Microbial Transcription Factor-Based Biosensors: Innovations from Design to Applications in Synthetic Biology
Abstract
1. Introduction
2. Overview of Genetically Encoded Biosensors
3. TF-Based Biosensor Systems
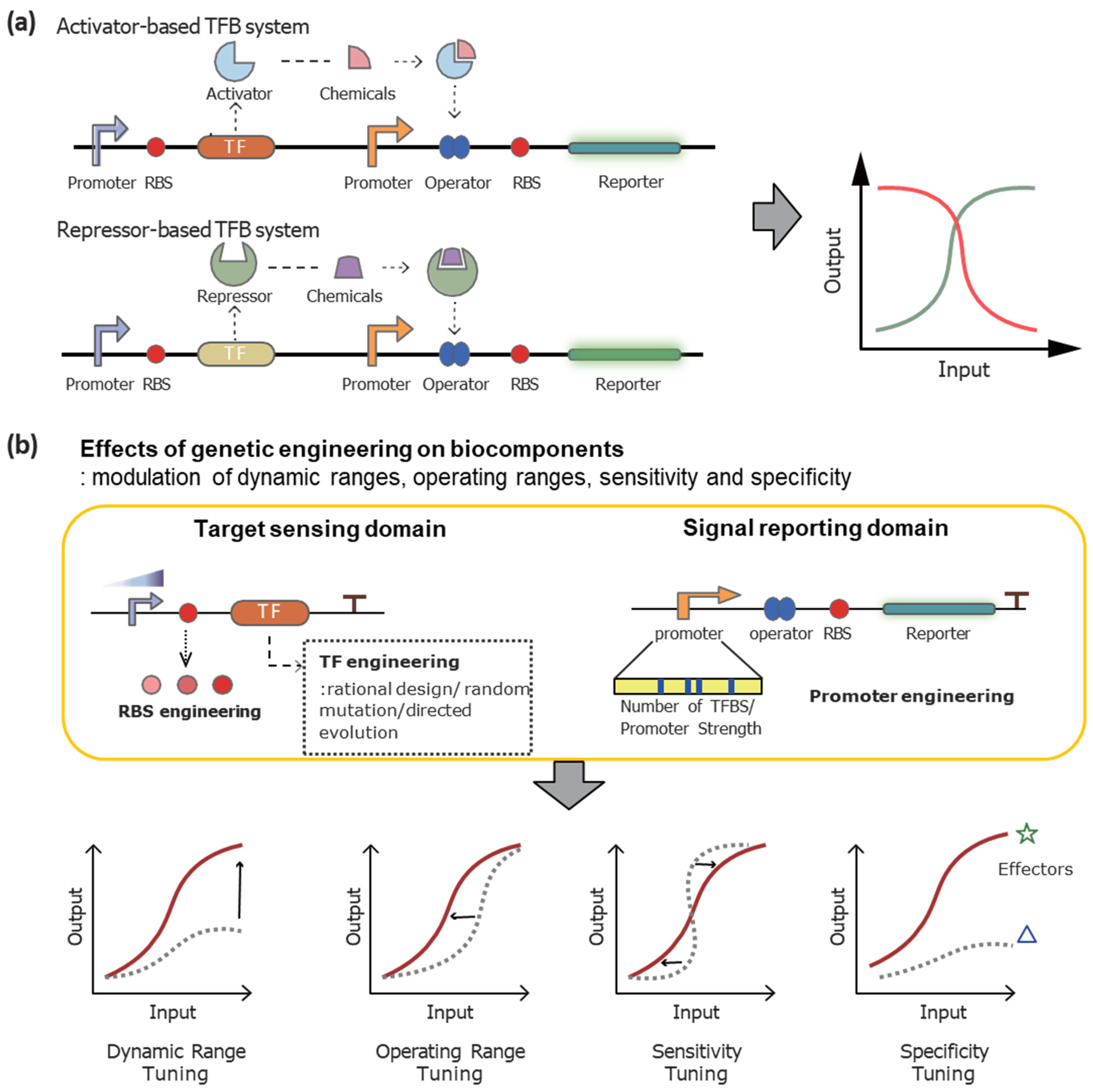
3.1. The Mechanisms of Action
3.2. Engineering Strategies
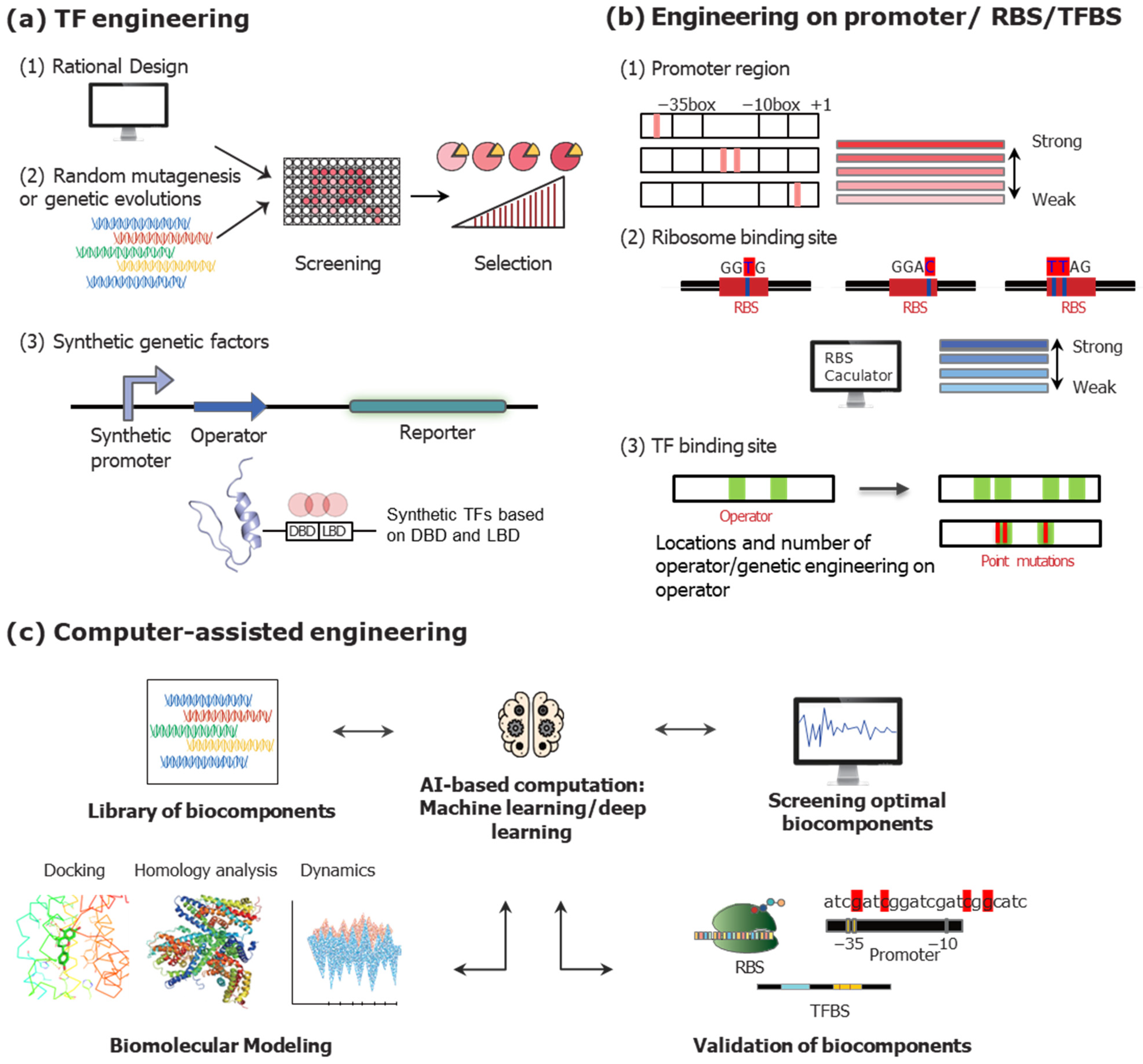
3.2.1. Genetic Engineering on TFs to Modulate TFB Systems
3.2.2. Engineering on DNA Sequences to Optimize TFB Systems
3.2.3. Recent Trends of Engineering Strategies to Enhance the TFB Systems
Design of Synthetic TFs
Computer-Assisted Engineering Strategies
4. Applications of TF-Based Biosensor Systems
4.1. TFB Systems on High-Throughput Screening
4.2. TFB Systems on Stain Evolution
4.3. Metabolic Engineering for Synthetic Biology
5. Conclusions and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, K. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [PubMed]
- Bidmanova, S.; Kotlanova, M.; Rataj, T.; Damborsky, J.; Trtilek, M.; Prokop, Z. Fluorescence-based biosensor for monitoring of environmental pollutants: From concept to field application. Biosens. Bioelectron. 2016, 84, 97–105. [Google Scholar]
- Ding, D.; Li, J.; Bai, D.; Fang, H.; Lin, J.; Zhang, D. Biosensor-based monitoring of the central metabolic pathway metabolites. Biosens. Bioelectron. 2020, 167, 112456. [Google Scholar]
- Rao Bommi, J.; Kummari, S.; Lakavath, K.; Sukumaran, R.A.; Panicker, L.R.; Marty, J.L.; Yugender Goud, K. Recent trends in biosensing and diagnostic methods for novel cancer biomarkers. Biosensors 2023, 13, 398. [Google Scholar] [CrossRef]
- Higgins, I.; Lowe, C. Introduction to the principles and applications of biosensors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1987, 316, 3–11. [Google Scholar]
- Cammann, K.; Lemke, U.; Rohen, A.; Sander, J.; Wilken, H.; Winter, B. Chemical sensors and biosensors—Principles and applications. Angew. Chem. Int. Ed. Engl. 1991, 30, 516–539. [Google Scholar]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 8. [Google Scholar]
- Song, Y.; Lin, B.; Tian, T.; Xu, X.; Wang, W.; Ruan, Q.; Guo, J.; Zhu, Z.; Yang, C. Recent progress in microfluidics-based biosensing. Anal. Chem. 2018, 91, 388–404. [Google Scholar]
- Singh, P. SPR biosensors: Historical perspectives and current challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar]
- Zamani, M.; Klapperich, C.M.; Furst, A.L. Recent advances in gold electrode fabrication for low-resource setting biosensing. Lab Chip 2023, 23, 1410–1419. [Google Scholar]
- Karnwal, A.; Kumar Sachan, R.S.; Devgon, I.; Devgon, J.; Pant, G.; Panchpuri, M.; Ahmad, A.; Alshammari, M.B.; Hossain, K.; Kumar, G. Gold nanoparticles in nanobiotechnology: From synthesis to biosensing applications. ACS Omega 2024, 9, 29966–29982. [Google Scholar] [PubMed]
- Jianrong, C.; Yuqing, M.; Nongyue, H.; Xiaohua, W.; Sijiao, L. Nanotechnology and biosensors. Biotechnol. Adv. 2004, 22, 505–518. [Google Scholar] [PubMed]
- Farrokhnia, M.; Amoabediny, G.; Ebrahimi, M.; Ganjali, M.; Arjmand, M. Ultrasensitive early detection of insulin antibody employing novel electrochemical nano-biosensor based on controllable electro-fabrication process. Talanta 2022, 238, 122947. [Google Scholar] [PubMed]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar]
- Hashem, A.; Hossain, M.M.; Marlinda, A.R.; Mamun, M.A.; Sagadevan, S.; Shahnavaz, Z.; Simarani, K.; Johan, M.R. Nucleic acid-based electrochemical biosensors for rapid clinical diagnosis: Advances, challenges, and opportunities. Crit. Rev. Clin. Lab. Sci. 2022, 59, 156–177. [Google Scholar]
- Mahr, R.; Frunzke, J. Transcription factor-based biosensors in biotechnology: Current state and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 79–90. [Google Scholar]
- Aghdam, E.M.; Hejazi, M.S.; Barzegar, A. Riboswitches: From living biosensors to novel targets of antibiotics. Gene 2016, 592, 244–259. [Google Scholar]
- Hallberg, Z.F.; Su, Y.; Kitto, R.Z.; Hammond, M.C. Engineering and in vivo applications of riboswitches. Annu. Rev. Biochem. 2017, 86, 515–539. [Google Scholar]
- Ge, H.; Marchisio, M.A. Aptamers, riboswitches, and ribozymes in S. cerevisiae synthetic biology. Life 2021, 11, 248. [Google Scholar] [CrossRef]
- Schmidt, C.M.; Smolke, C.D. RNA switches for synthetic biology. Cold Spring Harb. Perspect. Biol. 2019, 11, a032532. [Google Scholar]
- Ding, N.; Zhou, S.; Deng, Y. Transcription-factor-based biosensor engineering for applications in synthetic biology. ACS Synth. Biol. 2021, 10, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Zinkus-Boltz, J.; Dickinson, B.C. Recent advances in developing and applying biosensors for synthetic biology. Nano Futures 2019, 3, 042002. [Google Scholar] [CrossRef]
- Kavita, K.; Breaker, R.R. Discovering riboswitches: The past and the future. Trends Biochem. Sci. 2023, 48, 119–141. [Google Scholar]
- Liu, C.; Yu, H.; Zhang, B.; Liu, S.; Liu, C.-g.; Li, F.; Song, H. Engineering whole-cell microbial biosensors: Design principles and applications in monitoring and treatment of heavy metals and organic pollutants. Biotechnol. Adv. 2022, 60, 108019. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Lee, Y.; Kim, Y.; Park, C.; Choi, H.; Jang, G.; Yoon, Y. Development of novel Escherichia coli cell-based biosensors to monitor Mn (II) in environmental systems. Front. Microbiol. 2022, 13, 1051926. [Google Scholar] [CrossRef]
- Hynninen, A.; Tõnismann, K.; Virta, M. Improving the sensitivity of bacterial bioreporters for heavy metals. Bioeng. Bugs 2010, 1, 132–138. [Google Scholar] [CrossRef]
- Qin, L.; Liu, X.; Xu, K.; Li, C. Mining and design of biosensors for engineering microbial cell factory. Curr. Opin. Biotechnol. 2022, 75, 102694. [Google Scholar] [CrossRef]
- Zhou, G.J.; Zhang, F. Applications and tuning strategies for transcription factor-based metabolite biosensors. Biosensors 2023, 13, 428. [Google Scholar] [CrossRef]
- Mannan, A.A.; Liu, D.; Zhang, F.; Oyarzún, D.A. Fundamental design principles for transcription-factor-based metabolite biosensors. ACS Synth. Biol. 2017, 6, 1851–1859. [Google Scholar]
- Li, J.; Qin, Z.; Zhang, B.; Wu, X.; Wu, J.; Peng, L.; Xiao, Y. Development of transcriptional factor-based whole-cell biosensors to monitor and degrade antibiotics using mutant cells obtained via adaptive laboratory evolution. J. Hazard. Mater. 2024, 473, 134536. [Google Scholar] [CrossRef]
- Tellechea-Luzardo, J.; Stiebritz, M.T.; Carbonell, P. Transcription factor-based biosensors for screening and dynamic regulation. Front. Bioeng. Biotechnol. 2023, 11, 1118702. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Huo, Y.-X. Application Evaluation and Performance-Directed Improvement of the Native and Engineered Biosensors. ACS Sens. 2024, 9, 5002–5024. [Google Scholar] [PubMed]
- Flachbart, L.K.; Gertzen, C.G.W.; Gohlke, H.; Marienhagen, J. Development of a biosensor platform for phenolic compounds using a transition ligand strategy. ACS Synth. Biol. 2021, 10, 2002–2014. [Google Scholar]
- Kasey, C.M.; Zerrad, M.; Li, Y.; Cropp, T.A.; Williams, G.J. Development of transcription factor-based designer macrolide biosensors for metabolic engineering and synthetic biology. ACS Synth. Biol. 2018, 7, 227–239. [Google Scholar] [PubMed]
- Dabirian, Y.; Li, X.; Chen, Y.; David, F.; Nielsen, J.; Siewers, V. Expanding the dynamic range of a transcription factor-based biosensor in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 1968–1975. [Google Scholar] [PubMed]
- Zhang, Y.; Shi, S. Transcription factor-based biosensor for dynamic control in yeast for natural product synthesis. Front. Bioeng. Biotechnol. 2021, 9, 635265. [Google Scholar]
- Umeno, D.; Kimura, Y.; Kawai-Noma, S. Transcription factors as evolvable biosensors. Anal. Sci. 2021, 37, 699–703. [Google Scholar]
- Zhu, C.; Feng, Z.; Qin, H.; Chen, L.; Yan, M.; Li, L.; Qu, F. Recent progress of SELEX methods for screening nucleic acid aptamers. Talanta 2024, 266, 124998. [Google Scholar] [CrossRef]
- Gan, Z.; Roslan, M.A.M.; Abd Shukor, M.Y.; Halim, M.; Yasid, N.A.; Abdullah, J.; Md Yasin, I.S.; Wasoh, H. Advances in aptamer-based biosensors and cell-internalizing SELEX technology for diagnostic and therapeutic application. Biosensors 2022, 12, 922. [Google Scholar] [CrossRef]
- Dwidar, M.; Seike, Y.; Kobori, S.; Whitaker, C.; Matsuura, T.; Yokobayashi, Y. Programmable artificial cells using histamine-responsive synthetic riboswitch. J. Am. Chem. Soc. 2019, 141, 11103–11114. [Google Scholar]
- Kim, H.; Ju, J.; Lee, H.N.; Chun, H.; Seong, J. Genetically encoded biosensors based on fluorescent proteins. Sensors 2021, 21, 795. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Da, Y.; Tian, Y. Fluorescent proteins and genetically encoded biosensors. Chem. Soc. Rev. 2023, 52, 1189–1214. [Google Scholar] [PubMed]
- Zhang, J.; Jensen, M.K.; Keasling, J.D. Development of biosensors and their application in metabolic engineering. Curr. Opin. Chem. Biol. 2015, 28, 1–8. [Google Scholar]
- Baya, G.; Muhindi, S.; Ngendahimana, V.; Caguiat, J. Potential whole-cell biosensors for detection of metal using MerR family proteins from Enterobacter sp. YSU and Stenotrophomonas maltophilia OR02. Micromachines 2021, 12, 142. [Google Scholar] [CrossRef]
- Fernandez-López, R.; Ruiz, R.; de la Cruz, F.; Moncalián, G. Transcription factor-based biosensors enlightened by the analyte. Front. Microbiol. 2015, 6, 648. [Google Scholar]
- Williamson, L.L.; Borlee, B.R.; Schloss, P.D.; Guan, C.; Allen, H.K.; Handelsman, J. Intracellular screen to identify metagenomic clones that induce or inhibit a quorum-sensing biosensor. Appl. Environ. Microbiol. 2005, 71, 6335–6344. [Google Scholar]
- Jeon, Y.; Lee, Y.; Kim, K.; Jang, G.; Yoon, Y. Transcription Factor-Based Biosensors for Detecting Pathogens. Biosensors 2022, 12, 470. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, Y.; Song, K.; Ji, H.; Hwang, S.-J.; Yoon, Y. Development of an Escherichia coli Cell-Based Biosensor for Aspirin Monitoring by Genetic Engineering of MarR. Biosensors 2024, 14, 547. [Google Scholar] [CrossRef]
- Libis, V.; Delépine, B.; Faulon, J.-L. Sensing new chemicals with bacterial transcription factors. Curr. Opin. Microbiol. 2016, 33, 105–112. [Google Scholar]
- Guo, Y.; Hui, C.-y.; Liu, L.; Chen, M.-p.; Huang, H.-y. Development of a bioavailable Hg (II) sensing system based on MerR-regulated visual pigment biosynthesis. Sci. Rep. 2021, 11, 13516. [Google Scholar]
- Din, G.; Hasan, F.; Conway, M.; Denney, B.; Ripp, S.; Shah, A.A. Engineering a bioluminescent bioreporter from an environmentally sourced mercury-resistant Enterobacter cloacae strain for the detection of bioavailable mercury. J. Appl. Microbiol. 2019, 127, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Ren, X.; Li, J.; Liang, F.; Rao, X.; Gao, Y.; Wu, W.; Li, D.; Wang, J.; Zhao, J. Development of a sensitive Escherichia coli bioreporter without antibiotic markers for detecting bioavailable copper in water environments. Front. Microbiol. 2020, 10, 3031. [Google Scholar] [CrossRef] [PubMed]
- Riether, K.; Dollard, M.-A.; Billard, P. Assessment of heavy metal bioavailability using Escherichia coli zntAp:: Lux and copAp:: Lux-based biosensors. Appl. Microbiol. Biotechnol. 2001, 57, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, E.; Hosseinkhani, S.; Nedaeinia, R.; Khoshdel-Sarkarizi, H.; Nedaeinia, M.; Ranjbar, M.; Ebrahimi, N.; Farjami, Z.; Nourmohammadi, M.; Mahmoudi, A. Construction of a sensitive and specific lead biosensor using a genetically engineered bacterial system with a luciferase gene reporter controlled by pbr and cadA promoters. BioMed. Eng. OnLine 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Alam, K.K.; Jung, J.K.; Verosloff, M.S.; Clauer, P.R.; Lee, J.W.; Capdevila, D.A.; Pastén, P.A.; Giedroc, D.P.; Collins, J.J.; Lucks, J.B. Rapid, low-cost detection of water contaminants using regulated in vitro transcription. BioRxiv 2019, 619296. [Google Scholar] [CrossRef]
- Uchiyama, T.; Miyazaki, K. Product-induced gene expression, a product-responsive reporter assay used to screen metagenomic libraries for enzyme-encoding genes. Appl. Environ. Microbiol. 2010, 76, 7029–7035. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, Y.; Jang, G.; Kim, B.-G.; Yoon, Y. A novel Escherichia coli cell–based bioreporter for quantification of salicylic acid in cosmetics. Appl. Microbiol. Biotechnol. 2024, 108, 148. [Google Scholar] [CrossRef]
- Raman, S.; Rogers, J.K.; Taylor, N.D.; Church, G.M. Evolution-guided optimization of biosynthetic pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 17803–17808. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Wang, L.; Zhao, J.; Li, S.; Yi, L.; Jiang, S.; Lu, Z.; Zhang, G. A new yeast-based bioreporter for simple, sensitive, and cost-effective detection of dioxin-like compounds. Sens. Actuators B Chem. 2025, 423, 136730. [Google Scholar] [CrossRef]
- Siedler, S.; Stahlhut, S.G.; Malla, S.; Maury, J.; Neves, A.R. Novel biosensors based on flavonoid-responsive transcriptional regulators introduced into Escherichia coli. Metab. Eng. 2014, 21, 2–8. [Google Scholar] [CrossRef]
- Massai, F.; Imperi, F.; Quattrucci, S.; Zennaro, E.; Visca, P.; Leoni, L. A multitask biosensor for micro-volumetric detection of N-3-oxo-dodecanoyl-homoserine lactone quorum sensing signal. Biosens. Bioelectron. 2011, 26, 3444–3449. [Google Scholar] [PubMed]
- Wu, Y.; Wang, C.-W.; Wang, D.; Wei, N. A Whole-Cell Biosensor for Point-of-Care Detection of Waterborne Bacterial Pathogens. ACS Synth. Biol. 2021, 10, 333–344. [Google Scholar] [CrossRef]
- Winson, M.K.; Swift, S.; Fish, L.; Throup, J.P.; Jørgensen, F.; Chhabra, S.R.; Bycroft, B.W.; Williams, P.; Stewart, G.S. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 1998, 163, 185–192. [Google Scholar]
- Raut, N.; Pasini, P.; Daunert, S. Deciphering bacterial universal language by detecting the quorum sensing signal, autoinducer-2, with a whole-cell sensing system. Anal. Chem. 2013, 85, 9604–9609. [Google Scholar]
- Kim, H.; Jang, G.; Kim, B.-G.; Yoon, Y. Modulation of the metal (loid) specificity of whole-cell bioreporters by genetic engineering of ZntR metal-binding loops. J. Microbiol. Biotechnol. 2020, 30, 681. [Google Scholar]
- Kang, Y.; Lee, W.; Jang, G.; Kim, B.-G.; Yoon, Y. Modulating the sensing properties of Escherichia coli-based bioreporters for cadmium and mercury. Appl. Microbiol. Biotechnol. 2018, 102, 4863–4872. [Google Scholar] [CrossRef]
- FM Machado, L.; Currin, A.; Dixon, N. Directed evolution of the PcaV allosteric transcription factor to generate a biosensor for aromatic aldehydes. J. Biol. Eng. 2019, 13, 91. [Google Scholar]
- Teng, Y.; Gong, X.; Zhang, J.; Obideen, Z.; Yan, Y. Investigating and engineering an 1, 2-propanediol-responsive transcription factor-based biosensor. ACS Synth. Biol. 2024, 13, 2177–2187. [Google Scholar]
- Wang, Z.; Doshi, A.; Chowdhury, R.; Wang, Y.; Maranas, C.D.; Cirino, P.C. Engineering sensitivity and specificity of AraC-based biosensors responsive to triacetic acid lactone and orsellinic acid. Protein Eng. Des. Sel. 2020, 33, gzaa027. [Google Scholar]
- Wu, J.; Jiang, P.; Chen, W.; Xiong, D.; Huang, L.; Jia, J.; Chen, Y.; Jin, J.-M.; Tang, S.-Y. Design and application of a lactulose biosensor. Sci. Rep. 2017, 7, 45994. [Google Scholar]
- Chen, S.-Y.; Zhang, Y.; Li, R.; Wang, B.; Ye, B.-C. De novo design of the ArsR regulated P ars promoter enables a highly sensitive whole-cell biosensor for arsenic contamination. Anal. Chem. 2022, 94, 7210–7218. [Google Scholar] [PubMed]
- Zou, Y.; Li, C.; Zhang, R.; Jiang, T.; Liu, N.; Wang, J.; Wang, X.; Yan, Y. Exploring the tunability and dynamic properties of MarR-PmarO sensor system in Escherichia coli. ACS Synth. Biol. 2021, 10, 2076–2086. [Google Scholar]
- Xu, P.; Wang, W.; Li, L.; Bhan, N.; Zhang, F.; Koffas, M.A. Design and kinetic analysis of a hybrid promoter–regulator system for malonyl-CoA sensing in Escherichia coli. ACS Chem. Biol. 2014, 9, 451–458. [Google Scholar]
- Liang, C.; Zhang, X.; Wu, J.; Mu, S.; Wu, Z.; Jin, J.-M.; Tang, S.-Y. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit. Metab. Eng. 2020, 57, 239–246. [Google Scholar] [PubMed]
- Ding, N.; Yuan, Z.; Zhang, X.; Chen, J.; Zhou, S.; Deng, Y. Programmable cross-ribosome-binding sites to fine-tune the dynamic range of transcription factor-based biosensor. Nucleic Acids Res. 2020, 48, 10602–10613. [Google Scholar]
- Khalil, A.S.; Lu, T.K.; Bashor, C.J.; Ramirez, C.L.; Pyenson, N.C.; Joung, J.K.; Collins, J.J. A synthetic biology framework for programming eukaryotic transcription functions. Cell 2012, 150, 647–658. [Google Scholar] [PubMed]
- Chen, Y.; Zheng, H.; Yang, J.; Cao, Y.; Zhou, H. Development of a synthetic transcription factor-based S-adenosylmethionine biosensor in Saccharomyces cerevisiae. Biotechnol. Lett. 2023, 45, 255–262. [Google Scholar]
- Bhat, S.; Banerjee, A.; Alagesan, S. AraC-Based Biosensor for the Detection of Isoprene in E. coli. ACS Omega 2023, 8, 26806–26815. [Google Scholar]
- Zhou, Y.; Yuan, Y.; Wu, Y.; Li, L.; Jameel, A.; Xing, X.-H.; Zhang, C. Encoding genetic circuits with DNA barcodes paves the way for machine learning-assisted metabolite biosensor response curve profiling in yeast. ACS Synth. Biol. 2022, 11, 977–989. [Google Scholar]
- de Almeida, B.P.; Reiter, F.; Pagani, M.; Stark, A. DeepSTARR predicts enhancer activity from DNA sequence and enables the de novo design of synthetic enhancers. Nat. Genet. 2022, 54, 613–624. [Google Scholar]
- Yoon, Y.; Kang, Y.; Lee, W.; Oh, K.-C.; Jang, G.; Kim, B.-G. Modulating the properties of metal-sensing whole-cell bioreporters by interfering with Escherichia coli metal homeostasis. J. Microbiol. Biotechnol. 2018, 28, 323–329. [Google Scholar] [PubMed]
- Qu, G.; Liu, B.; Zhang, K.; Jiang, Y.; Guo, J.; Wang, R.; Miao, Y.; Zhai, C.; Sun, Z. Computer-assisted engineering of the catalytic activity of a carboxylic acid reductase. J. Biotechnol. 2019, 306, 97–104. [Google Scholar] [PubMed]
- Siedhoff, N.E.; Schwaneberg, U.; Davari, M.D. Machine learning-assisted enzyme engineering. Methods Enzymol. 2020, 643, 281–315. [Google Scholar]
- Tellechea-Luzardo, J.; Martín Lázaro, H.; Moreno López, R.; Carbonell, P. Sensbio: An online server for biosensor design. BMC Bioinform. 2023, 24, 71. [Google Scholar]
- Kim, H.; Seong, W.; Rha, E.; Lee, H.; Kim, S.K.; Kwon, K.K.; Park, K.-H.; Lee, D.-H.; Lee, S.-G. Machine learning linked evolutionary biosensor array for highly sensitive and specific molecular identification. Biosens. Bioelectron. 2020, 170, 112670. [Google Scholar]
- Ding, N.; Yuan, Z.; Ma, Z.; Wu, Y.; Yin, L. AI-Assisted Rational Design and Activity Prediction of Biological Elements for Optimizing Transcription-Factor-Based Biosensors. Molecules 2024, 29, 3512. [Google Scholar] [CrossRef]
- Cheng, F.; Tang, X.L.; Kardashliev, T. Transcription factor-based biosensors in high-throughput screening: Advances and applications. Biotechnol. J. 2018, 13, 1700648. [Google Scholar]
- Zhao, M.; Shang, J.; Chen, J.; Zabed, H.M.; Qi, X. Fine-Tuning the Expression of the Glycolate Biosynthetic Pathway in Escherichia coli Using Synthetic Promoters. Fermentation 2024, 10, 67. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, X.; Lv, X.; Liu, L.; Li, J.; Du, G.; Chen, J.; Liu, Y. Construction of short synthetic promoters for optimization of ovalbumin expression level in Saccharomyces cerevisiae. Syst. Microbiol. Biomanuf. 2024, 4, 996–1005. [Google Scholar]
- Huang, Y.-K.; Yu, C.-H.; Ng, I.-S. Precise strength prediction of endogenous promoters from Escherichia coli and J-series promoters by artificial intelligence. J. Taiwan Inst. Chem. Eng. 2024, 160, 105211. [Google Scholar]
- Yasmeen, E.; Wang, J.; Riaz, M.; Zhang, L.; Zuo, K. Designing artificial synthetic promoters for accurate, smart, and versatile gene expression in plants. Plant Commun. 2023, 4, 100558. [Google Scholar]
- Kumaran, A.; Jude Serpes, N.; Gupta, T.; James, A.; Sharma, A.; Kumar, D.; Nagraik, R.; Kumar, V.; Pandey, S. Advancements in CRISPR-based biosensing for next-gen point of care diagnostic application. Biosensors 2023, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, F.; Yan, W.; Dai, Z.; Dong, W.; Zhou, J.; Zhang, W.; Xin, F.; Jiang, M. Recent advances of CRISPR/Cas9-based genetic engineering and transcriptional regulation in industrial biology. Front. Bioeng. Biotechnol. 2020, 7, 459. [Google Scholar]
- Chen, W.C.; Gaidukov, L.; Lai, Y.; Wu, M.-R.; Cao, J.; Gutbrod, M.J.; Choi, G.C.; Utomo, R.P.; Chen, Y.-C.; Wroblewska, L. A synthetic transcription platform for programmable gene expression in mammalian cells. Nat. Commun. 2022, 13, 6167. [Google Scholar]
- Vaknin, I.; Amit, R. Molecular and experimental tools to design synthetic enhancers. Curr. Opin. Biotechnol. 2022, 76, 102728. [Google Scholar]
- Perez-Pinera, P.; Ousterout, D.G.; Brunger, J.M.; Farin, A.M.; Glass, K.A.; Guilak, F.; Crawford, G.E.; Hartemink, A.J.; Gersbach, C.A. Synergistic and tunable human gene activation by combinations of synthetic transcription factors. Nat. Methods 2013, 10, 239–242. [Google Scholar]
- Yaghmai, R.; Cutting, G.R. Optimized regulation of gene expression using artificial transcription factors. Mol. Ther. 2002, 5, 685–694. [Google Scholar]
- Crampon, K.; Giorkallos, A.; Deldossi, M.; Baud, S.; Steffenel, L.A. Machine-learning methods for ligand–protein molecular docking. Drug Discov. Today 2022, 27, 151–164. [Google Scholar]
- Wang, H.; Fu, T.; Du, Y.; Gao, W.; Huang, K.; Liu, Z.; Chandak, P.; Liu, S.; Van Katwyk, P.; Deac, A. Scientific discovery in the age of artificial intelligence. Nature 2023, 620, 47–60. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar]
- Jang, W.D.; Kim, G.B.; Kim, Y.; Lee, S.Y. Applications of artificial intelligence to enzyme and pathway design for metabolic engineering. Curr. Opin. Biotechnol. 2022, 73, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Poon, M.N.; Zeng, X.; Zhang, P.; Wei, Z.; Wang, H.; Wang, Y.; Wei, L.; Wang, X. Synthetic promoter design in Escherichia coli based on multinomial diffusion model. iScience 2024, 27, 111207. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Höllerer, S.; Papaxanthos, L.; Gumpinger, A.C.; Fischer, K.; Beisel, C.; Borgwardt, K.; Benenson, Y.; Jeschek, M. Large-scale DNA-based phenotypic recording and deep learning enable highly accurate sequence-function mapping. Nat. Commun. 2020, 11, 3551. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, R.; Liu, Y.; Chen, S.; Liu, Q.; Zeng, W. Applications of transformer-based language models in bioinformatics: A survey. Bioinform. Adv. 2023, 3, vbad001. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Gao, Y.; Palsson, B.O.; Lee, S.Y. DeepTFactor: A deep learning-based tool for the prediction of transcription factors. Proc. Natl. Acad. Sci. USA 2021, 118, e2021171118. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Xue, N.; Wang, L.; Zhang, X.; Zhao, L.; Guo, Y.; Zhang, Y.; Wang, M. Modulating sensitivity of an erythromycin biosensor for precise high-throughput screening of strains with different characteristics. ACS Synth. Biol. 2023, 12, 1761–1771. [Google Scholar] [CrossRef]
- Binder, S.; Schendzielorz, G.; Stäbler, N.; Krumbach, K.; Hoffmann, K.; Bott, M.; Eggeling, L. A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level. Genome Biol. 2012, 13, R40. [Google Scholar] [CrossRef]
- d’Oelsnitz, S.; Nguyen, V.; Alper, H.S.; Ellington, A.D. Evolving a generalist biosensor for bicyclic monoterpenes. ACS Synth. Biol. 2022, 11, 265–272. [Google Scholar] [CrossRef]
- Chen, B.; Tang, X.; Zhang, Y.; Zabed, H.M.; Ravikumar, Y.; Iqbal, M.W.; Wang, J.; Zhao, M.; Qi, X. Biosensor-assisted evolution of RhaD for enhancing the biosynthetic yield of d-allulose. Food Biosci. 2024, 60, 104426. [Google Scholar] [CrossRef]
- Ding, N.; Sun, L.; Zhou, X.; Zhang, L.; Deng, Y.; Yin, L. Enhancing glucaric acid production from myo-inositol in Escherichia coli by eliminating cell-to-cell variation. Appl. Environ. Microbiol. 2024, 90, e00149-24. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, J.M.; Wagner, J.M.; Tu, C.C.; Tong, A.; Liu, Y.; Alper, H.S. Biosensor-enabled directed evolution to improve muconic acid production in Saccharomyces cerevisiae. Biotechnol. J. 2017, 12, 1600687. [Google Scholar]
- Chen, C.; Liu, J.; Yao, G.; Bao, S.; Wan, X.; Wang, F.; Wang, K.; Song, T.; Han, P.; Liu, T. A novel, genetically encoded whole-cell biosensor for directed evolution of myrcene synthase in Escherichia coli. Biosens. Bioelectron. 2023, 228, 115176. [Google Scholar]
- Dietrich, J.A.; Shis, D.L.; Alikhani, A.; Keasling, J.D. Transcription factor-based screens and synthetic selections for microbial small-molecule biosynthesis. ACS Synth. Biol. 2013, 2, 47–58. [Google Scholar]
- Tong, Y.; Li, N.; Zhou, S.; Zhang, L.; Xu, S.; Zhou, J. Improvement of Chalcone Synthase Activity and High-Efficiency Fermentative Production of (2 S)-Naringenin via In Vivo Biosensor-Guided Directed Evolution. ACS Synth. Biol. 2024, 13, 1454–1466. [Google Scholar]
- Chou, H.H.; Keasling, J.D. Programming adaptive control to evolve increased metabolite production. Nat. Commun. 2013, 4, 2595. [Google Scholar] [PubMed]
- Zhang, F.; Carothers, J.M.; Keasling, J.D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 2012, 30, 354–359. [Google Scholar]
- Zhu, Y.; Li, Y.; Xu, Y.; Zhang, J.; Ma, L.; Qi, Q.; Wang, Q. Development of bifunctional biosensors for sensing and dynamic control of glycolysis flux in metabolic engineering. Metab. Eng. 2021, 68, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, H.; Zhang, J.; Zhao, J.; Qi, Q.; Wang, Q. De novo design of the global transcriptional factor Cra-regulated promoters enables highly sensitive glycolysis flux biosensor for dynamic metabolic control. Microb. Biotechnol. 2023, 16, 605–617. [Google Scholar]
- Xu, X.; Li, X.; Liu, Y.; Zhu, Y.; Li, J.; Du, G.; Chen, J.; Ledesma-Amaro, R.; Liu, L. Pyruvate-responsive genetic circuits for dynamic control of central metabolism. Nat. Chem. Biol. 2020, 16, 1261–1268. [Google Scholar]
- Verma, B.K.; Mannan, A.A.; Zhang, F.; Oyarzún, D.A. Trade-offs in biosensor optimization for dynamic pathway engineering. ACS Synth. Biol. 2021, 11, 228–240. [Google Scholar]
- Moon, T.S.; Lou, C.; Tamsir, A.; Stanton, B.C.; Voigt, C.A. Genetic programs constructed from layered logic gates in single cells. Nature 2012, 491, 249–253. [Google Scholar] [CrossRef]
- Nielsen, A.A.; Der, B.S.; Shin, J.; Vaidyanathan, P.; Paralanov, V.; Strychalski, E.A.; Ross, D.; Densmore, D.; Voigt, C.A. Genetic circuit design automation. Science 2016, 352, aac7341. [Google Scholar] [PubMed]
- O’Connor, E.; Micklefield, J.; Cai, Y. Searching for the optimal microbial factory: High-throughput biosensors and analytical techniques for screening small molecules. Curr. Opin. Biotechnol. 2024, 87, 103125. [Google Scholar]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed evolution: Methodologies and applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar]
- Romero, P.A.; Arnold, F.H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 2009, 10, 866–876. [Google Scholar]
- Long, C.P.; Antoniewicz, M.R. How adaptive evolution reshapes metabolism to improve fitness: Recent advances and future outlook. Curr. Opin. Chem. Eng. 2018, 22, 209–215. [Google Scholar] [PubMed]
- Leavitt, J.M.; Tong, A.; Tong, J.; Pattie, J.; Alper, H.S. Coordinated transcription factor and promoter engineering to establish strong expression elements in Saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 866–876. [Google Scholar]
- De Paepe, B.; Peters, G.; Coussement, P.; Maertens, J.; De Mey, M. Tailor-made transcriptional biosensors for optimizing microbial cell factories. J. Ind. Microbiol. Biotechnol. 2017, 44, 623–645. [Google Scholar]
- Yu, W.; Xu, X.; Jin, K.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Genetically encoded biosensors for microbial synthetic biology: From conceptual frameworks to practical applications. Biotechnol. Adv. 2023, 62, 108077. [Google Scholar]
- Choe, D.; Lee, J.H.; Yoo, M.; Hwang, S.; Sung, B.H.; Cho, S.; Palsson, B.; Kim, S.C.; Cho, B.-K. Adaptive laboratory evolution of a genome-reduced Escherichia coli. Nat. Commun. 2019, 10, 935. [Google Scholar]
- Teng, Y.; Zhang, J.; Jiang, T.; Zou, Y.; Gong, X.; Yan, Y. Biosensor-enabled pathway optimization in metabolic engineering. Curr. Opin. Biotechnol. 2022, 75, 102696. [Google Scholar]
- Saltepe, B.; Kehribar, E.Ş.; Su Yirmibeşoğlu, S.S.; Şafak Şeker, U.Ö. Cellular biosensors with engineered genetic circuits. ACS Sens. 2018, 3, 13–26. [Google Scholar] [PubMed]
- Xiang, Y.; Dalchau, N.; Wang, B. Scaling up genetic circuit design for cellular computing: Advances and prospects. Nat. Comput. 2018, 17, 833–853. [Google Scholar]
| Targets | TFs | Origin | Dynamic Range and DL | Ref. | |
|---|---|---|---|---|---|
| Heavy metals | Hg(II) | MerR | E. coli P. luminescens | 0.78–12.5 μM; 0.39 μM 0.4–1600 μg/L; 0.2 µg/L | [50] [51] |
| Cu(II) | CueR | E. coli | 0.39–78.68 μM | [52] | |
| As(III), As(V) | ArsR | E. coli | 10 µg/L | [53] | |
| Zn(II), Hg(II), Cd(II) | ZntR | E. coli | 3–30, 30–300, 0.01–1 μM | [24] | |
| Pb(II) | PbrR | C. metallidurans | 0.2 to 0.05 μg/mL | [54] | |
| Mn(II) | MntR | E. coli | 0.01–10 µM | [25] | |
| Organic chemicals | 3-HBA Tetracycline | MobR TetR | C. testosterone E. coli | 2 mM 1.25 μM | [55] |
| 3-MBz | BenR | E. coli | 0.1–1.0 mM | [56] | |
| Salicylic acid | MarR | E. coli | 5 µM | [57] | |
| tetracycline | TetR | E. coli | 0.05–0.15 µM | [58] | |
| TCDD | AhR-ARNT | human | 10 fM | [59] | |
| Flavonoids | Kaempferol Quercetin | QdoR | E. coli | 0.01–0.05 mM 0.01–0.05 mM | [60] |
| Naringenin | FdeR | E. coli | 0.01–0.05 mM | [60] | |
| Phloretin Genistein | TtgR | E. coli | 0.01–0.1 mM 0.001–0.1 mM | [58] | |
| Quorum sensing molecules | HSLs and AHLs | LasR QscR LuxR RhlR | P. aeruginosa P. aeruginosa V. fischeri P. aeruginosa | pM—μM 0.01–0.1 μM - - | [61] [62] [63] |
| Autoinducer-2 | LuxR | V. harveyi BB170 | 0.25 pM | [64] |
| Genetic System | Strategies | Effects on Performances of TFB Systems | Ref. | |
|---|---|---|---|---|
| TF engineering | ZntR-PzntA | Replacing the MBLs Rational design-based mutagenesis on ZntR | Broad specificity of TFB modulated to Hg and Cd specific Enhancing Cd and Hg sensitivity | [65] [66] |
| PcaV-PPV | Direct evolution on PcaV | Selectivity shifted from PCA to vanillin | [67] | |
| MarR-marO | Rational design-based mutagenesis on MarR | Modulating the specificity and selectivity of TFB system to aspirin | [48] | |
| PocR-Pcob | Mutation on PocR to modulate the interaction with activator | Interaction with activator altered the level of RNA polymerase recruiting, regulating sensitivity, and dynamic ranges of TFB system | [68] | |
| AraC | Direct evolution of AraC and TetA-based dual-selection by introducing OA as a ‘decoy’ ligand | Improvement of selectivity and sensitivity of TFB systems toward ligands about 24-fold compared with native TFB systems | [69] | |
| LacI | LacI engineering by saturation mutagenesis | Altering effector specificity to lactulose and applied to C2E evolution to enhance lactulose production | [70] | |
| Engineering on DNA sequences | ArsR-Pars | Promoter sequence optimization and TFBS adjustment | Enhancing the sensitivity (9.38 ppb of DL) and expansion of the dynamic range (0–5 ppb) | [71] |
| MarR- marO | Modulating the strength of promoters | Modulating the dynamic ranges of TFB system toward SA | [72] | |
| FapR-fapO FapR | Insertion of lacO between promoter and fapO Modification and re-localization of TFBS on promoter | Enhancing biosynthesis of malonyl-CoA-derived compounds by controlling the dynamic range and optimized carbon flux The dynamic ranges of TFB system modulated by types of promoters and the number of TFBS | [73] [35] | |
| HucR-PhucR | Mutation on HucR and modifying promoter sequences | Increase sensitivity to vanillin about 27-fold and 10-fold increase in vanillin production by engineering | [74] | |
| CdaR | Library screening of RBSn and RBSm for TFs and reporter genes | Evaluating the effects of combining both RBSs and constructing powerful platform to tune the dynamic range of biosensors by deep learning | [75] | |
| Synthetic TFs | ZFs-synthetic operators | Construction of TFB systems based on various combinations of ZF-based TFs and operators | TFB systems showed different dynamic ranges upon the sequences of sTF and operators; the outputs were modulated by genetic components | [76] |
| MetJ-hER-VP16 | Construction of sTF by conjugating MetJ, hER, and VP16 | TFB system based on synthetic TF responds to SAM in a dose-dependent manner | [77] | |
| Acla-PAraC | Replacing the LBD of AraC with IsoA to construct chimeric TF | Modulating sensitivity and specificity of TFB system toward isoprene by employing chimeric TF | [78] | |
| Computer-assisted engineering | RBS | Construction of CLM-RDR by deep learning of large datasets cRBSs | AI-based RBSs design and verifying the prediction accuracy using arabinose and glycolate biosensors | [75] |
| Enhancer/ Operator | Construction of MLalgorithm to predict dose-response relationship | Prediction the genotype-phenotype relationships based on biocomponents | [79] | |
| Enhancer | a deep learning model, DeepSTARR, to predict activity of enhancers | de novo design and functional validation of synthetic enhancers with desired activities | [80] |
| Targets | TFs | Origin | Roles of TFB Systems | Outcome | Ref. | |
|---|---|---|---|---|---|---|
| HTS | erythromycin | MphR | S. erythraea | Screening strain libraries of RBS engineering | A total of 6.8-fold increase in erythromycin production | [107] |
| Lysine | LysG | C. glutamicum | Screening strain from library generated by MNNG treatment | A total of 21% improvement in lysine production | [108] | |
| BMP | CamR | P. putida | Promoter, operator, and RBS engineering and CamR evolution | increased the system’s signal-to-noise ratio to 150-fold. | [109] | |
| D-allulose | PsiR | A. tumefaciens | Selecting RhaD mutants from directed evolution | Two superior strains isolated from 40,000 colonies | [110] | |
| GA | CdaR | E. coil | Selecting high GA-producing strain | A total of 17-fold increase in GA production | [111] | |
| Directed/adapted evolution | AAA | ARO80 | S. cerevisiae | High AAA-producing strain selection from ALE | the highest MA-producing titer reported to date | [112] |
| β-myrcene | MyrR | Pseudomonas sp. | Applied for directed evolution of myrcene synthase | the highest titer reported to date: 510.38 mg/L of myrcene | [113] | |
| 1-butanol | BmoR | P. butanovora | Selection of high 1-butanal-producing strain | A total of 120-fold enrichment for a 1-butanol | [114] | |
| Kaempf | QdoR | B. subtills | Selection of high kaempferol-producing strains from library | A total of 56 mM of kaempferol produced per OD600 in E. coli | [60] | |
| Naringenin | TtgR | P. putida | Selecting enhanced CHS from directed evolution | increasing the naringenin titer by 65.34% | [115] | |
| IPP | sTF | E. coli | Selection of high IPP-producing strains induced by mutD5 | Increase the IPP production by E. coli evolved by FREP | [116] | |
| Metabolic engineering | Fatty acid | FadR | E. coli | Regulation of FAEE production pathway by the DSRS | A total of 1.5 g/L and 3-fold yield increase in FAEE | [117] |
| FBP | Cra | E. coli | Dynamic control of glycolysis flux in E. coli | A total of 111.3 g/L of mevalonate without generating by-products | [118] | |
| FBP | Cra | E. coli | Dynamic control of the following: (1) Target ATP Synthesis Gene (2) Membrane Synthesis Gene | Increasing production of the following: (1) Pyruvate (9.66 g/L) (2) Lycopene (100.3 mg/L) | [119] | |
| pyruvate | PdhR | B. subtills | Design genetic circuits for dynamic dual control (activation and inhibition) | Four-fold increase in glucaric acid production | [120] | |
| Programming genetic circuits | Model to optimize performance trade-off in the design of metabolite biosensors | Optimizing the flux-versus-burden trade-off | Design a kinetic model for dynamic control circuits | [121] | ||
| Ara, IPTG, aTC, and etc. | AraC, TetR, Laci, Sica, InvF, and etc. | Transducing the input signals to layering logic gates | Construction of logic gates and a design strategy for integrated circuits | [122] | ||
| Computational tool, Cello, to construct in silico design for genetic circuits | A genetic module to regulate input and output signals | Forty-five out of sixty designed circuits for E. coli performed | [123] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Ji, H.; Lee, J.; Yoon, Y. Microbial Transcription Factor-Based Biosensors: Innovations from Design to Applications in Synthetic Biology. Biosensors 2025, 15, 221. https://doi.org/10.3390/bios15040221
Song K, Ji H, Lee J, Yoon Y. Microbial Transcription Factor-Based Biosensors: Innovations from Design to Applications in Synthetic Biology. Biosensors. 2025; 15(4):221. https://doi.org/10.3390/bios15040221
Chicago/Turabian StyleSong, Kyeongseok, Haekang Ji, Jiwon Lee, and Youngdae Yoon. 2025. "Microbial Transcription Factor-Based Biosensors: Innovations from Design to Applications in Synthetic Biology" Biosensors 15, no. 4: 221. https://doi.org/10.3390/bios15040221
APA StyleSong, K., Ji, H., Lee, J., & Yoon, Y. (2025). Microbial Transcription Factor-Based Biosensors: Innovations from Design to Applications in Synthetic Biology. Biosensors, 15(4), 221. https://doi.org/10.3390/bios15040221





