A Brief Review of Aptamer-Based Biosensors in Recent Years
Abstract
1. Introduction
2. Aptamer-Based Biosensors
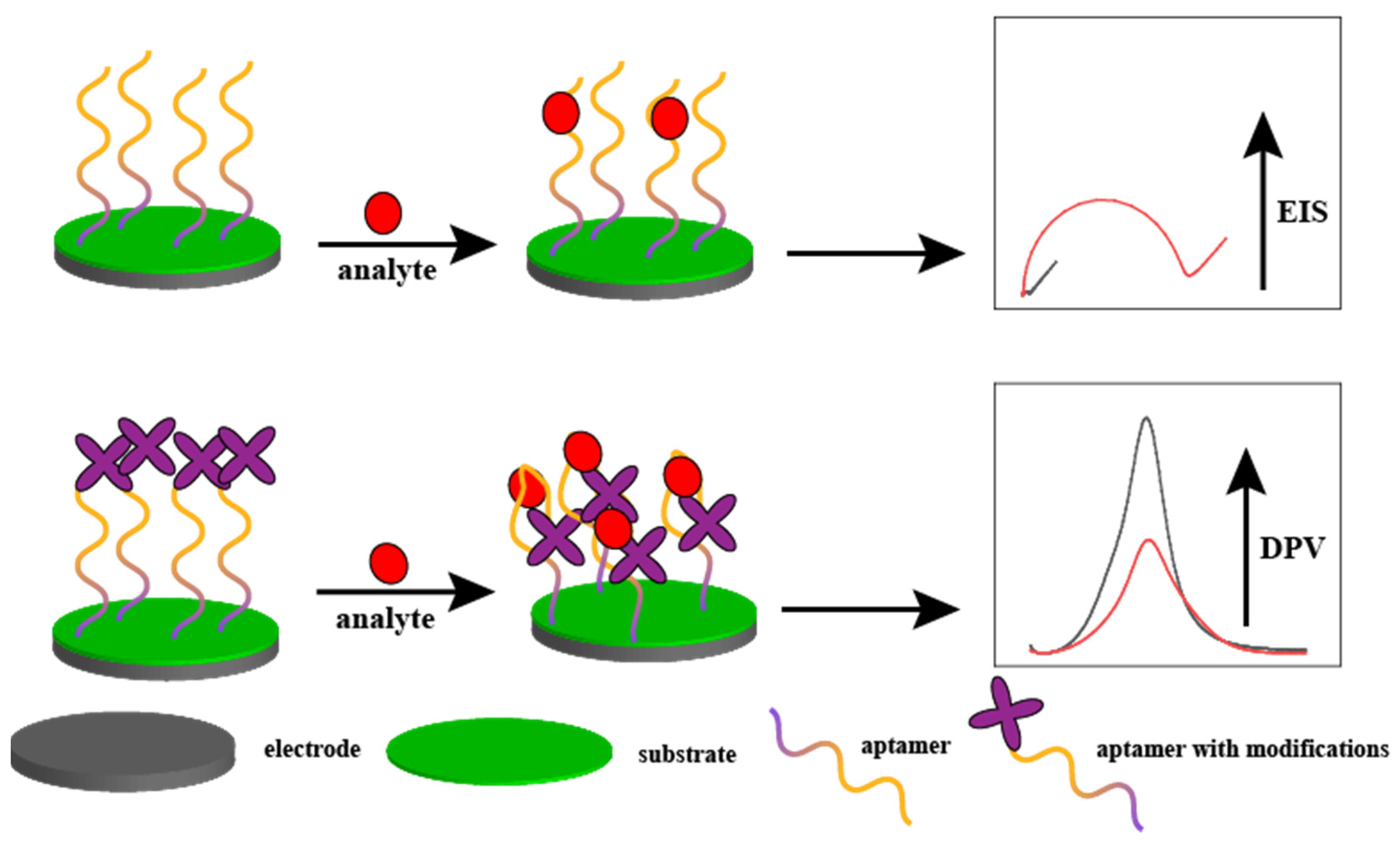
2.1. Electrochemical Sensors
2.2. Colorimetric or Fluorescent Sensors
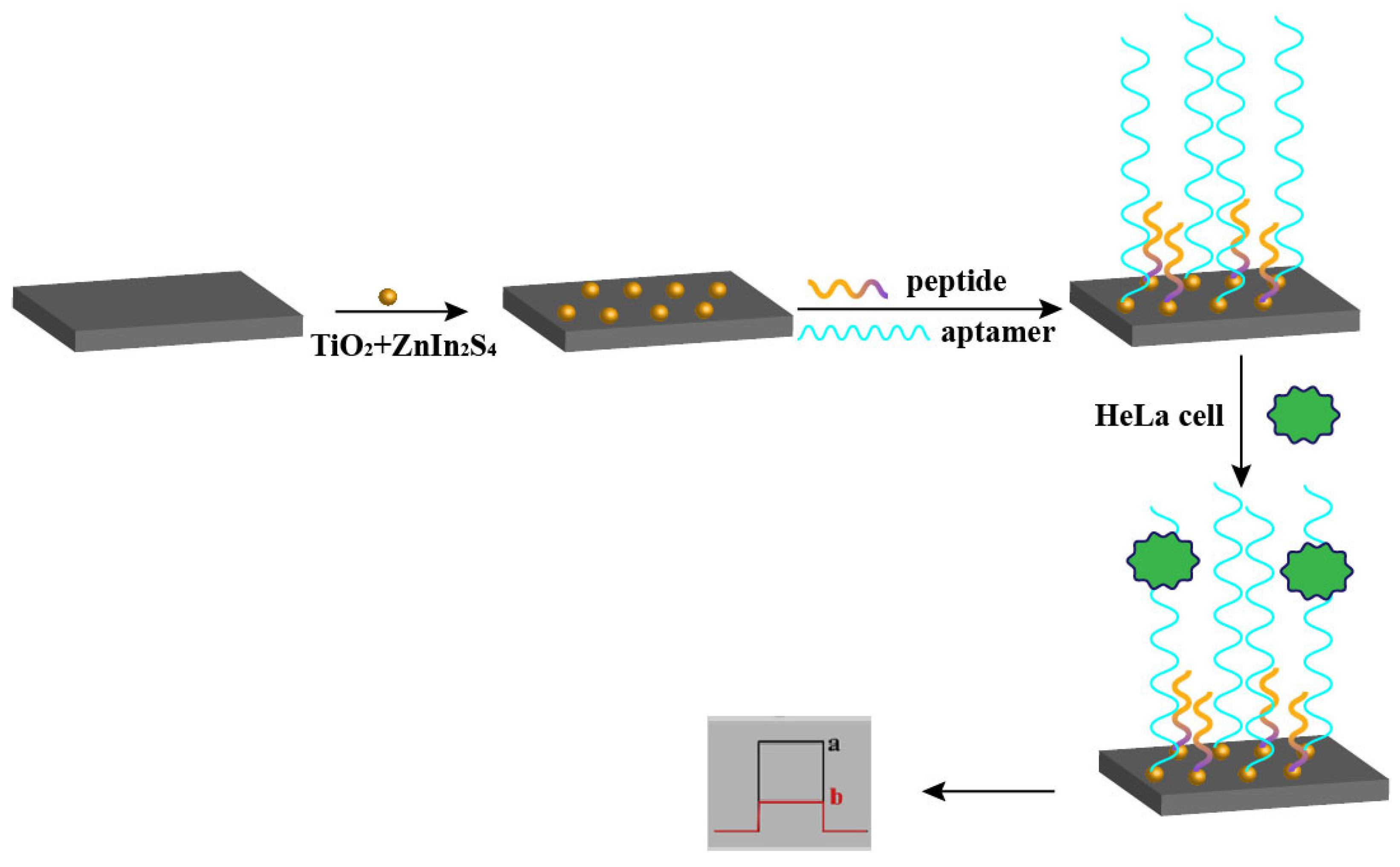
2.3. Electroluminescent Sensors
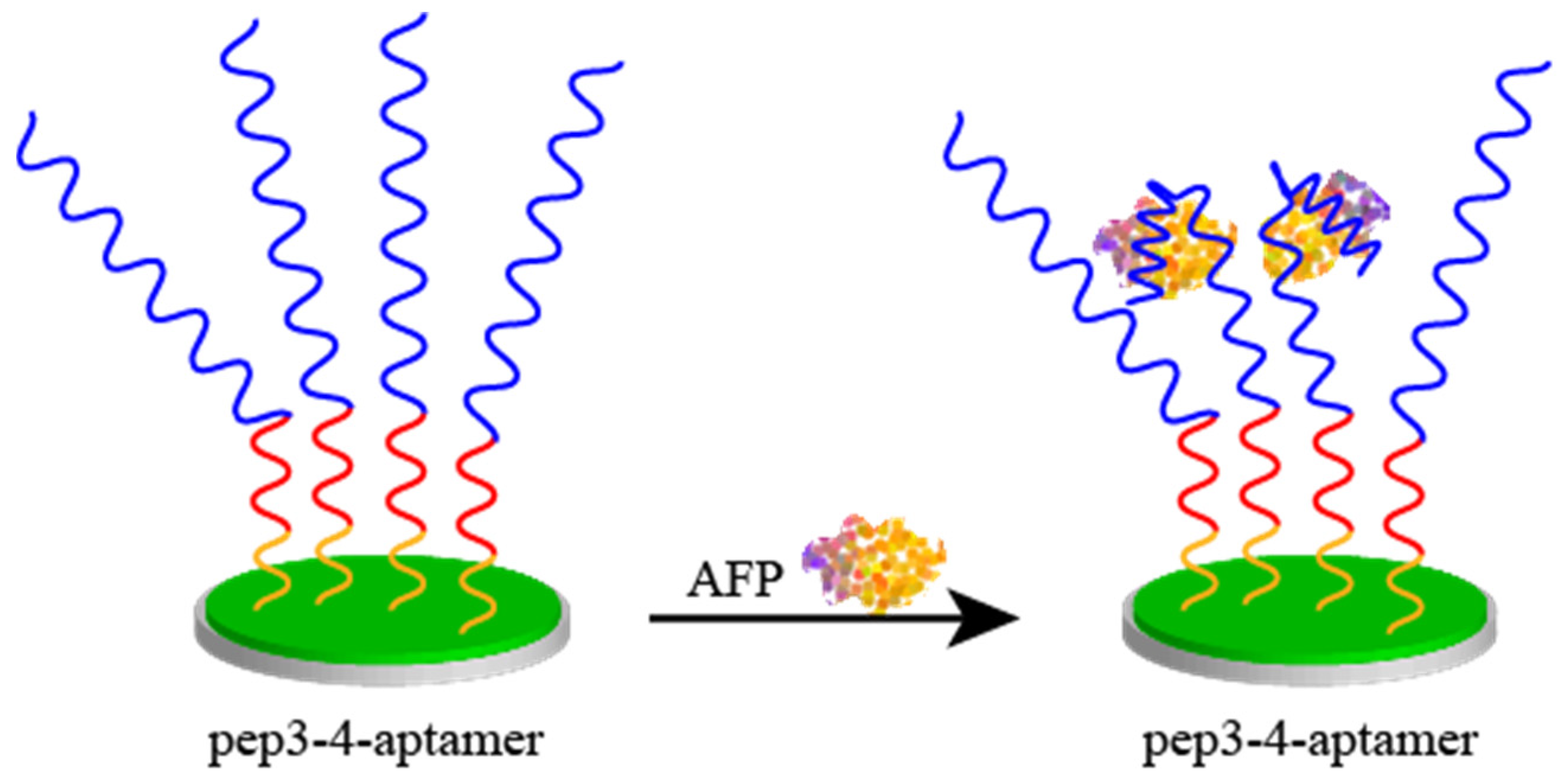
2.4. Peptides as an Antipollution Layer
3. Conclusion and Future Perspective
3.1. Discussion and Conclusions
3.2. Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Malinee, M.; Dhiman, A.; Kumar, A.; Sharma, T.K. Aptamer Technology for the Detection of Foodborne Pathogens and Toxins. In Advanced Biosensors for Health Care Applications; Inamuddin, K.R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 45–69. [Google Scholar]
- Wolter, O.; Mayer, G. Aptamers as Valuable Molecular Tools in Neurosciences. J. Neurosci. 2017, 37, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA Aptamers Against Epithelial Cell Adhesion Molecule for Cancer Cell Imaging and Circulating Tumor Cell Capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Berti, F. Short Peptides as Biosensor Transducers. Anal. Bioanal. Chem. 2012, 402, 3055–3070. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Guardia, M. Aptamer-Based Assay of Biomolecules: Recent Advances in Electro-Analytical Approach. TRAC-Trend Anal. Chem. 2017, 89, 119–132. [Google Scholar] [CrossRef]
- Rothlisberger, P.; Hollenstein, M. Aptamer Chemistry. Adv. Drug Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Tomilin, F.N.; Moryachkov, R.; Shchugoreva, I.; Zabluda, V.N.; Peters, G.; Platunov, M.; Spiridonova, V.; Melnichuk, A.; Atrokhova, A.; Zamay, S.S.; et al. Four Steps for Revealing and Adjusting the 3d Structure of Aptamers in Solution by Small-Angle X-Ray Scattering and Computer Simulation. Anal. Bioanal. Chem. 2019, 411, 6723–6732. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhu, M.; Chen, C.; Li, Y.; Cui, H.; Liu, S.; Zhao, Q. Implantable Flexible Sensors for Health Monitoring. Adv. Healthc. Mater. 2023, 13, 2302460. [Google Scholar] [CrossRef]
- Sun, H.; Zu, Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Wang, Y.; Wen, C.; Davis, B.; Wang, X.; Lee, K.; Wang, Y. High-affinity One-step Sptamer Selection Using a Non-fouling Porous Hydrogel. Nat. Biotechnol. 2023, 42, 1224–1231. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Boyd, B.J. Peptide-based Biosensors. Talanta 2015, 136, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Katsoyannis, P.G. Peptide Synthesis And Protein Structure. J. Polym. Sci. 1961, XLLX, 51–74. [Google Scholar] [CrossRef]
- Boyle, A.L.; Bromley, E.H.; Bartlett, G.J.; Sessions, R.B.; Sharp, T.H.; Williams, C.L.; Curmi, P.M.; Forde, N.R.; Linke, H.; Woolfson, D.N. Squaring the Circle in Peptide Assembly: From Fibers to Discrete Nanostructures by De Novo Design. J. Am. Chem. Soc. 2012, 134, 15457–15467. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Melchionna, M.; Bellotto, O.; Kralj, S.; Semeraro, S.; Parisi, E.; Iglesias, D.; D’Andrea, P.; De Zorzi, R.; Vargiu, A.V.; et al. Nanoscale Assembly of Functional Peptides with Divergent Programming Elements. ACS Nano 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; Guardia, M.d.l. Peptide Based Biosensors. TRAC-Trend Anal. Chem. 2018, 107, 1–20. [Google Scholar] [CrossRef]
- Wang, G.; Han, R.; Su, X.; Li, Y.; Xu, G.; Luo, X. Zwitterionic Peptide Anchored to Conducting Polymer PEDOT for the Development of Antifouling and Ultrasensitive Electrochemical DNA Sensor. Biosens. Bioelectron. 2017, 92, 396–401. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Jiang, S. Ultra-Low Fouling Peptide Surfaces Derived from Natural Amino Acids. Biomaterials 2009, 30, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Sfragano, P.S.; Moro, G.; Polo, F.; Palchetti, I. The Role of Peptides in the Design of Electrochemical Biosensors for Clinical Diagnostics. Biosensors 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Fan, W.; Zhou, P.; Xing, R.; Cao, S.; Yan, X. High-entropy Non-covalent Cyclic Peptide Glass. Nat. Nanotechnol. 2024, 19, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, C.; Zhou, L.; Wu, L.; Bian, K.; Zeng, J.; Wang, J.; Feng, Z.; Yin, Y.; Cao, Z. Highly Sensitive Determination of L-Tyrosine in Pig Serum Based On Ultrathin CuS Nanosheets Composite Electrode. Biosens. Bioelectron. 2019, 140, 111356. [Google Scholar] [CrossRef]
- Xing, R.C.Y.; Fan, W.; Ren, X.; Yan, X. Biomolecular Glass With Amino Acid and Peptide Nanoarchitectonics. Sci. Adv. 2023, 9, eadd8105. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Lee, J.W.; Ellington, A.D. Applications of Aptamers as Sensors. Annu Rev. Anal. Chem. 2009, 2, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, Y.; Chen, S.; Liu, J. Controlling Dopamine Binding by the New Aptamer for A FRET-Based Biosensor. Biosens. Bioelectron. 2020, 173, 112798. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Weng, C.C.; Li, B.R.; Li, Y.K. An Antifouling Peptide-Based Biosensor for Determination of Streptococcus Pneumonia Markers in Human Serum. Biosens. Bioelectron. 2020, 151, 111969. [Google Scholar] [CrossRef]
- Zhu, K.J.; Zhou, L.; Wu, L.; Feng, S.F.; Hu, H.Y.; He, J.L.; He, Y.M.; Feng, Z.M.; Yin, Y.L.; Yu, D.; et al. An Enzyme-Free Amperometric Sensor Based on Self-Assembling Ferrocene-Conjugated Oligopeptide for Specific Determination of L-Arginine. Chin. J. Chem. 2021, 39, 2755–2762. [Google Scholar] [CrossRef]
- He, Y.; Zhou, L.; Deng, L.; Feng, Z.; Cao, Z.; Yin, Y. An Electrochemical Impedimetric Sensing Platform Based on A Peptide Aptamer Identified by High-Throughput Molecular Docking for Sensitive L-Arginine Detection. Bioelectrochemistry 2021, 137, 107634. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kim, M.W.; Park, C.Y.; Choi, C.S.; Kailasa, S.K.; Park, J.P.; Park, T.J. Development of A Rapid and Sensitive Electrochemical Biosensor for Detection of Human Norovirus via Novel Specific Binding Peptides. Biosens. Bioelectron. 2019, 123, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Kim, J.H.; Song, D.K.; Park, T.J.; Park, J.P. An Affinity Peptide-Incorporated Electrochemical Biosensor for the Detection of Neutrophil Gelatinase-Associated Lipocalin. Biosens. Bioelectron. 2019, 142, 111482. [Google Scholar] [CrossRef]
- Alvarez-Martos, I.; Moller, A.; Ferapontova, E.E. Dopamine Binding and Analysis in Undiluted Human Serum and Blood by the RNA-Aptamer Electrode. ACS Chem. Neurosci. 2019, 10, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Konari, M.; Heydari-Bafrooei, E.; Dinari, M. Efficient Immobilization of Aptamers on The Layered Double Hydroxide Nanohybrids for The Electrochemical Proteins Detection. Int. J. Biol. Macromol. 2021, 166, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, N.; Soltani, N.; Mohammadi, F. A Nanoporous Gold-Based Electrochemical Aptasensor for Sensitive Detection of Cocaine. RSC Adv. 2019, 9, 14296–14301. [Google Scholar] [CrossRef]
- Li, J.; Si, Y.; Park, Y.E.; Choi, J.S.; Jung, S.M.; Lee, J.E.; Lee, H.J. A Serotonin Voltammetric Biosensor Composed of Carbon Nanocomposites and DNA Aptamer. Mikrochim. Acta 2021, 188, 146. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Hashkavayi, A.; Raoof, J.B. Ultrasensitive and Reusable Electrochemical Aptasensor for Detection of Tryptophan Using of [Fe(Bpy)3](P-Ch3c6h4so2)2 as An Electroactive Indicator. J. Pharm. Biomed. Anal. 2019, 163, 180–187. [Google Scholar] [CrossRef]
- Ok, J.; Park, S.; Jung, Y.H.; Kim, T.I. Wearable and Implantable Cortisol-Sensing Electronics for Stress Monitoring. Adv. Mater. 2023, 36, 2211595. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, L.; Liu, S.; Sheng, X.; Yin, L. Recent Development of Implantable Chemical Sensors Utilizing Flexible and Biodegradable Materials for Biomedical Applications. ACS Nano 2024, 18, 3969–3995. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dai, J.; Zhu, M.; Arroyo-Currás, N.; Li, H.; Wang, Y.; Wang, Q.; Lou, X.; Kippin, T.E.; Wang, S.; et al. Implantable Hydrogel-Protective DNA Aptamer-Based Sensor Supports Accurate, Continuous Electrochemical Analysis of Drugs at Multiple Sites in Living Rats. ACS Nano 2023, 17, 18525–18538. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, N.; Matarasso, A.; Heck, I.; Li, H.; Lu, W.; Phaup, J.G.; Schneider, M.J.; Wu, Y.; Weng, Z.; et al. Implantable Aptamer-Graphene Microtransistors for Real-Time Monitoring of Neurochemical Release in Vivo. Nano Lett. 2022, 22, 3668–3677. [Google Scholar] [CrossRef]
- Reynoso, M.; Chang, A.-Y.; Wu, Y.; Murray, R.; Suresh, S.; Dugas, Y.; Wang, J.; Arroyo-Currás, N. 3D-printed, Aptamer-based Microneedle Sensor Arrays Using Magnetic Placement on Live Rats for Pharmacokinetic Measurements in Interstitial Fluid. Biosens. Bioelectron. 2024, 244, 115802. [Google Scholar] [CrossRef]
- Fernández-Vega, L.; Meléndez-Rodríguez, D.E.; Ospina-Alejandro, M.; Casanova, K.; Vázquez, Y.; Cunci, L. Development of a Neuropeptide Y-Sensitive Implantable Microelectrode for Continuous Measurements. ACS Sens. 2024, 9, 2645–2652. [Google Scholar] [CrossRef]
- Zhao, C.; Man, T.; Cao, Y.; Weiss, P.S.; Monbouquette, H.G.; Andrews, A.M. Flexible and Implantable Polyimide Aptamer-Field-Effect Transistor Biosensors. ACS Sens. 2022, 7, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Papani, R.; Li, Y.; Wang, S. Soft Mechanical Sensors for Wearable and Implantable Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1961. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Wu, Y.; Guo, M.; Gu, C.; Dai, C.; Kong, D.; Wang, Y.; Zhang, C.; Qu, D.; et al. Rapid and Ultrasensitive Electromechanical Detection of Ions, Biomolecules and SARS-CoV-2 RNA in Unamplified Samples. Nat. Biomed. Eng. 2022, 6, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, G.; Song, Y.; Li, H.; Zhang, Y.; Schneider, M.J.; Qiang, Y.; Kaszas, J.; Weng, Z.; Sun, H.; et al. Multiplexed Monitoring of Neurochemicals via Electrografting-Enabled Site-Selective Functionalization of Aptamers on Field-Effect Transistors. Anal. Chem. 2022, 94, 8605–8617. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dai, Z.; Tang, X.; Lin, Z.; Lo, P.K.; Meyyappan, M.; Lai, K.W.C. Graphene Field-Effect Transistors for the Sensitive and Selective Detection of Escherichia coli Using Pyrene-Tagged DNA Aptamer. Adv. Healthc. Mater. 2017, 6, 1700736. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.; Zhuang, Q. Highly Sensitive and Selective Biosensor for Heparin Detection with Rhodamine B-Labelled Peptides as Fluorescent Bioreceptors. Sens. Actuat. B-Chem. 2019, 299, 126873. [Google Scholar] [CrossRef]
- Tang, Y.; Kang, A.; Yang, X.; Hu, L.; Tang, Y.; Li, S.; Xie, Y.; Miao, Q.; Pan, Y.; Zhu, D. A Robust OFF-ON Fluorescent Biosensor for Detection and Clearance of Bacterial Endotoxin by Specific Peptide Based Aggregation Induced Emission. Sens. Actuat. B-Chem. 2020, 304, 127300. [Google Scholar] [CrossRef]
- Parnsubsakul, A.; Oaew, S.; Surareungchai, W. Zwitterionic Peptide-Capped Gold Nanoparticles for Colorimetric Detection of Ni2+. Nanoscale 2018, 10, 5466–5473. [Google Scholar] [CrossRef]
- Lim, S.K.; Chen, P.; Lee, F.L.; Moochhala, S.; Liedberg, B. Peptide-Assembled Graphene Oxide as A Fluorescent Turn-On Sensor for Lipopolysaccharide (Endotoxin) Detection. Anal. Chem. 2015, 87, 9408–9412. [Google Scholar] [CrossRef]
- Gong, M.S.; Oh, G.; Chung, J.; Jang, H.-S.; Lee, B.Y.; Chung, W.-J. Hierarchically Structured Peptide Nanofibers for Colorimetric Detection of Gaseous Aldehydes. Sens. Actuat. B-Chem. 2018, 282, 868–875. [Google Scholar] [CrossRef]
- Lee, J.I.; Jang, S.C.; Chung, J.; Choi, W.-K.; Hong, C.; Ahn, G.R.; Kim, S.H.; Lee, B.Y.; Chung, W.-J. Colorimetric Allergenic Fungal Spore Detection Using Peptide-Modified Gold Nanoparticles. Sens. Actuat. B-Chem. 2020, 327, 128894. [Google Scholar] [CrossRef]
- Mu, F.; He, J.; Fan, F.; Shi, G. Dual-Emission Fluorescence Biosensing of Vancomycin Based on Aiegen-Peptide Conjugates and Aptamer-Modified Au Nanoclusters. Anal. Chim. Acta 2021, 1150, 238177. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Wang, D.; Feng, L.; Li, G.; Xu, M. An Improved Structure-Switch Aptamer-Based Fluorescent Pb(2+) Biosensor Utilizing the Binding Induced Quenching of AMT to G-Quadruplex. Chem Commun 2020, 56, 10517–10520. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Y.; Yang, H.; Dong, Y.; Zhang, K.; Lu, Y.; Deng, R.; He, Q. Enzyme-Free Amplified and Ultrafast Detection of Aflatoxin B1 Using Dual-Terminal Proximity Aptamer Probes. Food Chem. 2019, 283, 32–38. [Google Scholar] [CrossRef]
- Mehta, P.K.; Lee, J.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Ratiometric Fluorescence Sensing System for Lead Ions Based on Self-Assembly of Bioprobes Triggered by Specific Pb2+–Peptide Interactions. ACS Appl. Mater. Interfaces 2023, 15, 14131–14145. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, Y.; He, S.; Liu, X.; Gui, Q.-w.; Deng, L.; Wang, H.; Cao, Z.; Feng, Z.; Xiong, B.; et al. Peptide Aptamer-Based Colorimetric Sensor for the Detection of L-Tryptophan in Porcine Serum. Microchem. J. 2024, 197, 109896. [Google Scholar] [CrossRef]
- Kim, D.-H.; Seong, J.; Lee, H.; Lee, K.-H. Ratiometric Fluorescence Detection of Hg(II) in Aqueous Solutions at Physiological pH and Live Cells with a Chemosensor Based on Tyrosine. Sens. Actuat. B-Chem. 2014, 196, 421–428. [Google Scholar] [CrossRef]
- Lee, J.Y.; Mehta, P.K.; Subedi, S.; Lee, K.-H. Development of ratiometric fluorescent probes based on peptides for sensing Pb2+ in aquatic environments and human serum. Spectrochim. Acta A 2023, 294, 122502. [Google Scholar] [CrossRef]
- Peng, K.; Liu, X.; Yuan, H.; Li, M.; Wu, X.; Wang, Z.; Hao, L.; Xu, F. A Novel Fluorescent Biosensor Based on Affinity-enhanced Aptamer-peptide Conjugate for Sensitive Detection of Lead(II) in Aquatic Products. Anal. Bioanal. Chem. 2023, 415, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Zhang, F.R.; Zou, W.Z.; Huang, W.T.; Guo, Z. Peptide-based System for Sensing Pb2+ and Molecular Logic Computing. Anal. Biochem. 2021, 630, 114333. [Google Scholar] [CrossRef]
- Poudineh, M.; Maikawa, C.L.; Ma, E.Y.; Pan, J.; Mamerow, D.; Hang, Y.; Baker, S.W.; Beirami, A.; Yoshikawa, A.; Eisenstein, M.; et al. A Fluorescence Sandwich Immunoassay for the Real-time Continuous Detection of Glucose and Insulin in Live Animals. Nat. Biomed. Eng. 2020, 5, 53–63. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, K.; Xue, S.; Wu, B.; Xiao, Z.; Feng, Z.; Yin, Y.; Li, J.; Yu, D.; Cao, Z. Dual-Mode Arginine Assay Based on the Conformation Switch of a Ferrocene-Grafted Polypeptide. Anal. Chem. 2024, 96, 10943–10952. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wei, P.; He, F.; Gou, Y.; Wang, P.; Yang, X. Rational Design of Dual-modalitye Peptide-based Probe for Detection of Cu(II) and -histidine in 100% Aqueous Solution and Its Application for Living Cells, Test Dtrips and Smartphone. J. Photochem. Photobiol. A 2023, 442, 114762. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, M.; Xue, S.; Chen, B.; Wang, P. Rational Development of a Peptide-based Probe for Fluorescence and Colorimetric Dual-mode Detection of Cu2+ and S2− ions: Real Application in Cell Imaging and Test Strips. Spectrochim. Acta A 2023, 302, 123006. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Ding, C.; Luo, X. Ratiometric Antifouling Electrochemiluminescence Biosensor Based on Bi-Functional Peptides and Low Toxic Quantum Dots. Sens. Actuat. B-Chem. 2020, 322, 128613. [Google Scholar] [CrossRef]
- Fan, X.; Li, Z.; Wang, S.; Wang, Y.; Yu, L.; Fan, X. An Electrochemiluminescence Sandwich Biosensor for the Detection of Lipopolysaccharide. Quim. Nova 2020, 43, 747–751. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, Y.; Zhang, F.; Yi, L.; Shang, Y.; Ren, D.; Ge, Z. Electrochemiluminescence Sensor Based on Cyclic Peptides-Recognition and Au Nanoparticles Assisted Graphitic Carbon Nitride for Glucose Determination. Mikrochim. Acta 2021, 188, 151. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Liu, H.; Li, Z.; Sun, Q.; Wang, Y.; Fan, X. A Sensitive Electrochemiluminescence Biosensor for Assay of Cancer Biomarker (Mmp-2) Based on Ngqds-Ru@Sio2 Luminophore. Talanta 2022, 236, 122830. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, G.; Ni, J.; Wang, Q.; Lin, Z. From Signal Amplification to Restrained Background: Magnetic Graphene Oxide Assisted Homogeneous Electrochemiluminescence Aptasensor for Highly Sensitive Detection of Okadaic Acid. Sens. Actuat. B-Chem. 2021, 327, 128872. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Ding, C.; Luo, X. Highly Selective Ratiometric Electrogenerated Chemiluminescence Assay of DNA Methyltransferase Activity via Polyaniline and Anti-Fouling Peptide Modified Electrode. Biosens. Bioelectron. 2019, 142, 111553. [Google Scholar] [CrossRef]
- Khonsari, Y.N.; Sun, S. A Novel Label Free Electrochemiluminescent Aptasensor for the Detection of Lysozyme. Mater. Sci. Eng. C Mater. 2019, 96, 146–152. [Google Scholar] [CrossRef]
- Sha, H.; Zhang, Y.; Wang, Y.; Ke, H.; Xiong, X.; Xue, H.; Jia, N. Electroluminescent Aptasensor Based on Rusio(2) Nanoparticles for Detection Cytochrome C Using Ferrocene as Quenching Probe. Biosens. Bioelectron. 2019, 132, 203–209. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Y.; Tan, X.; Wu, J.; Huang, K.; Mi, Y.; Ou, P.; Wei, F. Flower-Like Titanium Dioxide as Novel Co-Reaction Accelerator for Ultrasensitive “OFF–ON” Electrochemiluminescence Aptasensor Construction Based on 2d G-C3n4 Layer for Thrombin Detection. J. Solid. State Electr. 2022, 26, 959–971. [Google Scholar] [CrossRef]
- Aili, D.; Stevens, M.M. Bioresponsive Peptide-inorganic Hybrid Nanomaterials. Chem. Soc. Rev. 2010, 39, 3358–3370. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, B.E.; van Hest, J.C.; Lowik, D.W. Molecular Tools for the Construction of Peptide-based Materials. Chem. Soc. Rev. 2014, 43, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiao, X.; Chen, M.; Li, Y.; Wang, X.; Xu, Z.; Wu, Y.; Luo, X. d-Amino Acid-Based Antifouling Peptides for the Construction of Electrochemical Biosensors Capable of Assaying Proteins in Serum with Enhanced Stability. ACS Sens. 2022, 7, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Accardo, F.; Prandi, B.; Dellafiora, L.; Tedeschi, T.; Sforza, S. How D-Amino Acids Embedded in the Protein Sequence Modify Its Digestibility: Behaviour of Digestive Enzymes Tested on A Model Peptide Used as Target. Food Chem. 2024, 458, 140175. [Google Scholar] [CrossRef]
- Bolduc, O.R.; Clouthier, C.M.; Pelletier, J.N.; Masson, J.F. Peptide Self-Assembled Monolayers for Label-Free and Unamplified Surface Plasmon Resonance Biosensing in Crude Cell Lysate. Anal. Chem. 2009, 81, 6779–6788. [Google Scholar] [CrossRef]
- Xu, C.; Hu, X.; Wang, J.; Zhang, Y.-M.; Liu, X.-J.; Xie, B.-B.; Yao, C.; Li, Y.; Li, X.-S. A Library of Antifouling Surfaces Derived From Natural Amino Acids by Click Reaction. ACS Appl. Mater. Interfaces 2015, 7, 17337–17345. [Google Scholar] [CrossRef] [PubMed]
- Hucknall, A.; Rangarajan, S.; Chilkoti, A. In Pursuit of Zero: Polymer Brushes that Resist the Adsorption of Proteins. Adv. Mater. 2009, 21, 2441–2446. [Google Scholar] [CrossRef]
- Bolduc, O.R.; Pelletier, J.N.; Masson, J.-F.o. SPR Biosensing in Crude Serum Using Ultralow Fouling Binary Patterned Peptide SAM. Anal. Chem. 2010, 82, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Khatayevich, D.; Gungormus, M.; Yazici, H.; So, C.; Cetinel, S.; Ma, H.; Jen, A.; Tamerler, C.; Sarikaya, M. Biofunctionalization of Materials for Implants Using Engineered Peptides. Acta Biomater. 2010, 6, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Song, J.; Lu, Y.; Davis, J.J.; Gao, F.; Luo, X. Electrochemical Aptasensor for Ultralow Fouling Cancer Cell Quantification in Complex Biological Media Based on Designed Branched Peptides. Anal. Chem. 2019, 91, 8334–8340. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Huang, Y.; Duan, X.; Wei, X.; Fan, Y.; Gan, D.; Yue, S.; Cheng, W.; Chen, T. Fiber Optic Surface Plasmon Resonance Biosensor for Detection of PDGF-BB in Serum Based on Self-Assembled Aptamer and Antifouling Peptide Monolayer. Biosens. Bioelectron. 2019, 140, 111350. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Fan, X.; Hou, H.; Gao, F.; Luo, X. Electrochemical Sensing Interfaces Based on Hierarchically Architectured Zwitterionic Peptides for Ultralow Fouling Detection of Alpha Fetoprotein in Serum. Anal. Chim. Acta 2021, 1146, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, J.; Gao, C.; Sheng, L.; Meng, A. A Glassy Carbon Electrode Modified with Graphene Oxide, Poly(3,4-Ethylenedioxythiophene), an Antifouling Peptide and An Aptamer for Ultrasensitive Detection of Adenosine Triphosphate. Mikrochim. Acta 2019, 186, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, J.; Gao, C.; Qiu, G.; Meng, A.; Li, Q. Construction of Electrochemical Aptasensor Based on Coral-Like Poly-Aniline and Au Nano-Particles for the Sensitive Detection of Prostate Specific Antigen. Sens. Actuat. B-Chem. 2019, 295, 93–100. [Google Scholar] [CrossRef]
- Fan, G.-C.; Li, Z.; Lu, Y.; Ma, L.; Zhao, H.; Luo, X. Robust Photoelectrochemical Cytosensor in Biological Media Using Antifouling Property of Zwitterionic Peptide. Sens. Actuat. B-Chem. 2019, 299, 126996. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, L.; Niu, S.; Ding, C.; Luo, X. Ratiometric Electrogenerated Chemiluminescence Sensor Based on A Designed Anti-Fouling Peptide for the Detection of Carcinoembryonic Antigen. Anal. Chim. Acta 2020, 1136, 134–140. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.-Q.; Ye, B.-C. Colorimetric Assay for Parallel Detection of Cd2+, Ni2+ and Co2+ Using Peptide-Modified Gold Nanoparticles. Analyst 2012, 137, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Neupane, L.N.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Selective and Sensitive Detection of Heavy Metal Ions in 100% Aqueous Solution and Cells with a Fluorescence Chemosensor Based on Peptide Using Aggregation-Induced Emission. Anal. Chem. 2016, 88, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, M.; Wang, D.; Chen, K.; Lin, P.; Ge, Y.; Liu, W.; Wu, J. Highly Selective Fluorescence Probe with Peptide Backbone for Imaging Mercury Ions in Living Cells Based on Aggregation-Induced Emission Effect. J. Hazard. Mater. 2021, 415, 125712. [Google Scholar] [CrossRef] [PubMed]
- Ranallo, S.; Bracaglia, S.; Sorrentino, D.; Ricci, F. Synthetic Antigen-Conjugated DNA Systems for Antibody Detection and Characterization. ACS Sens. 2023, 8, 2415–2426. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, M.; Deng, W.; Kong, N.; Hu, J.; Wang, P.; Yang, X. A Highly Sensitive and Selective Fluorescent “On–Off-On” Peptide-Based Probe for Sequential Detection of Hg2+ and S2− Ions: Applications In Living Cells and Zebrafish Imaging. Spectrochim. Acta A 2024, 318, 124514. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, H.; Ji, C.; Zhang, L.; Zhao, C.; Tang, L.; Zhang, C.; Sun, Z.; Tan, W.; Yuan, Q. Electron Transfer-Triggered Imaging of EGFR Signaling Activity. Nat. Commun. 2022, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Z.; Wei, Y.; Wang, W.; Wang, F.; Yang, Y.; Song, H.; Yuan, Q. Multiplexed Identification of Bacterial Biofilm Infections Based on Machine-Learning-Aided Lanthanide Encoding. ACS Nano 2022, 16, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Huang, W.; Wan, G.; Xia, M.; Chen, D.; Zhang, Y.; Wang, Y.; Guo, F.; Tan, J.; et al. Integrated Urinalysis Devices Based on Interface-Engineered Field-Effect Transistor Biosensors Incorporated With Electronic Circuits. Adv. Mater. 2022, 34, e2203224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, M.; Ji, C.; Wei, J.; Yang, Y.; Huang, Z.; Tan, W.; Tan, J.; Yuan, Q. In Situ Visualization of Epidermal Growth Factor Receptor Nuclear Translocation with Circular Bivalent Aptamer. Anal. Chem. 2022, 94, 17413–17421. [Google Scholar] [CrossRef]
- Chen, N.; Cheng, D.; He, T.; Yuan, Q. Real-Time Monitoring of Dynamic Chemical Processes in Microbial Metabolism with Optical Sensors. Chin. J. Chem. 2023, 41, 1836–1840. [Google Scholar] [CrossRef]




| Probe | Sequence | LOD | Linear Range | Analyte | Method | Ref. |
|---|---|---|---|---|---|---|
| Peptide | HHHHHHHGGGGGENIMPVLGC | 0.5 nM | 0.25–7.5 nM | UlaG | EIS | [26] |
| Peptide-Fc | GGGGGFGHIHEGYGGGGK | 31 pM | 0.0001–10 μM | L-Arg | DPV | [27] |
| Peptide | GGGGFGHIHEGY | 0.1 pM | 0.1 pM–0.1 mM | L-Arg | EIS | [28] |
| Peptide | QHKMHKPHKNTKGGGGSGGGGSC | 2.47 copies/mL | 10–105 copies/mL | Norovirus | EIS | [29] |
| Peptide | DRWVARDPASIF | 3.93 ng/mL | 0.0001–7.5 μg/mL | NGAL | SWV | [30] |
| Peptide-rhodamine B | RNRHTHLRTRPRK | 0.075 nM | 0.01–0.1 nM, 1.0–70.0 nM | Heparin | Fluorescence | [47] |
| CTPY-peptide | GCKPTFRRLKWKYKCG | 6.97 nM | 0.1–1 μM | LPS | Fluorescence | [48] |
| Au NPs-peptide | EKEKEKPPPPC | 30 nM | 60–160 nM | Ni2+ | Colorimetric | [49] |
| TMRho-peptide | KKNYSSSISSIHC | 130 pM | 0–20 nM | LPS | Fluorescence | [50] |
| NFs-peptide | GGGKKK | 300 ppm | - | Aldehydes | Colorimetric | [51] |
| Au NPs-peptide | FTPHPVGRPHTM | 50 spores/mL | 0–1.5 × 103 spores/mL | A. niger Spores | Colorimetric | [52] |
| PAMAM-QDs-peptide | DRDRDRDRSGRPVLG | 1.82 fM | 10.0 fM–1.0 nM | Thrombin | Electroluminescent | [66] |
| Peptide- Ru1@SiO2 | CIGKLHSAGK | 0.3 ng/mL | 1.0–500 ng/mL | LPS | electroluminescent | [67] |
| Au NPs/g-C3N4-peptide | cyclo-[−CNDNHCRDNDC−] | 0.57 nM | 1–100 mM | Glucose | electroluminescent | [68] |
| Ru(bpy)32+ -NGQDs-peptide | CGPLGVRGK | 6.5 pg/mL | 0.01–185 ng/mL | MMP-2 | electroluminescent | [69] |
| RNA | HO-C6-S-S-C6-5′-UCU CUG UGU GCG CCA GAG ACA GUG GGG CAG AUA UGG GCC AGC ACA GAA UGA GGC CC-3′ | 67 nM | 0.1–1 μM | Dopamine | CA | [31] |
| ssDNA | 5′-NH2-AGT CCG TGG TAG GGC AGG TTG GGG TGA CT-3′ | 0.1 fM | 0.005 pM–12 nM | Thrombin | DPV | [32] |
| ssDNA-DHBA | 5′-AGA CAA GGA AAA TCC TTC AA TGA AGT GGG TCG-3′ | 21 nM | 0.05–1, 1–35 mM | Cocaine | SWV | [33] |
| ssDNA-Tyr | 5′-NH2-CTC TCG GGA CGA CTG GTA GGC AGA TAG GGG AAG CTG ATT CGA TGC GTG GGT CGT CCC-3′ | 2 nM | 0.05–0.5, 1–20 μM | 5-HT | DPV | [34] |
| Hemin-ssDNA | 5′-AGC ACG TTG GTT AGG TCA GGT TTG GGT TTC GTG C-3′ | 1.0 nM | 3.0–100,000.0 nM | Tryptophan | DPV | [35] |
| ssDNA-Au NCs | 5′-NH2-CGA GGG TAC CGC AAT AGT ACT TAT TGT TCG CCT ATT GTG GGT GGG-3′. | 2.79 ng/mL | 0.01–100 mg/mL | Van | fluorescence | [53] |
| AMT-ssDNA | 5′-GGG TGG GTG GGT GGG T-3′ | 3.6 nM | 0.1–1.0 μM | Pb2+ | fluorescence | [54] |
| FAM-ssDNA | 5′-GTT GGG CAC GTG TTG TCT CTC TGT GTC TCG TGC CCT TCG CTA GGC CCA CA-3′ | 0.91 ng/mL | 1–200 ng/mL | AFB1 | fluorescence | [55] |
| Ru(bpy)32+- ssDNA | 5′-GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG-3′ | 4 pg/mL | 0.01–10.0 ng/mL | OA | electroluminescent | [70] |
| Au@luminol- ssDNA | 5′-HOOC-TTT TTT GAA GGA GGG GCG ATC TTT TTG ATC TTT TT-(CH2)6-SH-3′ | 0.02 U/mL | 0.05–100 U/mL | MTase | electroluminescent | [71] |
| NGQD-S2O82−- ssDNA | 5′-HS-(CH2)6-ATC TAC GAA TTC ATC AGG GCT AAA GAG TGC AGA GTT ACT TAG-3′ | 0.8 fM | 10 fM–10 nM | Lysozyme | electroluminescent | [72] |
| Ru(bpy)32+-RuSiO2-ssDNA | 5′-CCG TGT CTG GGG CCG ACC GGC GCA TTG GGT ACG TTG C(CH2)6-NH2-3′ | 0.48 pM | 0.001–100 nM | Cyt C | electroluminescent | [73] |
| g-C3N4- ssDNA | 5′-HOOC-(CH2)6-ATT TGG CCA ACC ACA CCA ACC-3′ | 8.9 × 10−12 M | 10−11–10−5 M | Thrombin | electroluminescent | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; He, Y.; He, S.; Deng, L.; Wang, H.; Cao, Z.; Feng, Z.; Xiong, B.; Yin, Y. A Brief Review of Aptamer-Based Biosensors in Recent Years. Biosensors 2025, 15, 120. https://doi.org/10.3390/bios15020120
Wang W, He Y, He S, Deng L, Wang H, Cao Z, Feng Z, Xiong B, Yin Y. A Brief Review of Aptamer-Based Biosensors in Recent Years. Biosensors. 2025; 15(2):120. https://doi.org/10.3390/bios15020120
Chicago/Turabian StyleWang, Wenjing, Yumin He, Suxiang He, Lei Deng, Hui Wang, Zhong Cao, Zemeng Feng, Benhai Xiong, and Yulong Yin. 2025. "A Brief Review of Aptamer-Based Biosensors in Recent Years" Biosensors 15, no. 2: 120. https://doi.org/10.3390/bios15020120
APA StyleWang, W., He, Y., He, S., Deng, L., Wang, H., Cao, Z., Feng, Z., Xiong, B., & Yin, Y. (2025). A Brief Review of Aptamer-Based Biosensors in Recent Years. Biosensors, 15(2), 120. https://doi.org/10.3390/bios15020120








