivity), they often lack feasibility for wearable or practical medical applications. Finally, the MSRRMA exhibits a sensitivity of 24.6 MHz/mg/dL, showcasing its ability to detect minor glucose concentration variations. While the work in [
5] shows a higher sensitivity (36.86 MHz/mg/dL), the MSRRMA strikes a balance between high sensitivity and other critical factors like compactness, safety, and practical implementation.
The proposed multiband sensor targets specific frequency ranges where glucose exhibits distinctive dielectric responses. This selective sensitivity to glucose-related changes, as opposed to other biological variations, improves the sensor’s specificity and reduces interference from other blood components or physiological factors. Existing designs may lack this fine-tuned specificity, leading to higher susceptibility to noise or non-glucose-related variations in the measurements. Additionally, this approach involves designing and fabricating a custom packaging case using 3D printing technology, providing the flexibility needed to optimize sensor performance.
Selecting lower operating frequencies from 1 to 5 GHz also offers significant practical advantages over high frequency. Sensors within this frequency range are easier to integrate with existing electronic devices and systems. In addition, sensitivity should increase with frequency due to the enhanced electromagnetic interaction at higher frequencies. Otherwise operating in a low-frequency range, the proposed sensor achieves superior sensitivity compared to high-frequency designs.
In future work, evaluating the impact of sweat, movement, temperature, blood type, and finger pressure on measurement accuracy may be necessary to incorporate compensation techniques. The sensor may require calibration for different finger sizes or the ability to adapt to a wide range of tissue volumes. Advanced signal processing techniques or machine learning algorithms could be employed to account for finger size variations and enhance measurement accuracy across different users. More work will be conducted on the selectivity of glucose compared to other composites and electrolytes mixed in blood, and designing additional circuits to complement the operation of the proposed system.