An Innovative Enzymatic Surface Plasmon Resonance-Based Biosensor Designed for Precise Detection of Glycine Amino Acid
Abstract
1. Introduction
2. Methodology
2.1. Materials and Reagents
2.2. SPR Platform
2.3. Immobilization of the Enzyme by Covalent Binding
2.4. SPR Measurements for Glycine Detection
3. Results and Discussion
3.1. Immobilization of the Enzyme by Covalent Binding
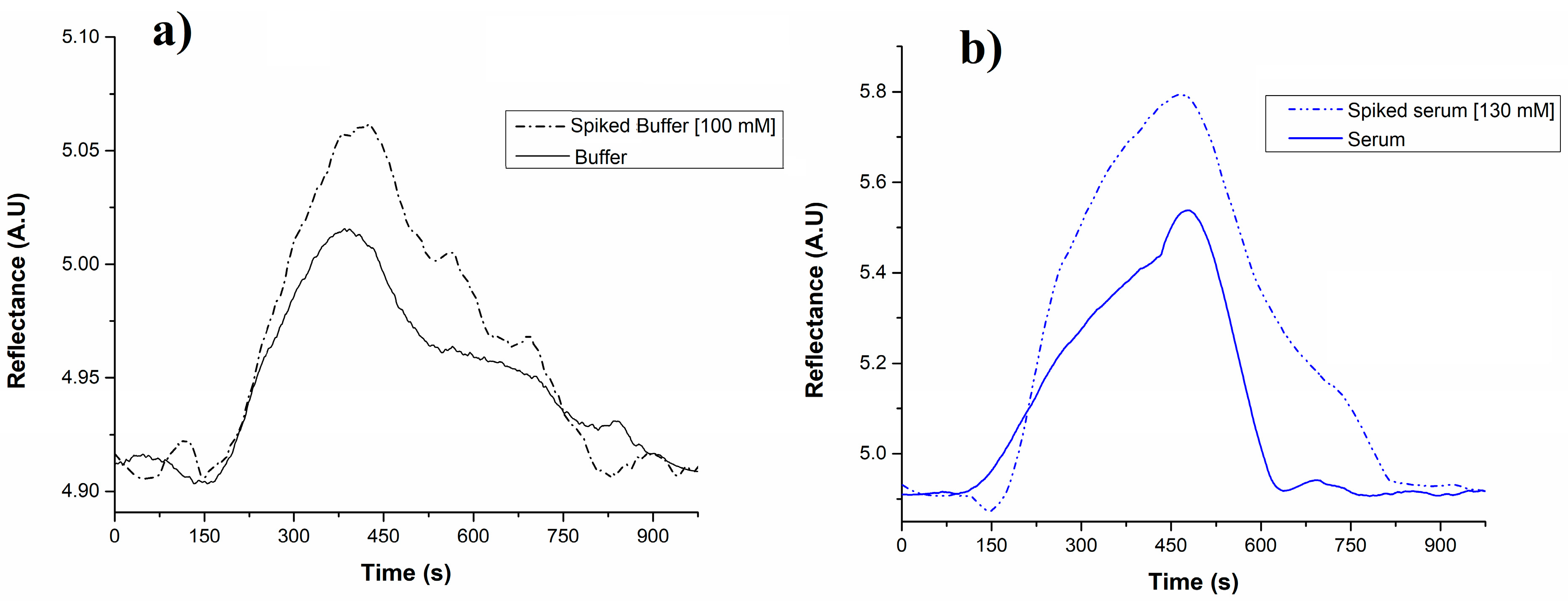
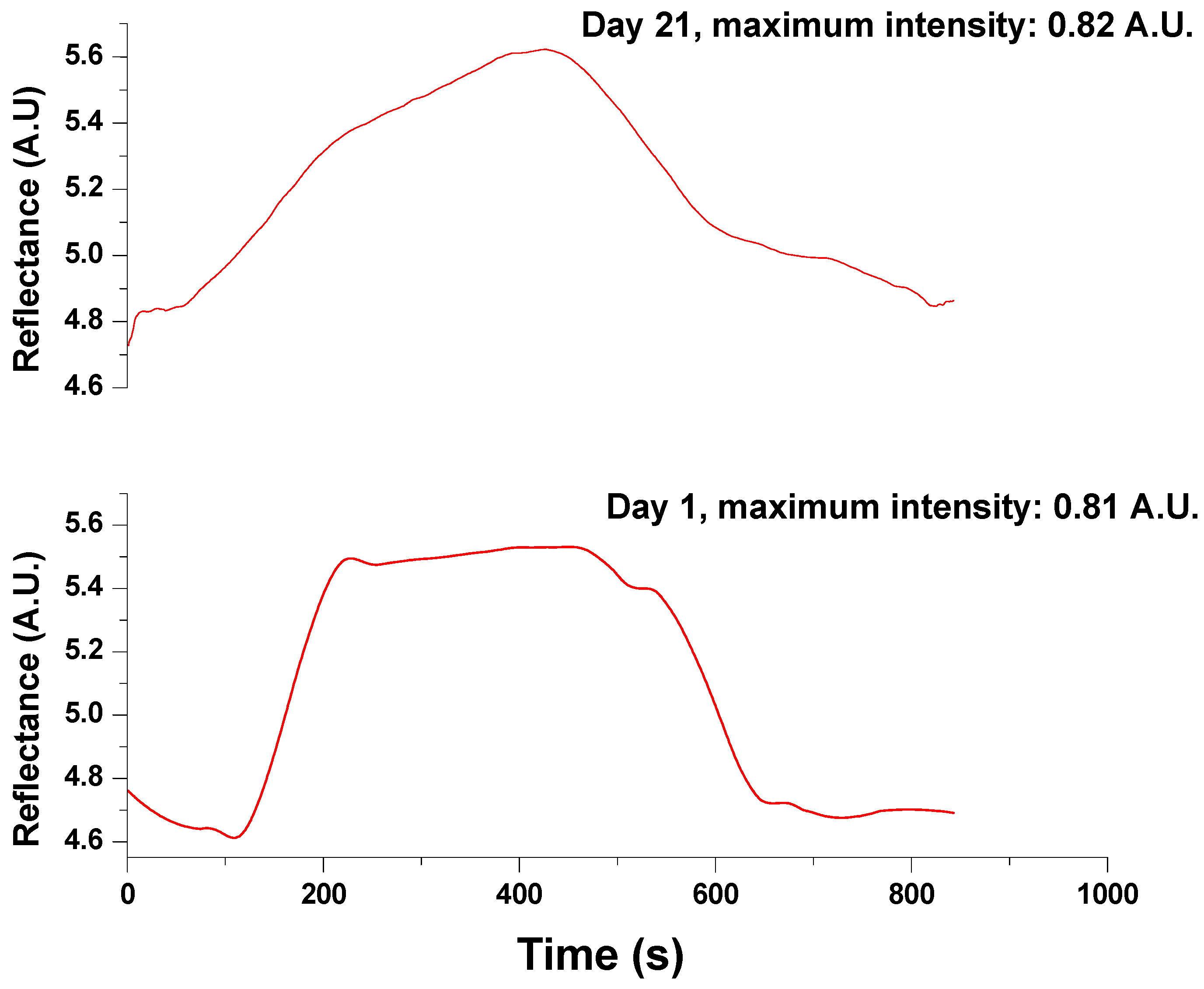
3.2. SPR Measurements for Glycine Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar] [PubMed]
- Fajar, A.; Syarifuddin, E.; Hendarto, J.; Labeda, I.; Lusikooy, R.E.; Dani, M.I.; Sampetoding, S.; Kusuma, M.I.; Uwuratuw, J.A.; Faruk, M. The relationship between glycine levels in collagen in the anterior rectus sheath tissue and the onset of indirect inguinal hernia: A cross-sectional study. Ann. Med. Surg. 2022, 73, 103166. [Google Scholar] [CrossRef] [PubMed]
- Halper, J.; Kjaer, M. Progress in Heritable Soft Connective Tissue Diseases. Adv. Exp. Med. Biol. 2013, 802, 31–47. [Google Scholar]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; de Paz-Lugo, P. Branch-point stoichiometry can generate weak links in metabolism: The case of glycine biosynthesis. J. Biosci. 2008, 33, 771–780. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; de Paz-Lugo, P.; Cornish-Bowden, A.; Cárdenas, M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009, 34, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Cerón, K.A.; Sánchez-Muñoz, F.; Gutierrez-Rojas, R.A.; Acevedo-Villavicencio, L.N.; Flores-Zarate, A.V.; Huang, F.; Giacoman-Martinez, A.; Villafaña, S.; Romero-Nava, R. Glycine: The Smallest Anti-Inflammatory Micronutrient. Int. J. Mol. Sci. 2023, 24, 11236. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Raventhiran, S.; Lee, J.H.; Lim, Z.X.; Goh, J.; Kennedy, B.K.; Maier, A.B. The effect of glycine administration on the characteristics of physiological systems in human adults: A systematic review. GeroScience 2024, 46, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin resistance and glycine metabolism in humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, T.; Anil, A.E.; Jin, D.; Jayathilake, K.; Lee, M.; Meltzer, H.Y. Plasma glycine and serine levels in schizophrenia compared to normal controls and major depression: Relation to negative symptoms. Int. J. Neuropsychopharmacol. 2004, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.N.; Paragomi, P.; Wang, R.; Huang, J.Y.; Adams-Haduch, J.; Midttun, Ø.; Ulvik, A.; Nguyen, T.C.; Brand, R.E.; Gao, Y.; et al. The Association between Serum Serine and Glycine and Related-Metabolites with Pancreatic Cancer in a Prospective Cohort Study. Cancers 2022, 14, 2199. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Masaka, S.; Fukada, C.; Houzaki, M.; Endo, T.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Salivary Glycine Is a Significant Predictor for the Attenuation of Polyp and Tumor Microenvironment Formation by Fucoxanthin in AOM/DSS Mice. Vivo 2019, 33, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.; Kim, E.; Osorio-Vasquez, V.; Doll, S.; Bustraan, S.; Liang, R.J.; Luengo, A.; Davidson, S.M.; Ali, A.; Ferraro, G.B.; et al. Limited Environmental Serine and Glycine Confer Brain Metastasis Sensitivity to PHGDH Inhibition. Cancer Discov. 2020, 10, 1352–1373. [Google Scholar] [CrossRef]
- Jiang, J.; James, C.A.; Wong, P. Bioanalytical method development and validation for the determination of glycine in human cerebrospinal fluid by ion-pair reversed-phase liquid chromatography—Tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 128, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, E.; Kapaki, E.; Kokotis, P.; Paraskevas, G.P.; Katsaros, N.; Libitaki, G.; Petropoulou, O.; Zis, V.; Sfagos, C.; Vassilopoulos, D. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis. Vivo 2008, 22, 137–141. [Google Scholar]
- Yamaguchi, H.; Miyazaki, M. Enzyme-immobilized microfluidic devices for biomolecule detection. TrAC Trends Anal. Chem. 2023, 159, 116908. [Google Scholar] [CrossRef]
- Jabbari, S.; Dabirmanesh, B.; Arab, S.S.; Amanlou, M.; Daneshjou, S.; Gholami, S.; Khajeh, K. A novel enzyme based SPR-biosensor to detect bromocriptine as an ergoline derivative drug. Sens. Actuators B Chem. 2017, 240, 519–527. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Shimizu, F.M.; Mejía-Salazar, J.R.; Oliveira, O.N., Jr.; Ferreira, M. Surface plasmon resonance biosensor for enzymatic detection of small analytes. Nanotechnology 2017, 28, 145501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuators B Chem. 2022, 366, 131984. [Google Scholar] [CrossRef]
- Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Blanco-Gámez, E.A.; Rodríguez-Delgado, J.M.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. A Novel Enzyme-Based SPR Strategy for Detection of the Antimicrobial Agent Chlorophene. Biosensors 2021, 11, 43. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Awasthi, S.; Mishra, P.K.; Srivastava, P.K. Biosensors/molecular tools for detection of waterborne pathogens. Waterborne Pathog. 2020, 2020, 237–277. [Google Scholar] [CrossRef]
- Bianco, M.; Sonato, A.; De Girolamo, A.; Pascale, M.; Romanato, F.; Rinaldi, R.; Arima, V. An aptamer-based SPR-polarization platform for high sensitive OTA detection. Sens. Actuators B Chem. 2017, 241, 314–320. [Google Scholar] [CrossRef]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. FO-SPR based dextrose sensor using Ag/ZnO nanorods/GOx for insulinoma detection. Biosens. Bioelectron. 2016, 85, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Shpacovitch, V.; Hergenröder, R. Surface Plasmon Resonance (SPR)-Based Biosensors as Instruments with High Versatility and Sensitivity. Sensors 2020, 20, 3010. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Chen, W.J.; Huang, Y.; Xiao, Y.; Wu, S.; Lei, Q.; Zhong, J.; Zhu, X.; Chen, S. Bioremediation potential of laccase for catalysis of glyphosate, isoproturon, lignin, and parathion: Molecular docking, dynamics, and simulation. J. Hazard. Mater. 2023, 443, 130319. [Google Scholar] [CrossRef] [PubMed]
- Do, L.T.T.; Le, P.H.D.; Nguyen, Q.M.; Luong, U.B. Solid-state production of laccase by Pleurotus sp. applied in glyphosate degradation. Sci. Technol. Dev. J. 2017, 20, 147–151. [Google Scholar] [CrossRef]
- Swe, T.M.; Nandar, W.; Ei, H.H.; Win, N.N.; Swe, K.K.; Ko, T.K.; Win, T.T. Bio-removal Efficiency of Glyphosate by Using Indigenous Laccase Producing Fungi. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 249–256. [Google Scholar] [CrossRef]
- Sánchez-Alvarez, A.; Luna-Moreno, D.; Hernández-Morales, J.A.; Zaragoza-Zambrano, J.O.; Castillo-Guerrero, D.H. Control of Stepper Motor Rotary Stages applied to optical sensing technique using LabView. Optik 2018, 164, 65–71. [Google Scholar] [CrossRef]
- Wong, C.L.; Olivo, M. Surface plasmon resonance imaging sensors: A review. Plasmonics 2014, 9, 809–824. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pérez-Ràfols, C.; Liu, Y.; Wang, Q.; Cuartero, M.; Crespo, G.A. Why not glycine electrochemical biosensors? Sensors 2020, 20, 4049. [Google Scholar] [CrossRef] [PubMed]
- Sota, H.; Hasegawa, Y.; Iwakura, M. Detection of Conformational Changes in an Immobilized Protein Using Surface Plasmon Resonance. Anal. Chem. 1998, 70, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.-C.; Belenguer, J.; Gomez-Montes, S.; Miralles, J.; Escuela, A.M.; Montoya, A.; Lechuga, L.M. Indirect competitive immunoassay for the detection of fungicide Thiabendazole in whole orange samples by Surface Plasmon Resonance. Analyst 2012, 137, 5659–5665. [Google Scholar] [CrossRef]
- Ranimol, G.; Sunkar, S. A comparative study of free laccase and laccase immobilized in copper alginate. Indian J. Biochem. Biophys. 2022, 59, 214–223. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Nasseri, S.; Alimohammadi, M.; Mahvi, A.H.; Faramarzi, M.A. Application of free and immobilized laccase for removal and detoxification of fluoroquinolones from aqueous solution. Glob. NEST J. 2020, 22, 240–249. [Google Scholar] [CrossRef]
- Rouhani, S.; Azizi, S.; Kibechu, R.W.; Mamba, B.B.; Msagati, T.A.M. Laccase Immobilized Fe3O4-Graphene Oxide Nanobiocatalyst Improves Stability and Immobilization Efficiency in the Green Preparation of Sulfa Drugs. Catalysts 2020, 10, 459. [Google Scholar] [CrossRef]
- Atiroğlu, V.; Atiroğlu, A.; Atiroğlu, A.; Al-Hajri, A.S.; Özacar, M. Green immobilization: Enhancing enzyme stability and reusability on eco-friendly support. Food Chem. 2024, 448, 138978. [Google Scholar] [CrossRef] [PubMed]
- Rosini, E.; Piubelli, L.; Molla, G.; Frattini, L.; Valentino, M.; Varriale, A.; Pollegioni, L. Novel biosensors based on optimized glycine oxidase. FEBS J. 2014, 281, 3460–3472. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Hermanowicz, A.; Grycz, A.; Łukaszewski, Z.; Gorodkiewicz, E. An SPR imaging immunosensor for leptin determination in blood plasma. Anal. Methods 2021, 13, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Veettil, T.C.P.; Wood, B.R. A combined near-infrared and mid-infrared spectroscopic approach for the detection and quantification of Glycine in human serum. Sensors 2022, 22, 4528. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, M.; de Torresi, S.I.C.; Kubota, L.T. Electrochemical oxidation of glycine by doped nickel hydroxide modified electrode. Sens. Actuators B Chem. 2008, 135, 245–249. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karim-Nezhad, G.; Shadjou, N.; Hajjizadeh, M.; Khalilzadeh, B.; Saghatforoush, L.; Abnosi, M.H.; Babaei, A.; Ershad, S. Cobalt hydroxide nanoparticles modified glassy carbon electrode as a biosensor for electrooxidation and determination of some amino acids. Anal. Biochem. 2009, 389, 130–137. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Campillo-Brocal, J.C.; Jiménez-Quero, A.; Crespo, G.A.; Cuartero, M. Electrochemical biosensor for glycine detection in biological fluids. Biosens. Bioelectron. 2021, 182, 113154. [Google Scholar] [CrossRef]
- Saini, N.; Yadav, D.; Shirolkar, M.; Murugappan, S.; Thorat, N.; Kulkarni, A. Chitosan lecithin nanocomposite based electrochemical biosensor for glycine detection in biological matrices. Colloids Surf. B Biointerfaces 2024, 238, 113901. [Google Scholar] [CrossRef] [PubMed]
- Şaylan, M.; Er, E.Ö.; Tekin, Z.; Bakırdere, S. An accurate and sensitive analytical method for the simultaneous determination of glycine, methionine and homocysteine in biological matrices by matrix matching strategy and LC—Quadrupole-time-of-flight-MS/MS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118394. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Kosciuk, T.; Yang, M.; Price, I.R.; Lin, H. Binding Affinity Determines Substrate Specificity and Enables Discovery of Substrates for N-Myristoyltransferases. ACS Catal. 2021, 11, 14877–14883. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [PubMed]



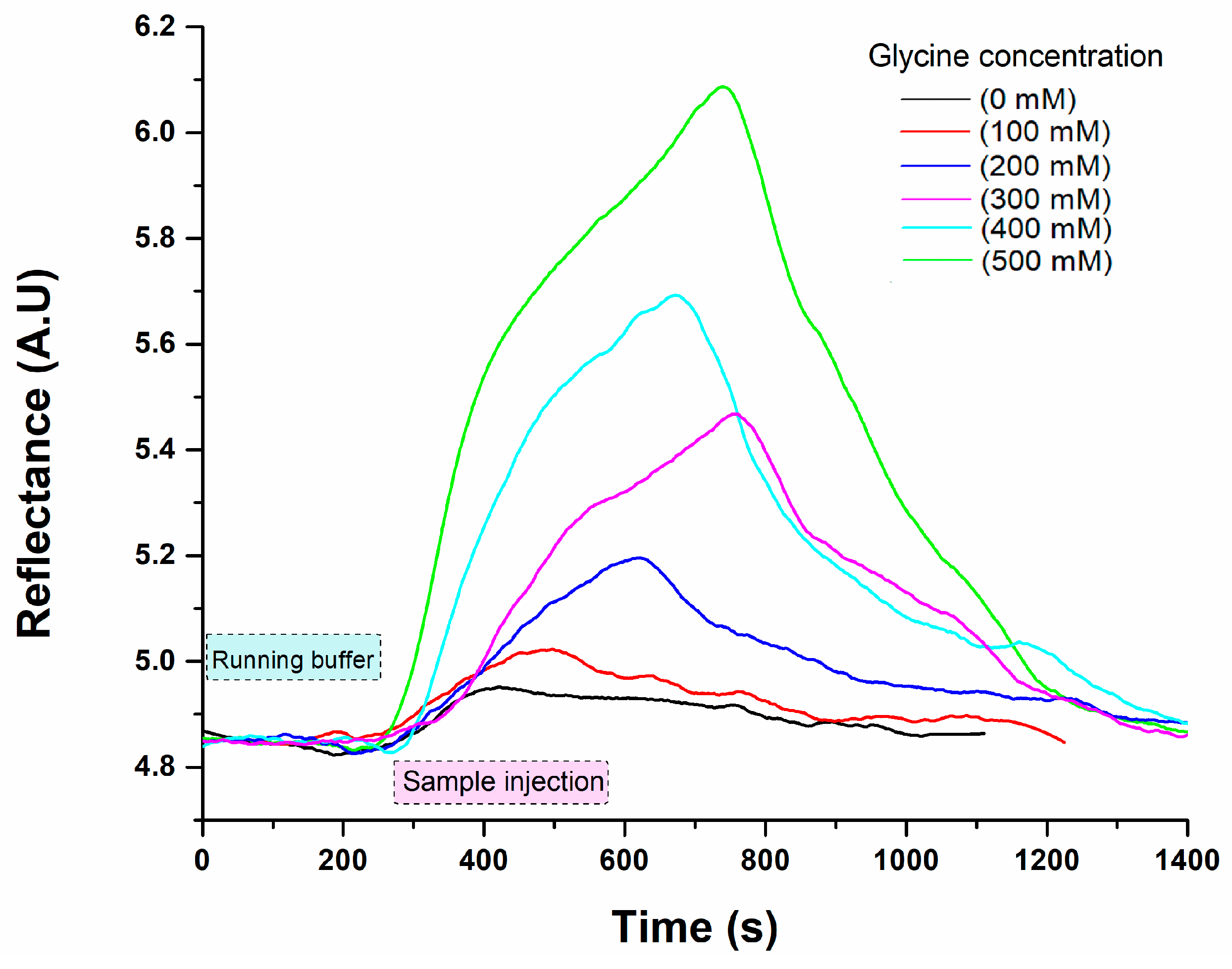
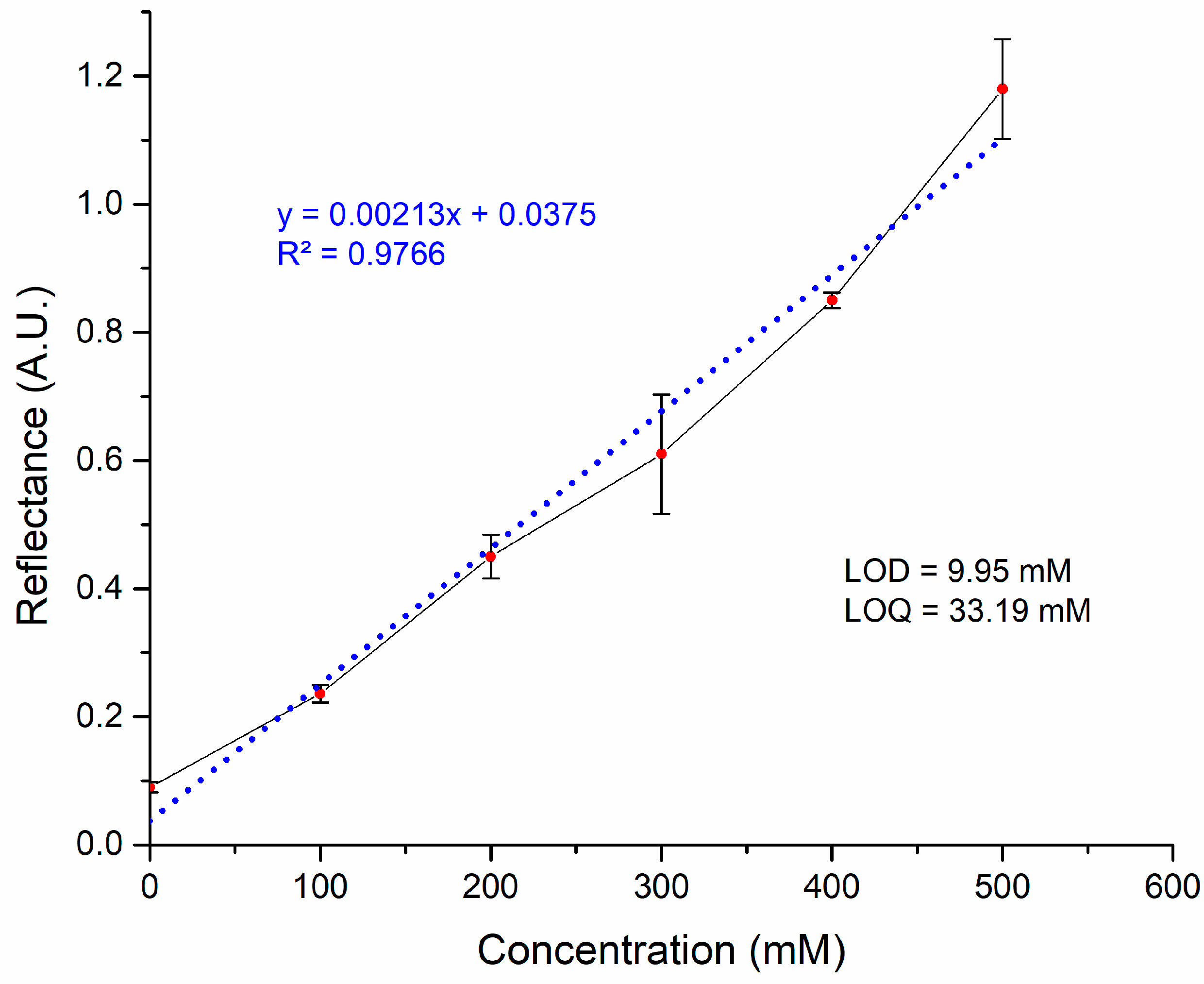
| Methods | LOD (mM) | Dynamic Range (mM) | Reference |
|---|---|---|---|
| Laccase-based surface plasmon resonance | 9.953 | 0–500 | This study |
| Attenuated total reflection (ATR) spectroscopy | 3.463 | 0–666 | [45] |
| Near-infrared (NIR) spectroscopy | 2.930 | 0–666 | [45] |
| Amperometry with electrode of Ni(OH)2 | 0.030 | 0.10–1.2 | [46] |
| Differential pulse voltammetry with glassy carbon electrode-modified Co(OH)O nanoparticles | 0.010 | 0.020–1.5 | [47] |
| Amperometric sensor with carbon electrode modified with glycine oxidase | 0.011 | 0.020–0.50 | [48] |
| Cyclic voltammetry with carbon electrode modified with Prussian Blue -Chitosan–lecithin nanocomposite | 0.008 | 0.007–0.240 | [49] |
| Liquid chromatography (LC)–quadrupole-time-of-flight–tandem mass spectrometry (MS/MS) | 0.009 | 0.033–0.666 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Villanueva, G.E.; Rodríguez-Quiroz, O.; Sánchez-Álvarez, A.; Rodríguez-Delgado, J.M.; Villarreal-Chiu, J.F.; Luna-Moreno, D.; Rodríguez-Delgado, M.M. An Innovative Enzymatic Surface Plasmon Resonance-Based Biosensor Designed for Precise Detection of Glycine Amino Acid. Biosensors 2025, 15, 81. https://doi.org/10.3390/bios15020081
Quintanilla-Villanueva GE, Rodríguez-Quiroz O, Sánchez-Álvarez A, Rodríguez-Delgado JM, Villarreal-Chiu JF, Luna-Moreno D, Rodríguez-Delgado MM. An Innovative Enzymatic Surface Plasmon Resonance-Based Biosensor Designed for Precise Detection of Glycine Amino Acid. Biosensors. 2025; 15(2):81. https://doi.org/10.3390/bios15020081
Chicago/Turabian StyleQuintanilla-Villanueva, Gabriela Elizabeth, Osvaldo Rodríguez-Quiroz, Araceli Sánchez-Álvarez, José Manuel Rodríguez-Delgado, Juan Francisco Villarreal-Chiu, Donato Luna-Moreno, and Melissa Marlene Rodríguez-Delgado. 2025. "An Innovative Enzymatic Surface Plasmon Resonance-Based Biosensor Designed for Precise Detection of Glycine Amino Acid" Biosensors 15, no. 2: 81. https://doi.org/10.3390/bios15020081
APA StyleQuintanilla-Villanueva, G. E., Rodríguez-Quiroz, O., Sánchez-Álvarez, A., Rodríguez-Delgado, J. M., Villarreal-Chiu, J. F., Luna-Moreno, D., & Rodríguez-Delgado, M. M. (2025). An Innovative Enzymatic Surface Plasmon Resonance-Based Biosensor Designed for Precise Detection of Glycine Amino Acid. Biosensors, 15(2), 81. https://doi.org/10.3390/bios15020081






