Machine Learning-Based VO2 Estimation Using a Wearable Multiwavelength Photoplethysmography Device
Abstract
1. Introduction
2. Materials and Methods
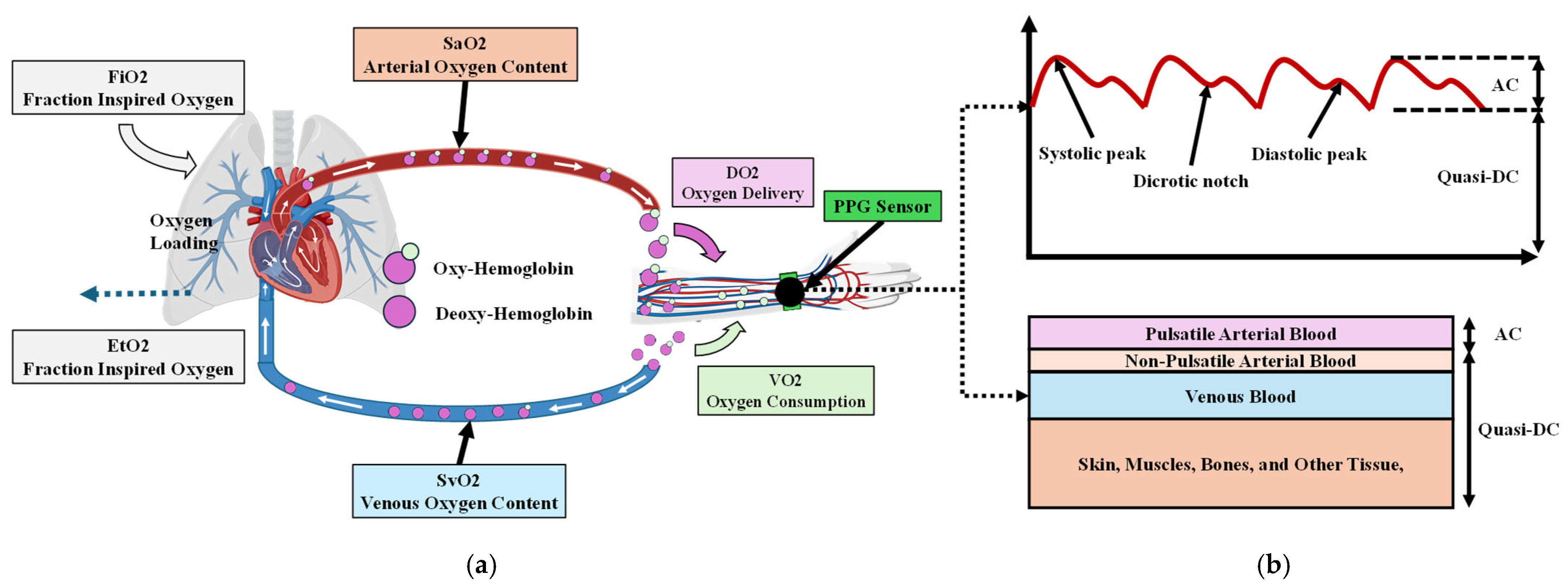
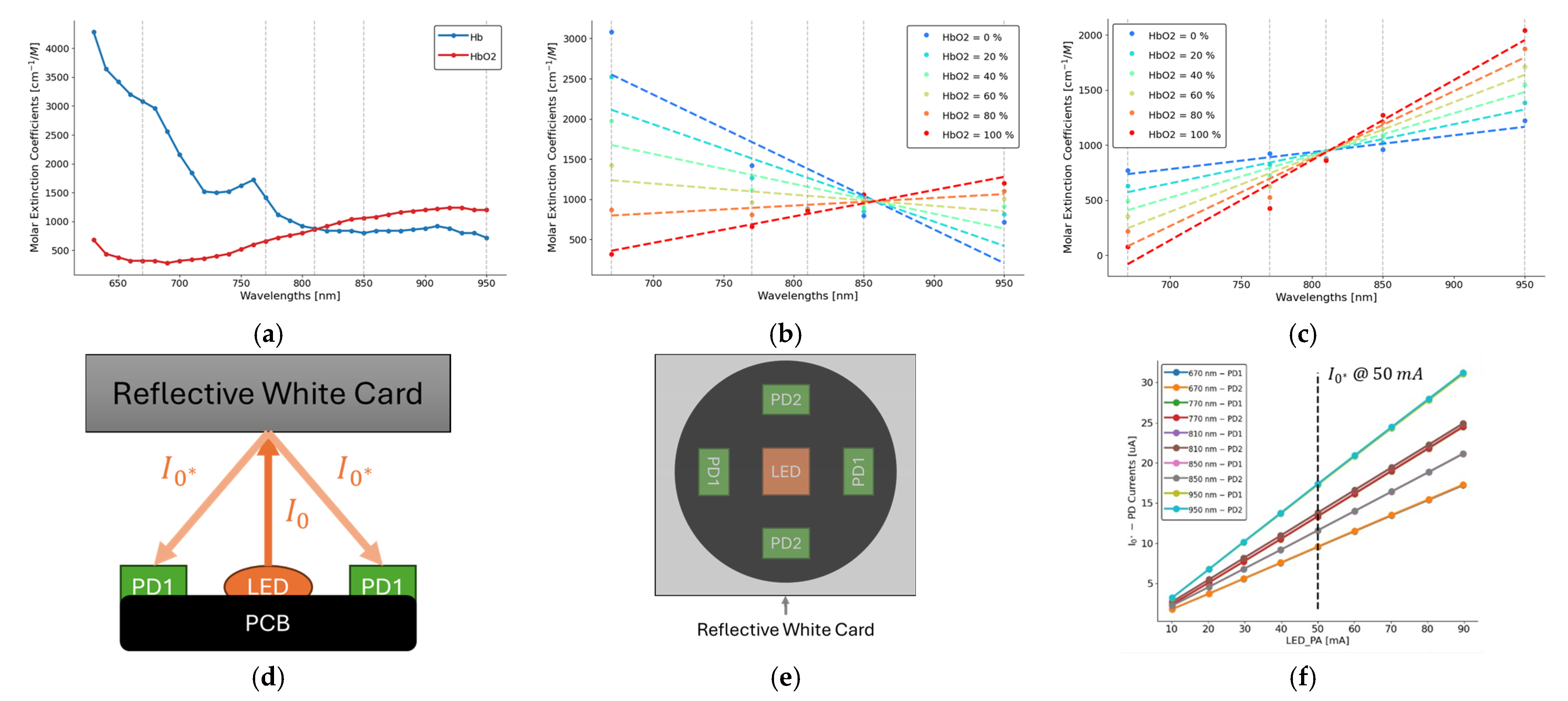
2.1. Beer–Lambert Law
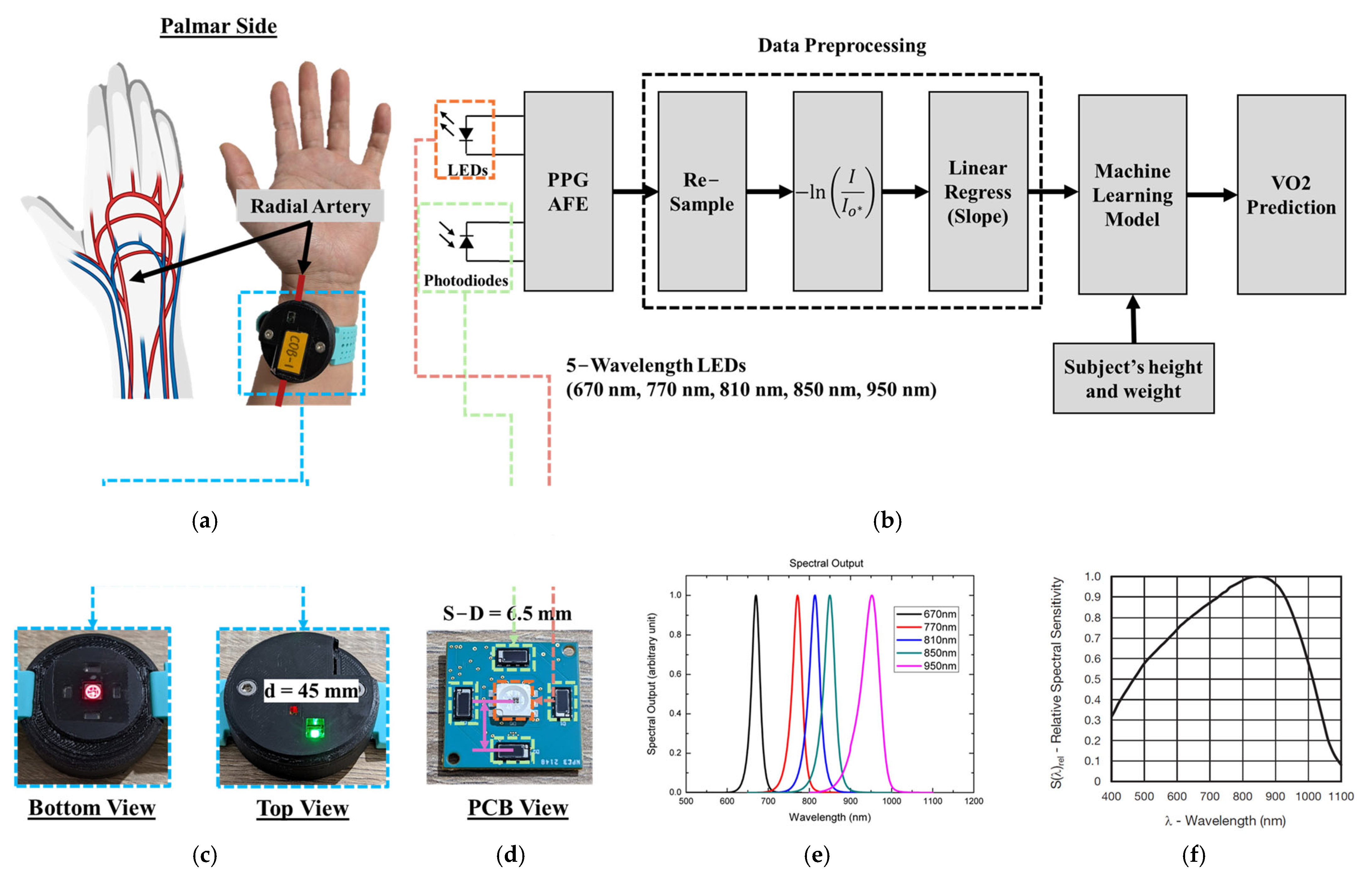
2.2. PPG Sensor System
2.3. Human Subject Study Protocol
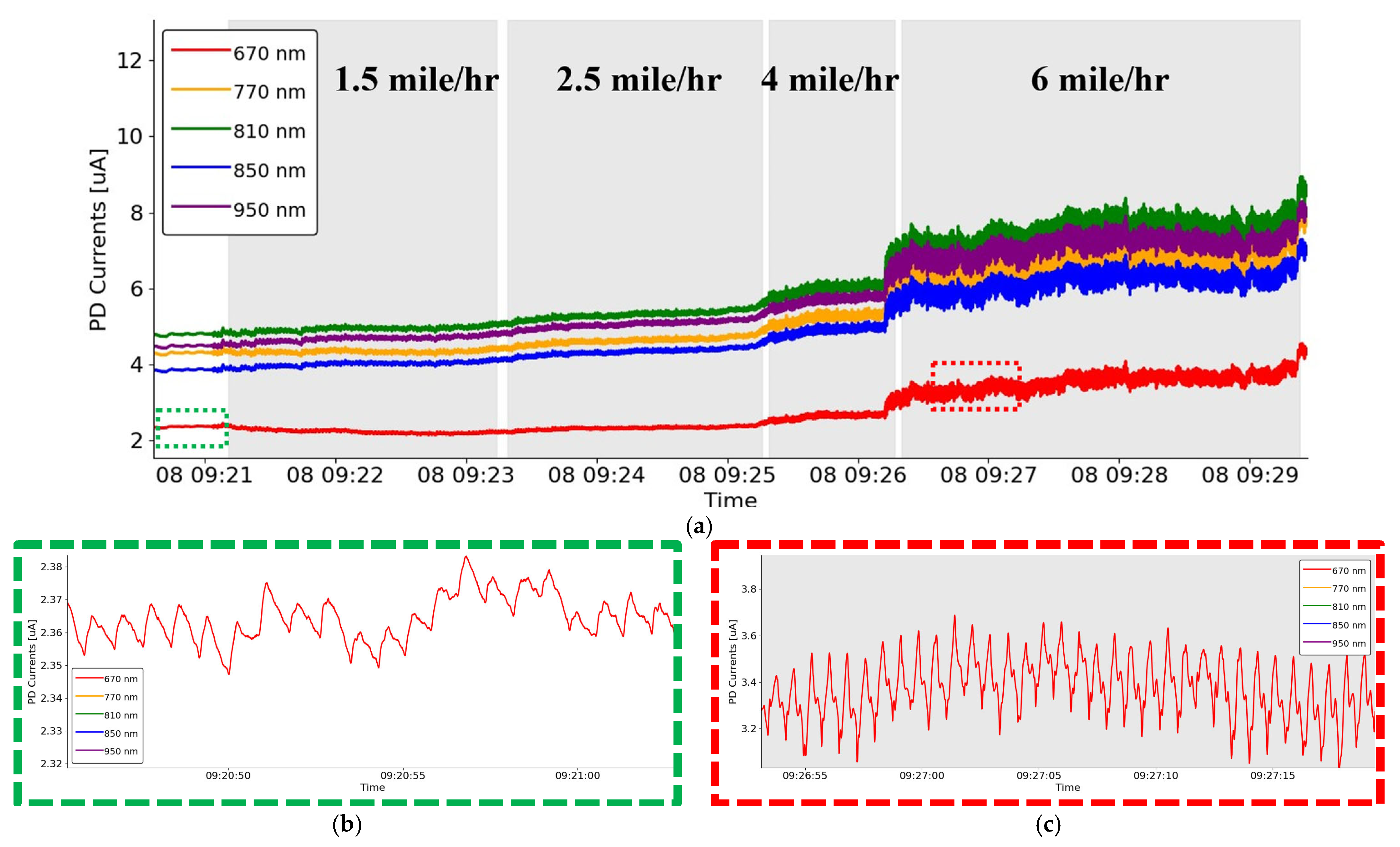
3. Results
4. Discussion
4.1. Comparison with Existing Wearable VO2_max Estimation Methods
4.2. Advantages of DC Analysis
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, S.; Tamrakar, S.; Nallathambi, N.; Vrindavanam, S.A.; Prasad, R.; Kothari, R. Assessment of Maximal Oxygen Uptake (VO2 Max) in Athletes and Nonathletes Assessed in Sports Physiology Laboratory. Cureus 2024, 16, e61124. [Google Scholar] [CrossRef] [PubMed]
- Herdy, A.H.; Ritt, L.E.; Stein, R.; Araújo, C.G.; Milani, M.; Meneghelo, R.S.; Ferraz, A.S.; Hossri, C.; Almeida, A.E.; Fernandes-Silva, M.M.; et al. Cardiopulmonary Exercise Test: Background, Applicability and Interpretation. Arq. Bras. Cardiol. 2016, 107, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Divakaruni, A.S.; Jastroch, M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat. Metab. 2022, 4, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.-P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Grafton, G.; Cascino, T.M.; Perry, D.; Ashur, C.; Koelling, T.M. Resting Oxygen Consumption and Heart Failure: Importance of Measurement for Determination of Cardiac Output with the Use of the Fick Principle. J. Card. Fail. 2020, 26, 664–672. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Silverthorn, D.U.; Johnson, B.R.; Ober, W.C.; Garrison, C.W.; Silverthorn, A.C. Human Physiology: An Integrated Approach, 6th ed.; Pearson Education: Boston, MA, USA, 2013; pp. 436–439. [Google Scholar]
- Fuentes, S.; Chowdhury, Y.S. Fraction of Inspired Oxygen; StatPearls Publishing LLC: Treasure Island, Fl, USA, 2024. [Google Scholar]
- Schumacker, P.T.; Samsel, R.W. Analysis of oxygen delivery and uptake relationships in the Krogh tissue model. J. Appl. Physiol. 1989, 67, 1234–1244. [Google Scholar] [CrossRef]
- Abay, T.Y.; Kyriacou, P.A. Photoplethysmography for blood volumes and oxygenation changes during intermittent vascular occlusions. J. Clin. Monit. Comput. 2018, 32, 447–455. [Google Scholar] [CrossRef]
- Ray, D.; Collins, T.; Woolley, S.; Ponnapalli, P. A Review of Wearable Multi-Wavelength Photoplethysmography. IEEE Rev. Biomed. Eng. 2023, 16, 136–151. [Google Scholar] [CrossRef]
- Kim, K.B.; Baek, H.J. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics 2023, 12, 2923. [Google Scholar] [CrossRef]
- Sabeti, E.; Reamaroon, N.; Mathis, M.; Gryak, J.; Sjoding, M.; Najarian, K. Signal quality measure for pulsatile physiological signals using morphological features: Applications in reliability measure for pulse oximetry. Inform. Med. Unlocked 2019, 16, 100222. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Coté, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H. Heart rate extraction from PPG signals using variational mode decomposition. Biocybern. Biomed. Eng. 2019, 39, 75–86. [Google Scholar] [CrossRef]
- Park, J.; Seok, H.S.; Kim, S.-S.; Shin, H. Photoplethysmogram Analysis and Applications: An Integrative Review. Front. Physiol. 2022, 12, 808451. [Google Scholar] [CrossRef]
- Charlton, P.H.; Celka, P.; Farukh, B.; Chowienczyk, P.; Alastruey, J. Assessing mental stress from the photoplethysmogram: A numerical study. Physiol. Meas. 2018, 39, 054001. [Google Scholar] [CrossRef]
- Charlton, P.H.; Kotzen, K.; Mejía-Mejía, E.; Aston, P.J.; Budidha, K.; Mant, J.; Pettit, C.; Behar, J.A.; Kyriacou, P.A. Detecting beats in the photoplethysmogram: Benchmarking open-source algorithms. Physiol. Meas. 2022, 43, 085007. [Google Scholar] [CrossRef]
- Rohr, M.; Tarvainen, M.; Miri, S.; Güney, G.; Vehkaoja, A.; Hoog Antink, C. An extensive quantitative analysis of the effects of errors in beat-to-beat intervals on all commonly used HRV parameters. Sci. Rep. 2024, 14, 2498. [Google Scholar] [CrossRef]
- Hosanee, M.; Chan, G.; Welykholowa, K.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Cuffless Single-Site Photoplethysmography for Blood Pressure Monitoring. J. Clin. Med. 2020, 9, 723. [Google Scholar] [CrossRef]
- Mahardika, T.N.; Fuadah, Y.N.; Jeong, D.U.; Lim, K.M. PPG Signals-Based Blood-Pressure Estimation Using Grid Search in Hyperparameter Optimization of CNN-LSTM. Diagnostics 2023, 13, 2566. [Google Scholar] [CrossRef]
- Qin, C.; Wang, X.; Xu, G.; Ma, X. Advances in Cuffless Continuous Blood Pressure Monitoring Technology Based on PPG Signals. BioMed Res. Int. 2022, 2022, 8094351. [Google Scholar] [CrossRef]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A. Measurement of energy expenditure. Public Health Nutr. 2005, 8, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Wilkerson, D.P.; Jones, A.M. Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur. J. Appl. Physiol. 2008, 102, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef]
- Hsiao, C.-T.; McMurray, J.; Branan, K.; Coté, G. Changes in Morphology of Multiwavelength Photoplethysmography Across and Down Major Arteries in the Forearm; SPIE: Bellingham, WA, USA, 2023; Volume 12387. [Google Scholar]
- Beatty, P.C.W. Design of Pulse Oximeters (Medical Science Series). Physiol. Meas. 1998, 19, 40. [Google Scholar] [CrossRef]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices Evid. Res. 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Nitzan, M.; Engelberg, S. Three-wavelength technique for the measurement of oxygen saturation in arterial blood and in venous blood. J. Biomed. Opt. 2009, 14, 024046. [Google Scholar] [CrossRef]
- Marktech Optoelectronics. MTMD6788594SMT6 Datasheet. Available online: https://specs.marktechopto.com/pdf/products/datasheet/MTMD6788594SMT6.pdf (accessed on 28 December 2024).
- Vishay. VEMD8080 Product Information. Available online: https://www.vishay.com/en/product/84565/ (accessed on 28 December 2024).
- Tannen, T. Robert A Bruce. Lancet 2004, 363, 1403. [Google Scholar] [CrossRef]
- Cooper, K.D.; Shafer, A.B. Validity and Reliability of the Polar A300’s Fitness Test Feature to Predict VO2max. Int. J. Exerc. Sci. 2019, 12, 393–401. [Google Scholar] [CrossRef]
- Freeberg, K.A.; Baughman, B.R.; Vickey, T.; Sullivan, J.A.; Sawyer, B.J. Assessing the ability of the Fitbit Charge 2 to accurately predict VO2max. Mhealth 2019, 5, 39. [Google Scholar] [CrossRef]
- Caserman, P.; Yum, S.; Göbel, S.; Reif, A.; Matura, S. Assessing the Accuracy of Smartwatch-Based Estimation of Maximum Oxygen Uptake Using the Apple Watch Series 7: Validation Study. JMIR Biomed. Eng. 2024, 9, e59459. [Google Scholar] [CrossRef]
- Klepin, K.; Wing, D.; Higgins, M.; Nichols, J.; Godino, J.G. Validity of Cardiorespiratory Fitness Measured with Fitbit Compared to VO2max. Med. Sci. Sports Exerc. 2019, 51, 2251–2256. [Google Scholar] [CrossRef]
- Spathis, D.; Perez-Pozuelo, I.; Gonzales, T.I.; Wu, Y.; Brage, S.; Wareham, N.; Mascolo, C. Longitudinal cardio-respiratory fitness prediction through wearables in free-living environments. npj Digit. Med. 2022, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- Amelard, R.; Hedge, E.T.; Hughson, R.L. Temporal convolutional networks predict dynamic oxygen uptake response from wearable sensors across exercise intensities. npj Digit. Med. 2021, 4, 156. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Park, H.K.; Kim, I.Y. Motion Artifact Reduction in Wearable Photoplethysmography Based on Multi-Channel Sensors with Multiple Wavelengths. Sensors 2020, 20, 1493. [Google Scholar] [CrossRef]
- Gautam, Y.; Jebelli, H. Autoencoder-based Photoplethysmography (PPG) signal reliability enhancement in construction health monitoring. Autom. Constr. 2024, 165, 105537. [Google Scholar] [CrossRef]
- Dao, D.; Salehizadeh, S.M.A.; Noh, Y.; Chong, J.W.; Cho, C.H.; McManus, D.; Darling, C.E.; Mendelson, Y.; Chon, K.H. A Robust Motion Artifact Detection Algorithm for Accurate Detection of Heart Rates from Photoplethysmographic Signals Using Time–Frequency Spectral Features. IEEE J. Biomed. Health Inform. 2017, 21, 1242–1253. [Google Scholar] [CrossRef]
- Vicente-Samper, J.M.; Tamantini, C.; Ávila-Navarro, E.; De La Casa-Lillo, M.Á.; Zollo, L.; Sabater-Navarro, J.M.; Cordella, F. An ML-Based Approach to Reconstruct Heart Rate from PPG in Presence of Motion Artifacts. Biosensors 2023, 13, 718. [Google Scholar] [CrossRef]
- Pollreisz, D.; TaheriNejad, N. Detection and Removal of Motion Artifacts in PPG Signals. Mob. Netw. Appl. 2022, 27, 728–738. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S.; Vullings, R.; Biswas, D.; Simões-Capela, N.; van Helleputte, N.; van Hoof, C.; Groenendaal, W. Motion Artifact Reduction for Wrist-Worn Photoplethysmograph Sensors Based on Different Wavelengths. Sensors 2019, 19, 673. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, 8. [Google Scholar] [CrossRef]
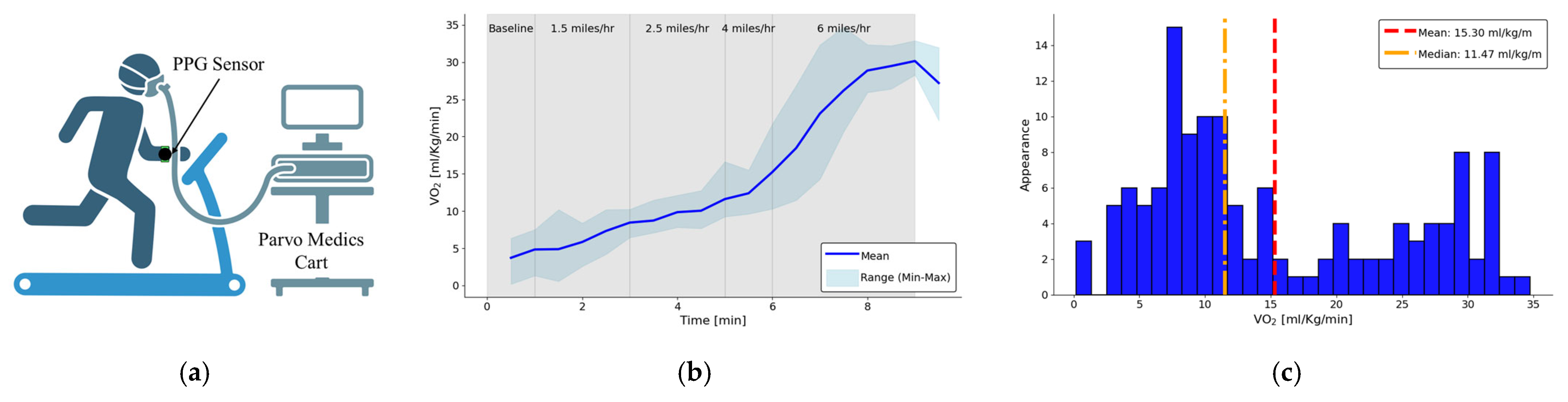

| Exercise Protocol | Duration | Comment |
|---|---|---|
| Sitting Sedentary | 0.5 min | Establish PPG baseline |
| Walking | 2 min | 1.5 miles/h |
| Walking | 2 min | 2.5 miles/h |
| Running | 1 min | 4 miles/h |
| Running | 3 min | 6 miles/h |
| Total | ~10 min |
| Study | Methodology | Sample Size | Advantages | Limitations |
|---|---|---|---|---|
| Fitbit Charge 2 (Freeberg et al., 2019) [35] | HR + activity data estimation | 30 | Convenient; Large user base | Relies on static formulas; less precise |
| Apple Watch Series 7 (Caserman et al., 2024) [36] | HR-based estimation during exercise | 19 | Widely accessible; User-friendly | Lower precision; Relies on demographic and HR data only |
| Neural Network model (Spathis et al., 2022) [38] | HR + accelerometer data | 11,059 | Large, diverse dataset; Effective in daily life settings | High computational cost; No oxygenation data |
| Temporal convolutional network (Amelard et al., 2021) [39] | Wearable sensors with advanced ML | 22 | Captures dynamics at varying intensities; Robust | Requires extensive data and computational power |
| This study | Multiwavelength PPG + Random Forest | 8 | Direct oxygenation measurement; Real-time; Non-invasive; High accuracy | Small sample; Preliminary validation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-T.; Tong, C.; Coté, G.L. Machine Learning-Based VO2 Estimation Using a Wearable Multiwavelength Photoplethysmography Device. Biosensors 2025, 15, 208. https://doi.org/10.3390/bios15040208
Hsiao C-T, Tong C, Coté GL. Machine Learning-Based VO2 Estimation Using a Wearable Multiwavelength Photoplethysmography Device. Biosensors. 2025; 15(4):208. https://doi.org/10.3390/bios15040208
Chicago/Turabian StyleHsiao, Chin-To, Carl Tong, and Gerard L. Coté. 2025. "Machine Learning-Based VO2 Estimation Using a Wearable Multiwavelength Photoplethysmography Device" Biosensors 15, no. 4: 208. https://doi.org/10.3390/bios15040208
APA StyleHsiao, C.-T., Tong, C., & Coté, G. L. (2025). Machine Learning-Based VO2 Estimation Using a Wearable Multiwavelength Photoplethysmography Device. Biosensors, 15(4), 208. https://doi.org/10.3390/bios15040208





