Biomass-Derived Carbon Dots as Fluorescent Probes for Label-Free Sensing of Hemin and as Radical Scavengers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Instrumentation
2.3. Preparation of MPCDs
2.4. Quantum Yield (QY) Measurement
2.5. Radical Scavenging Potential of MPCDs
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. Detection of Hemin by MPCDs
2.7. Serum Sample Analysis
3. Results and Discussion
3.1. Synthesis and Optical Characteristics of MPCDs
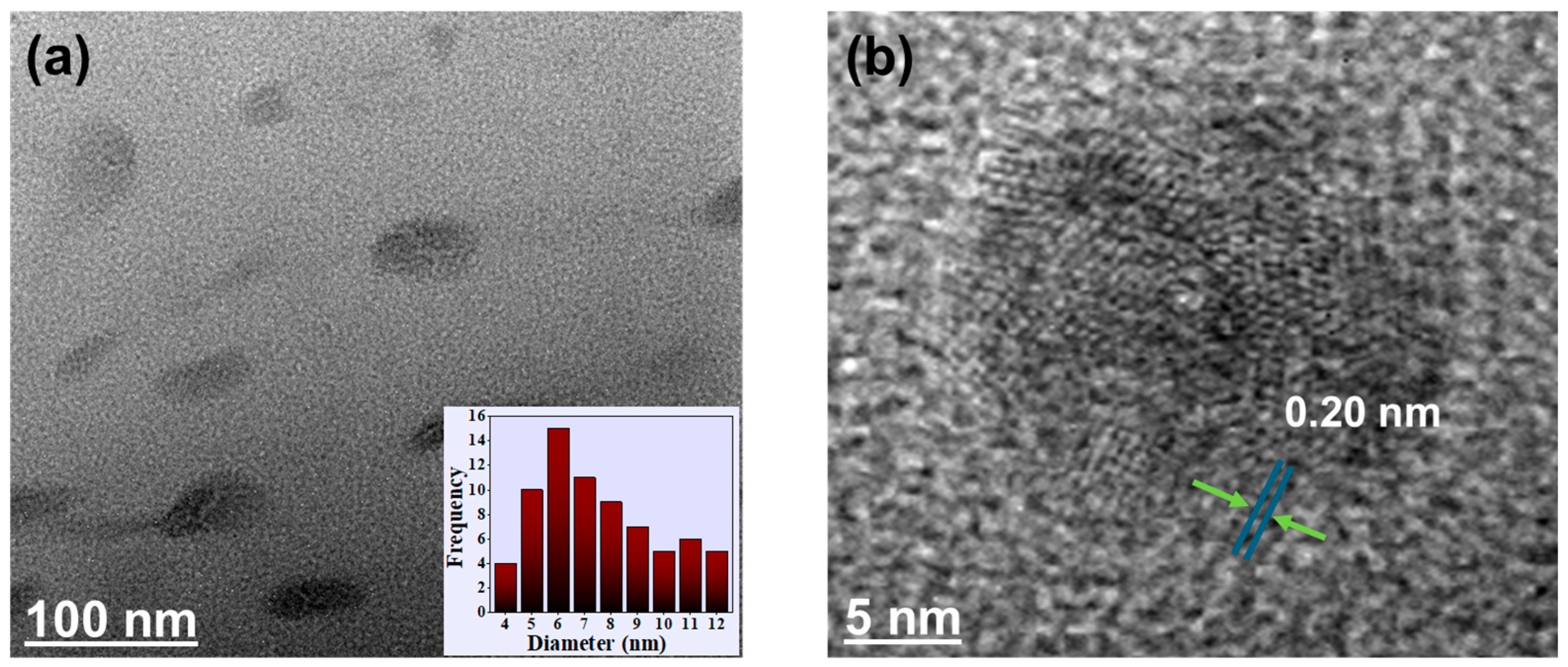
3.2. Morphological Characteristics of MPCDs
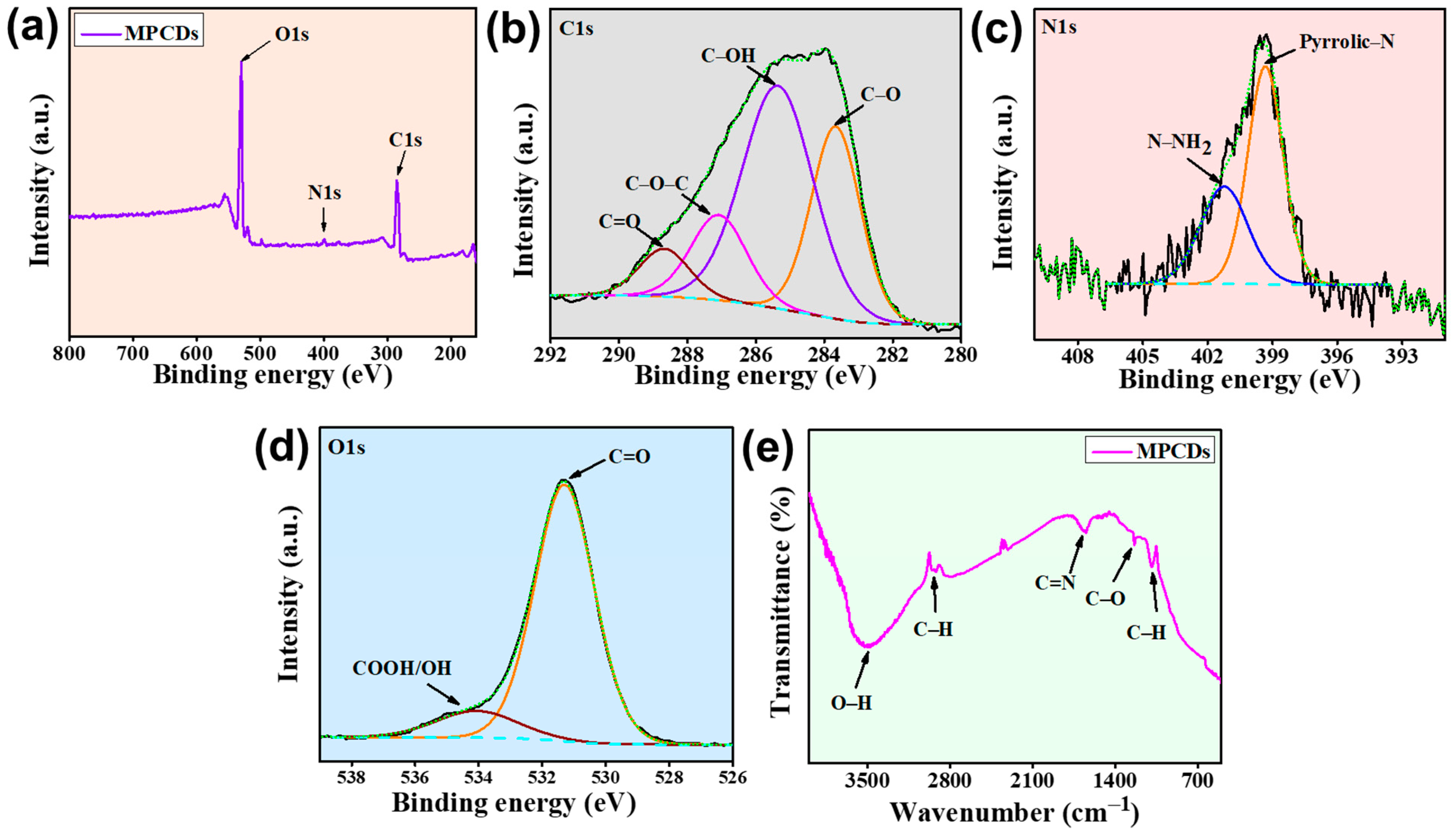
3.3. Elemental Characteristics of MPCDs
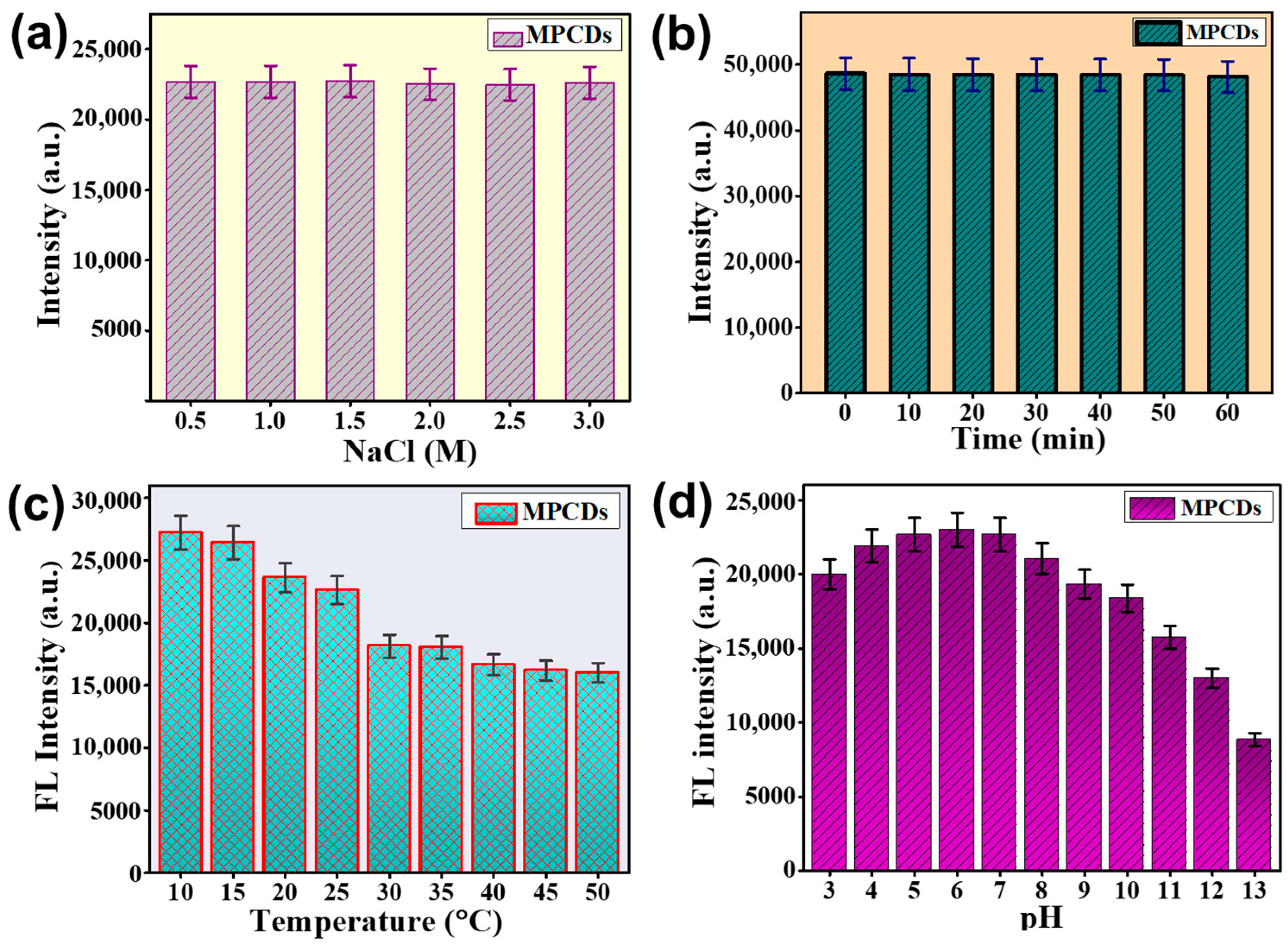
3.4. Stability of MPCDs
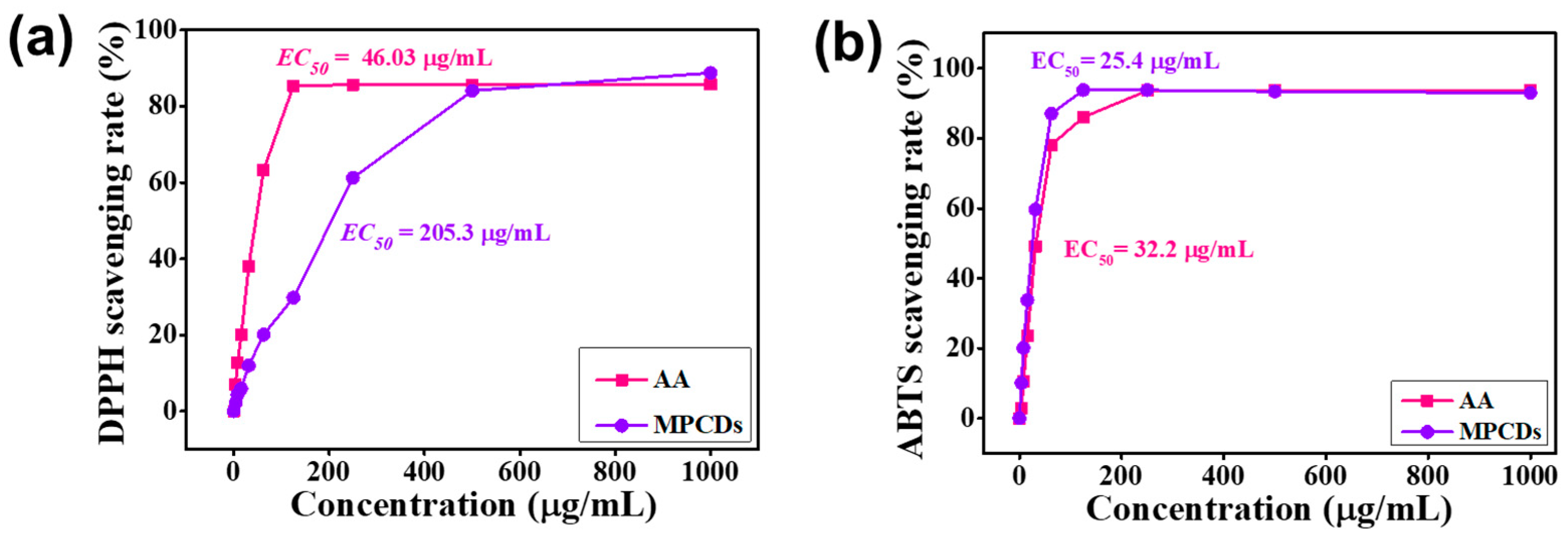
3.5. Antioxidant Activity
3.5.1. DPPH
3.5.2. ABTS
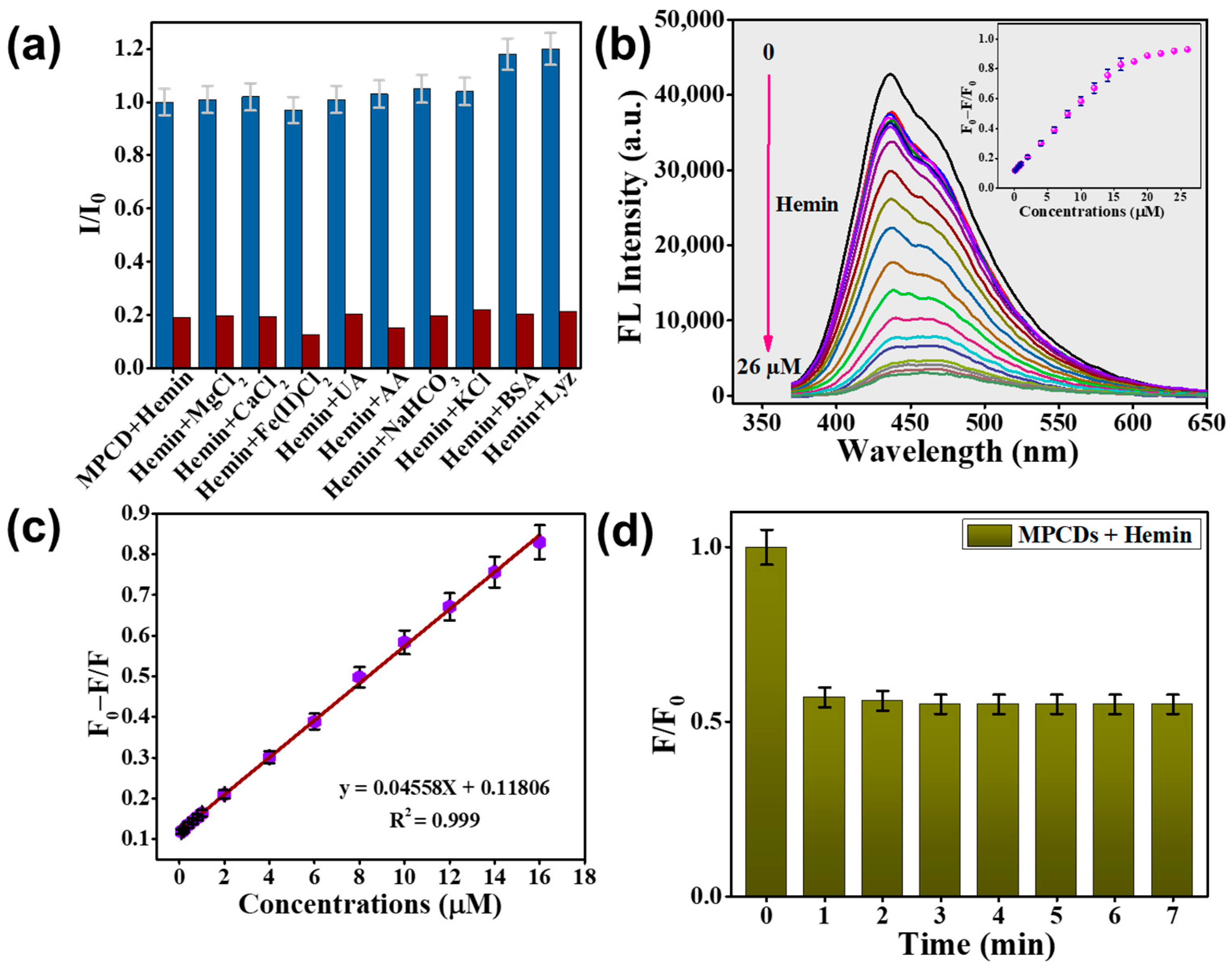
3.6. Hemin Detection by MPCDs
3.7. Possible Mechanisms of Hemin Sensing by MPCDs
3.8. Detection of Hemin in Commercial Serum Samples by MPCDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estarreja, J.; Caldeira, G.; Silva, I.; Mendes, P.; Mateus, V. The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: Protocol for a Systematic Review. JMIR Res. Protoc. 2023, 12, e48368. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Yao, D.; Chen, C.; Zhang, C. An efficient human serum albumin-assisted fluorescence approach for hemin detection. Environ. Technol. Innov. 2023, 29, 102969. [Google Scholar] [CrossRef]
- Napoli, E.; Morin, D.; Bernhardt, R.; Buckpitt, A.; Cortopassi, G. Hemin rescues adrenodoxin, heme a and cytochrome oxidase activity in frataxin-deficient oligodendroglioma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 773–780. [Google Scholar] [CrossRef]
- Gao, Y.-T.; Chang, S.; Chen, B.-B.; Li, D.-W. Dual-Exciting Central Carbon Nanoclusters for the Dual-Channel Detection of Hemin. Inorganics 2023, 11, 226. [Google Scholar] [CrossRef]
- Desai, C. Meyler’s side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. Indian J. Pharmacol. 2016, 48, 224. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Gu, Z.; Pan, H.; Zhou, P.; Gan, Q.; Yuan, Y.; Liu, C. Multifunctional carbon dots for biomedical applications: Diagnosis, therapy, and theranostic. Small 2024, 20, 2303773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; El-Ramady, H.; Prokisch, J. Food safety aspects of carbon dots: A review. Environ. Chem. Lett. 2024, 23, 337–360. [Google Scholar] [CrossRef]
- Liu, C.; Lin, X.; Liao, J.; Yang, M.; Jiang, M.; Huang, Y.; Du, Z.; Chen, L.; Fan, S.; Huang, Q. Carbon dots-based dopamine sensors: Recent advances and challenges. Chin. Chem. Lett. 2024, 35, 109598. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Gong, X. The Emerging Star of Carbon Luminescent Materials: Exploring the Mysteries of the Nanolight of Carbon Dots for Optoelectronic Applications. Small 2024, 20, 2400107. [Google Scholar] [CrossRef]
- Wang, L.; Gu, C.; Wu, L.; Tan, W.; Shang, Z.; Tian, Y.; Ma, J. Recent advances in carbon dots for electrochemical sensing and biosensing: A systematic review. Microchem. J. 2024, 207, 111687. [Google Scholar] [CrossRef]
- Wang, F.; Dong, X.; Zuo, Y.; Xie, Z.; Guan, R. Effectively enhancing red fluorescence strategy and bioimaging applications of carbon dots. Mater. Today Phys. 2024, 41, 101332. [Google Scholar] [CrossRef]
- Li, Q.; Fan, J.; Mu, H.; Chen, L.; Yang, Y.; Yu, S. Nucleus-targeting orange-emissive carbon dots delivery adriamycin for enhanced anti-liver cancer therapy. Chin. Chem. Lett. 2024, 35, 108947. [Google Scholar] [CrossRef]
- Kumar, J.V.; Alam, M.W.; Selvaraj, M.; Almutairi, H.H.; Albuhulayqah, M.; Sadaf, S.; Dhananjaya, M.; Joo, S.W. Fluorescent carbon dots for biodiesel production: A comprehensive review (2019–2024). Inorg. Chem. Commun. 2024, 162, 112247. [Google Scholar] [CrossRef]
- Thara, C.R.; Mathew, B. Microwave synthesized N-doped carbon dots for dual mode detection of Hg (II) ion and degradation of malachite green dye. Talanta 2024, 268, 125278. [Google Scholar] [CrossRef] [PubMed]
- Seesuea, C.; Sansenya, S.; Thangsunan, P.; Wechakorn, K. Green synthesis of elephant manure-derived carbon dots and multifunctional applications: Metal sensing, antioxidant, and rice plant promotion. Sustain. Mater. Technol. 2024, 39, e00786. [Google Scholar] [CrossRef]
- Ju, Y.J.; Li, N.; Liu, S.G.; Liang, J.Y.; Gao, X.; Fan, Y.Z.; Luo, H.Q.; Li, N.B. Proton-controlled synthesis of red-emitting carbon dots and application for hematin detection in human erythrocytes. Anal. Bioanal. Chem. 2019, 411, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huo, Z.; Sun, X.; Shen, J. Facile and green synthesis of N, Cl-dual-doped carbon dots as a label-free fluorescent probe for hematin and temperature sensing. Microchem. J. 2020, 153, 104528. [Google Scholar] [CrossRef]
- Yu, Z.-y.; Duo, S.-w. Facile, ultrafast synthesis of up-and down-conversion fluorescent carbon dots for highly sensitive detection of hematin. Opt. Mater. 2020, 107, 110029. [Google Scholar] [CrossRef]
- Singh, P.; Bhankar, V.; Kumar, S.; Kumar, K. Biomass-derived carbon dots as significant biological tools in the medicinal field: A review. Adv. Colloid Interface Sci. 2024, 328, 103182. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, B.; Qu, X.; Li, S.; Huang, J.; Li, J.; Lu, S.; Zhou, N. State-of-the-art of biomass-derived carbon dots: Preparation, properties, and applications. Chin. Chem. Lett. 2024, 35, 108423. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, B.; Zhuang, Q.; Wang, Y.; Luo, X.; Xie, Y.; Zhou, D. Green synthesis of fluorescent nitrogen–sulfur Co-doped carbon dots from scallion leaves for hemin sensing. Anal. Lett. 2020, 53, 1704–1718. [Google Scholar] [CrossRef]
- Bhatt, S.C.; Kumar, V.; Gupta, A.K.; Mishra, S.; Naik, B.; Rustagi, S.; Preet, M.S. Insights on bio-functional properties of Myrica esculenta plant for nutritional and livelihood security. Food Chem. Adv. 2023, 3, 100434. [Google Scholar] [CrossRef]
- Kabra, A.; Martins, N.; Sharma, R.; Kabra, R.; Baghel, U.S. Myrica esculenta Buch.-Ham. ex D. Don: A natural source for health promotion and disease prevention. Plants 2019, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Khan, S.U.; Mir, S.A.; Iqbal, M.J.; Pottoo, F.H.; Dhiman, N.; Malik, F.; Ali, A. Myrica esculenta Buch.-Ham. (ex D. Don): A review on its phytochemistry, pharmacology and nutritional potential. Comb. Chem. High Throughput Screen. 2022, 25, 2372–2386. [Google Scholar] [PubMed]
- Das, L.; Das, M.; Barkalita, L.M.; Borah, P. Comprehensive Analysis of Antioxidant Properties, GC-MS, and FTIR Profiles of Myrica esculenta Fruit Extracts from Western East Khasi Hills of Meghalaya. Chem. Biodivers. 2024, 21, e202401006. [Google Scholar] [CrossRef] [PubMed]
- Phanjom, P.; Zoremi, E.; Mazumder, J.; Saha, M.; Baruah, S.B. Green synthesis of silver nanoparticles using leaf extract of Myrica esculenta. Int. J. Nanosci. Nanotechnol. 2012, 3, 73–79. [Google Scholar]
- Lal, S.; Verma, R.; Chauhan, A.; Dhatwalia, J.; Guleria, I.; Ghotekar, S.; Thakur, S.; Mansi, K.; Kumar, R.; Kumari, A. Antioxidant, antimicrobial, and photocatalytic activity of green synthesized ZnO-NPs from Myrica esculenta fruits extract. Inorg. Chem. Commun. 2022, 141, 109518. [Google Scholar] [CrossRef]
- Chauhan, A.; Dhatwalia, J.; Dutta, V.; Gopalakrishnan, C.; Rana, G.; Hikku, G.S.; Kumari, A.; Duglet, R.; Ghotekar, S. An investigation of the antimicrobial and antioxidant efficacy of copper oxide (I) nanoparticles: A green approach from Myrica esculenta fruit extract. Chem. Phys. Impact 2023, 7, 100390. [Google Scholar] [CrossRef]
- Shukla, M.K.; Kaushik, H.; Tiwari, H.; Behera, C.; Tonk, R.K.; Husain, A.; Singh, J.; Kesari, K.K.; Kumar, D. Development and characterization of Myrica esculenta plant extract-based albumin nanoparticles for anticancer activity. Ind. Crop. Prod. 2024, 218, 118815. [Google Scholar] [CrossRef]
- Yadav, A.; Raghav, S.; Jangid, N.K.; Srivastava, A.; Jadoun, S.; Srivastava, M.; Dwivedi, J. Myrica esculenta Leaf Extract—Assisted Green Synthesis of Porous Magnetic Chitosan Composites for Fast Removal of Cd (II) from Water: Kinetics and Thermodynamics of Adsorption. Polymers 2023, 15, 4339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Lee, H.-J. Multimodal applications of green carbon dots derived from Potentilla indica (mock strawberry): Antioxidant, antimicrobial, and quenching based quercetin sensor. J. Environ. Chem. Eng. 2024, 12, 114216. [Google Scholar] [CrossRef]
- Sharma, N.; Das, G.S.; Yun, K. Green synthesis of multipurpose carbon quantum dots from red cabbage and estimation of their antioxidant potential and bio-labeling activity. Appl. Microbiol. Biotechnol. 2020, 104, 7187–7200. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Pandey, F.P.; Kumari, P.; Basu, N.; Tiwari, M.K.; Lahiri, J.; Kharwar, R.N.; Parmar, A.S. Label-Free Fluorometric Detection of Adulterant Malachite Green Using Carbon Dots Derived from the Medicinal Plant Source Ocimum tenuiflorum. ChemistrySelect 2019, 4, 4839–4847. [Google Scholar] [CrossRef]
- Paul, A.; Kurian, M. Facile synthesis of nitrogen doped carbon dots from waste biomass: Potential optical and biomedical applications. Clean. Eng. Technol. 2021, 3, 100103. [Google Scholar] [CrossRef]
- Carolan, D.; Rocks, C.; Padmanaban, D.B.; Maguire, P.; Svrcek, V.; Mariotti, D. Environmentally friendly nitrogen-doped carbon quantum dots for next generation solar cells. Sustain. Energy Fuels 2017, 1, 1611–1619. [Google Scholar] [CrossRef]
- Tian, H.; Ju, G.; Li, M.; Fu, W.; Dai, Y.; Liang, Z.; Qiu, Y.; Qin, Z.; Yin, X. Fluorescent “on–off–on” sensor based on N, S co-doped carbon dots from seaweed (Sargassum carpophyllum) for specific detection of Cr (VI) and ascorbic acid. RSC Adv. 2021, 11, 35946–35953. [Google Scholar] [CrossRef]
- Godavarthi, S.; Kumar, K.M.; Vélez, E.V.; Hernandez-Eligio, A.; Mahendhiran, M.; Hernandez-Como, N.; Aleman, M.; Gomez, L.M. Nitrogen doped carbon dots derived from Sargassum fluitans as fluorophore for DNA detection. J. Photochem. Photobiol. B Biol. 2017, 172, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens. Actuators B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Watcharamongkol, T.; Khaopueak, P.; Seesuea, C.; Wechakorn, K. Green hydrothermal synthesis of multifunctional carbon dots from cassava pulps for metal sensing, antioxidant, and mercury detoxification in plants. Carbon Resour. Convers. 2024, 7, 100206. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, A.; Park, M.; Lee, H.-J. Silkworm-derived carbon nano rods (swCNR) for detection of bismuth ions (Bi3+) in aquatic medium and their antiradical properties. Heliyon 2024, 10, e33572. [Google Scholar] [CrossRef]
- Deng, W.-w.; Zang, C.-r.; Li, Q.-c.; Sun, B.; Mei, X.-p.; Bai, L.; Shang, X.-m.; Deng, Y.; Xiao, Y.-q.; Ghiladi, R.A. Hydrothermally derived green carbon dots from broccoli water extracts: Decreased toxicity, enhanced free-radical scavenging, and anti-inflammatory performance. ACS Biomater. Sci. Eng. 2023, 9, 1307–1319. [Google Scholar] [CrossRef]
- Dinç, S.; Tjardts, T.; Maschkowitz, G.; Lukaszczuk, V.; Gharibzahedi, S.M.T.; Altintas, Z. Highly sensitive detection of Campylobacter jejuni using a carbon dot-embedded nanoMIPs fluorescent sensor. Microchem. J. 2024, 208, 112301. [Google Scholar] [CrossRef]
- Mondal, I.; Samanta, D.; Shaw, M.; Shaik, M.A.S.; Mahto, M.K.; Basu, R.; Bhattacharya, A.; Pathak, A. Pyridinic-N-rich carbon dots in IFE-based Turn-off Fluorometric detection of nerve agent Mimic–Diethyl chlorophosphate and multicolor cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 294, 122530. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, X.; Bai, H.; Jia, P.; Zhao, Y.; Liu, Y.; Wang, L.; Zhuang, Y.; Yue, T. Fluorescent detection of tetracycline in foods based on carbon dots derived from natural red beet pigment. LWT 2022, 157, 113100. [Google Scholar] [CrossRef]
- Guo, G.; Li, T.; Zheng, Q.; Tang, S.; Hu, H.; Wang, X.; Chen, D. Dual-excitation carbon dots-based lateral flow visual sensing device for ratiometric determination and discrimination of tetracyclines in food. Sens. Actuators B Chem. 2023, 395, 134523. [Google Scholar] [CrossRef]


| Source | Probe | Method | Linear Range (µM) | LOD (nM) | Year | Reference |
|---|---|---|---|---|---|---|
| o-phenylenediamine | N-doped CDs | Hydrothermal | 0.4–32 | 180 | 2019 | [16] |
| Hexadecylpyridinium chloride | N,Cl-doped CDs | Hydrothermal | 1–32 | 160 | 2020 | [17] |
| Citric acid, magnesium chloride, and ethylenediamine | Mg-CDs-0.1 Mg-CDs-0.5 | Microwave | 0.1–75 0.01–25 | 25 1 | 2020 | [18] |
| Scallion leaves | N,S-doped CDs | Hydrothermal | 0.5–10 | 100 | 2020 | [21] |
| Myrica esculenta fruit | MPCDs | Hydrothermal | 0.1–16 | 14.1 | - | This work |
| Hemin (μM) | Aexc | Aemm | Fobs | Fcor | CF Fcor/Fobs | Fcor,blank/Fcor | Eobs % | Ecor % |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.291 | 0.116 | 34,542 | 53,325 | 1.54 | 1 | 0 | 0 |
| 1 | 0.359 | 0.141 | 31,278 | 53,076 | 1.72 | 1.00 | 9.44 | 0.46 |
| 3 | 0.454 | 0.172 | 26,388 | 50,722 | 1.92 | 1.05 | 23.60 | 4.88 |
| 6 | 0.491 | 0.205 | 22,498 | 46,343 | 2.05 | 1.15 | 34.86 | 13.09 |
| 9 | 0.531 | 0.227 | 19,985 | 43,712 | 2.18 | 1.21 | 42.14 | 18.28 |
| 12 | 0.627 | 0.275 | 15,469 | 38,777 | 2.50 | 1.37 | 55.21 | 27.28 |
| 15 | 0.664 | 0.319 | 12,649 | 34,278 | 2.71 | 1.55 | 63.38 | 35.71 |
| Samples | Spiked (µM) | Found | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1 | 1.07 | 107.3 | 1.5 |
| 2 | 8 | 7.89 | 98.7 | 1.9 |
| 3 | 14 | 14.06 | 100.4 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Lee, H.-J. Biomass-Derived Carbon Dots as Fluorescent Probes for Label-Free Sensing of Hemin and as Radical Scavengers. Biosensors 2025, 15, 105. https://doi.org/10.3390/bios15020105
Sharma N, Lee H-J. Biomass-Derived Carbon Dots as Fluorescent Probes for Label-Free Sensing of Hemin and as Radical Scavengers. Biosensors. 2025; 15(2):105. https://doi.org/10.3390/bios15020105
Chicago/Turabian StyleSharma, Neha, and Hae-Jeung Lee. 2025. "Biomass-Derived Carbon Dots as Fluorescent Probes for Label-Free Sensing of Hemin and as Radical Scavengers" Biosensors 15, no. 2: 105. https://doi.org/10.3390/bios15020105
APA StyleSharma, N., & Lee, H.-J. (2025). Biomass-Derived Carbon Dots as Fluorescent Probes for Label-Free Sensing of Hemin and as Radical Scavengers. Biosensors, 15(2), 105. https://doi.org/10.3390/bios15020105






