DNA-Engineered Coating for Protecting the Catalytic Activity of Platinum Nanozymes in Biological Systems
Abstract
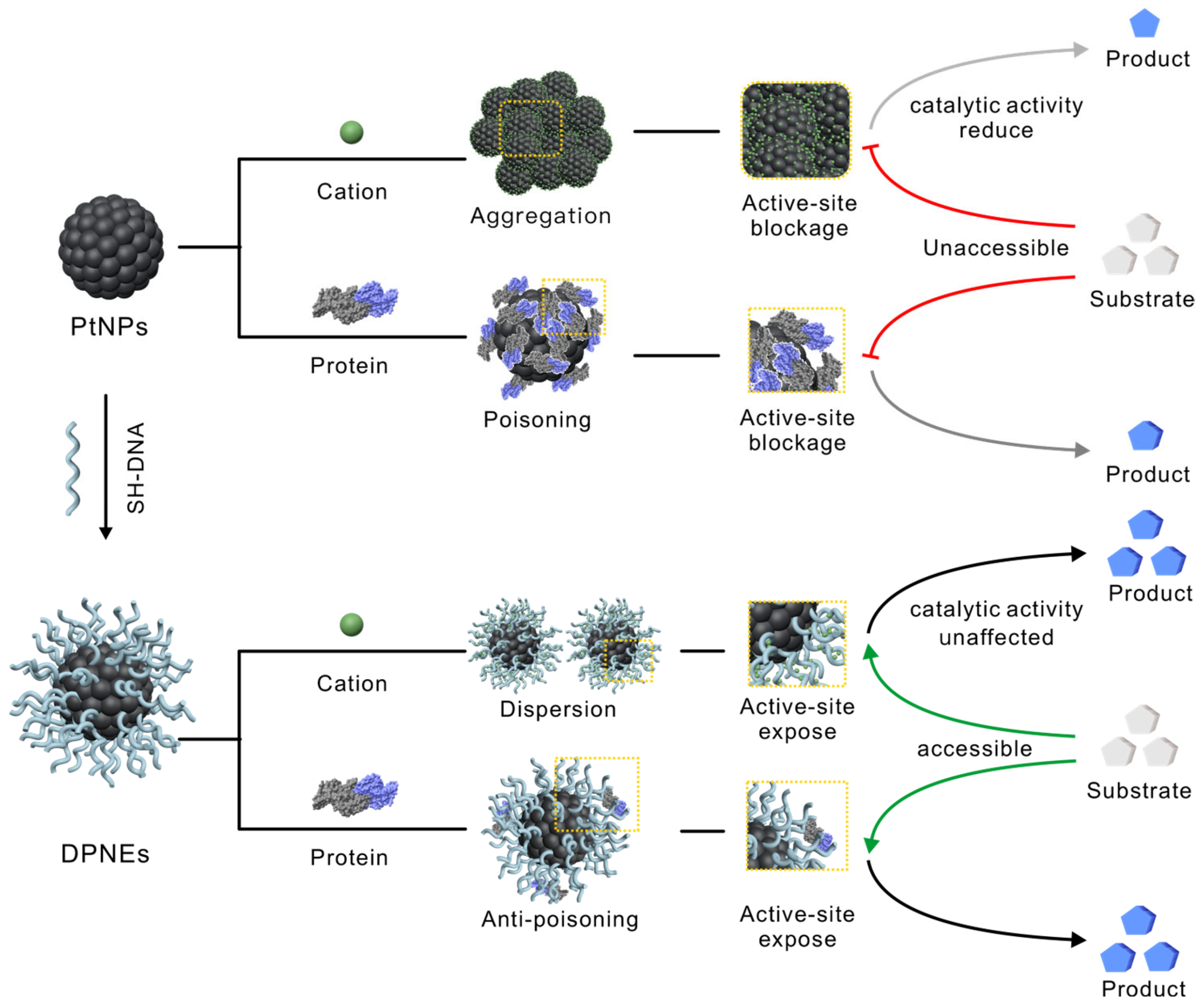
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of DNA-Coated Platinum Nanozymes (DPNEs)
2.3. Transmission Electron Microscopy (TEM) Characterization
2.4. Metal Salt Ion, BSA and Serum Treatment of PtNPs/DPNEs
2.5. Peroxidase Activity Measurement Method
2.6. Methods and Procedures for miRNAs Detection
3. Results and Discussion
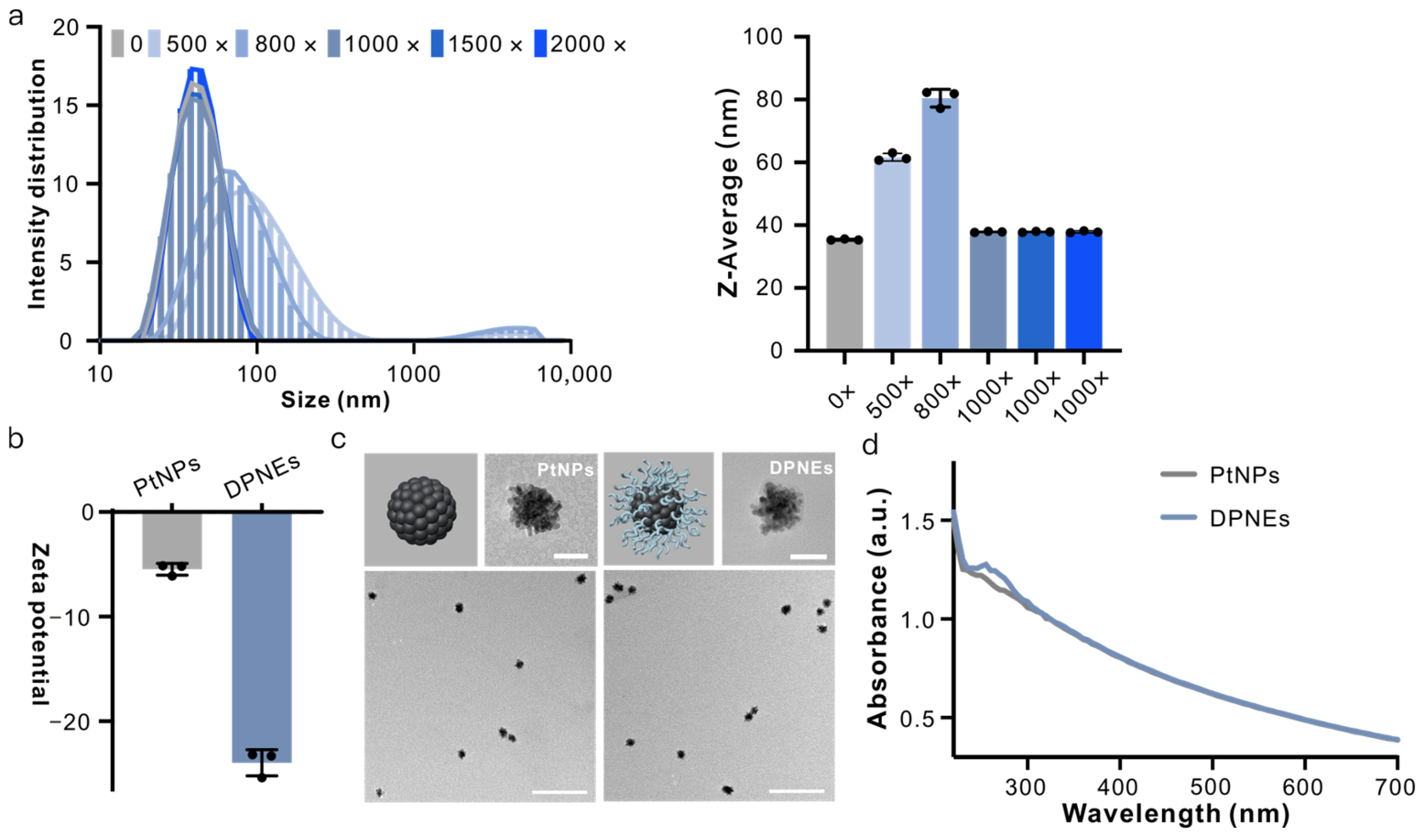
3.1. Design, Synthesis and Structural Characterization of DNA Coating on PtNPs
3.2. DNA-Coated PtNPs Can Resist High Salt Concentrations
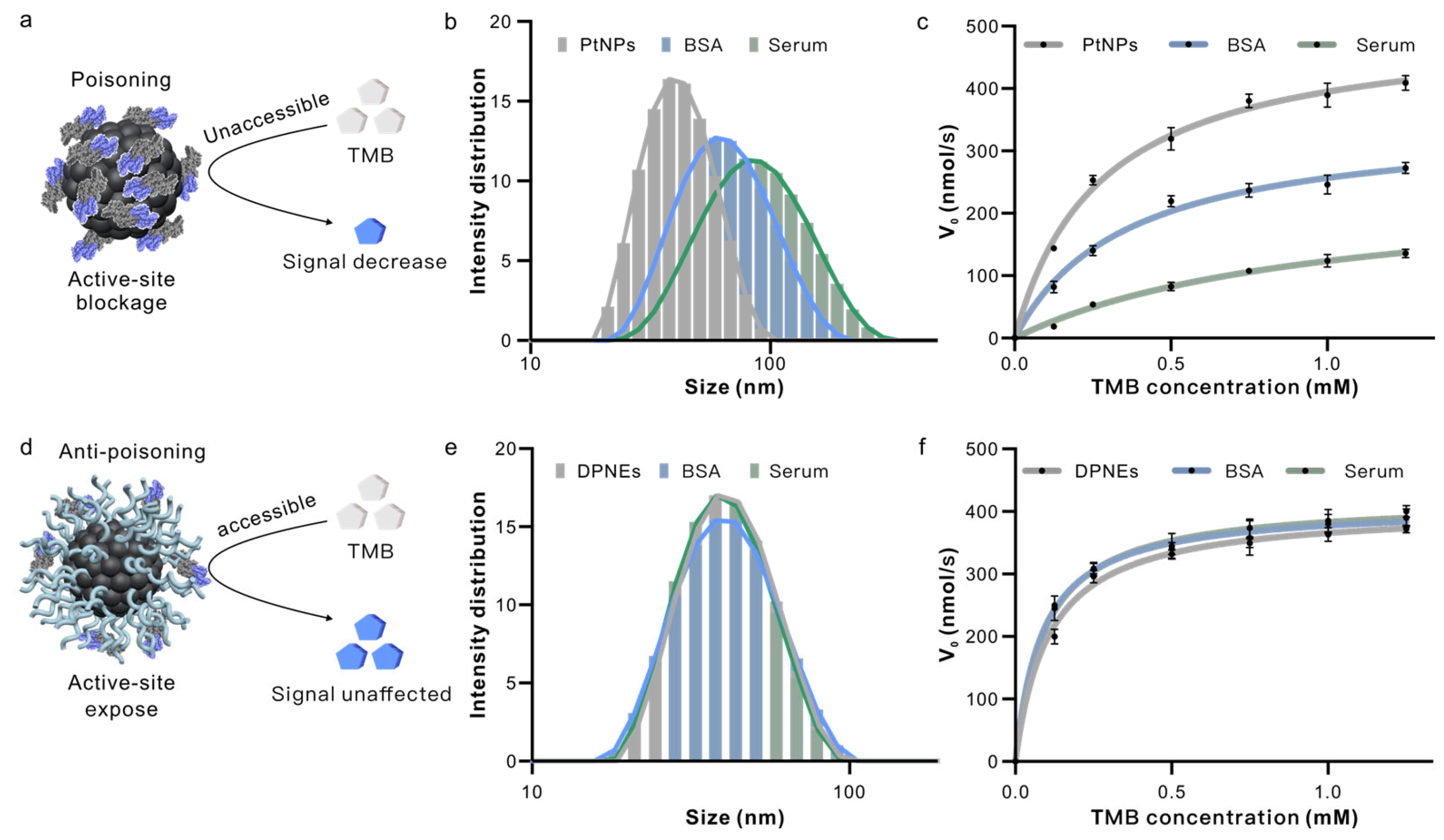
3.3. DNA Coating Serves as a Molecular Sieve in Serum
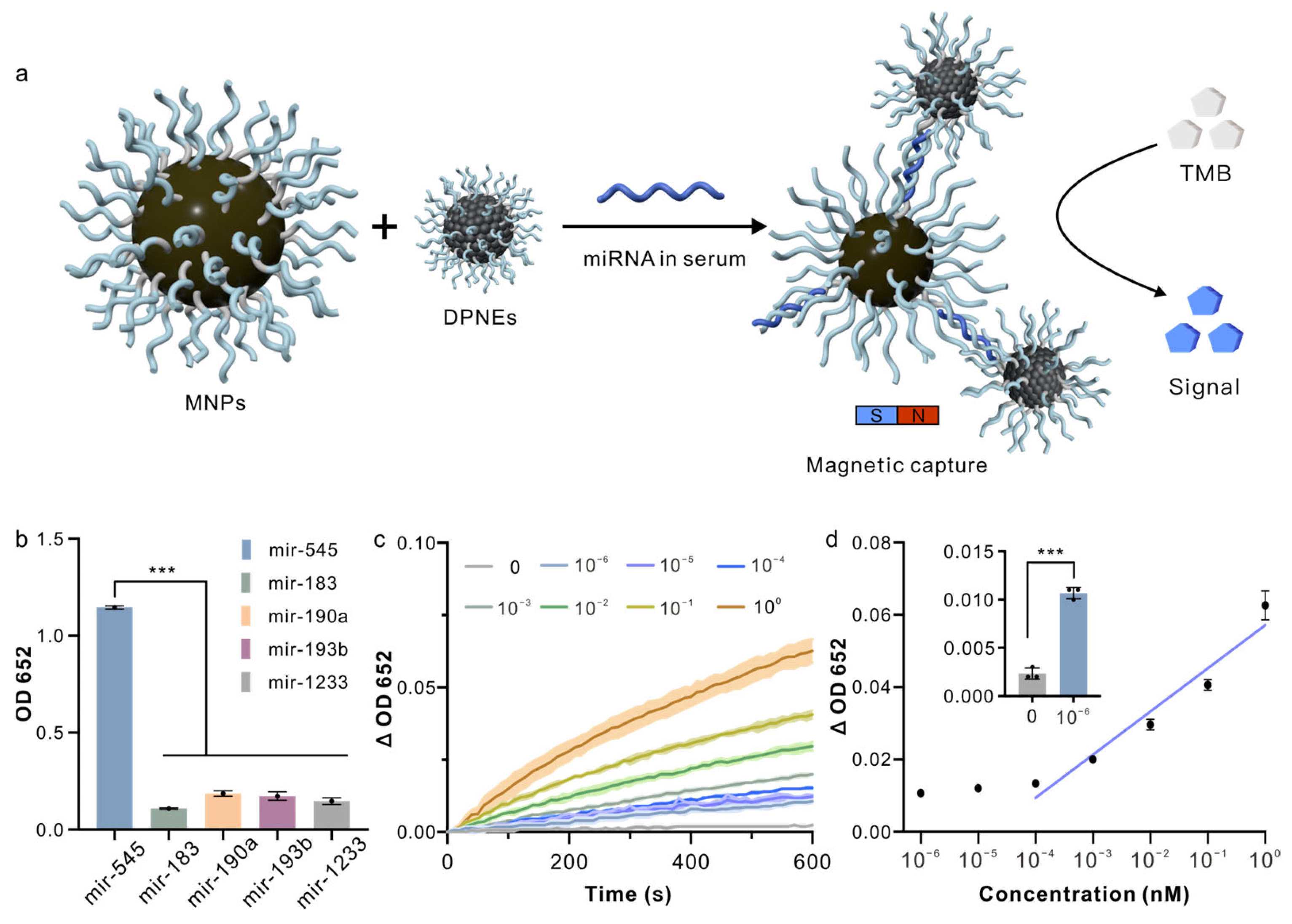
3.4. Detection of miRNAs in Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SNAs | Spherical nucleic acids |
| DPNEs | DNA-coated platinum nanozymes |
| PtNPs | Platinum nanoparticles |
| DLS | Dynamic light scattering |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| BSA | Bovine serum albumin |
| HF | Heart failure |
| HFpEF | Heart failure with preserved ejection fraction |
| MNPs | Magnetic nanoparticles |
References
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A clear definition with fuzzy edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically Synthesized Prussian Blue Nanoparticles Defeating Natural Enzyme Peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat. Commun. 2020, 11, 357. [Google Scholar] [CrossRef]
- Samuel, E.L.G.; Marcano, D.C.; Berka, V.; Bitner, B.R.; Wu, G.; Potter, A.; Fabian, R.H.; Pautler, R.G.; Kent, T.A.; Tsai, A.-L.; et al. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc. Natl. Acad. Sci. USA 2015, 112, 2343–2348. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef]
- Tonga, G.Y.; Jeong, Y.; Duncan, B.; Mizuhara, T.; Mout, R.; Das, R.; Kim, S.T.; Yeh, Y.-C.; Yan, B.; Hou, S.; et al. Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat. Chem. 2015, 7, 597–603. [Google Scholar] [CrossRef]
- Natalio, F.; André, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley Iii, W.C.; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal–organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- Cao, X.; Yue, L.; Wang, C.; Luo, X.; Zhang, C.; Zhao, X.; Wu, F.; White, J.C.; Wang, Z.; Xing, B. Foliar Application with Iron Oxide Nanomaterials Stimulate Nitrogen Fixation, Yield, and Nutritional Quality of Soybean. ACS Nano 2022, 16, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.J.; Mitchell, S.; Mondelli, C.; Jaydev, S.; Pérez-Ramírez, J. Unifying views on catalyst deactivation. Nat. Catal. 2022, 5, 854–866. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.; Lee, D.Y. Aurozyme: A Revolutionary Nanozyme in Colitis, Switching Peroxidase-Like to Catalase-Like Activity. Small 2023, 19, 2302331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, Z.; Zhang, X.; Duan, Q.; Zhang, Y.; Midgley, A.C.; Jiao, L.; Liu, R.; Zhu, M.; Kong, D.; et al. Assembly of Genetically Engineered Ionizable Protein Nanocage-based Nanozymes for Intracellular Superoxide Scavenging. Nat. Commun. 2025, 16, 1123. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Wang, Y.; Zhang, Y.; Li, S.; Liu, W.; Liu, S.; Liu, Y.; Xing, H.; Otake, K.-i.; et al. Microenvironmental modulation breaks intrinsic pH limitations of nanozymes to boost their activities. Nat. Commun. 2024, 15, 10861. [Google Scholar] [CrossRef]
- Cutler, J.I.; Auyeung, E.; Mirkin, C.A. Spherical Nucleic Acids. J. Am. Chem. Soc. 2012, 134, 1376–1391. [Google Scholar] [CrossRef]
- Zhu, D.; Song, P.; Shen, J.; Su, S.; Chao, J.; Aldalbahi, A.; Zhou, Z.; Song, S.; Fan, C.; Zuo, X.; et al. PolyA-Mediated DNA Assembly on Gold Nanoparticles for Thermodynamically Favorable and Rapid Hybridization Analysis. Anal. Chem. 2016, 88, 4949–4954. [Google Scholar] [CrossRef]
- Pei, H.; Li, F.; Wan, Y.; Wei, M.; Liu, H.; Su, Y.; Chen, N.; Huang, Q.; Fan, C. Designed Diblock Oligonucleotide for the Synthesis of Spatially Isolated and Highly Hybridizable Functionalization of DNA–Gold Nanoparticle Nanoconjugates. J. Am. Chem. Soc. 2012, 134, 11876–11879. [Google Scholar] [CrossRef]
- Yan, J.; Tan, Y.-L.; Lin, M.-J.; Xing, H.; Jiang, J.-H. A DNA-mediated crosslinking strategy to enhance cellular delivery and sensor performance of protein spherical nucleic acids. Chem. Sci. 2021, 12, 1803–1809. [Google Scholar] [CrossRef]
- Jiao, K.; Yan, Q.; Guo, L.; Qu, Z.; Cao, S.; Chen, X.; Li, Q.; Zhu, Y.; Li, J.; Wang, L.; et al. Poly-Adenine-Based Spherical Nucleic Acids for Efficient Live-Cell MicroRNA Capture. Angew. Chem. Int. Ed. 2021, 60, 14438–14445. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, X.; Wang, D.; Cai, J.; Wang, Y.; Chen, P.; Ren, M.; Lu, H.; Union, J.; Zhang, L.; et al. Spherical Nucleic Acids for Topical Treatment of Hyperpigmentation. J. Am. Chem. Soc. 2021, 143, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, J.; Guo, L.; Zhu, S.; Hou, X.; Cao, S.; Zhou, M.; Shi, J.; Li, J.; Liu, K.; et al. DNA-Engineered Degradable Invisibility Cloaking for Tumor-Targeting Nanoparticles. J. Am. Chem. Soc. 2024, 146, 25253–25262. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.-H.; Lin, X.-L.; Liu, Y.-H.; Li, K.-L.; Zhuang, Q.-Q.; Peng, H.-P.; Liu, A.-L.; Xia, X.-H.; Chen, W. Chitosan-stabilized platinum nanoparticles as effective oxidase mimics for colorimetric detection of acid phosphatase. Nanoscale 2017, 9, 10292–10300. [Google Scholar] [CrossRef]
- Shao, N.; Han, X.; Song, Y.; Zhang, P.; Qin, L. CRISPR-Cas12a Coupled with Platinum Nanoreporter for Visual Quantification of SNVs on a Volumetric Bar-Chart Chip. Anal. Chem. 2019, 91, 12384–12391. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Freezing Directed Construction of Bio/Nano Interfaces: Reagentless Conjugation, Denser Spherical Nucleic Acids, and Better Nanoflares. J. Am. Chem. Soc. 2017, 139, 9471–9474. [Google Scholar] [CrossRef]
- Owan Theophilus, E.; Hodge David, O.; Herges Regina, M.; Jacobsen Steven, J.; Roger Veronique, L.; Redfield Margaret, M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; Hernandez, A.F.; Fonarow, G.C. Trends in Patients Hospitalized with Heart Failure and Preserved Left Ventricular Ejection Fraction. Circulation 2012, 126, 65–75. [Google Scholar] [CrossRef]
- Wong, L.L.; Zou, R.; Zhou, L.; Lim, J.Y.; Phua, D.C.Y.; Liu, C.; Chong, J.P.C.; Ng, J.Y.X.; Liew, O.W.; Chan, S.P.; et al. Combining Circulating MicroRNA and NT-proBNP to Detect and Categorize Heart Failure Subtypes. J. Am. Coll. Cardiol. 2019, 73, 1300–1313. [Google Scholar] [CrossRef]
- Ntelios, D.; Georgiou, E.; Alexouda, S.; Malousi, A.; Efthimiadis, G.; Tzimagiorgis, G. A critical approach for successful use of circulating microRNAs as biomarkers in cardiovascular diseases: The case of hypertrophic cardiomyopathy. Heart Fail. Rev. 2022, 27, 281–294. [Google Scholar] [CrossRef]
- Nair, N.; Kumar, S.; Gongora, E.; Gupta, S. Circulating miRNA as novel markers for diastolic dysfunction. Mol. Cell. Biochem. 2013, 376, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Xu, L.; Deng, T.; Zheng, J.; Li, J. Catalytic Hairpin Self-Assembly-Based SERS Sensor Array for the Simultaneous Measurement of Multiple Cancer-Associated miRNAs. ACS Sens. 2020, 5, 4009–4016. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, H.; Wang, X.; Tu, W.; Dai, Z. Green light excited ultrasensitive photoelectrochemical biosensing for microRNA at a low applied potential based on the dual role of Au NPs in TiO2 nanorods/Au NPs composites. Nanoscale 2018, 10, 16474–16478. [Google Scholar] [CrossRef]
- Liu, M.X.; Liang, S.; Tang, Y.; Tian, J.; Zhao, Y.; Zhao, S. Rapid and label-free fluorescence bioassay for microRNA based on exonuclease III-assisted cycle amplification. RSC Adv. 2018, 8, 15967–15972. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, G.; Wang, W.; Huang, X.; Peng, H.; Pu, Q.; Du, F.; Cui, X.; Deng, Y.; Tang, Z. Colorimetric PCR-Based microRNA Detection Method Based on Small Organic Dye and Single Enzyme. Anal. Chem. 2018, 90, 7107–7111. [Google Scholar] [CrossRef]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Wei, S.; Li, A.; Huang, L.; Liu, J. A signal amplification system constructed by bi-enzymes and bi-nanospheres for sensitive detection of norepinephrine and miRNA. Biosens. Bioelectron. 2019, 124–125, 224–232. [Google Scholar] [CrossRef]
- Pan, M.; Liang, M.; Sun, J.; Liu, X.; Wang, F. Lighting Up Fluorescent Silver Clusters via Target-Catalyzed Hairpin Assembly for Amplified Biosensing. Langmuir 2018, 34, 14851–14857. [Google Scholar] [CrossRef]
- Tian, R.; Ning, W.; Chen, M.; Zhang, C.; Li, Q.; Bai, J. High performance electrochemical biosensor based on 3D nitrogen-doped reduced graphene oxide electrode and tetrahedral DNA nanostructure. Talanta 2019, 194, 273–281. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Liu, X.; Tang, S.; Wang, Y.; Yang, M.; Guo, L.; Li, J.; Jiao, K.; Wang, L. DNA-Engineered Coating for Protecting the Catalytic Activity of Platinum Nanozymes in Biological Systems. Biosensors 2025, 15, 205. https://doi.org/10.3390/bios15040205
Ren L, Liu X, Tang S, Wang Y, Yang M, Guo L, Li J, Jiao K, Wang L. DNA-Engineered Coating for Protecting the Catalytic Activity of Platinum Nanozymes in Biological Systems. Biosensors. 2025; 15(4):205. https://doi.org/10.3390/bios15040205
Chicago/Turabian StyleRen, Lei, Xia Liu, Shuai Tang, Yue Wang, Miao Yang, Linjie Guo, Jiang Li, Kai Jiao, and Lihua Wang. 2025. "DNA-Engineered Coating for Protecting the Catalytic Activity of Platinum Nanozymes in Biological Systems" Biosensors 15, no. 4: 205. https://doi.org/10.3390/bios15040205
APA StyleRen, L., Liu, X., Tang, S., Wang, Y., Yang, M., Guo, L., Li, J., Jiao, K., & Wang, L. (2025). DNA-Engineered Coating for Protecting the Catalytic Activity of Platinum Nanozymes in Biological Systems. Biosensors, 15(4), 205. https://doi.org/10.3390/bios15040205





