Abstract
This study provides a comprehensive analysis of the complex interaction between heavy metals (HMs) and the gut microbiota, adopting a bidirectional approach that explores both the influence of HMs on the gut microbiota populations and the potential role of probiotics in modulating these changes. By examining these interconnected aspects, the study aims to offer a deeper understanding of how HMs disrupt microbial balance and how probiotic interventions may mitigate or reverse these effects, promoting detoxification processes and overall gut health. In addition, the review highlights innovative tools, such as biosensors, for the rapid, precise, and non-invasive detection of HMs in urine. These advanced technologies enable the real-time monitoring of the effectiveness of probiotic-based interventions, offering critical insights into their role in promoting the elimination of HMs from the body and improving detoxification.
1. Introduction
Epidemiological evidence suggests that heavy metals (HMs) may play a role in the progression of various metabolic disorders [1], with HM-induced disruptions to the gut microbiota being a contributing factor in their onset and development [2]. Notably, the gut microbiota serves as the first line of defense against the toxic effects of HMs, and there is a dynamic, bidirectional interaction between the two [3]. On one hand, HMs can cause significant alterations in the structure, abundance, and diversity of intestinal microbial communities, while simultaneously influencing their metabolic profiles [4]. On the other hand, the gut microbiota plays a crucial role in modulating the bioavailability of HMs [5], impacting their absorption and metabolism through mechanisms such as altering intestinal pH, redox potential, and the expression of the genes involved in detoxification processes [6]. Through bioaccumulation, binding, and enzymatic transformation, the microbiota can facilitate the excretion of HMs, offering protective effects for the host [7,8]. Furthermore, exposure to HMs, particularly toxic ones, can disturb the gut microbiota, potentially impairing metabolic and physiological functions [9]. Such disruption may contribute to the onset or progression of a wide range of conditions, including cardiovascular diseases, neurodegenerative disorders, ulcerative colitis, cirrhosis, allergies, diabetes, autism, and other inflammatory diseases [10,11].
Recent research has significantly expanded our understanding of the relationship between HMs and the gut microbiota utilizing various models beyond rodents, such as chickens, fish, crayfish, and Bufo gargarizans.
The intricate interactions between the gut microbiota and HMs have been increasingly recognized. For instance, cadmium (Cd)-induced dysbiosis in the gut microbiota has been shown to exacerbate liver injury by increasing intestinal permeability in murine models [1]. Studies have consistently reported that HM exposure leads to significant shifts in microbial composition, with a decrease in the abundance of Proteobacteria and Firmicutes and an increase in Bacteroidetes at the phylum level [12].
Probiotics, defined as the mono- or mixed cultures of viable microorganisms that promote health by improving intestinal microbial balance, have shown promise in mitigating HM-induced dysbiosis. Common probiotic species include Bifidobacterium, Lactobacillus, Streptococcus, Enterococcus, Clostridium, Bacillus, and Escherichia coli. Among these, certain strains, particularly those from Lactobacillus, Bacillus, Bifidobacterium, and Clostridium species, have demonstrated strong resistance to HMs. This resistance is achieved by altering physiological conditions or expressing HM-binding peptides/proteins or detoxification enzymes involved in HM biotransformation. These processes not only reverse HM-induced dysbiosis but also provide protection against HM toxicity [12]. The impact of probiotics on HM elimination can be validated using advanced detection methods for HMs in urine. Various technologies have been developed to detect the concentration and toxicity of heavy metals, including electrothermal atomic absorption spectrometry [13], inductively coupled plasma-optical emission spectrometry [14], and high-pressure liquid chromatography inductively coupled plasma-mass spectrometry, among others [15]. While high-precision methods are valuable, biosensors, point-of-care devices, and similar technologies stand out for their cost-effectiveness, versatility, and robustness, offering rapid, non-invasive, and user-friendly solutions that do not require specialized personnel [16,17,18]. Biosensors operate by detecting signals generated from interactions between analytes and biological components, providing a response proportional to the heavy metal concentration [19]. As defined by the International Union of Pure and Applied Chemistry (IUPAC), biosensors are self-integrated devices that offer quantitative or semi-quantitative data through direct interaction with transducer elements [13]. These methods not only facilitate the assessment of probiotic interventions and HMs status but also provide insights into other health indicators. For example, the presence of copper (Cu) in urine may be associated with Wilson’s disease [20] or urinary tract infections, which have been shown to exert inhibitory effects on certain bacteria [21].
This study offers a comprehensive analysis of the bidirectional interaction between HMs and the gut microbiota. It highlights both the adverse effects of HMs on microbial communities and the potential for probiotics to modulate gut microbiota and elimination of HMs. Unlike previous studies that address these issues separately, our review integrates these findings to provide a deeper understanding of the mechanisms underlying microbial disruption and probiotic-mediated detoxification. In addition, a key innovative aspect of this study is the inclusion of advanced biosensor technologies for rapid, accurate, and non-invasive detection of HM in urine. These cutting-edge tools not only allow real-time monitoring of probiotic interventions, but also improve diagnostic capabilities for HM-related health conditions. By combining knowledge of microbial modulation with state-of-the-art detection methods, this review aims to address critical gaps and provide new perspectives on mitigating HM toxicity and improving gut health.
2. Effects of Heavy Metal Exposure on the Gut Microbiota
Exposure to HMs has been linked to significant health risks in both humans and animals particularly through its impact on the gut microbiota [22]. Approximately 60% of the ingested HMs are absorbed in the intestine, where they can cause oxidative stress and damage to the intestinal barrier, which subsequently can lead to increased intestinal inflammation [23,24,25]. While all the studied metals cause microbiota dysbiosis, the specific changes in microbial composition and functional consequences vary between them.
2.1. Arsenic
Chronic arsenic (As) exposure has been associated with a range of gastrointestinal disorders including dyspepsia, gastroenteritis, and chronic diarrhea, as well as compromised gut barrier integrity [26,27,28]. Subchronic exposure to As results in significant changes to colonic epithelial structure and impairs intestinal barrier function, primarily through damage to the intestinal microvilli [29]. This disruption of the intestinal barrier is accompanied by an increased production of inflammatory cytokines, such as IL-6, IL-8, and TNF-α, and the generation of oxidative stress in colonic epithelial cells [30,31].
As exposure has been shown to correlate with an increase in pathogenic bacterial populations and a concomitant decrease in commensal bacteria, highlighting its significant impact on gut microbial balance [32,33].
Experimental studies in As-exposed mice showed a marked reduction in the alpha diversity of the gut microbiota, along with a shift in microbiota composition including an increase in Bacteroidetes and a decrease in Firmicutes [34]. For example, a study on 8-week-old mice, exposed to As in drinking water at a concentration of 0.5 ppm and 5 ppm As, found that As exposure induced intestinal inflammation and a decrease in microbial diversity, particularly in the 5 ppm group. Compared to controls, the 5 ppm As exposure decreased the relative abundance of Bacteroidetes and Firmicutes, while Verrucomicrobia significantly increased. Additionally, there was a reduction in the abundance of Muribaculaceae, Alistipes, Lachnospiraceae, Prevotellaceae, Lactobacillus, and Alloprevotella, while the abundance of Akkermansia, Bacteroides, Bacteroidales, Muribaculum, Ruminococcaceae, Parabacteroides, and Helicobacter increased following As exposure during the developmental period [35]. In another study by Li et al. [36], exposure to 50 ppm sodium arsenite in drinking water for two weeks in five-week-old laboratory mice led to a significant decrease in the abundance of Bacteroidetes and Tenericutes. At the genus level, the arsenite-exposed mice showed a notable increase in the abundance of Acetivibrio, Pelotomaculum, Anaerovorax, Anoxybacillus, Alistipes, Alkalitalea, Chryseobacterium, Bosea, and Curvibacter compared to the control mice. Conversely, a significant decrease was observed in the abundance of Clostridium, Syntrophococcus, Fusicatenibacter, Cellulosilyticum, Blautia, Oribacterium, Fastidiosipila, Gemmiger, Intestinimonas, Butyncicoccus, Geosporobacter, Anaerovibrio, Escherichia Shigella, Parasutterella, and Anaeroplasma.
These findings collectively highlight the profound and multifaceted impact of As on the gut microbiota. The As-induced dysbiosis not only disrupts microbial diversity and abundance but also exacerbates intestinal inflammation and oxidative stress, contributing to broader systemic effects.
2.2. Cadmium
Exposure to cadmium (Cd) is associated with significant disruptions in intestinal barrier integrity and gut microbiota composition. Cd exposure has been shown to reduce the expression and cause abnormal localization of key cell adhesion proteins, such as ZO-1, ZO-2, junctional adhesion molecule A (JAM-A), occludin, and claudin-1, in intestinal epithelial cells. This disruption leads to increased intestinal permeability, which compromises gut integrity and function [8,37]. Notably, Pb exposure mirrors some of the effects of As and Cd by reducing microbial diversity and promoting pathogenic bacteria, yet its effects on the gut microbiota appear more consistent across different studies. For example, Li et al. [36] demonstrated that administering 50 ppm Cd chloride in drinking water to laboratory mice for two weeks significantly decreased bacterial diversity in the intestinal microbiota, compared to controls with a marked reduction in the abundance of Bacteroidetes and Proteobacteria. At the genus level, Cd exposure led to an increase in the abundance of certain bacteria such as Anaerosporobacter, Acidaminobacter, Prevotella, Barnesiella, Parabacteroides, and Alistipes, while the abundance of bacteria like Syntrophococcus, Clostridium, Eisenbergiella, Coprococcus, Blautia, Anaerostipes, Hespellia, Cellulosilyticum, Ruminococcus, Intestinimonas, Gemmiger, Caloramator, Alkaliphilus, Thermotalea, Filifactor, Gracilibacter, Anaerovorax, Peptococcus, Kandleria, Anaerovibrio, Desulfovibrio, Klebsiella, Parasutterella, Brevundimonas, Acinetobacter, Mucispirillum, Anaeroplasma, Arthrobacter, and Clostridium XIVb, III, IV, XI, and XVIII was significantly reduced.
Yang et al. [38] further investigated the effects of varying doses of Cd exposure in rats. They reported that Cd exposure led to a disruption of the microbiota composition, with significant reductions in the abundance of Prevotella and Lachnoclostridium, while Escherichia coli–Shigella populations increased. A 2023 study [39] supported these findings, showing similar changes in the intestinal microbiota composition, with increased levels of pathogenic bacteria such as Helicobacter and Campylobacter in a mouse model exposed to 3.6 mg/L oral Cd for 23 weeks. Interestingly, unlike As, which primarily shifts microbial populations toward Bacteroidetes, Cd exposure appears to drive a decline in beneficial microbes across multiple phyla while promoting opportunistic pathogens. For instance, a 2020 study [37] reported a significant reduction in the abundance of Akkermansia muciniphila, a commensal bacterium known for its role in maintaining intestinal barrier integrity and regulating metabolic homeostasis. Mice exposed to drinking water containing Cd chloride exhibited decreased Akkermansia muciniphila populations, further emphasizing the detrimental effects of Cd on gut health. The disruption of the gut microbiota by Cd highlights the compound’s role in promoting intestinal inflammation, microbial dysbiosis, and loss of gut barrier function. These findings underline the importance of further research into mitigating Cd toxicity and exploring strategies to restore the gut microbial balance following exposure.
2.3. Mercury
Exposure to mercury (Hg) has been shown to significantly compromise intestinal barrier integrity by reducing the expression of important intercellular junction proteins, leading to increased intestinal permeability and facilitating the absorption of other toxic metals [9]. As such, Hg exposure significantly downregulates the expression of claudin 1, occludin, ZO-1, and JAM1 in colon epithelial cells. Moreover, Hg exposure increases the volume of intestinal cells and membrane permeability without affecting cell viability, thereby enhancing the uptake of other toxic metals under similar physiological conditions [40]. Studies have shown that mercury chloride (HgCl2) administration in mice results in significant shifts in gut microbiota composition. For example, a significant increase in the abundance of Butyricimonas, Dehalobacterium, Coprococcus, Oscillospira, and Bilophila was shown while the abundance of Sporosarcina, Jeotgailcoccus, Staphylococcus, and Acinetobacter, was significantly reduced [41]. Further, exposure to 0.4 μg/mL inorganic mercury (IHg) has been shown to induce a phylum-level increase in Tenericutes and a decrease in Verrucomicrobia, indicating significant microbiota alterations compared to controls. At the genus and species levels, this exposure led to a significant increase in the abundance of intestinal pathogenic bacteria, including Streptococcus, Enterococcus faecalis, Peptostreptococcus, Staphylococcus aureus, Pneumococcus, Erysipelas, Bacillus anthracis, Tetanus, Spirochetes, Actinomycetes, and Tuberculosis, as well as an increase in sulfate-reducing bacteria compared to controls [42].
Finally, exposure of rats to methylmercury, a more toxic form of Hg (0.4 μg/mL, equivalent to 10 μg/kg body weight), led to significant changes in the microbiota composition. Specifically, there was a decrease in the abundance of Lactobacillaceae, Bacteroidaceae, Streptococcaceae, and Sutterellaceae, and an increase in Desulfovibrionaceae, Helicobacteraceae, Peptococcaceae, and Rhodospirillaceae [43].
These findings highlight the severe impact of Hg on intestinal homeostasis, with broad implications for metabolic, immune, and inflammatory processes. Given its ability to disrupt gut microbiota composition, Hg exposure may contribute to a heightened risk of gastrointestinal and systemic inflammatory diseases.
2.4. Lead
Exposure to lead (Pb) has been shown to significantly decrease the expression of the MUC2 gene and tight junction proteins (ZO-1, claudin 1, and occludin) in intercellular junctions, leading to increased intestinal permeability in both mice exposed to low doses over the long term and those exposed to high doses over a short period [44]. A study examining the effects of exposing mice to 10 ppm lead chloride in drinking water (equivalent to 2 mg/kg body weight/day) for 13 weeks found that after just 4 weeks of exposure, there was a reduction in the abundance of Ruminococcus, Clostridiales, and Oscillospira. By the end of the 13-week exposure period, a significant decrease in the abundance of Lachnospiraceae, Blautia, Coprococcus, and Ruminococcus was observed [4]. Similarly, Xia et al. [45] examined the effects of 15 weeks of exposure to 0.1 mg/L Pb in drinking water in laboratory mice. Using quantitative real-time PCR of cecal contents, they showed a reduction in the abundance of the Bacteroidetes and Firmicutes phyla. Further, 16S rRNA gene sequencing showed a decrease in Firmicutes and an increase in Bacteroidetes and Proteobacteria phyla. At the genus level, Pb exposure was linked to an increase in Parabacteroides and a decrease in Dehalobacterium.
Another study showed that the exposure of mice to 1.83 g/L lead acetate ((CH3COO)2Pb·3H2O) in drinking water led to intestinal dysbiosis, characterized by a decrease in beneficial species such as Akkermansia muciniphila, Faecalibacterium prausnitzii, and Oscillibacter ruminantium [46]. Population-based human studies further support these findings. A cross-sectional study involving 696 participants examined urinary Pb concentrations and their association with gut microbiota composition. The results demonstrated a strong correlation between elevated urinary Pb levels and an increased abundance of Desulfovibrio, Eubacterium, and Ruminococcus. Conversely, there was a notable decline in Clostridium, Coprococcus, and Pediococcus [47]. These findings collectively suggest that Pb exposure disrupts gut microbial homeostasis, favoring the proliferation of pro-inflammatory and pathogenic bacteria while depleting beneficial commensal species. This dysbiosis may play a role in Pb-induced systemic toxicity, contributing to inflammatory and metabolic disturbances.
HMs are known to interfere with signaling pathways affecting a variety of cellular processes, including cell growth, proliferation, survival, metabolism, and apoptosis. Their bioaccumulation can lead to diverse toxic effects on different body tissues and organ systems, primarily through their capacity to disrupt antioxidant defense mechanisms, induce oxidative stress, and substitute essential metals in biological processes, leading to cellular damage and increased risk of diseases such as cancer, neurodegenerative disorders, and cardiovascular complications. Table 1 presents the specific effects of some heavy metals on the human body.

Table 1.
Effects of HMs on human body.
3. The Role of Probiotics in Reducing Heavy Metal Toxicity
HMs, once accumulated in the body, can cause various chronic conditions and increase the risk of cancer. Due to their persistent nature in the environment, these metals contaminate the soil and enter the food chain, affecting both human health and ecosystems [54,55]. Although traditional detoxification methods exist, they are often costly and may have undesirable side effects. A promising and less invasive alternative is the use of probiotics, live microorganisms that, when administered in appropriate doses, provide health benefits to the host by aiding in the elimination of metals from the body [56,57].
The As resistance genes of certain bacterial genera, such as Bacteroides, Clostridium, Alistipes, and Bilophila, enable them to chemically modify As. Through methylation, these bacteria transform As into a less toxic form, thereby ensuring their survival and simultaneously protecting the host organism from the harmful effects of this element [58,59]. The results from a study on the management of As poisoning using probiotics showed that they ameliorated the negative effects of As on oxidative stress and reproductive dysfunction in female rats. The probiotic mixture, containing 137.14 million cells of each Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, and Bifidobacterium bifidum and 28.57 million cells of Saccharomyces boulardii, led to the restoration of antioxidant activities (superoxide dismutase (SOD), catalase, and NPSH), a reduction in malondialdehyde (MDA) and Cd levels, and improved vitamin B12 and estradiol levels. Additionally, they decreased the expression of inflammatory markers and tissue lesions, providing protection against DNA damage and uterine–ovarian necrosis [60]. Another study investigating the effects of probiotics on As toxicity in Wistar rats showed that As increased MDA, myeloperoxidase (MPO), and inflammatory cytokine levels while reducing serum antioxidant defense parameters. Probiotics containing Lactobacillus and Bifidobacterium lactis demonstrated a significant protective effect, reducing genotoxicity and improving SOD levels, TNF-α, and interferon-gamma (IFN-γ). These beneficial effects were attributed to the probiotics’ ability to decrease the production of reactive oxygen species (ROS) and reduce intestinal inflammation [61].
The protective effects of probiotics against Cd toxicity have been extensively studied. In one study, Wistar rats exposed to Cd exhibited significant metal accumulation in the liver and kidneys, increased oxidative stress (elevated MDA and 8-hydroxy-2′-deoxyguanosine [8-OHdG]), and altered glutathione (GSH) and metallothionein levels. Treatment with Lactobacillus- and Bifidobacterium-based probiotics and nano-probiotics significantly reversed these effects, with the nano-formulation showing greater efficacy in reducing GSH and 8-OHdG levels [62]. In another study, Djurasevic et al. [63] demonstrated that the administration of Lactobacillus rhamnosus Rosell-11, Lactobacillus acidophilus Rosell-52, and Bifidobacterium longum Rosell-175 significantly increased Cd excretion through feces, reducing its levels in the blood, liver, and kidneys, while improving liver function. Similarly, Lactobacillus plantarum CCFM8610, a strain with high Cd-binding capacity and antioxidant properties, demonstrated a significant reduction in Cd accumulation in tissues, attenuation of inflammation, and improved gut barrier integrity Cd [8]. Pediococcus pentosaceus GS4 was also effective in reducing Cd deposition in the liver and intestines, improving membrane integrity, and mitigating Cd-induced intestinal lesions [64]. A comparative study evaluating 13 probiotic strains for their Cd detoxification potential found that Streptococcus thermophilus exhibited the highest resistance to Cd and potent antioxidant properties, significantly reducing Cd levels in the bloodstream [65].
Regarding the effects of probiotics on Hg-induced toxicity, Majlesi et al. [66] investigated the efficacy of Lactobacillus plantarum and Bacillus coagulans in a rat model. Probiotic administration provided significant protection against Hg toxicity by reducing Hg levels in the liver and kidneys and preventing changes in glutathione peroxidase (GPx) and SOD levels. Probiotics also led to a significant reduction in creatinine, urea, bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels. In another study, Lactobacillus brevis 23017 protected the intestinal barrier, reducing weight loss and intestinal lesions caused by Hg. It also modulated tight junction proteins and reduced inflammation and oxidative stress through the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) pathways [67]. Exposure to Hg chloride caused oxidative stress and renal damage, including tubular necrosis, in laboratory mice. Supplementation with a probiotic product containing Lactobacillus plantarum, Lactobacillus delbrueckii spp. Bulgaricus PXN39, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Streptococcus salivarius spp. Thermophilus, and Enterococcus faecium significantly modulated MDA levels and antioxidant enzyme activities, having a beneficial effect on the histopathological lesions induced by Hg toxicity [68].
Probiotic-based interventions have also shown promise in mitigating Pb toxicity. Zhai et al. [69] demonstrated that both a supplement containing grape seed extract, tea polyphenols, and Lactobacillus plantarum CCFM8661, as well as another supplement including vitamin C, calcium carbonate, zinc acetate, and L. plantarum CCFM8661, effectively reduced Pb levels, protecting antioxidant enzyme activities and recovering oxidative stress markers and cognitive deficits in mice. These supplements offered superior protection compared to chelator treatments, and administration during Pb exposure provided a significantly greater protective effect than subsequent administration. Additionally, Lactobacillus plantarum CCFM8661 protected against Pb toxicity by restoring antioxidant enzyme activity and reducing Pb levels in blood and tissues [70]. The treatment’s efficacy was significantly higher when the probiotic was administered throughout the duration of Pb exposure. Another probiotic studied in Pb toxicity in mice was Lactobacillus delbrueckii subsp. bulgaricus KLDS1.0207, which was shown to reduce mortality, increase Pb excretion through feces, prevent Pb accumulation in tissues, and improve antioxidant functions in the liver and kidneys [71]. The role of gut microbiota in heavy metal detoxification, highlighting eight key microbial mechanisms is illustrated in Figure 1. Biosorption allows gut bacteria to bind metals on their surface, reducing absorption, while bioprecipitation by sulfate-reducing bacteria (SRB) converts Cd, Hg, and Pb into insoluble salts. Bioassimilation involves siderophore production by Lactobacillus and Bacillus, limiting Fe, Pb, and Cd uptake, whereas bioaccumulation enables temporary metal storage, though it is limited in the gut. Biotransformation, performed by Bacteroides and Clostridium, modifies metal toxicity through enzymatic processes such as methylation, reduction, and oxidation [11]. For instance, arsenate reductase reduces arsenate to arsenite, which is more soluble and can be more easily excreted [72], while mercuric reductase converts toxic mercury into elemental mercury, reducing its bioavailability and toxicity [73]. These enzymatic reactions are influenced by various factors such as pH, redox potential, and the availability of cofactors like NADPH and S-adenosylmethionine [74]. Furthermore, bioleaching mechanisms utilize efflux pumps to expel toxic metals back into the intestinal lumen. Lastly, metal solubilization occurs through the bacterial secretion of lactic and acetic acids, which influence metal bioavailability. These mechanisms collectively help regulate metal toxicity and reduce heavy metal absorption in the human body.
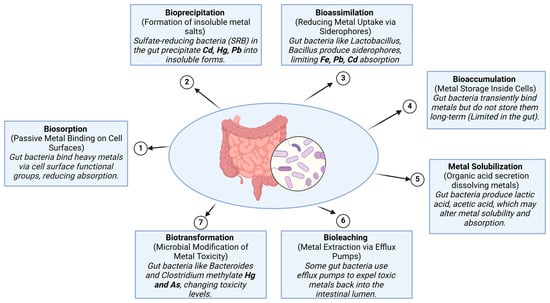
Figure 1.
The role of gut microbiota in heavy metal detoxification.
The growing body of evidence underscores the potential of probiotics as a natural and effective strategy for mitigating HM toxicity. Through various mechanisms such as metal binding, biotransformation, competitive exclusion, immune modulation, and gut barrier protection, probiotic bacteria offer a safe and accessible means of detoxification. While further clinical studies are needed to validate these findings in humans, probiotics represent a promising adjunctive therapy for reducing the toxic burden of HMs and minimizing their long-term health consequences (Figure 2).
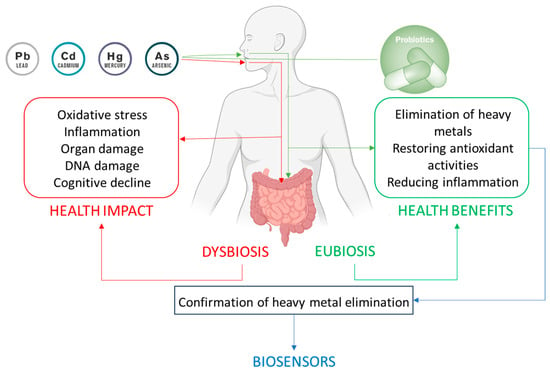
Figure 2.
Interaction between heavy metals and gut microbiota, probiotic modulation, and the biosensor-based detection of heavy metals. The figure depicts the dual impact of heavy metal exposure on the body. On one hand, heavy metals exert direct toxic effects on various organs and physiological systems, leading to detrimental health outcomes. On the other hand, they disrupt the balance of the gut microbiota, inducing dysbiosis that exacerbates health issues. Probiotic administration aids in detoxification by reducing metal bioavailability, improving overall health, restoring eubiosis, and enhancing health. Biosensors enable the rapid, accurate, and non-invasive monitoring of heavy metals in urine, providing real-time confirmation of the detoxification process and the efficacy of probiotic-based interventions.
Limitations of Probiotic Therapies in HM Detoxification
While probiotic therapies are widely recognized for their health benefits, they also present limitations, particularly in vulnerable populations. Kimse et al. [75] highlight that although generally safe, probiotics can cause mild gastrointestinal symptoms such as bloating, flatulence, or diarrhea, especially during initial supplementation. Rare allergic reactions, manifesting as rashes, itching, or swelling, have also been reported. More concerningly, in immunocompromised individuals, infants, and the elderly, probiotics pose a risk of severe infections, including bacteremia, fungemia, and endocarditis.
Beyond these adverse effects, several other challenges impact the effectiveness of probiotic therapies. One key issue is survivability within the gastrointestinal tract, as probiotics must withstand harsh conditions like acidity and bile salts to be effective [76]. Furthermore, probiotic effects are highly strain-specific, requiring careful selection to ensure therapeutic efficacy. Individual variability in gut microbiota composition complicates standardization, as responses to probiotic treatment can differ significantly between individuals. Regulatory and safety concerns, particularly regarding genetically engineered probiotics, further hinder widespread application, alongside the need for advanced delivery mechanisms to maintain probiotic viability [76,77]. Similarly, other papers [76] highlight not only the challenges in ensuring probiotics survive gastrointestinal conditions, but also their strain-specific nature, and the variability in individual responses due to differences in diet, genetics, and microbiota composition. Variations in manufacturing practices also lead to inconsistencies in probiotic potency and purity, potentially affecting their safety and effectiveness. These challenges underscore the need for continued research and technological advancements to improve the efficacy and reliability of probiotic treatments. Another critical limitation in probiotic prescription practices is the lack of standardized guidelines, leading to inconsistent recommendations among healthcare professionals. Insufficient clinical evidence on the efficacy of many probiotic products raises concerns about their therapeutic benefits. Additionally, variations in regulatory frameworks across different regions contribute to discrepancies in probiotic quality and labeling, complicating prescribing decisions. The vast diversity of probiotic strains makes individualized therapy difficult, often resulting in suboptimal outcomes. Addressing these limitations requires the development of standardized protocols, rigorous clinical research, and improved regulatory frameworks to ensure their safe and effective use.
In the context of probiotics use in mitigating the deleterious effects of heavy metals, several unresolved issues persist. The absence of long-term clinical studies assessing probiotic efficacy and safety remains a concern, while variability in individual responses due to differences in microbiome composition and genetic factors makes treatment outcomes unpredictable [78]. Furthermore, determining optimal dosages and selecting the most effective probiotic strains remains a significant challenge. These issues highlight the necessity for further research to establish strong clinical evidence and standardized therapeutic recommendations. For example, in colorectal cancer therapy that involves monitoring environmental and dietary exposure to heavy metals, given their role in carcinogenesis, probiotics face additional hurdles. Native probiotics often exhibit weak therapeutic efficacy and uncontrolled physiological behavior, limiting their effectiveness against malignancies [79]. Their simple metabolic activities and single therapeutic functions make them less suited for addressing the complexities of cancer treatment. Moreover, the use of viable microorganisms carries risks, including infections, inflammatory responses, and even potential carcinogenic effects. To overcome these obstacles, genetic modifications and synthetic biology approaches have been suggested as means to enhance the therapeutic properties of probiotics, making them safer and more effective for CRC therapy.
4. Biosensors for Heavy Metals Detection from Urine
HMs, once accumulated in the body, pose serious health risks contributing to chronic conditions and increasing the risk of cancer [54,55]. These toxic elements are commonly found in bodily fluids such as blood, sweat, and urine, playing a key role in their detoxification and excretion. Thus, monitoring HM levels provides valuable insights into an individual’s toxic exposure and overall health [80]. Given its abundance and ease of collection, urine is an ideal non-invasive diagnostic medium for detecting various human diseases, particularly those affecting the urinary tract. Electrochemical biosensors have emerged as powerful tools for urinary diagnostics due to their exceptional sensitivity, cost-effectiveness, and capability to detect a broad range of target molecules, including nucleic acids and protein biomarkers [81]. These biosensors offer a rapid, portable, and user-friendly alternative to traditional laboratory methods, making them especially valuable in point-of-care (POC) applications. Biosensors are increasingly recognized as highly selective, cost-efficient analytical tools, playing a critical role in early-stage diagnostics and personalized healthcare management. Recent advancements in nanotechnology have significantly improved biosensor performance by enhancing device design, optimizing sensing interfaces, and increasing detection sensitivity. These technological innovations have enabled the development of biosensors tailored for real-time, on-site monitoring, thereby supporting precision medicine approaches [82].
Traditionally, the determination of metal concentrations in body fluids requires sophisticated analytical techniques such as atomic absorption spectrometry (AAS), inductively coupled plasma-mass spectrometry (ICP-MS), and inductively coupled plasma atomic emission spectrometry (ICP-AES) [83,84,85,86]. However, these methods have several limitations, including high operational costs, the need for specialized personnel, and reliance on centralized laboratories. Shipping samples to these labs introduces delays and increases costs, making these techniques impractical for rapid assessment, particularly in low-resource settings and developing countries [87]. Biosensor technology overcomes these limitations by offering a portable, cost-effective, and user-friendly alternative for HM detection. Many metals in urine have a short half-life, reflecting recent exposure and allowing for the real-time monitoring of detoxification interventions, including the effects of probiotic administration [88]. The classification of heavy metal biosensors into three main types: electrochemical, optical, and mass-based biosensors, each with distinct sensitivity, advantages, and limitations, is presented in Figure 3.
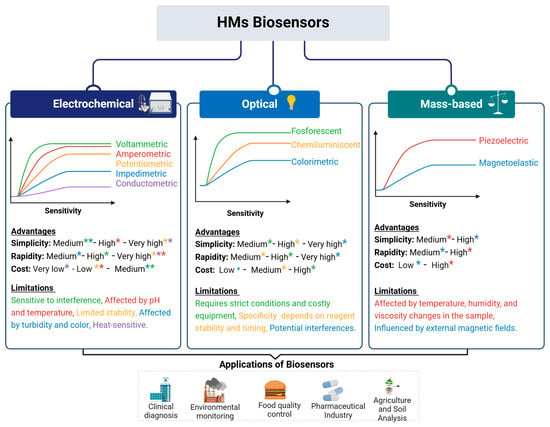
Figure 3.
Classification, characteristics, and application of biosensors. Electrochemical biosensors (voltammetric, amperometric, potentiometric, impedimetric, and conductometric) offer high sensitivity and low cost but can be affected by pH, temperature, and sample turbidity. Optical biosensors (fluorescent, chemiluminescent, and colorimetric) provide a lower sensitivity compared with electrochemical biosensors and require strict conditions and costly equipment. Mass-based biosensors (piezoelectric and magnetoelastic) detect metal accumulation based on mass changes, offering high simplicity and rapidity, but can be affected by environmental conditions such as temperature, humidity, and external magnetic fields. At the bottom of the Figure, the key applications of biosensors are highlighted, including clinical diagnosis, environmental monitoring, food quality control, pharmaceuticals, and agriculture. The colors used in the figure correspond to the types of biosensors according to the sensitivity graphs and are later used to highlight the advantages and limitations of each method.
Urine samples for HM determination are preferably collected as first-morning urine using sterile polyethylene or polypropylene containers to minimize the risk of contamination. After collection, the samples are transported to the laboratory in plastic containers and either refrigerated at 4 °C for immediate analysis or aliquoted and frozen at −70 °C for long-term storage. Prior to analysis, the samples undergo acid digestion using concentrated nitric acid (HNO3) and hydrogen peroxide (H2O2) to break down the organic matrix and release HMs. All the equipment used, including laboratory containers, is rigorously cleaned with detergents and distilled water to prevent contamination, ensuring the accuracy and reliability of analytical results [89,90,91]. Figure 4. provides a classification of the techniques and sensing platforms used for detecting HMs (Cu, Hg, Cd, and Pb) in urine samples, categorized into electrochemical and optical methods.
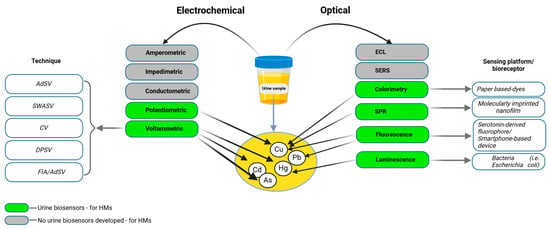
Figure 4.
Electrochemical and optical biosensors for urine analysis. Electrochemical techniques, including adsorptive stripping voltammetry (AdSV), square wave anodic stripping voltammetry (SWASV), cyclic voltammetry (CV), differential pulse stripping voltammetry (DPSV), and flow injection analysis combined with adsorptive stripping voltammetry (FIA/AdSV), utilize sensing mechanisms such as amperometric, impedimetric, conductometric, potentiometric, and voltammetric approaches. The green color indicates techniques where urine biosensors for heavy metals are developed, while the gray color signifies the lack of biosensors. Optical techniques include colorimetry, electrochemiluminescence (ECL), Surface-Enhanced Raman Spectroscopy (SERS), surface plasmon resonance (SPR), fluorescence, luminescence, and Förster resonance energy transfer (FRET), which rely on visual or spectral changes for detection. The sensing platforms or bioreceptors associated with these optical methods include paper-based dyes, molecularly imprinted nanofilms, serotonin-derived fluorophores, bacteria (Escherichia coli), and smartphone-based devices.
In recent years, a significant number of biosensors have been developed for the detection of HMs in urine, providing innovative, non-invasive solutions for real-time monitoring. A comprehensive summary of these biosensors and their respective methodologies is provided in Table 2.

Table 2.
Biosensors for heavy metals in urine.
4.1. Arsenic Detection in Urine Using Advanced Biosensors
As, a highly toxic metal linked to severe health issues such as cancer, cardiovascular diseases, and neurological disorders requires effective detection methods to monitor exposure accurately. Advanced biosensors have emerged as promising tools for As detection due to their high sensitivity, selectivity, and low detection limits [109]. Several studies have explored the detection mechanisms for As ions, highlighting significant advances achieved through the development of biosensors that involve aptamers, nanomaterials, and engineered biological systems. Colorimetric biosensors have emerged as simple, cost-effective, and portable and easy-to-use solutions for on-site As detection, making it particularly suitable for in situ applications. They utilize gold or silver nanoparticles, which aggregate and change color upon interaction with As ions [110]. Recent innovations include a 3D-printed device for As (III) detection, further enhancing the practicality of these biosensors [111]. Additionally, inorganic As species (As+3 and As+5) also exhibit redox properties, enabling spontaneous reactions with chromogenic reagents for precise colorimetric detection [112].
Electrochemical biosensors are widely used for their precision in As detection. These devices utilize modified electrodes incorporating materials such as graphene oxide and gold nanoparticles, which enhance conductivity and surface interactions. Voltammetric techniques, such as square wave anodic stripping voltammetry (SWASV), enable highly accurate detection by pre-concentrating As on nanotextured electrode surfaces and measuring oxidation currents [113].
Molecular biosensors leveraging DNA aptamers provide another powerful approach to As detection. Merulla et al. [114] explored DNA-based biosensors that employ aptamers to target As ions selectively. These biosensors generate detectable changes in response to As binding, such as variations in optical or electrical properties. Functionalization with nanomaterials, such as gold nanoparticles, further enhances sensitivity and selectivity, making them suitable for both environmental and biological assays [115]. Innovative biosensing approaches include the development of genetically engineered bacteria designed to produce measurable signals in response to As exposure. These biosensors utilize biological pathways to detect As, providing a robust and scalable detection platform. Merulla et al. [114] demonstrated that engineered microbial strains can be programmed to fluoresce or change metabolic activity upon As binding, making them highly adaptable for environmental and medical testing.
Jia et al. [116] detailed the development of an advanced whole-cell biosensor using Escherichia coli DH5α. Which integrates a positive feedback amplifier to enhance As ion detection. This biosensor utilizes an As-inducible promoter (Pars), the regulatory gene (arsR), and the reporter gene mCherry creating a fluorescence-based detection system. A positive feedback loop via LuxR autoregulatory elements significantly amplifies output signals, improving sensitivity by an order of magnitude compared to earlier designs. The application of nanotechnology in As biosensing has led to the development of highly sensitive and selective detection methods. Recent studies have demonstrated the effectiveness of carbon dot-MnO2 nanocomposites and fluorescent test papers which exhibit high sensitivity and low detection limits for As detection [117]. Functionalization of carbon-based nanomaterials with recognition elements such as aptamers, antibodies, or chemical ligands enables As-induced changes in electrical conductivity, fluorescence, or optical properties, allowing for versatile and efficient detection.
4.2. Mercury Detection in Urine Using Advanced Biosensors
Hg concentrations in blood and urine are widely used as biomarkers to assess Hg exposure. A 24 h urine sample is particularly valuable for evaluating the extent of exposure. According to various studies, urine Hg levels below 10 µg L⁻1 and blood Hg levels below 20 µg L⁻1 are generally regarded as within the normal range [118,119]. Biomonitoring Hg levels in urine can provide valuable information on environmental exposure to inorganic and elemental Hg. This process is particularly important before and during interventions, such as probiotic administration, aimed at enhancing Hg excretion. Additionally, continued monitoring during probiotic consumption is essential to assess whether there is an increase in urinary Hg levels, indicating enhanced Hg excretion [120]. Nanotechnology-based sensors designed for the detection of mercury ions (Hg2⁺) include various nanomaterials, such as silver and gold nanoparticles, silica-based materials, magnetic nanoparticles, quantum dots, carbon dots, and electrochemical sensors. These sensors highlight unique properties and mechanisms that enable highly sensitive and selective Hg detection [121]. These technologies leverage the unique properties of nanomaterials, enabling the highly sensitive and selective detection of Hg, a toxic metal that poses significant risks to human health and the environment. Advanced biosensors further enhance detection capabilities by utilizing molecular recognition elements, nanomaterials, and innovative transduction mechanisms.
Aptamer- and antibody-based biosensors are among the most frequently used for detecting Hg2⁺. These systems employ recognition elements such as aptamers or antibodies specific to Hg2⁺ and rely on transduction methods like electrochemical impedance spectroscopy (EIS) and fluorescence. The integration of nanomaterials, including quantum dots and graphene, improves electron transfer and amplifies signals, allowing for highly sensitive and selective detection. Hg binding triggers optical or electrical changes, which are then measured to quantify Hg levels [115].
Another innovative approach involves peptide-based fluorescent biosensors. A novel biosensor was presented by Sosnowska et al. [122] that utilizes a seven-amino-acid peptide, FY7, that detects Hg2⁺ through a 2:1 complex formed between Hg2⁺ ions and the tyrosine residue in the peptide. This interaction causes a decrease in fluorescence emission proportional to the Hg concentration. This method enables rapid, sensitive, and selective detection with a low detection limit, requiring minimal sample volumes and being particularly suited for micro-volume analysis using a glass capillary fluorometer. Rajasekar et al. [123] described photoresponsive biosensors which represent an additional advancement, offering exceptional sensitivity and selectivity by exploiting optical changes induced by Hg binding. For instance, azobenzene-based sensors detect Hg2⁺ through UV-Vis or fluorescence shifts caused by photoisomerization, while coumarin- and fluorescein-based systems exhibit fluorescence quenching or colorimetric changes upon Hg interaction, facilitating applications such as live-cell imaging and environmental monitoring. Pyrene sensors rely on fluorescence quenching due to Hg2⁺ interaction, while quinoline-based sensors detect Hg by altering fluorescence properties, making them ideal for wearable technologies and Internet of Things (IoT) applications [123]. Gold nanoparticle (AuNP)-based colorimetric biosensors are another tool that provides a simple yet effective mechanism for Hg detection. Functionalized AuNPs aggregate in the presence of Hg2⁺, leading to a visible color change from red to blue. This method is rapid, cost-efficient, and particularly well suited for on-site and field applications, making it a valuable tool for practical Hg detection [124].
4.3. Cadmium Detection in Urine Using Advanced Biosensors
Cd is a widespread HM pollutant that poses significant threats to food safety and human health even at low concentrations. Traditional detection methods are expensive, labor-intensive, and unsuitable for real-time or on-site analysis [125]. This underscores the need for innovative techniques, such as biosensors, which can provide more precise detection and identification of metal compounds like Cd [126]. Monitoring Cd levels in urine is an important biomarker for assessing an individual’s total body burden and potential toxic effects [99]. Genetically engineered biosensors have been developed by Hu et al. [127] to detect HMs such as Cd and Hg with high specificity and sensitivity. One mechanism utilizes a dual-colored bacterial biosensor incorporating CadR and MerR regulators. CadR responds to Cd and Pb by producing prodeoxyviolacein (PDV), emitting a gray-green color, while MerR converts PDV to deoxyviolacein (DV), producing a purple color in the presence of Hg. This biosensor provides dose-dependent dual signals, where gray-green indicates Cd concentrations from nanomolar to micromolar levels, and purple is specific to Hg detection.
Another biosensing approach employs genetically engineered bacterial cells containing artificial Cad operons derived from the natural Cd resistance system of Pseudomonas putida. In these systems, CadR, a Cd-responsive transcriptional regulator, activates a promoter upon Cd2⁺ binding, leading to the expression of reporter genes like mCherry (red fluorescence), eGFP (green fluorescence), or lacZα (enzymatic activity). These signals indicate Cd concentration. Some designs include a Cd-binding domain on the bacterial surface, enabling the simultaneous detection and removal of Cd [128]. A dual-sensing bacterial bioreporter system utilizes two Cd-responsive regulators, CadC and CadR, coupled with fluorescent reporters such as eGFP for green fluorescence and mCherry for red fluorescence. Upon exposure to Cd, these regulators activate their respective reporters, producing distinct fluorescence signals. This integration allows the simultaneous detection and quantification of bioavailable Cd with enhanced specificity and reduced interference from other metals [129].
Another bacterial biosensor engineered to detect Cd utilizes the production of a visible blue-purple pigment, violacein. This biosensor leverages the Cd-responsive regulator CadR to trigger violacein biosynthesis in the presence of Cd, producing a color change detectable by the naked eye. The violacein signal is stable and measurable at 578 nm, offering a cost-effective, minimal-equipment method for Cd monitoring [130]. Joe M. et al. [131] developed a biosensing mechanism using genetically engineered Deinococcus radiodurans that produces a red pigment, deinoxanthin, in response to Cd. The promoter of the Cd-sensitive gene DR_0659 is activated in the presence of Cd, controlling the expression of the crtI gene responsible for red pigment synthesis. Upon Cd exposure, the bacteria visibly change color from light yellow to bright red, detectable at Cd concentrations ranging from 50 nM to 1 mM. This system relies on a stable, Cd-specific colorimetric reaction with minimal interference from other metals. Similarly to Hg biosensors, Cd biosensors also use aptamers or whole-cell systems to respond to Cd2⁺. Nanomaterials such as gold nanoparticles or graphene oxide enhance these systems by improving conductivity and surface area. Electrochemical methods, including differential pulse voltammetry (DPV), are commonly employed for detection, providing high sensitivity and selectivity [115].
4.4. Copper Detection in Urine Using Advanced Biosensors
Cu is an essential trace element that plays a crucial role in various biochemical processes and the regulation of physiological functions across all forms of life. It contributes to key physiological activities such as oxidative phosphorylation, angiogenesis, blood coagulation, and antioxidation. Additionally, as a cofactor for numerous enzymes, Cu is indispensable in processes like erythropoiesis, cellular respiration, cholesterol and glucose metabolism, pigment production, and hormone synthesis [132]; however, a high level of Cu is toxic [21]. In addition to the methods presented in Table 1, Cu in urine can also be determined using flow injection analysis with electrochemical detection at platinum disk microelectrodes [133]. The determination of Cu is also highly important for Wilson’s disease (WND). This condition is characterized by increased urinary Cu excretion, which can sometimes exceed 100 µg/day, compared to the normal upper limit of 50 µg/day. In untreated WND patients, urinary Cu excretion (UCE), a reflection of serum non-ceruloplasmin-bound Cu (NCC), is elevated. Consequently, measuring Cu levels in a 24 h urine collection is a common diagnostic test for WND. Urinary Cu excretion exceeding 100 µg/24 h is considered diagnostic for the disease [134].
Beyond its metabolic roles, Cu plays a crucial role in defending against urinary tract infections (UTIs) by being mobilized into urine to limit bacterial growth, restricting iron availability through ceruloplasmin, and directly impairing the virulence of uropathogenic Escherichia coli (UPEC), highlighting a novel host defense mechanism [135]. Figure 5 shows an integrated biosensor system for detecting Cu levels in urine. It highlights a biorecognition layer incorporating enzymes, proteins, aptamers, functionalized nanomaterials (e.g., graphene), and chemical reagents to ensure selectivity and sensitivity. Transducers such as optical (colorimetric, surface plasmon resonance (SPR), fluorescence, and luminescence) and electrochemical convert interactions into measurable signals. The output is displayed through response–time curves or directly on electronic devices, enabling real-time, accurate, and non-invasive Cu monitoring for diagnostic and therapeutic applications. In addition, it emphasizes its association with probiotics, Wilson’s disease, and urinary tract infections.
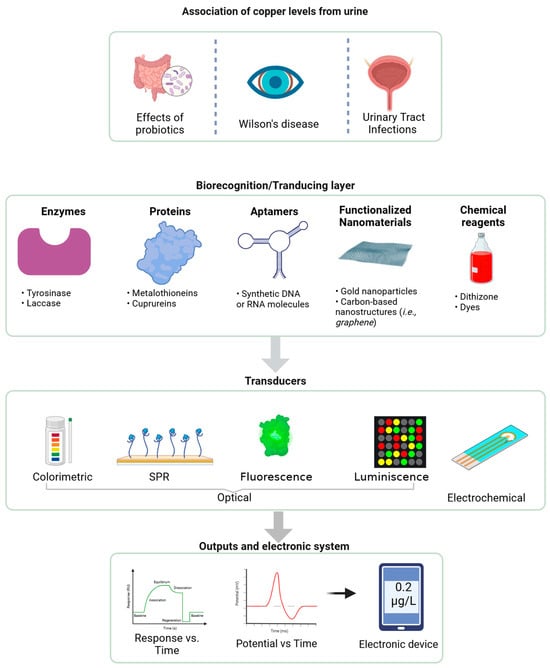
Figure 5.
Integrated biosensor system for copper detection in urine (generated with BioRender).
4.5. Lead Detection in Urine Using Advanced Biosensors
Exposure to Pb in the general population primarily occurs through diet, environmental dust, and contaminated drinking water, with additional contributions from inhaling cigarette smoke and Pb-contaminated air. Blood Pb levels serve as indicators of both recent exposure and the total body burden of Pb. Pb is eliminated from the body through feces and urine, with urinary Pb occasionally used as a biomarker for exposure [91]. Biosensors for Pb detection have emerged as effective tools for environmental and health monitoring. These devices frequently rely on DNAzymes, which catalyze specific reactions in the presence of Pb2⁺ ions. Common configurations include electrochemical sensors with modified electrodes, such as those incorporating gold nanoparticles, and optical sensors that detect fluorescence or colorimetric changes. Polyadenine aptamers and split DNAzymes are widely used as recognition elements, as they undergo detectable structural changes upon binding with Pb2⁺ ions, allowing for highly selective detection [115]. Other advanced sensors utilize anodic stripping voltammetry techniques incorporating Hg-film electrodes or novel nanostructured materials. Examples include self-assembled monolayers on mesoporous supports and carbon nanotubes, which enhance sensitivity and detection performance through their high surface area and conductivity [100]. A significant breakthrough in Pb biosensing technology is the development of a portable fluorescence resonance energy transfer (FRET)-based biosensor named pMet-Pb. This device features a genetically encoded FRET-based Pb biosensor, Met-Pb 1.44 M1, integrated into a biochip. Upon exposure to Pb2⁺, the biosensor undergoes a conformational change that modifies the FRET signal, enabling the quantification of Pb concentrations [101].
5. Conclusions and Future Perspectives
HMs exert a profound influence on gut microbiota composition and function, contributing to dysbiosis and a cascade of adverse health effects. The bidirectional relationship between the gut microbiota and HMs underscores the importance of maintaining microbial homeostasis to mitigate toxicity. Probiotic interventions have emerged as a promising strategy to counteract HM-induced dysbiosis by restoring microbial balance, enhancing detoxification processes, and reinforcing intestinal barrier integrity. These findings highlight the therapeutic potential of probiotics not only in HM detoxification but also in broader applications related to metabolic and inflammatory disorders. Beyond microbiome modulation, the integration of advanced biosensing technologies marks a significant step forward in real-time HM detection and exposure monitoring. Biosensors provide a non-invasive, rapid, and highly sensitive means of detecting HMs in urine, allowing for the continuous monitoring of exposure levels and the effectiveness of detoxification interventions. By leveraging biosensors, healthcare providers and researchers can assess the real-time impact of probiotic and dietary interventions, facilitating precision medicine approaches tailored to individual detoxification needs.
Future research should focus on the development of targeted probiotic therapies specifically designed to modulate the gut microbiota in response to HM exposure. Identifying bacterial strains with enhanced HM-binding capabilities will be crucial for optimizing probiotic formulations. Additionally, expanding research on synbiotics, combinations of probiotics and prebiotics, could further enhance the detoxification potential of microbial interventions by providing substrates that promote the growth of beneficial bacteria involved in metal chelation and excretion.
Another key area for advancement lies in the refinement and miniaturization of biosensor technologies. Integrating graphene-based nanomaterials, molecularly imprinted polymers, and artificial intelligence-driven data analysis into biosensor platforms will improve sensitivity, accuracy, and ease of use. The development of wearable or point-of-care biosensors could revolutionize real-time exposure monitoring, allowing individuals to track HM levels in their urine and adjust detoxification strategies accordingly. A promising direction is the combination of microbiome profiling with biosensor data to develop personalized detoxification approaches. By tailoring probiotic treatments, dietary recommendations, and lifestyle modifications based on an individual’s gut microbiota composition and HM exposure levels, healthcare practitioners can implement precision medicine strategies that optimize detoxification outcomes. Furthermore, expanding clinical research into the long-term health effects of chronic low-dose HM exposure and microbiota-targeted interventions will provide deeper insights into preventive and therapeutic measures. Collaborative efforts between microbiologists, toxicologists, bioengineers, and clinicians will be essential in translating laboratory findings into practical applications for environmental health, public policy, and personalized medicine. Ultimately, the integration of microbiome modulation and biosensor-based monitoring offers a powerful and innovative approach to managing HM exposure. Advancements in these fields hold the potential to significantly reduce HM toxicity-related health risks, improve patient outcomes, and pave the way for next-generation detoxification strategies tailored to individual needs.
Author Contributions
Conceptualization, L.A.-N., O.C.I., A.L. and M.C.; methodology, L.A.-N., O.C.I., A.L. and M.C.; investigation, L.A.-N. and O.C.I.; resources, L.A.-N. and O.C.I.; writing—original draft preparation, L.A.-N. and O.C.I.; writing—review and editing, A.L. and M.C.; visualization, L.A.-N. and O.C.I.; supervision, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from the EU’s NextGenerationEU instrument through the National Recovery and Resilience Plan of Romania-Pillar III-C9-I8, managed by the Ministry of Research, Innovation and Digitalization, as part of the project titled “Metagenomics and Bioinformatics tools for Wastewater-based Genomic Surveillance of viral Pathogens for early prediction of public health risks (MetBio-WGSP)” contract no.760286//27.03.2024, code CF 167/31.07.2023.
Acknowledgments
This work was funded by the EU’s NextGenerationEU instrument through the National Recovery and Resilience Plan of Romania-Pillar III-C9-I8, managed by the Ministry of Research, Innovation and Digitalization, within the project entitled “Metagenomics and Bioinformatics tools for Wastewater-based Genomic Surveillance of viral Pathogens for early prediction of public health risks (MetBio-WGSP)”, contract no.760286//27.03.2024, code CF 167/31.07.2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut Microbiota: A Target for Heavy Metal Toxicity and a Probiotic Protective Strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Motta-Romero, H.A.; Perez-Donado, C.E.; Auchtung, J.M.; Rose, D.J. Toxicity of Cadmium on Dynamic Human Gut Microbiome Cultures and the Protective Effect of Cadmium-Tolerant Bacteria Autochthonous to the Gut. Chemosphere 2023, 338, 139581. [Google Scholar] [CrossRef]
- Gao, B.; Chi, L.; Mahbub, R.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Multi-Omics Reveals That Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol. 2017, 30, 996–1005. [Google Scholar] [CrossRef]
- Peng, Z.; Liao, Y.; Yang, W.; Liu, L. Metal(Loid)-Gut Microbiota Interactions and Microbiota-Related Protective Strategies: A Review. Environ. Int. 2024, 192, 109017. [Google Scholar] [CrossRef]
- Giambò, F.; Italia, S.; Teodoro, M.; Briguglio, G.; Furnari, N.; Catanoso, R.; Costa, C.; Fenga, C. Influence of Toxic Metal Exposure on the Gut Microbiota (Review). World Acad. Sci. J. 2021, 3, 19. [Google Scholar] [CrossRef]
- Claus, S.P.; Ellero, S.L.; Berger, B.; Krause, L.; Bruttin, A.; Molina, J.; Paris, A.; Want, E.J.; de Waziers, I.; Cloarec, O.; et al. Colonization-Induced Host-Gut Microbial Metabolic Interaction. mBio 2011, 2, e00271-10. [Google Scholar] [CrossRef]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef]
- Ghosh, S.; Nukavarapu, S.P.; Jala, V.R. Effects of Heavy Metals on Gut Barrier Integrity and Gut Microbiota. Microbiota Host 2024, 2, e230015. [Google Scholar] [CrossRef]
- Liu, Z.; Cascioli, V.; McCarthy, P.W. Healthcare Monitoring Using Low-Cost Sensors to Supplement and Replace Human Sensation: Does It Have Potential to Increase Independent Living and Prevent Disease? Sensors 2023, 23, 2139. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T. Toxic and Essential Metals: Metabolic Interactions with the Gut Microbiota and Health Implications. Front. Nutr. 2024, 11, 1448388. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tu, H.; Chen, T. Potential Application of Living Microorganisms in the Detoxification of Heavy Metals. Foods 2022, 11, 1905. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Rangasamy, G.; Nisha Pauline, J.M.; Ramaraju, P.; Mohanasundaram, S.; Nguyen Vo, D.-V. Biosensor for Heavy Metals Detection in Wastewater: A Review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, H.; Shin, W.H.; Oh, J.-M.; Koo, S.-M.; Kim, Y.; Lee, T.; Yu, B.J.; Park, C. Development of Colorimetric Whole-Cell Biosensor for Detection of Heavy Metals in Environment for Public Health. Int. J. Environ. Res. Public. Health 2021, 18, 12721. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, J.; Pan, X.; Shen, H.; Hu, Z.; Zhang, J.; Wang, L.; Wu, P.; Tan, Y. Development and Validation of a High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry Method for the Simultaneous Determination of Arsenic and Mercury Species in Human Urine. Chemosensors 2025, 13, 78. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, S.; Wang, Q.; Li, J. Recent Progress in Biosensor Regeneration Techniques. Nanoscale 2024, 16, 2834–2846. [Google Scholar] [CrossRef]
- Anchidin-Norocel, L.; Gutt, G.; Tătăranu, E.; Amariei, S. Electrochemical Sensors and Biosensors: Effective Tools for Detecting Heavy Metals in Water and Food with Possible Implications for Children’s Health. Int. J. Electrochem. Sci. 2024, 19, 100643. [Google Scholar] [CrossRef]
- Anchidin-Norocel, L.; Savage, W.K.; Nemțoi, A.; Dimian, M.; Cobuz, C. Recent Progress in Saliva-Based Sensors for Continuous Monitoring of Heavy Metal Levels Linked with Diabetes and Obesity. Chemosensors 2024, 12, 269. [Google Scholar] [CrossRef]
- Popenda, A.; Wiśniowska, E.; Manuel, C. Biosensors in Environmental Analysis of Microplastics and Heavy Metal Compounds—A Review on Current Status and Challenges. Desalination Water Treat. 2024, 319, 100456. [Google Scholar] [CrossRef]
- Stremmel, W.; Weiskirchen, R. Wilson Disease: More Complex than Just Simply a Copper Overload Condition?—A Narrative Review. AME Med. J. 2022, 7, 26. [Google Scholar] [CrossRef]
- Huang, W.-S.; Lee, Y.-J.; Wang, L.; Chen, H.-H.; Chao, Y.-J.; Cheng, V.; Liaw, S.-J. Copper Affects Virulence and Diverse Phenotypes of Uropathogenic Proteus Mirabilis. J. Microbiol. Immunol. Infect. 2024, 57, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Ye, Z.; Kakade, A.; Virk, A.K.; Li, X.; Liu, P. A Review on Gut Remediation of Selected Environmental Contaminants: Possible Roles of Probiotics and Gut Microbiota. Nutrients 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Assefa, S.; Köhler, G. Intestinal Microbiome and Metal Toxicity. Curr. Opin. Toxicol. 2020, 19, 21–27. [Google Scholar] [CrossRef]
- Shao, M.; Zhu, Y. Long-Term Metal Exposure Changes Gut Microbiota of Residents Surrounding a Mining and Smelting Area. Sci. Rep. 2020, 10, 4453. [Google Scholar] [CrossRef]
- Chiocchetti, G.M.; Domene, A.; Kühl, A.A.; Zúñiga, M.; Vélez, D.; Devesa, V.; Monedero, V. In Vivo Evaluation of the Effect of Arsenite on the Intestinal Epithelium and Associated Microbiota in Mice. Arch. Toxicol. 2019, 93, 2127–2139. [Google Scholar] [CrossRef]
- Chiocchetti, G.M.; Vélez, D.; Devesa, V. Inorganic Arsenic Causes Intestinal Barrier Disruption. Metallomics 2019, 11, 1411–1418. [Google Scholar] [CrossRef]
- Fernández Fernández, N.; Estevez Boullosa, P.; Gómez Rodríguez, A.; Rodríguez Prada, J.I. A Rare Cause of Gastric Injury: Arsenic Intake. Am. J. Gastroenterol. 2019, 114, 1193. [Google Scholar] [CrossRef]
- Chiocchetti, G.M.; Vélez, D.; Devesa, V. Effect of Subchronic Exposure to Inorganic Arsenic on the Structure and Function of the Intestinal Epithelium. Toxicol. Lett. 2018, 286, 80–88. [Google Scholar] [CrossRef]
- Calatayud, M.; Devesa, V.; Vélez, D. Differential Toxicity and Gene Expression in Caco-2 Cells Exposed to Arsenic Species. Toxicol. Lett. 2013, 218, 70–80. [Google Scholar] [CrossRef]
- Calatayud, M.; Gimeno-Alcañiz, J.V.; Vélez, D.; Devesa, V. Trivalent Arsenic Species Induce Changes in Expression and Levels of Proinflammatory Cytokines in Intestinal Epithelial Cells. Toxicol. Lett. 2014, 224, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Brabec, J.L.; Wright, J.; Ly, T.; Wong, H.T.; McClimans, C.J.; Tokarev, V.; Lamendella, R.; Sherchand, S.; Shrestha, D.; Uprety, S.; et al. Arsenic Disturbs the Gut Microbiome of Individuals in a Disadvantaged Community in Nepal. Heliyon 2020, 6, e03313. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luo, Y.; Li, C.; Wang, J.; Chen, L.; Zhong, X.; Zhang, B.; Zhu, Q.; Zou, R.; Guo, X.; et al. Sub-Chronic Low-Dose Arsenic in Rice Exposure Induces Gut Microbiome Perturbations in Mice. Ecotoxicol. Environ. Saf. 2021, 227, 112934. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chi, L.; Liu, C.-W.; Hsiao, Y.-C.; Lu, K. Chronic Arsenic Exposure Perturbs Gut Microbiota and Bile Acid Homeostasis in Mice. Chem. Res. Toxicol. 2023, 36, 1037–1043. [Google Scholar] [CrossRef]
- Wu, H.; Wu, R.; Chen, X.; Geng, H.; Hu, Y.; Gao, L.; Fu, J.; Pi, J.; Xu, Y. Developmental Arsenic Exposure Induces Dysbiosis of Gut Microbiota and Disruption of Plasma Metabolites in Mice. Toxicol. Appl. Pharmacol. 2022, 450, 116174. [Google Scholar] [CrossRef]
- Li, X.; Brejnrod, A.D.; Ernst, M.; Rykær, M.; Herschend, J.; Olsen, N.M.C.; Dorrestein, P.C.; Rensing, C.; Sørensen, S.J. Heavy Metal Exposure Causes Changes in the Metabolic Health-Associated Gut Microbiome and Metabolites. Environ. Int. 2019, 126, 454–467. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Xia, Y.; Liu, K.; Ren, L.; Ji, Y. The Dysbiosis of Gut Microbiota Caused by Low-Dose Cadmium Aggravate the Injury of Mice Liver through Increasing Intestinal Permeability. Microorganisms 2020, 8, 211. [Google Scholar] [CrossRef]
- Yang, J.; Chen, W.; Sun, Y.; Liu, J.; Zhang, W. Effects of Cadmium on Organ Function, Gut Microbiota and Its Metabolomics Profile in Adolescent Rats. Ecotoxicol. Environ. Saf. 2021, 222, 112501. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, H.; Deng, P.; Tan, M.; Chen, C.; Tang, B.; Li, J.; Chen, F.; Zhao, Q.; Li, L.; et al. Environmental Cadmium Exposure Facilitates Mammary Tumorigenesis via Reprogramming Gut Microbiota-Mediated Glutamine Metabolism in MMTV-Erbb2 Mice. Sci. Total Environ. 2023, 897, 165348. [Google Scholar] [CrossRef]
- Aduayom, I.; Denizeau, F.; Jumarie, C. Multiple Effects of Mercury on Cell Volume Regulation, Plasma Membrane Permeability, and Thiol Content in the Human Intestinal Cell Line Caco-2. Cell Biol. Toxicol. 2005, 21, 163–179. [Google Scholar] [CrossRef]
- Ruan, Y.; Wu, C.; Guo, X.; Xu, Z.; Xing, C.; Cao, H.; Zhang, C.; Hu, G.; Liu, P. High Doses of Copper and Mercury Changed Cecal Microbiota in Female Mice. Biol. Trace Elem. Res. 2019, 189, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, W.; He, L.; Xie, H.; Feng, B.; Zhu, H.; Zhao, J.; Cui, L.; Li, B.; Li, Y.-F. Understanding the Hepatoxicity of Inorganic Mercury through Guts: Perturbance to Gut Microbiota, Alteration of Gut-Liver Axis Related Metabolites and Damage to Gut Integrity. Ecotoxicol. Environ. Saf. 2021, 225, 112791. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, J.; Zhang, W.; He, L.; Wang, L.; Chang, D.; Cui, L.; Gao, Y.; Li, B.; Chen, C.; et al. Acute Oral Methylmercury Exposure Perturbs the Gut Microbiome and Alters Gut-Brain Axis Related Metabolites in Rats. Ecotoxicol. Environ. Saf. 2020, 190, 110130. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Wang, J.; Cen, S.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Modulation of the Gut Microbiota by a Galactooligosaccharide Protects against Heavy Metal Lead Accumulation in Mice. Food Funct. 2019, 10, 3768–3781. [Google Scholar] [CrossRef]
- Xia, J.; Jin, C.; Pan, Z.; Sun, L.; Fu, Z.; Jin, Y. Chronic Exposure to Low Concentrations of Lead Induces Metabolic Disorder and Dysbiosis of the Gut Microbiota in Mice. Sci. Total Environ. 2018, 631, 439–448. [Google Scholar] [CrossRef]
- Zhai, Q.; Li, T.; Yu, L.; Xiao, Y.; Feng, S.; Wu, J.; Zhao, J.; Zhang, H.; Chen, W. Effects of Subchronic Oral Toxic Metal Exposure on the Intestinal Microbiota of Mice. Sci. Bull. 2017, 62, 831–840. [Google Scholar] [CrossRef]
- Eggers, S.; Safdar, N.; Sethi, A.K.; Suen, G.; Peppard, P.E.; Kates, A.E.; Skarlupka, J.H.; Kanarek, M.; Malecki, K.M.C. Urinary Lead Concentration and Composition of the Adult Gut Microbiota in a Cross-Sectional Population-Based Sample. Environ. Int. 2019, 133, 105122. [Google Scholar] [CrossRef]
- Nucera, S.; Serra, M.; Caminiti, R.; Ruga, S.; Passacatini, L.C.; Macrì, R.; Scarano, F.; Maiuolo, J.; Bulotta, R.; Mollace, R.; et al. Non-Essential Heavy Metal Effects in Cardiovascular Diseases: An Overview of Systematic Reviews. Front. Cardiovasc. Med. 2024, 11, 1332339. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P. Microalgae for Biofuel Production and Removal of Heavy Metals: A Review. Environ. Chem. Lett. 2020, 18, 1905–1923. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.; Mtei, K. A Comprehensive Review on the Decontamination of Lead( <scp>ii</Scp> ) from Water and Wastewater by Low-Cost Biosorbents. RSC Adv. 2022, 12, 11233–11254. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of Heavy Metals from Wastewater: A Current Perspective on Microalgae-Based Future. Lett. Appl. Microbiol. 2022, 75, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Moukadiri, H.; Noukrati, H.; Ben Youcef, H.; Iraola, I.; Trabadelo, V.; Oukarroum, A.; Malka, G.; Barroug, A. Impact and Toxicity of Heavy Metals on Human Health and Latest Trends in Removal Process from Aquatic Media. Int. J. Environ. Sci. Technol. 2024, 21, 3407–3444. [Google Scholar] [CrossRef]
- Rama Jyothi, N. Heavy Metal Sources and Their Effects on Human Health. In Heavy Metals—Their Environmental Impacts and Mitigation; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/74650 (accessed on 30 January 2025).
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals--Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Kibret, M. Recent Trends in Microbial Biosorption of Heavy Metals: A Review. Biochem. Mol. Biol. 2013, 1, 19. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of Siderophore-Producing Bacteria for Improving Heavy Metal Phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Huang, L.; Xie, J.; Lv, B.; Shi, X.; Li, G.; Liang, F.; Lian, J. Optimization of Nutrient Component for Diesel Oil Degradation by Acinetobacter Beijerinckii ZRS. Mar. Pollut. Bull. 2013, 76, 325–332. [Google Scholar] [CrossRef]
- Yu, H.; Wu, B.; Zhang, X.-X.; Liu, S.; Yu, J.; Cheng, S.; Ren, H.-Q.; Ye, L. Arsenic Metabolism and Toxicity Influenced by Ferric Iron in Simulated Gastrointestinal Tract and the Roles of Gut Microbiota. Environ. Sci. Technol. 2016, 50, 7189–7197. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, Z.; Cai, X.; Du, H.; Sun, G.; Cui, Y. In Vitro Method To Assess Soil Arsenic Metabolism by Human Gut Microbiota: Arsenic Speciation and Distribution. Environ. Sci. Technol. 2015, 49, 10675–10681. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Khatun, S.; Maity, M.; Jana, S.; Perveen, H.; Dash, M.; Dey, A.; Jana, L.R.; Maity, P.P. Association of Vitamin B12, Lactate Dehydrogenase, and Regulation of NF-ΚB in the Mitigation of Sodium Arsenite-Induced ROS Generation in Uterine Tissue by Commercially Available Probiotics. Probiotics Antimicrob. Proteins 2019, 11, 30–42. [Google Scholar] [CrossRef]
- Saeed Alahmari, A. Immunotoxic and Genotoxic Effects of Arsenic and Ameliorative Potential of Quercetin and Probiotics in Wistar Rat. Am. J. Life Sci. 2017, 5, 108. [Google Scholar] [CrossRef][Green Version]
- Mohmmad Monadi Al-Enazi, A.; Virk, P.; Hindi, A.; Awad, M.A.; Elobeid, M.; Qindeel, R. Protective Effect of Probiotic Bacteria and Its Nanoformulation against Cadmium-Induced Oxidative Stress in Male Wistar Rat. J. King Saud. Univ. Sci. 2020, 32, 3045–3051. [Google Scholar] [CrossRef]
- Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic, D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The Protective Effects of Probiotic Bacteria on Cadmium Toxicity in Rats. J. Med. Food 2017, 20, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Mishra, A.K.; Ghosh, A.R.; Mandal, B.K. Probiotic Pediococcus Pentosaceus GS4 Shields Brush Border Membrane and Alleviates Liver Toxicity Imposed by Chronic Cadmium Exposure in Swiss Albino Mice. J. Appl. Microbiol. 2019, 126, 1233–1244. [Google Scholar] [CrossRef]
- G Allam, N.; M Ali, E.M.; Shabanna, S.; Abd-Elrahman, E. Protective Efficacy of Streptococcus Thermophilus Against Acute Cadmium Toxicity in Mice. Iran. J. Pharm. Res. 2018, 17, 695–707. [Google Scholar]
- Majlesi, M.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Effect of Probiotic Bacillus Coagulans and Lactobacillus Plantarum on Alleviation of Mercury Toxicity in Rat. Probiotics Antimicrob. Proteins 2017, 9, 300–309. [Google Scholar] [CrossRef]
- Jiang, X.; Gu, S.; Liu, D.; Zhao, L.; Xia, S.; He, X.; Chen, H.; Ge, J. Lactobacillus Brevis 23017 Relieves Mercury Toxicity in the Colon by Modulation of Oxidative Stress and Inflammation Through the Interplay of MAPK and NF-ΚB Signaling Cascades. Front. Microbiol. 2018, 9, 2425. [Google Scholar] [CrossRef]
- Assumaidaee, A.A.M.; Ali, N.M.; Akutbi, S.H.; Fadhil, A.A. Efficacy Of Probiotic (Protoxine) On Mercury-Induced Nephrotoxicity And Lipid Peroxidation In Rats. Diyala Agric. Sci. J. (DASJ) 2018, 10, 114–126. [Google Scholar]
- Zhai, Q.; Yang, L.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Protective Effects of Dietary Supplements Containing Probiotics, Micronutrients, and Plant Extracts Against Lead Toxicity in Mice. Front. Microbiol. 2018, 9, 2134. [Google Scholar] [CrossRef]
- Tian, F.; Zhai, Q.; Zhao, J.; Liu, X.; Wang, G.; Zhang, H.; Zhang, H.; Chen, W. Lactobacillus Plantarum CCFM8661 Alleviates Lead Toxicity in Mice. Biol. Trace Elem. Res. 2012, 150, 264–271. [Google Scholar] [CrossRef]
- Yi, Y.-J.; Lim, J.-M.; Gu, S.; Lee, W.-K.; Oh, E.; Lee, S.-M.; Oh, B.-T. Potential Use of Lactic Acid Bacteria Leuconostoc Mesenteroides as a Probiotic for the Removal of Pb(II) Toxicity. J. Microbiol. 2017, 55, 296–303. [Google Scholar] [CrossRef]
- Hu, C.; Yu, C.; Liu, Y.; Hou, X.; Liu, X.; Hu, Y.; Jin, C. A Hybrid Mechanism for the Synechocystis Arsenate Reductase Revealed by Structural Snapshots during Arsenate Reduction. J. Biol. Chem. 2015, 290, 22262–22273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, T.; Lu, B.; Li, X.; Jiang, L. Application of Cofactors in the Regulation of Microbial Metabolism: A State of the Art Review. Front. Microbiol. 2023, 14, 1145784. [Google Scholar] [CrossRef]
- Ķimse, L.; Reinis, A.; Miķelsone-Jansone, L.; Gintere, S.; Krūmiņa, A. A Narrative Review of Psychobiotics: Probiotics That Influence the Gut–Brain Axis. Medicina 2024, 60, 601. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Dharmasiddhi, I.P.W.; Chen, S.; Liu, Y.; Liu, H. Review of the Potential of Probiotics in Disease Treatment: Mechanisms, Engineering, and Applications. Processes 2024, 12, 316. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental Toxicity of Cadmium in Infants and Children: A Review. Environ. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef]
- Basnet, J.; Eissa, M.A.; Yanes Cardozo, L.L.; Romero, D.G.; Rezq, S. Impact of Probiotics and Prebiotics on Gut Microbiome and Hormonal Regulation. Gastrointest. Disord. 2024, 6, 801–815. [Google Scholar] [CrossRef]
- Han, H.; Zhang, Y.; Tang, H.; Zhou, T.; Khan, A. A Review of the Use of Native and Engineered Probiotics for Colorectal Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 3896. [Google Scholar] [CrossRef]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.-C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef]
- Pan, Y.; Sonn, G.A.; Sin, M.L.Y.; Mach, K.E.; Shih, M.-C.; Gau, V.; Wong, P.K.; Liao, J.C. Electrochemical Immunosensor Detection of Urinary Lactoferrin in Clinical Samples for Urinary Tract Infection Diagnosis. Biosens. Bioelectron. 2010, 26, 649–654. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, A.; Awasthi, K.K.; Awasthi, K.; Awasthi, A. Biosensors for Diagnosis of Urinary Tract Infections: Advances and Future Challenges. Mater. Lett. X 2021, 10, 100077. [Google Scholar] [CrossRef]
- Sani, A.; Abdullahi, I.L. Evaluation of Some Heavy Metals Concentration in Body Fluids of Metal Workers in Kano Metropolis, Nigeria. Toxicol. Rep. 2017, 4, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhu, Z.; Xiang, Y.; Yang, Q.; Yuan, Q.; Li, X.; Yu, G. Associations of Blood and Urinary Heavy Metals with Stress Urinary Incontinence Risk Among Adults in NHANES, 2003–2018. Biol. Trace Elem. Res. 2024, 203, 1327–1341. [Google Scholar] [CrossRef]
- Ogunfowokan, A.O.; Adekunle, A.S.; Oyebode, B.A.; Oyekunle, J.A.O.; Komolafe, A.O.; Omoniyi-Esan, G.O. Determination of Heavy Metals in Urine of Patients and Tissue of Corpses by Atomic Absorption Spectroscopy. Chem. Afr. 2019, 2, 699–712. [Google Scholar] [CrossRef]
- Hwang, C.; Lee, W.-J.; Kim, S.D.; Park, S.; Kim, J.H. Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors 2022, 12, 1020. [Google Scholar] [CrossRef]
- Andreasi Bassi, C.; Wu, Z.; Forst, L.; Papautsky, I. Determination of Mercury with a Miniature Sensor for Point-of-care Testing. Electroanalysis 2023, 35, e202200234. [Google Scholar] [CrossRef]
- Duan, W.; Xu, C.; Liu, Q.; Xu, J.; Weng, Z.; Zhang, X.; Basnet, T.B.; Dahal, M.; Gu, A. Levels of a Mixture of Heavy Metals in Blood and Urine and All-Cause, Cardiovascular Disease and Cancer Mortality: A Population-Based Cohort Study. Environ. Pollut. 2020, 263, 114630. [Google Scholar] [CrossRef]
- Alkufi, A.A.; Oleiwi, M.H.; Abojassim, A.A. Comparison of Heavy Metals in Urine Samples of Smoker and Non-Smoker Persons. Biol. Trace Elem. Res. 2024, 202, 5349–5355. [Google Scholar] [CrossRef]
- Men, Y.; Li, L.; Zhang, F.; Kong, X.; Zhang, W.; Hao, C.; Wang, G. Evaluation of Heavy Metals and Metabolites in the Urine of Patients with Breast Cancer. Oncol. Lett. 2020, 19, 1331–1337. [Google Scholar] [CrossRef]
- Sallsten, G.; Ellingsen, D.G.; Berlinger, B.; Weinbruch, S.; Barregard, L. Variability of Lead in Urine and Blood in Healthy Individuals. Environ. Res. 2022, 212, 113412. [Google Scholar] [CrossRef]
- Wu, B.; Ga, L.; Wang, Y.; Ai, J. Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments. Molecules 2023, 29, 34. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Vidal, L.; Costa-García, A.; Canals, A. Mercury Determination in Urine Samples by Gold Nanostructured Screen-Printed Carbon Electrodes after Vortex-Assisted Ionic Liquid Dispersive Liquid–Liquid Microextraction. Anal. Chim. Acta 2016, 915, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Pasini, P.; Mirasoli, M.; Guardigli, M.; Russo, C.; Musiani, M.; Baraldini, M. Sensitive determination of urinary mercury(ii) by a bioluminescent transgenic bacteria-based biosensor. Anal. Lett. 2001, 34, 29–41. [Google Scholar] [CrossRef]
- Ma, B.; Guo, Y.; Lin, Y.; Zhang, J.; Wang, X.; Zhang, W.; Luo, J.; Chen, Y.; Zhang, N.; Lu, Q.; et al. High-Throughput Screening of Human Mercury Exposure Based on a Low-Cost Naked Eye-Recognized Biosensing Platform. Biosens. Bioelectron. 2024, 248, 115961. [Google Scholar] [CrossRef]
- Valera, D.; Sánchez, M.; Domínguez, J.R.; Alvarado, J.; Espinoza-Montero, P.J.; Carrera, P.; Bonilla, P.; Manciati, C.; González, G.; Fernández, L. Electrochemical Determination of Lead in Human Blood Serum and Urine by Anodic Stripping Voltammetry Using Glassy Carbon Electrodes Covered with Ag–Hg and Ag–Bi Bimetallic Nanoparticles. Anal. Methods 2018, 10, 4114–4121. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Lin, Y.; Ma, B.; Ge, X.; Zhang, W.; Zhang, N.; Yang, S.; Hui, C. Detection of Cadmium in Human Biospecimens by a Cadmium-Selective Whole-Cell Biosensor Based on Deoxyviolacein. ACS Biomater. Sci. Eng. 2024, 10, 4046–4058. [Google Scholar] [CrossRef]
- Gazica, K.; FitzGerald, E.; Dangel, G.; Haynes, E.N.; Yadav, J.; Alvarez, N.T. Towards On-Site Detection of Cadmium in Human Urine. J. Electroanal. Chem. 2020, 859, 113808. [Google Scholar] [CrossRef]
- Argun, A.A.; Banks, A.M.; Merlen, G.; Tempelman, L.A.; Becker, M.F.; Schuelke, T.; Dweik, B.M. Highly Sensitive Detection of Urinary Cadmium to Assess Personal Exposure. Anal. Chim. Acta 2013, 773, 45–51. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Hongsirikarn, K.; Fryxell, G.E.; Addleman, R.; Timchalk, C. Electrochemical Sensors for the Detection of Lead and Other Toxic Heavy Metals: The Next Generation of Personal Exposure Biomonitors. Environ. Health Perspect. 2007, 115, 1683–1690. [Google Scholar] [CrossRef]
- Lai, W.-Q.; Chang, Y.-F.; Chou, F.-N.; Yang, D.-M. Portable FRET-Based Biosensor Device for On-Site Lead Detection. Biosensors 2022, 12, 157. [Google Scholar] [CrossRef]
- Lettieri, M.; Scarano, S.; Caponi, L.; Bertolini, A.; Saba, A.; Palladino, P.; Minunni, M. Serotonin-Derived Fluorophore: A Novel Fluorescent Biomaterial for Copper Detection in Urine. Sensors 2023, 23, 3030. [Google Scholar] [CrossRef] [PubMed]
- Gerdan, Z.; Saylan, Y.; Denizli, A. Recent Advances of Optical Sensors for Copper Ion Detection. Micromachines 2022, 13, 1298. [Google Scholar] [CrossRef]
- Chopra, T.; Sasan, S.; Devi, L.; Parkesh, R.; Kapoor, K.K. A Comprehensive Review on Recent Advances in Copper Sensors. Coord. Chem. Rev. 2022, 470, 214704. [Google Scholar] [CrossRef]
- Sorouraddin, M.H.; Saadati, M. Determination of Copper in Urine and Water Samples Using a Simple Led-Based Colorimeter. J. Anal. Chem. 2010, 65, 423–428. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; del Pilar Cañizares-Macías, M. A Prototype Microfluidic Paper-Based Chromatic Device for Simultaneous Determination of Copper(II) and Zinc(II) in Urine. Talanta Open 2023, 7, 100178. [Google Scholar] [CrossRef]
- Geetha, M.; Sadasivuni, K.K.; Al-Ejji, M.; Sivadas, N.; Bhattacharyya, B.; Musthafa, F.N.; Alfarwati, S.; Promi, T.J.; Ahmad, S.A.; Alabed, S.; et al. Design and Development of Inexpensive Paper-Based Chemosensors for Detection of Divalent Copper. J. Fluoresc. 2023, 33, 2327–2338. [Google Scholar] [CrossRef]
- Jerónimo, P.C.A.; Araújo, A.N.; Montenegro, M.C.B.S.M.; Pasquini, C.; Raimundo, I.M. Direct Determination of Copper in Urine Using a Sol–Gel Optical Sensor Coupled to a Multicommutated Flow System. Anal. Bioanal. Chem. 2004, 380, 108–114. [Google Scholar] [CrossRef]
- Mir, T.U.G.; Wani, A.K.; Akhtar, N.; Katoch, V.; Shukla, S.; Kadam, U.S.; Hong, J.C. Advancing Biological Investigations Using Portable Sensors for Detection of Sensitive Samples. Heliyon 2023, 9, e22679. [Google Scholar] [CrossRef]
- Shalvi; Gautam, V.; Lata Verma, K.; Suman; Jain, V.K.; Kumar, A. An Overview of Advanced Approaches for Detecting Arsenic at Trace Levels. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100730. [Google Scholar] [CrossRef]
- Bonthula, S.; Devarajan, S.; Maurya, M.R.; Al-Maadeed, S.; Maalej, R.; Chaari, M.Z.; Sadasivuni, K.K. Advancing Rapid Arsenic (III) Detection Through Device-Integrated Colorimetry. Chem. Afr. 2024, 7, 4381–4391. [Google Scholar] [CrossRef]
- Gebremedhin, K.H.; Kahsay, M.H.; Wegahita, N.K.; Teklu, T.; Berhe, B.A.; Gebru, A.G.; Tesfay, A.H.; Asgedom, A.G. Nanomaterial-Based Optical Colorimetric Sensors for Rapid Monitoring of Inorganic Arsenic Species: A Review. Discov. Nano 2024, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Babar, N.-U.-A.; Joya, K.S.; Tayyab, M.A.; Ashiq, M.N.; Sohail, M. Highly Sensitive and Selective Detection of Arsenic Using Electrogenerated Nanotextured Gold Assemblage. ACS Omega 2019, 4, 13645–13657. [Google Scholar] [CrossRef] [PubMed]
- Merulla, D.; Buffi, N.; Beggah, S.; Truffer, F.; Geiser, M.; Renaud, P.; van der Meer, J.R. Bioreporters and Biosensors for Arsenic Detection. Biotechnological Solutions for a World-Wide Pollution Problem. Curr. Opin. Biotechnol. 2013, 24, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Salek Maghsoudi, A.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Jia, X.; Bu, R.; Zhao, T.; Wu, K. Sensitive and Specific Whole-Cell Biosensor for Arsenic Detection. Appl. Environ. Microbiol. 2019, 85, e00694-19. [Google Scholar] [CrossRef]
- Bhat, A.; Tian, F.; Singh, B. Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives. Sensors 2024, 24, 3889. [Google Scholar] [CrossRef]
- Caragea, G.; Vãrzaru, A.C.; Avram, O.; Macovei, R.; Costea, A.; Popescu, D.M.; Smarandache, A.M. An Overview of the Complications of Acute and Chronic Mercury Exposures. Past, Present, and Future. Rom. J. Mil. Med. 2021, 124, 411. [Google Scholar] [CrossRef]
- Lensoni, L.; Adlim, M.; Kamil, H.; Karma, T.; Suhendrayatna, S. Identification and Correlation Test of Mercury Levels in Community Urine at Traditional Gold Processing Locations. J. Ecol. Eng. 2023, 24, 357–365. [Google Scholar] [CrossRef]
- De Craemer, S.; Baeyens, W.; Leermakers, M. Biomonitoring of Total Mercury in Urine: Method Validation and Sample Stability. Biomonitoring 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Nikkey; Swami, S.; Sharma, N.; Saini, A. Captivating Nano Sensors for Mercury Detection: A Promising Approach for Monitoring of Toxic Mercury in Environmental Samples. RSC Adv. 2024, 14, 18907–18941. [Google Scholar] [CrossRef]
- Sosnowska, M.; Pitula, E.; Janik, M.; Bruździak, P.; Śmietana, M.; Olszewski, M.; Nidzworski, D.; Gromadzka, B. Peptide-Based Rapid and Selective Detection of Mercury in Aqueous Samples with Micro-Volume Glass Capillary Fluorometer. Biosens 2024, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, M.; Narendran, C.; Mary, J.; Meenambigai, S. Recent Trends and Future Perspectives of Photoresponsive-Based Mercury (II) Sensors and Their Biomaterial Applications. Heliyon 2024, 10, e35826. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Yang, D.; Wang, M.; Yue, Q. A Colorimetric Detection of Hg2+ Based on Gold Nanoparticles Synthesized Oxidized N-Methylpyrrolidone as a Reducing Agent. Sci. Rep. 2023, 13, 22208. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gong, J.; Rong, P.; Liu, C.; Chen, J. A Portable and Quantitative Biosensor for Cadmium Detection Using Glucometer as the Point-of-Use Device. Talanta 2019, 198, 412–416. [Google Scholar] [CrossRef]
- Yoshii, T.; Nishitsugu, F.; Kikawada, K.; Maehashi, K.; Ikuta, T. Identification of Cadmium Compounds in a Solution Using Graphene-Based Sensor Array. Sensors 2023, 23, 1519. [Google Scholar] [CrossRef]
- Hu, S.; Hui, C.; Wu, C.; Gao, C.; Huang, Z.; Guo, Y. Dual-Colored Bacterial Biosensor Responsive to Cadmium, Mercury, and Lead for Detecting Heavy Metal Pollution in Seawater. Ecol. Indic. 2024, 166, 112244. [Google Scholar] [CrossRef]
- Guo, Y.; Hui, C.; Zhang, N.; Liu, L.; Li, H.; Zheng, H. Development of Cadmium Multiple-Signal Biosensing and Bioadsorption Systems Based on Artificial Cad Operons. Front. Bioeng. Biotechnol. 2021, 9, 585617. [Google Scholar] [CrossRef]
- Hui, C.; Guo, Y.; Wu, J.; Liu, L.; Yang, X.; Guo, X.; Xie, Y.; Yi, J. Detection of Bioavailable Cadmium by Double-Color Fluorescence Based on a Dual-Sensing Bioreporter System. Front. Microbiol. 2021, 12, 696195. [Google Scholar] [CrossRef]
- Hui, C.; Guo, Y.; Li, H.; Gao, C.; Yi, J. Detection of Environmental Pollutant Cadmium in Water Using a Visual Bacterial Biosensor. Sci. Rep. 2022, 12, 6898. [Google Scholar] [CrossRef]
- Joe, M.-H.; Lee, K.-H.; Lim, S.-Y.; Im, S.-H.; Song, H.-P.; Lee, I.S.; Kim, D.-H. Pigment-Based Whole-Cell Biosensor System for Cadmium Detection Using Genetically Engineered Deinococcus Radiodurans. Bioprocess. Biosyst. Eng. 2012, 35, 265–272. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, L.; Zeng, Q.; Zhu, H.; Luo, J. High Serum Copper as a Risk Factor of All-Cause and Cause-Specific Mortality among US Adults, NHANES 2011–2014. Front. Cardiovasc. Med. 2024, 11, 1340968. [Google Scholar] [CrossRef] [PubMed]
- Luscombe, D.L.; Bond, A.M.; Davey, D.E.; Bixler, J.W. Copper Determination in Urine by Flow Injection Analysis with Electrochemical Detection at Platinum Disk Microelectrodes of Various Radii. Anal. Chem. 1990, 62, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Grycan, M.; Przybyłkowski, A.M. Monitoring of Copper in Wilson Disease. Diagnostics 2023, 13, 1830. [Google Scholar] [CrossRef]
- Hyre, A.N.; Kavanagh, K.; Kock, N.D.; Donati, G.L.; Subashchandrabose, S. Copper Is a Host Effector Mobilized to Urine during Urinary Tract Infection To Impair Bacterial Colonization. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).