A New Chitosan-Modified Paper-Based SERS Glucose Sensor with Enhanced Reproducibility, Stability, and Sensitivity for Non-Enzymatic Label-Free Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
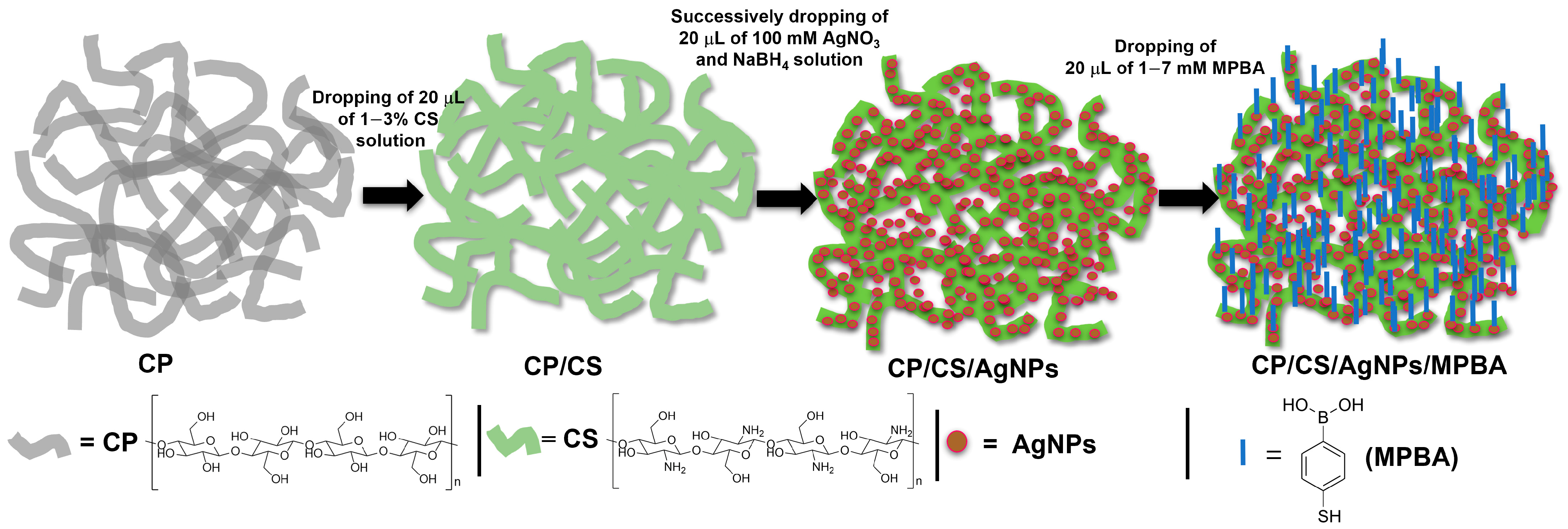
2.3. Fabrication of CP/CS/AgNPs/MPBA-Based SERS Sensor Substrate
3. Results
3.1. Preparation of CP/CS/AgNPs/MPBA SERS Sensor Substrate
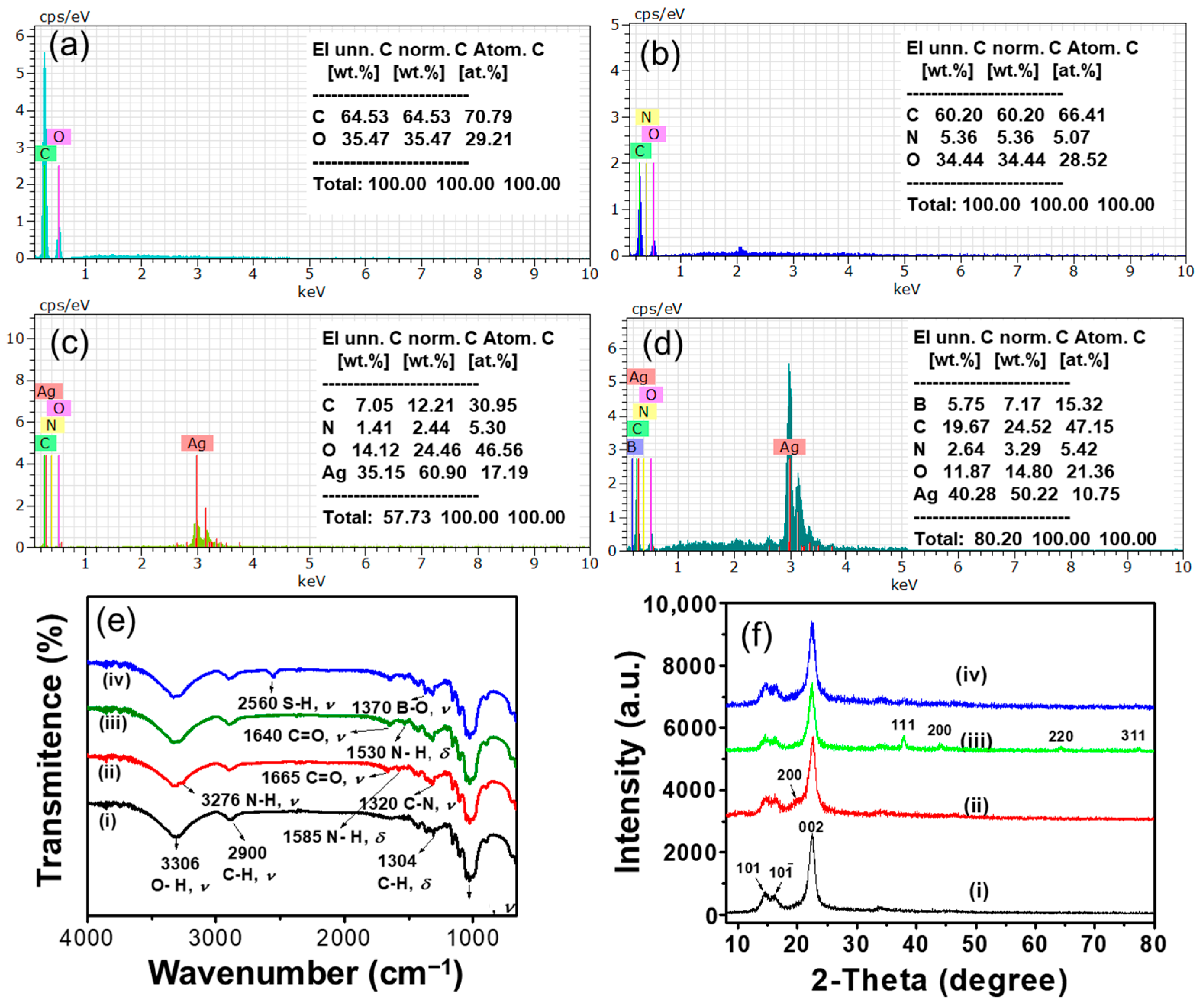
3.2. Characterization of CP/CS/AgNPs/MPBA-Based SERS Substrate
3.3. Determination of Analytical Enhancement Factor of CP/CS/AgNPs Sensor Substrate
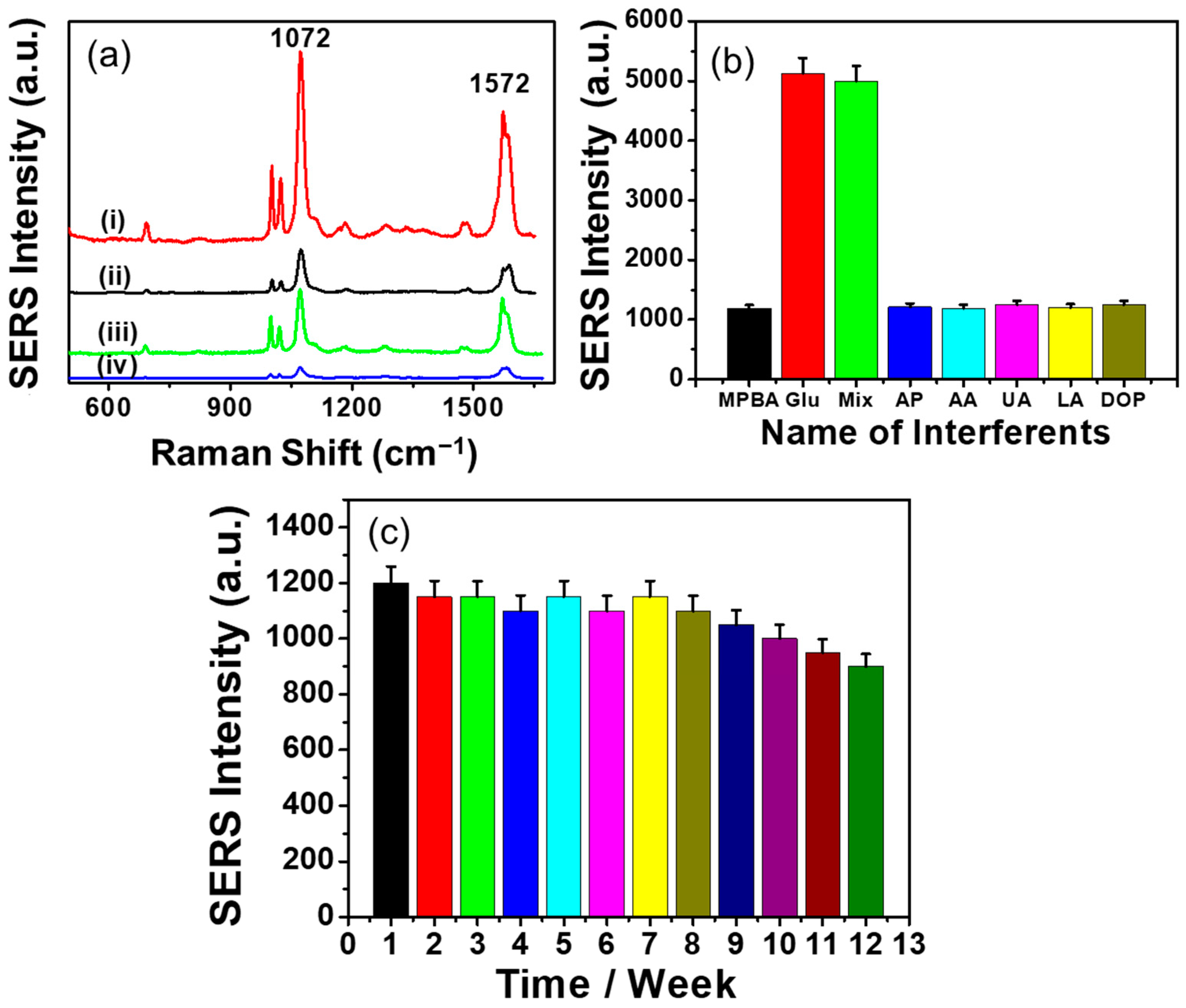
3.4. SERS Responses of CP/CS/AgNPs/MPBA-Based Sensor Substrate
3.5. Optimization of the Experimental Conditions for SERS Measurements
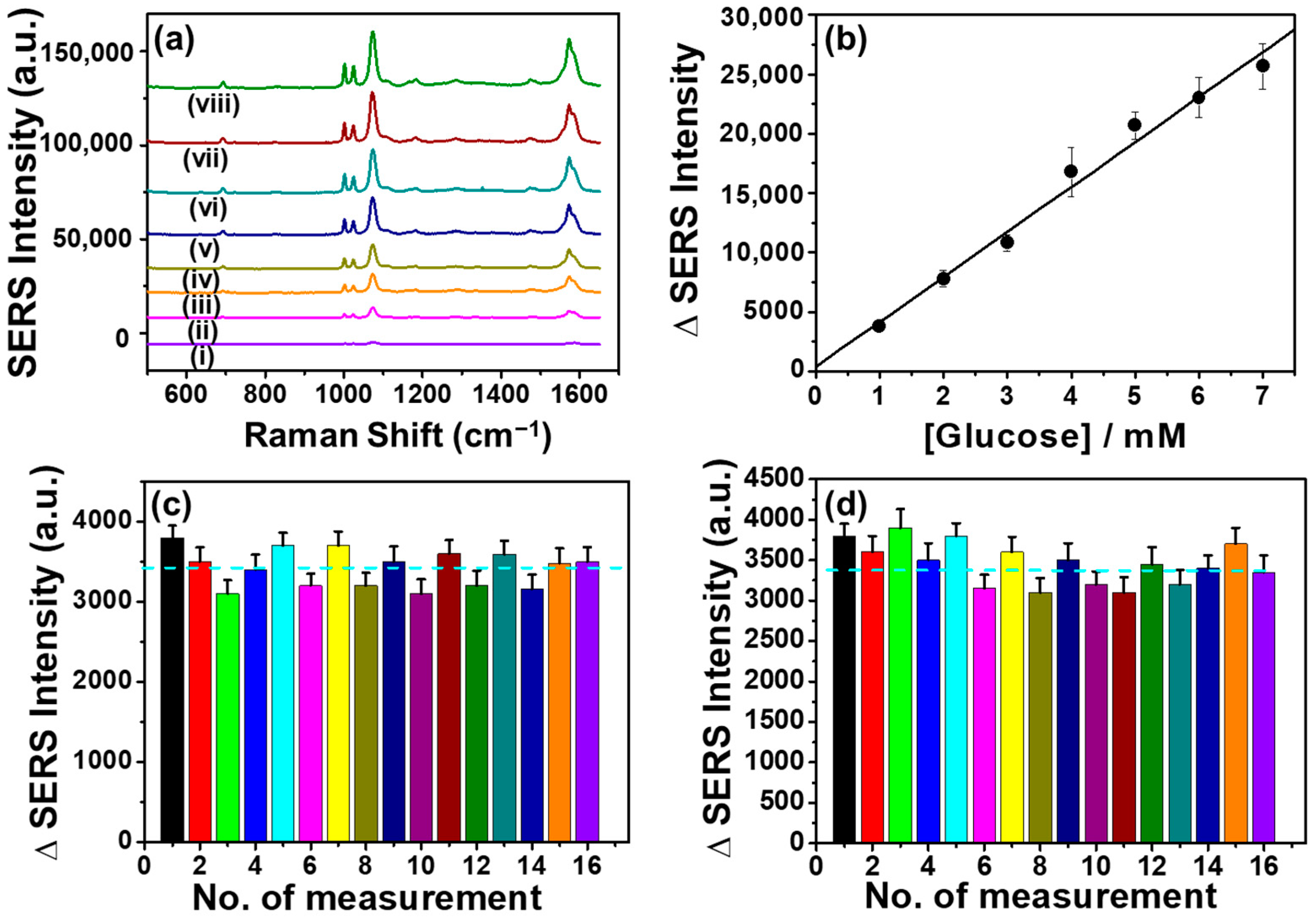
3.6. Analytical Evaluation of the Glucose Detection
3.7. Reproducibility
3.8. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- America Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [CrossRef] [PubMed]
- Davidson, M.B. Historical review of the diagnosis of prediabetes/intermediate hyperglycemia: Case for the international criteria. Diabetes Res. Clin. Pract. 2022, 185, 109219. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Work, T.M.; Bushway, A.A.; Bushway, R.J. HPLC determination of fructose, glucose, and sucrose in potatoes. J. Food Sci. 1981, 46, 300–301. [Google Scholar] [CrossRef]
- Musto, C.J.; Suslick, K.S. Differential sensing of sugars by colorimetric arrays. Curr. Opin. Chem. Biol. 2010, 14, 758–766. [Google Scholar] [CrossRef]
- Mu, J.; He, Y.; Wang, Y. Copper-incorporated SBA-15 with peroxidase-like activity and its application for colorimetric detection of glucose in human serum. Talanta 2016, 148, 22–28. [Google Scholar] [CrossRef]
- Radhakumary, C.; Sreenivasan, K. Naked eye detection of glucose in urine using glucose oxidase immobilized gold nanoparticles. Anal. Chem. 2011, 83, 2829–2833. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Lin, Y.; Zhu, N.; Ma, Y.; Mao, L. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 4800–4804. [Google Scholar] [CrossRef]
- Li, C.; Hu, J.; Liu, T.; Liu, S. Stimuli-triggered off/on switchable complexation between a novel type of charge-generation polymer (CGP) and gold nanoparticles for the sensitive colorimetric detection of hydrogen peroxide and glucose. Macromolecules 2011, 44, 429–431. [Google Scholar] [CrossRef]
- Takahashi, S.; Anzai, J.-I. Phenylboronic acid monolayer-modified electrodes sensitive to sugars. Langmuir 2005, 21, 5102–5107. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, P.; Zong, N.; Li, F. A sensitive non-enzyme sensing platform for glucose based on boronic acid–diol binding. Sens. Actuators B 2012, 161, 832–837. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Z.; Yang, L.; Ma, T.; Yang, L.; Guo, Q.; Huang, S. A glucose-responsive pH-switchable bioelectrocatalytic sensor based on phenylboronic acid-diol specificity. Electrochim. Acta 2015, 151, 370–377. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.-Y.; Liang, R.-P.; Li, Y.-H.; Qiu, J.-D. Boron-doped graphene quantum dots for selective glucose sensing based on the “abnormal” aggregation-induced photoluminescence enhancement. Anal. Chem. 2014, 86, 4423–4430. [Google Scholar] [CrossRef] [PubMed]
- Wannajuk, K.; Jamkatoke, M.; Tuntulani, T.; Tomapatanaget, B. Highly specific-glucose fluorescence sensing based on boronic anthraquinone derivatives via the GOx enzymatic reaction. Tetrahedron 2012, 68, 8899–8904. [Google Scholar] [CrossRef]
- Yan, J.; Fang, H.; Wang, B. Boronolectins and fluorescent boronolectins: An examination of the detailed chemistry issues important for the design. Med. Res. Rev. 2005, 25, 490–520. [Google Scholar] [CrossRef]
- Chen, R.; Xu, W.; Xiong, C.; Zhou, X.; Xiong, S.; Nie, Z.; Mao, L.; Chen, Y.; Chang, H.-C. High-salt-tolerance matrix for facile detection of glucose in rat brain microdialysates by MALDI mass spectrometry. Anal. Chem. 2012, 84, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Gu, L.; Wang, J.; Li, X.; Liang, G.; Zhou, J.; Zhang, Z. Novel and sensitive chemiluminescence sensors based on 2D-MOF nanosheets for one-step detection of glucose in human urine. J. Phys. Chem. C 2019, 123, 9388–9393. [Google Scholar] [CrossRef]
- Sun, X.; Stagon, S.; Huang, H.; Chen, J.; Lei, Y. Functionalized aligned silver nanorod arrays for glucose sensing through surface enhanced Raman scattering. RSC Adv. 2014, 4, 23382–23388. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Q.; Chu, W.; Zhao, W.; Zheng, J. Surface-enhanced Raman scattering behaviour of 4-mercaptophenyl boronic acid on assembled silver nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17638–17645. [Google Scholar] [CrossRef]
- Torul, H.; Ciftci, H.; Dudak, F.C.; Adguzel, Y.; Kulah, H.; Boyac, I.H.; Tamer, U. Glucose determination based on a two component self-assembled monolayer functionalized surface-enhanced Raman spectroscopy (SERS) probe. Anal. Methods. 2014, 6, 5097–5104. [Google Scholar] [CrossRef]
- Gupta, V.K.; Atar, N.; Yola, M.L.; Eryılmaz, M.; Torul, H.; Tamer, U.; Boyacı, İ.H.; Üstündağ, Z. A novel glucose biosensor platform based on Ag@AuNPs modified graphene oxide nanocomposite and SERS application. J. Colloid Interface Sci. 2013, 406, 231–237. [Google Scholar] [CrossRef]
- Pham, X.-H.; Shim, S.; Kim, T.-H.; Hahm, E.; Kim, H.-M.; Rho, W.-Y.; Jeong, D.H.; Lee, Y.-S.; Jun, B.-H. Glucose detection using 4-mercaptophenyl boronic acid-incorporated silver nanoparticles-embedded silica-coated graphene oxide as a SERS substrate. Bio. Chip. J. 2017, 11, 46–56. [Google Scholar] [CrossRef]
- Zhang, X.; Young, M.A.; Lyandres, O.; Van Duyne, R.P. Rapid detection of an anthrax biomarker by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2005, 127, 4484–4489. [Google Scholar] [CrossRef]
- Lyandres, O.; Shah, N.C.; Yonzon, C.R.; Walsh, J.T.; Glucksberg, M.R.; Van Duyne, R.P. Real-time glucose sensing by surface-enhanced Raman spectroscopy in bovine plasma facilitated by a mixed decanethiol/mercaptohexanol partition layer. Anal. Chem. 2005, 77, 6134–6139. [Google Scholar] [CrossRef]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for surface-enhanced Raman spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Braun, G.; Pavel, I.; Morrill, A.R.; Seferos, D.S.; Bazan, G.C.; Reich, N.O.; Moskovits, M. Chemically patterned microspheres for controlled nanoparticle assembly in the construction of SERS hot spots. J. Am. Chem. Soc. 2007, 129, 7760–7761. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Lane, L.A.; Qian, X.; Nie, S. SERS nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Zhao, J.; Van Duyne, R.P. Controlled plasmonic nanostructures for surface-enhanced spectroscopy and sensing. Acc. Chem. Res. 2008, 41, 1653–1661. [Google Scholar] [CrossRef]
- Kang, T.; Yoon, I.; Jeon, K.-S.; Choi, W.; Lee, Y.; Seo, K.; Yoo, Y.; Park, Q.; Ihee, H.; Suh, Y.D.; et al. Creating well-defined hot spots for surface-enhanced Raman scattering by single-crystalline noble metal nanowire pairs. J. Phys. Chem. C 2009, 113, 7492–7496. [Google Scholar] [CrossRef]
- Akter, R.; Lee, H.j.; Kim, T.; Choi, J.W.; Kim, H. A review on gold nanowire based SERS sensor for chemicals and biological molecules. Anal. Sci. Technol. 2024, 37, 201–210. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.H.; Kim, J.H.; Ahn, Y.J.; Kim, Y.-H.; Yu, J.S.; Choi, S. Paper-based surface-enhanced Raman spectroscopy for diagnosing prenatal diseases in women. ACS Nano 2018, 12, 7100–7108. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-J.; Jeong, K.-H. Glass nanopillar arrays with nanogap-rich silver nanoislands for highly intense surface enhanced Raman scattering. Adv. Mater. 2012, 24, 2234–2237. [Google Scholar] [CrossRef] [PubMed]
- Brolo, A.G.; Arctander, E.; Gordon, R.; Leathem, B.; Kavanagh, K.L. Nanohole-enhanced Raman scattering. Nano Lett. 2004, 4, 2015–2018. [Google Scholar] [CrossRef]
- Pastoria-Santos, I.; Kinnear, C.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Plasmonic polymer nanocomposites. Nat. Rev. Mater. 2018, 3, 375–391. [Google Scholar] [CrossRef]
- Cheng, C.-M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based ELISA. Angew. Chem. Int. Ed. 2010, 49, 771–774. [Google Scholar] [CrossRef]
- Cunningham, J.C.; Brenes, N.J.; Crooks, R.M. Paper electrochemical device for detection of DNA and thrombin by target-induced conformational switching. Anal. Chem. 2014, 86, 6166–6170. [Google Scholar] [CrossRef]
- He, M.; Liu, Z. Paper-based microfluidic device with upconversion fluorescence assay. Anal. Chem. 2013, 85, 11691–11694. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Ge, L.; Ge, S. A novel chemiluminescence paper microfluidic biosensor based on enzymatic reaction for uric acid determination. Biosens. Bioelectron. 2011, 26, 3284–3289. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Trinh, B.T.; Kim, K.H.; Moon, J.; Kang, H.; Jo, K.; Akter, R.; Jeong, J.; Lim, E.-K.; Jung, J.; et al. Au@ZIF-8 SERS paper for food spoilage detection. Biosens. Bioelectron. 2021, 179, 113063. [Google Scholar] [CrossRef]
- Chen, L.; Choo, J. Recent advances in surface-enhanced Raman scattering detection technology for microfluidic chips. Electrophoresis 2008, 29, 1815–1828. [Google Scholar] [CrossRef]
- Lee, M.; Oh, K.; Choi, H.-K.; Lee, S.G.; Youn, H.J.; Lee, H.L.; Jeong, D.H. Subnanomolar sensitivity of filter paper-based SERS sensor for pesticide detection by hydrophobicity Change of paper surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef]
- Lee, C.H.; Tian, L.; Singamaneni, S. Paper-based SERS swab for rapid trace detection on real-world surfaces. ACS Appl. Mater. Interfaces 2010, 2, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Hasi, W.-L.-J.; Lin, X.; Lou, X.-T.; Lin, S.; Yang, F.; Lin, D.-Y.; Lu, Z.-W. Chloride ion-assisted self-assembly of silver nanoparticles on filter paper as SERS substrate. Appl. Phys. A 2015, 118, 799–807. [Google Scholar] [CrossRef]
- Webb, J.A.; Aufrecht, J.; Hungerford, C.; Bardhan, R. Ultrasensitive analyte detection with plasmonic paper dipsticks and swabs integrated with branched nanoantennas. J. Mater. Chem. C 2014, 2, 10446–10454. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, J.; Xu, H.; Li, Y.; Ji, J.; Liu, B. Multifunctional paper strip based on self-assembled interfacial plasmonic nanoparticle arrays for sensitive SERS detection. ACS Appl. Mater. Interfaces 2015, 7, 16767–16774. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Zhao, J.; Ji, J.; Ji, C.; Liu, B. A three-dimensional silver nanoparticles decorated plasmonic paper strip for SERS detection of low-abundance molecules. Talanta 2016, 147, 493–500. [Google Scholar] [CrossRef]
- Meng, Y.; Lai, Y.; Jiang, X.; Zhao, Q.; Zhan, J. Silver nanoparticles decorated filter paper via self-sacrificing reduction for membrane extraction surface-enhanced Raman spectroscopy detection. Analyst 2013, 138, 2090–2095. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.-C.; Shin, J.-H.; Jin, K.-H.; Park, H.-K.; Choi, S. Instrument-free synthesizable fabrication of label-free optical biosensing paper strips for the early detection of infectious keratoconjunctivitides. Anal. Chem. 2016, 88, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, M.; Yu, D.; Yang, L. A novel paper rag as ‘D-SERS’ substrate for detection of pesticide residues at various peels. Talanta 2014, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rajapandiyan, P.; Yang, J. Photochemical method for decoration of silver nanoparticles on filter paper substrate for SERS application. J. Raman Spectrosc. 2014, 45, 574–580. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chou, S.-Y.; Tseng, Y.-C.; Tseng, S.-C.; Yen, Y.-T.; Chen, H.-L. Single-shot laser treatment provides quasi-three-dimensional paper-based substrates for SERS with attomolar sensitivity. Nanoscale 2015, 7, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Shin, J.-H.; Park, H.-K.; Choi, S. A low-cost, monometallic, surface-enhanced Raman scattering-functionalized paper platform for spot-on bioassays. Sens. Actuators B 2016, 222, 1112–1118. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Chen, L.; Lin, B. A fast and low-cost spray method for prototyping and depositing surface-enhanced Raman scattering arrays on microfluidic paper based device. Electrophoresis 2013, 34, 2162–2168. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. Inkjet printed surface enhanced Raman spectroscopy array on cellulose paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Tsai, B.-C.; Yang, J. Silver nanoparticle-treated filter paper as a highly sensitive surface-enhanced Raman scattering (SERS) substrate for detection of tyrosine in aqueous solution. Anal. Chim. Acta 2011, 708, 89–96. [Google Scholar] [CrossRef]
- Torul, H.; Çiftçi, H.; Çetin, D.; Suludere, Z.; Boyacı, I.H.; Tamer, U. Paper membrane-based SERS platform for the determination of glucose in blood samples. Anal. Bioanal. Chem. 2015, 407, 8243–8251. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Q.; Kutsanedzie, F.Y.H.; Yang, M.; Ouyang, Q.; Jiang, H. Highly sensitive and label-free determination of thiram residue using surface-enhanced Raman spectroscopy (SERS) coupled with paper-based microfluidics. Anal. Methods 2017, 9, 6186–6193. [Google Scholar] [CrossRef]
- Sun, X. Glucose detection through surface-enhanced Raman spectroscopy: A review. Anal. Chim. Acta 2022, 1206, 339226. [Google Scholar] [CrossRef]
- Choi, S.; Na, H.; Rahman, R.T.; Sim, J.; Chang, J.-B.; Nam, Y.S. Chitosan-coated mesoporous silica particles as a plastic-free platform for photochemical suppression and stabilization of organic ultraviolet filters. J. Photochem. Photobiol. B Biol. 2022, 235, 112565. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.T.; Shin, J.; Whang, C.-H.; Jung, W.; Yoo, D.; Seo, C.; Cho, B. -K.; Jon, S. Bilirubin nanomedicine rescues intestinal barrier destruction and restores mucosal immunity in colitis. ACS Nano 2023, 17, 10996–11013. [Google Scholar] [CrossRef] [PubMed]
- Supanakorn, G.; Taokaew, S.; Phisalaphong, M. Natural rubber films reinforced with cellulose and chitosan prepared by latex aqueous microdispersion. Cellulose 2021, 28, 8553–8566. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Yang, X.; Hou, Q.; Liu, S.; Zheng, W.; Long, Y.; Jiang, X. Mercaptophenylboronic acidactivated gold nanoparticles as nanoantibiotics against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 51148–51159. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Reddy, P.R.; Lee, Y.-I.; Kim, C. Synthesis and characterization of chitosan–PEG–Ag nanocomposites for antimicrobial application. Carbohydr. Polym. 2012, 87, 920–925. [Google Scholar] [CrossRef]
- Barriet, D.; Yam, C.M.; Shmakova, O.E.; Jamison, A.C.; Lee, T.R. 4-Mercaptophenylboronic acid SAMs on gold: comparison with SAMs derived from thiophenol, 4-mercaptophenol, and 4-mercaptobenzoic acid. Langmuir 2007, 23, 8866–8875. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Daviv, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Tegegne, W.A.; Su, W.-N.; Beyene, A.B.; Huang, W.-H.; Tsai, M.-C.; Hwang, B.J. Flexible hydrophobic filter paper-based SERS substrate using silver nanocubes for sensitive and rapid detection of adenine. Microchem. J. 2021, 168, 106349. [Google Scholar] [CrossRef]
- Su, H.; Wang, Y.; Yu, Z.; Liu, Y.; Zhang, X.; Wang, X.; Sui, H.; Sun, C.; Zhao, B. Surface-enhanced Raman spectroscopy study on the structure changes of 4-Mercaptophenylboronic Acid under different pH conditions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 185, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Shafer-Paltier, K.E.; Hayness, C.L.; Gluksberg, M.R.; Van Duyne, R.P. Toward a glucose biosensor based on surface-enhanced Raman scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.V.; Lam, Z.; Lau, W.K.O.; Leong, W.K.; Olivo, M. A transition metal carbonyl probe for use in a highly specific and sensitive SERS-based assay for glucose. J. Am. Chem. Soc. 2013, 135, 18028–18031. [Google Scholar] [CrossRef]


| Sample Name | Original Conc. (mM) | Added Conc. (mM) | Found Conc. (mM) | Recovery (%) |
|---|---|---|---|---|
| Two times diluted human serum | Unknown | 0 | 3.47 ± 0.3 (Standard addition) 3.3 ± 0.45 (Calibration) | |
| Spike 1 | 3.47 ± 0.3 | 1 | 4.5 ± 0.20 | 100.7 |
| Spike 2 | 3.47 ± 0.3 | 2 | 5.5 ± 0.35 | 100.5 |
| Spike 3 | 3.47 ± 0.3 | 3 | 6.4 ± 0.3 | 98.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, R.; Kim, T.; Choi, J.S.; Kim, H. A New Chitosan-Modified Paper-Based SERS Glucose Sensor with Enhanced Reproducibility, Stability, and Sensitivity for Non-Enzymatic Label-Free Detection. Biosensors 2025, 15, 153. https://doi.org/10.3390/bios15030153
Akter R, Kim T, Choi JS, Kim H. A New Chitosan-Modified Paper-Based SERS Glucose Sensor with Enhanced Reproducibility, Stability, and Sensitivity for Non-Enzymatic Label-Free Detection. Biosensors. 2025; 15(3):153. https://doi.org/10.3390/bios15030153
Chicago/Turabian StyleAkter, Rashida, Toeun Kim, Jong Seob Choi, and Hongki Kim. 2025. "A New Chitosan-Modified Paper-Based SERS Glucose Sensor with Enhanced Reproducibility, Stability, and Sensitivity for Non-Enzymatic Label-Free Detection" Biosensors 15, no. 3: 153. https://doi.org/10.3390/bios15030153
APA StyleAkter, R., Kim, T., Choi, J. S., & Kim, H. (2025). A New Chitosan-Modified Paper-Based SERS Glucose Sensor with Enhanced Reproducibility, Stability, and Sensitivity for Non-Enzymatic Label-Free Detection. Biosensors, 15(3), 153. https://doi.org/10.3390/bios15030153





