Bioluminescent Microbial Bioreporters: A Personal Perspective
Abstract
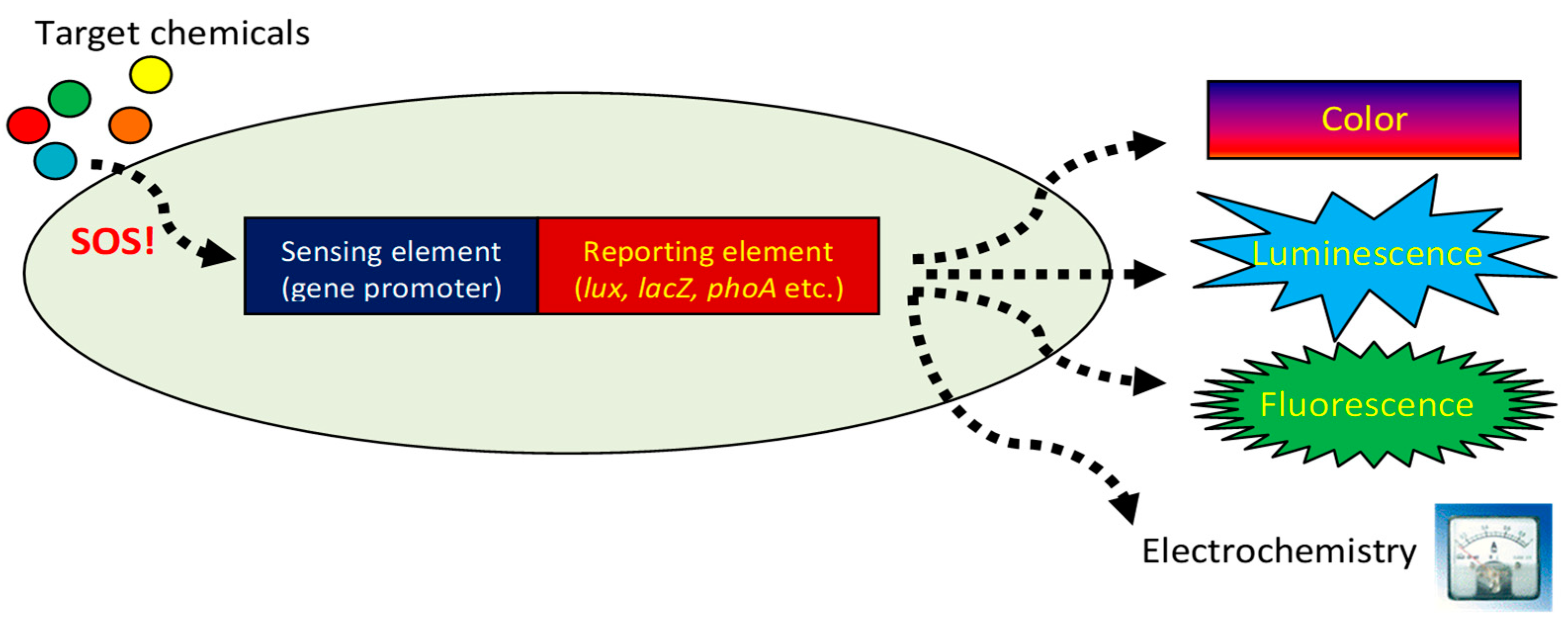
1. Introduction
2. The Basic Story: Selecting the Appropriate Sensing Element
3. How to Improve Your Sensor’s Performance
4. Just Having a Good Sensor Strain May Not Be Enough
5. Toxicity and Genotoxicity Assessment, the Panel Approach and Group Classification
6. Detection of Specific Compounds and Buried Explosives
7. Not Only Prokaryotes
8. Not Only Pollutants and Explosives
9. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- ISO 11348; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test). International Organization for Standardization: Geneva, Switzerland, 1998.
- Belkin, S. Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar] [CrossRef]
- Köhler, S.; Belkin, S.; Schmid, R.D. Reporter gene bioassays in environmental analysis. Fresenius’ J. Anal. Chem. 2000, 366, 769–779. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, J.R.; Belkin, S. Where microbiology meets microengineering: Design and applications of reporter bacteria. Nat. Rev. Microbiol. 2010, 8, 511–522. [Google Scholar] [CrossRef]
- King, J.M.H.; Digrazia, P.M.; Applegate, B.; Burlage, R.; Sanseverino, J.; Dunbar, P.; Larimer, F.; Sayler, G.S. Rapid, sensitive bioluminescent reporter technology for naphthalene exposure and biodegradation. Science 1990, 249, 778–781. [Google Scholar] [CrossRef]
- Selifonova, O.; Burlage, R.; Barkay, T. Bioluminescent sensors for detection of bioavailable Hg(II) in the environment. Appl. Environ. Microbiol. 1993, 59, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Belkin, S.; Smulski, D.R.; Vollmer, A.C.; Van Dyk, T.K.; LaRossa, R.A. Oxidative stress detection with Escherichia coli harboring a katG’::lux fusion. Appl. Environ. Microbiol. 1996, 62, 2252–2256. [Google Scholar] [CrossRef]
- Vollmer, A.C.; Belkin, S.; Smulski, D.R.; Van Dyk, T.K.; LaRossa, R.A. Detection of DNA damage by use of Escherichia coli carrying recA’::lux, uvrA’::lux or alkA’::lux reporter plasmids. Appl. Environ. Microbiol. 1997, 63, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Zaslaver, A.; Bren, A.; Ronen, M.; Itzkovitz, S.; Kikoin, I.; Shavit, S.; Liebermeister, W.; Surette, M.G.; Alon, U. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat. Methods 2006, 3, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Yagur-Kroll, S.; Lalush, C.; Rosen, R.; Bachar, N.; Moskovitz, Y.; Belkin, S. Escherichia coli bioreporters for the detection of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene. Appl. Microbiol. Biotechnol. 2014, 98, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Henshke, Y.; Shemer, B.; Belkin, S. The Escherichia coli azoR gene promoter: A new sensing element for microbial biodetection of trace explosives. Curr. Res. Biotechnol. 2021, 3, 21–28. [Google Scholar] [CrossRef]
- Davidov, Y.; Rozen, R.; Smulski, D.R.; Van Dyk, T.K.; Vollmer, A.C.; Elsemore, D.A.; LaRossa, R.A.; Belkin, S. Improved bacterial SOS promoter::lux fusions for genotoxicity detection. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 466, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rmailah, N.; Moscovici, L.; Riegraf, C.; Atias, H.; Buchinger, S.; Reifferscheid, G.; Belkin, S. Enhanced detection of estrogen-like compounds by genetically engineered yeast sensor strains. Biosensors 2024, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Shemer, B.; Shpigel, E.; Glozman, A.; Yagur-Kroll, S.; Kabessa, Y.; Agranat, A.J.; Belkin, S. Genome-wide gene-deletion screening identifies mutations that significantly enhance explosives’ vapor detection by a microbial sensor. New Biotechnol. 2020, 59, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shemer, B.; Shpigel, E.; Hazan, C.; Kabessa, Y.; Agranat, A.J.; Belkin, S. Detection of buried explosives with immobilized bacterial bioreporters. Microb. Biotechnol. 2020, 14, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Yagur-Kroll, S.; Belkin, S. Upgrading bioluminescent bacterial bioreporter performance by splitting the lux operon. Anal. Bioanal. Chem. 2011, 400, 1071–1082. [Google Scholar] [CrossRef]
- Palevsky, N.; Shemer, B.; Connolly, J.P.R.; Belkin, S. The highly conserved Escherichia coli transcription factor YhaJ regulates aromatic compound degradation. Front. Microbiol. 2016, 7, 1490. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Shemer, B.; Simanowitz, S.; Kabessa, Y.; Mizrachi, Y.; Gold, A.; Shpigel, E.; Agranat, A.J.; Belkin, S. Enhancing DNT detection by a bacterial bioreporter: Directed evolution of the transcriptional activator YhaJ. Front. Bioeng. Biotechnol. 2022, 10, 821835. [Google Scholar] [CrossRef]
- David, L.; Shpigel, E.; Levin, I.; Moshe, S.; Zimmerman, L.; Dadon-Simanowitz, S.; Shemer, B.; Levkovich, S.A.; Larush, L.; Magdassi, S.; et al. Performance upgrade of a microbial explosives’ sensor strain by screening a high throughput saturation library of a transcriptional regulator. Comput. Struct. Biotechnol. J. 2023, 21, 4252–4260. [Google Scholar] [CrossRef]
- Yagur-Kroll, S.; Amiel, E.; Rosen, R.; Belkin, S. Detection of 2,4-Dinitrotoluene and 2,4,6-Trinitrotoluene by an Escherichia coli bioreporter: Performance enhancement by directed evolution. Appl. Microbiol. Biotechnol. 2015, 99, 7177–7188. [Google Scholar] [CrossRef]
- Shpigel, E.; Shemer, B.; Elad, T.; Glozman, A.; Belkin, S. Bacterial bioreporters for the detection of trace explosives: Performance enhancement by DNA shuffling and random mutagenesis. Appl. Microbiol. Biotechnol. 2021, 105, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, E.; Nathansohn, S.; Glozman, A.; Rosen, R.; Shemer, B.; Yagur-Kroll, S.; Elad, T.; Belkin, S. Introduction of quorum sensing elements into bacterial bioreporter circuits enhances explosives’ detection capabilities. Eng. Life Sci. 2022, 22, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Yagur-Kroll, S.; Bilic, B.; Belkin, S. Strategies for enhancing bioluminescent bacterial sensor performance by promoter region manipulation. Microb. Biotechnol. 2010, 3, 300–310. [Google Scholar] [CrossRef] [PubMed]
- International Union of Pure and Applied Chemistry (IUPAC). Glossary for Chemists of Terms Used in Biotechnology. 1992. Available online: https://goldbook.iupac.org/terms/view/B00663 (accessed on 6 February 2025).
- Ben-Yoav, H.; Melamed, S.; Freeman, A.; Shacham-Diamand, Y.; Belkin, S. Whole-cell biochips for bio-sensing: Integration of live cells and inanimate surfaces. Crit. Rev. Biotechnol. 2011, 31, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-Y.; Belkin, S. Miniaturized bioluminescent whole-cell sensor systems. Curr. Opin. Biotechnol. 2023, 82, 102952. [Google Scholar] [CrossRef]
- Melamed, S.; Ceriotti, L.; Weigel, W.; Rossi, F.; Colpo, P.; Belkin, S. A printed nanolitre-scale bacterial sensor array. Lab Chip 2011, 11, 139–146. [Google Scholar] [CrossRef]
- Yagur-Kroll, S.; Schreuder, E.; Ingham, C.J.; Heideman, R.; Rosen, R.; Belkin, S. A miniature porous aluminum oxide-based flow-cell for online water quality monitoring using bacterial sensor cells. Biosens. Bioelectron. 2015, 64, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Almog, R.; Yagur-Kroll, S.; Levkov, K.; Melamed, S.; Shacham-Diamand, Y.; Belkin, S. Online monitoring of water toxicity by use of bioluminescent reporter bacterial biochips. Environ. Sci. Technol. 2011, 45, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Agranat, A.J.; Kabessa, Y.; Shemer, B.; Shpigel, E.; Schwartsglass, O.; Atamneh, L.; Uziel, Y.; Ejzenberg, M.; Mizrachi, Y.; Garcia, Y.; et al. An autonomous bioluminescent bacterial biosensor module for outdoor sensor networks, and its application for the detection of buried explosives. Biosens. Bioelectron. 2021, 185, 113253. [Google Scholar] [CrossRef]
- Tsai, H.; Tsai, Y.; Yagur-Kroll, S.; Palevsky, N.; Belkin, S.; Cheng, J.-Y. Water pollutant monitoring by a whole cell array through lens-free detection on CCD. Lab Chip 2015, 15, 1472–1480. [Google Scholar] [CrossRef]
- Kao, W.-C.; Belkin, S.; Cheng, J.-Y. Microbial biosensing of ciprofloxacin residues in food by a portable lens-free CCD-based analyzer. Anal. Bioanal. Chem. 2018, 410, 1257–1263. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Kao, W.-C.; Belkin, S.; Cheng, J.-Y. A smartphone-based whole-cell array sensor for detection of antibiotics in milk. Sensors 2019, 19, 3882. [Google Scholar] [CrossRef]
- Polyak, B.; Bassis, E.; Novodvorets, A.; Belkin, S.; Marks, R.S. Optical fiber bioluminescent whole-cell microbial biosensors to genotoxicants. Water Sci. Technol. 2000, 42, 305–311. [Google Scholar] [CrossRef]
- Polyak, B.; Bassis, E.; Novodvorets, A.; Belkin, S.; Marks, R.S. Bioluminescent whole cell optical fiber sensor to genotoxicants: System optimization. Sens. Actuators B Chem. 2001, 74, 18–26. [Google Scholar] [CrossRef]
- Lotan, O.; Bar-David, J.; Smith, C.L.C.; Yagur-Kroll, S.; Belkin, S.; Kristensen, A.; Levy, U. Nanoscale plasmonic V-groove waveguides for the interrogation of single fluorescent bacterial cells. Nano Lett. 2017, 17, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Prante, M.; Ude, C.; Große, M.; Raddatz, L.; Krings, U.; John, G.; Belkin, S.; Scheper, T. A portable biosensor for 2,4-dinitrotoluene vapors. Sensors 2018, 18, 4247. [Google Scholar] [CrossRef] [PubMed]
- Rajan Premkumar, J.; Lev, O.; Rosen, R.; Belkin, S. Encapsulation of luminous recombinant E. coli in sol-gel silicate films. Adv. Mater. 2001, 13, 1773–1775. [Google Scholar] [CrossRef]
- Rajan Premkumar, J.; Rosen, R.; Belkin, S.; Lev, O. Sol-gel luminescence biosensors: Encapsulation of recombinant E. coli reporters in thick silicate films. Anal. Chim. Acta 2002, 462, 11–23. [Google Scholar] [CrossRef]
- Rajan Premkumar, J.; Sagi, E.; Rozen, R.; Belkin, S.; Modestov, A.D.; Lev, O. Fluorescent bacteria encapsulated in sol-gel derived silicate films. Chem. Mater. 2002, 14, 2676–2686. [Google Scholar] [CrossRef]
- Tessema, D.A.; Rosen, R.; Pedazur, R.; Belkin, S.; Gun, J.; Ekeltchik, I.; Lev, O. Freeze-drying of sol–gel encapsulated recombinant bioluminescent E. coli by using lyo-protectants. Sens. Actuators B Chem. 2006, 113, 768–773. [Google Scholar] [CrossRef]
- Pedahzur, R.; Rosen, R.; Belkin, S. Stabilization of recombinant bioluminescent bacteria for biosensor applications. Cell Preserv. Technol. 2004, 2, 260–269. [Google Scholar] [CrossRef]
- Bjerketorp, J.; Håkansson, S.; Belkin, S.; Jansson, J.K. Advances in preservation methods: Keeping biosensor microorganisms alive and active. Curr. Opin. Biotechnol. 2006, 17, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.; Belkin, S.; Ulitzur, S.; Abeliovich, A. Fast assessment of toxicants’ adsorption on activated carbon using a luminous bacteria bioassay. Water Sci. Technol. 1993, 27, 113–120. [Google Scholar] [CrossRef]
- Belkin, S.; Stieber, M.; Tiehm, A.; Frimmel, F.H.; Abeliovich, A.; Werner, P.; Ulitzur, S. Toxicity and genotoxicity enhancement during polycyclic aromatic hydrocarbons’ biodegradation. Environ. Toxicol. Water Qual. 1994, 9, 303–309. [Google Scholar] [CrossRef]
- Brenner, A.; Belkin, S.; Ulitzur, S.; Abeliovich, A. Utilization of a bioluminescence toxicity assay for optimal design of biological and physico-chemical wastewater treatment processes. Environ. Toxicol. Water Qual. 1994, 9, 311–316. [Google Scholar] [CrossRef]
- Belkin, S.; Van Dyk, T.K.; Vollmer, A.C.; Smulski, D.R.; LaRossa, R.A. Monitoring subtoxic environmental hazards by stress-responsive luminous bacteria. Environ. Toxicol. Water Qual. 1996, 11, 179–185. [Google Scholar] [CrossRef]
- Belkin, S.; Smulski, D.R.; Dadon, S.; Vollmer, A.C.; Van Dyk, T.K.; LaRossa, R.A. A panel of stress-responsive luminous bacteria for the detection of selected classes of toxicants. Water Res. 1997, 31, 3009–3016. [Google Scholar] [CrossRef]
- Pedahzur, R.; Polyak, B.; Marks, R.S.; Belkin, S. Water toxicity detection by a panel of stress responsive luminescent bacteria. J. Appl. Toxicol. 2004, 24, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Woutersen, M.; Belkin, S.; Brouwer, B.; van Wezel, A.P.; Heringa, M.B. Are luminescent bacteria suitable for online detection and monitoring of toxic compounds in drinking water and its sources? Anal. Bioanal. Chem. 2011, 400, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-T.; Cajal, Y.; Skowronska, E.M.; Belkin, S.; Chen, J.; Van Dyk, T.K.; Sasser, M.; Jain, M.K. Cationic peptide antimicrobials induce selective transcription of micF and osmY in Escherichia coli. Biochim. Biophys. Acta Biomembr. 2000, 1463, 43–54. [Google Scholar] [CrossRef]
- Dukan, S.; Dadon, S.; Smulski, D.R.; Belkin, S. Hypochlorous acid activates the heat shock and soxRS systems of Escherichia coli. Appl. Environ. Microbiol. 1996, 62, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Bechor, O.; Smulski, D.R.; Van Dyk, T.K.; LaRossa, R.A.; Belkin, S. Recombinant microorganisms as environmental biosensors: Pollutant detection by Escherichia coli bearing fabA’::lux fusions. J. Biotechnol. 2002, 94, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, T.K.; Majarian, W.R.; Konstantinov, K.B.; Young, R.M.; Dhurjati, P.S.; LaRossa, R.A. Rapid and sensitive pollutant detection by induction of heat shock gene-bioluminescence gene fusions. Appl. Environ. Microbiol. 1994, 60, 1414–1420. [Google Scholar] [CrossRef]
- Ben-Israel, O.; Ben-Israel, H.; Ulitzur, S. Identification and quantification of toxic chemicals by use of Escherichia coli carrying lux genes fused to stress promoters. Appl. Environ. Microbiol. 1998, 64, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Benovich, E.; Magrisso, S.; Belkin, S. Toxicant identification by a luminescent bacterial bioreporter panel: Application of pattern classification algorithms. Environ. Sci. Technol. 2008, 42, 8486–8491. [Google Scholar] [CrossRef]
- Melamed, S.; Lalush, C.; Elad, T.; Yagur-Kroll, S.; Belkin, S.; Pedahzur, R. A bacterial reporter panel for the detection and classification of antibiotic substances. Microb. Biotechnol. 2012, 5, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Melamed, S.; Naftaly, S.; Belkin, S. Improved detection of antibiotic compounds by bacterial reporter strains achieved by manipulations of membrane permeability and efflux capacity. Appl. Microbiol. Biotechnol. 2014, 98, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Belkin, S. Broad spectrum detection and “barcoding” of water pollutants by a genome-wide bacterial sensor array. Water Res. 2013, 47, 3782–3790. [Google Scholar] [CrossRef]
- Elad, T.; Seo, H.B.; Belkin, S.; Gu, M.B. High-throughput prescreening of pharmaceuticals using a genome-wide bacterial bioreporter array. Biosens. Bioelectron. 2015, 68, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.-Y.; Marín-Benavides, R.; Belkin, S.; Cheng, J.-Y. Rapid printing of a bacterial array for a solid-phase assay (BacSPA) of heavy metal ions. Sens. Actuators B Chem. 2022, 359, 131540. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wei, C.-D.; Belkin, S.; Hsieh, T.-H.; Cheng, J.-Y. Machine-learning assisted antibiotic detection and categorization using a bacterial sensor array. Sens. Actuators B Chem. 2021, 355, 131257. [Google Scholar] [CrossRef]
- Elad, T.; Lee, J.H.; Belkin, S.; Gu, M.B. Microbial whole-cell arrays. Microb. Biotechnol. 2008, 1, 137–148. [Google Scholar] [CrossRef]
- Melamed, S.; Elad, T.; Belkin, S. Microbial sensor cell arrays. Curr. Opin. Biotechnol. 2012, 23, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Magrisso, S.; Erel, Y.; Belkin, S. Microbial reporters of metal bioavailability. Microb. Biotechnol. 2008, 1, 320–330. [Google Scholar] [CrossRef]
- Magrisso, S.; Belkin, S.; Erel, Y. Lead Bioavailability in soil and soil components. Water Air Soil Pollut. 2009, 202, 315–323. [Google Scholar] [CrossRef]
- Tauber, M.; Rosen, R.; Belkin, S. Whole-cell biodetection of halogenated organic acids. Talanta 2001, 55, 959–964. [Google Scholar] [CrossRef]
- Schweigert, N.; Belkin, S.; Leong-Morgenthaler, P.; Zehnder, A.J.B.; Eggen, R.I.L. Combinations of chlorocatechols and heavy metals cause DNA degradation in vitro but must not result in increased mutation rates in vivo. Environ. Mol. Mutagen. 1999, 33, 202–210. [Google Scholar] [CrossRef]
- Rozen, Y.; Nejidatl, A.; Gartemann, K.H.; Belkin, S. Specific detection of p-chlorobenzoic acid by Escherichia coli bearing a plasmid-borne fcbA’::lux fusion. Chemosphere 1999, 38, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Bachmann, T.T.; Schmitt, J.; Belkin, S.; Schmid, R.D. Detection of 4-chlorobenzoate using immobilized recombinant Escherichia coli reporter strains. Sens. Actuators B Chem. 2000, 70, 139–144. [Google Scholar] [CrossRef]
- Lifshitz, A.; Shemer, B.; Hazan, C.; Shpigel, E.; Belkin, S. A bacterial bioreporter for the detection of 1,3,5-trinitro-1,3,5-triazinane (RDX). Anal. Bioanal. Chem. 2022, 414, 5329–5336. [Google Scholar] [CrossRef]
- Grimm, A.-K.; Rozanes, D.; Shpigel, E.; Moscovici, L.; Belkin, S. A microbial cocaine bioreporter. Sensors 2024, 24, 6549. [Google Scholar] [CrossRef] [PubMed]
- Burlage, R.S.; Patek, D.R.; Everman, K.R. Method for Detection of Buried Explosives Using a Biosensor. U.S. Patent US005972638A, 26 October 1999. [Google Scholar]
- Meurer, H. Aerial-Supported Procedure for the Detection of Landmines. U.S. Patent USOO7673551B2, 9 March 2010. [Google Scholar]
- Belkin, S.; Yagur-Kroll, S.; Kabessa, Y.; Korouma, V.; Septon, T.; Anati, Y.; Zohar-Perez, C.; Rabinovitz, Z.; Nussinovitch, A.; Agranat, A.J. Remote detection of buried landmines using a bacterial sensor. Nat. Biotechnol. 2017, 35, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Shemer, B.; Yagur-Kroll, S.; Hazan, C.; Belkin, S. Aerobic transformation of 2,4-dinitrotoluene by Escherichia coli and its implications for the detection of trace explosives. Appl. Environ. Microbiol. 2018, 84, e01729-17. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, L.; Riegraf, C.; Abu-Rmailah, N.; Atias, H.; Shakibai, D.; Buchinger, S.; Reifferscheid, G.; Belkin, S. Yeast-based fluorescent sensors for the simultaneous detection of estrogenic and androgenic compounds, coupled with high-performance thin layer chromatography. Biosensors 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Riegraf, C.; Reifferscheid, G.; Moscovici, L.; Shakibai, D.; Hollert, H.; Belkin, S.; Buchinger, S. Coupling high-performance thin-layer chromatography with a battery of cell-based assays reveals bioactive components in wastewater and landfill leachates. Ecotoxicol. Environ. Saf. 2021, 214, 112092. [Google Scholar] [CrossRef]
- Shakibai, D.; Riegraf, C.; Moscovici, L.; Reifferscheid, G.; Buchinger, S.; Belkin, S. Coupling high-performance thin-layer chromatography with bacterial genotoxicity bioreporters. Environ. Sci. Technol. 2019, 53, 6410–6419. [Google Scholar] [CrossRef]
- Rozen, Y.; Belkin, S. Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 2001, 25, 513–529. [Google Scholar] [CrossRef]
- Rozen, Y.; Van Dyk, T.K.; LaRossa, R.A.; Belkin, S. Seawater activation of Escherichia coli gene promoter elements: Dominance of rpoS control. Microb. Ecol. 2001, 42, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Rozen, Y.; LaRossa, R.A.; Templeton, L.J.; Smulski, D.R.; Belkin, S. Gene expression analysis of the response by Escherichia coli to seawater. Antonie Leeuwenhoek 2002, 81, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.; Franchini, A.; Egli, T.; Belkin, S. Induction of the yjbEFGH operon is regulated by growth rate and oxygen concentration. Arch. Microbiol. 2008, 189, 219–226. [Google Scholar] [CrossRef]
- Ionescu, M.; Belkin, S. Overproduction of exopolysaccharides by an Escherichia coli K12 rpoS mutant in response to osmotic stress. Appl. Environ. Microbiol. 2009, 75, 483–492. [Google Scholar] [CrossRef]
- Ionescu, M.; Elgrably-Weiss, M.; Elad, T.; Rasouly, A.; Yagur-kroll, S.; Belkin, S. Negative regulation of σ70-driven promoters by σ70. Res. Microbiol. 2011, 162, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Harush, A.; Hadas, O.; Post, A.F.; Belkin, S. A Synechococcus PglnA::luxAB fusion for estimation of nitrogen bioavailability to freshwater cyanobacteria. Appl. Environ. Microbiol. 2003, 69, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Schreiter, P.P.-Y.; Gillor, O.; Post, A.F.; Belkin, S.; Schmid, R.D.; Bachmann, T.T. Monitoring of phosphorus bioavailability in water by an immobilized luminescent cyanobacterial reporter strain. Biosens. Bioelectron. 2001, 16, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Post, A.F.; Hadas, A.; Belkin, S.X. Phosphorous bioavailability monitoring by a bioluminescent cyanobacterial sensor strain. J. Phycol. 2001, 38, 107–115. [Google Scholar] [CrossRef]
- Gillor, O.; Hadas, O.; Post, A.F.; Belkin, S. Phosphorus and nitrogen in a monomictic freshwater lake: Employing cyanobacterial bioreporters to gain new insights into nutrient bioavailability. Freshw. Biol. 2010, 55, 1182–1190. [Google Scholar] [CrossRef]
- Senevirathna, W.; Kiro, R.; Rosen, R.; Popov, I.; Belkin, S.; Wells, M. CdSe quantum dots induce superoxide stress in engineered biosensor bacteria. Nanotoxicology 2009, 3, 98–108. [Google Scholar] [CrossRef]
- Miché, L.; Belkin, S.; Rozen, R.; Balandreau, J. Rice seedling whole exudates and extracted alkylresorcinols induce stress response in Escherichia coli biosensors. Environ. Microbiol. 2003, 5, 403–411. [Google Scholar] [CrossRef]
- Rosen, R.; Davidov, Y.; LaRossa, R.A.; Belkin, S. Microbial sensors of ultraviolet radiation based on recA’::lux fusions. Appl. Biochem. Biotechnol. 2000, 89, 151–160. [Google Scholar] [CrossRef]
- Gerchman, Y.; Cohen-Yaniv, V.; Betzalel, Y.; Yagur-Kroll, S.; Belkin, S.; Mamane, H. The involvement of superoxide radicals in medium pressure UV derived inactivation. Water Res. 2019, 161, 119–125. [Google Scholar] [CrossRef]
- Kessler, N.; Schauer, J.; Yagur-Kroll, S.; Melamed, S.; Tirosh, O.; Belkin, S.; Erel, Y. A bacterial bioreporter panel to assay the cytotoxicity of atmospheric particulate matter. Atmos. Environ. 2012, 63, 94–101. [Google Scholar] [CrossRef]
- Rosen, R.; Buchinger, S.; Pfänder, R.; Pedhazur, R.; Reifferscheid, G.; Belkin, S. SOS gene induction and possible mutagenic effects of freeze-drying in Escherichia coli and Salmonella typhimurium. Appl. Microbiol. Biotechnol. 2016, 100, 9255–9264. [Google Scholar] [CrossRef]
- Dukan, S.; Belkin, S.; Touati, D. Reactive oxygen species are partially involved in the bacteriocidal action of hypochlorous acid. Arch. Biochem. Biophys. 1999, 367, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Capin, J.; Chabert, E.; Zuñiga, A.; Bonnet, J. Microbial biosensors for diagnostics, surveillance and epidemiology: Today’s achievements and tomorrow’s prospects. Microb. Biotechnol. 2024, 17, e70047. [Google Scholar] [CrossRef] [PubMed]
- Moraskie, M.; Roshid, M.H.O.; O’Connor, G.; Dikici, E.; Zingg, J.-M.; Deo, S.; Daunert, S. Microbial whole-cell biosensors: Current applications, challenges, and future perspectives. Biosens. Bioelectron. 2021, 191, 113359. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.S.G.; Acosta, M.F.M.; Sánchez, J.A.M.; Vázquez, L.E.C. Principles and challenges of whole cell microbial biosensors in the food industry. J. Food Sci. 2024, 89, 5255–5269. [Google Scholar] [CrossRef]
- Tanna, T.; Ramachanderan, R.; Platt, R.J. Engineered bacteria to report gut function: Technologies and implementation. Curr. Opin. Microbiol. 2021, 59, 24–33. [Google Scholar] [CrossRef]
- Chemla, Y.; Sweeney, C.J.; Wozniak, C.A.; Voigt, C.A. Engineering bacteria for environmental release: Regulatory challenges and design strategies. Authorea 2024, preprint. [Google Scholar] [CrossRef]
- Elad, T.; Belkin, S. Reporter gene assays in ecotoxicology. In In Vitro Environmental Toxicology—Concepts, Application and Assessment; Reifferscheid, G., Buchinger, S., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2017; Volume 157, pp. 135–157. [Google Scholar] [CrossRef]
- Belkin, S.; Wang, B. Sense and sensibility: Of synthetic biology and the redesign of bioreporter circuits (Editorial). Microb. Biotechnol. 2021, 15, 103–106. [Google Scholar] [CrossRef]
- Li, X.; Daniel, R. Synthetic nonlinear computation for genetic circuit design. Curr. Opin. Biotechnol. 2022, 76, 102727. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkin, S. Bioluminescent Microbial Bioreporters: A Personal Perspective. Biosensors 2025, 15, 111. https://doi.org/10.3390/bios15020111
Belkin S. Bioluminescent Microbial Bioreporters: A Personal Perspective. Biosensors. 2025; 15(2):111. https://doi.org/10.3390/bios15020111
Chicago/Turabian StyleBelkin, Shimshon. 2025. "Bioluminescent Microbial Bioreporters: A Personal Perspective" Biosensors 15, no. 2: 111. https://doi.org/10.3390/bios15020111
APA StyleBelkin, S. (2025). Bioluminescent Microbial Bioreporters: A Personal Perspective. Biosensors, 15(2), 111. https://doi.org/10.3390/bios15020111




