The Application of Microfluidics in Traditional Chinese Medicine Research
Abstract
1. Introduction
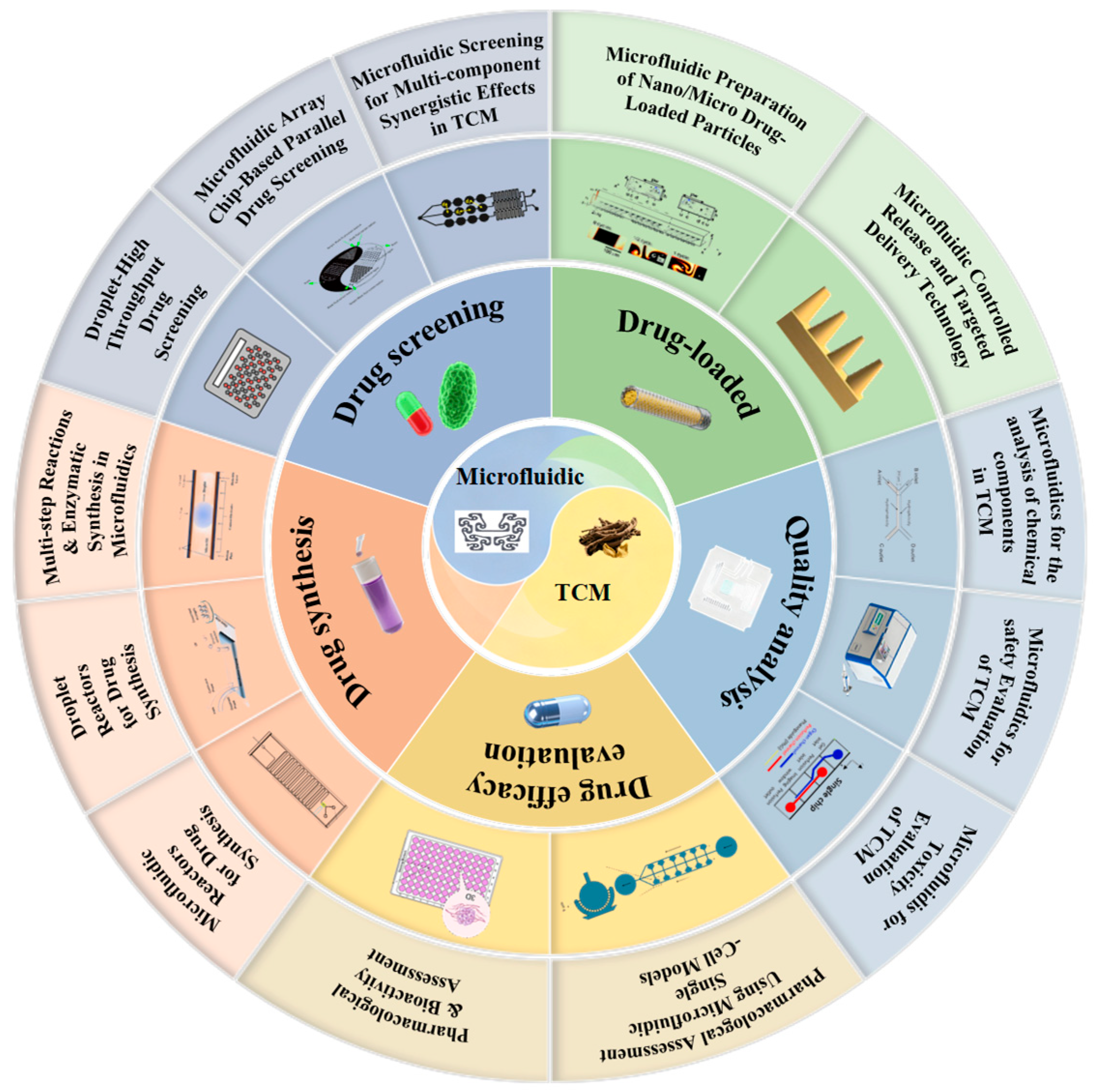
2. Applications of Microfluidic Technology in TCM Research
2.1. Quality Analysis
2.1.1. Microfluidics for the Analysis of Chemical Components in TCM
2.1.2. Microfluidics for Safety Evaluation of TCM
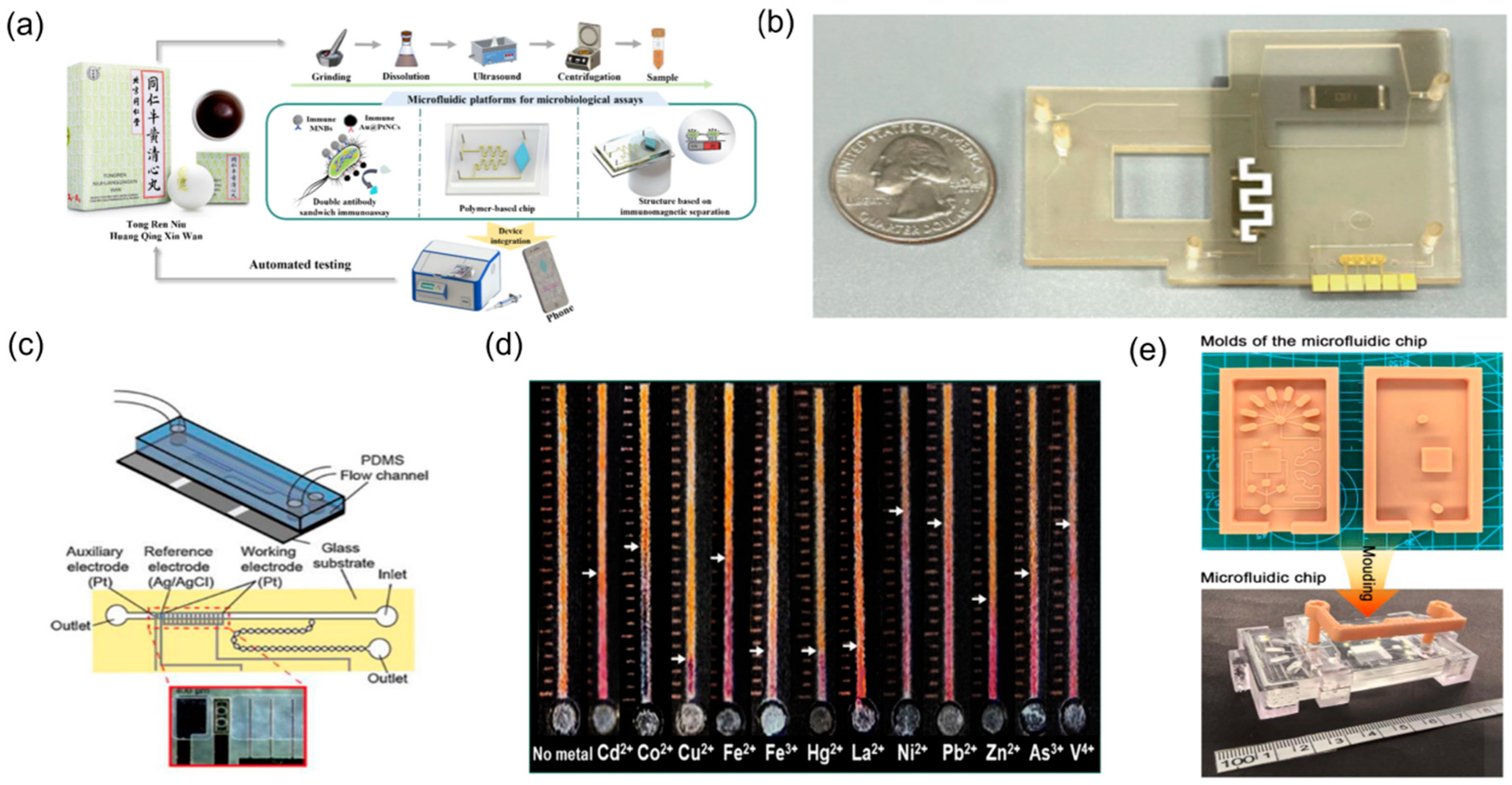
2.1.3. Microfluidics for Toxicity Evaluation of TCM
2.2. Pharmacological Evaluation
2.2.1. Pharmacological Assessment Using Microfluidic Single-Cell Models
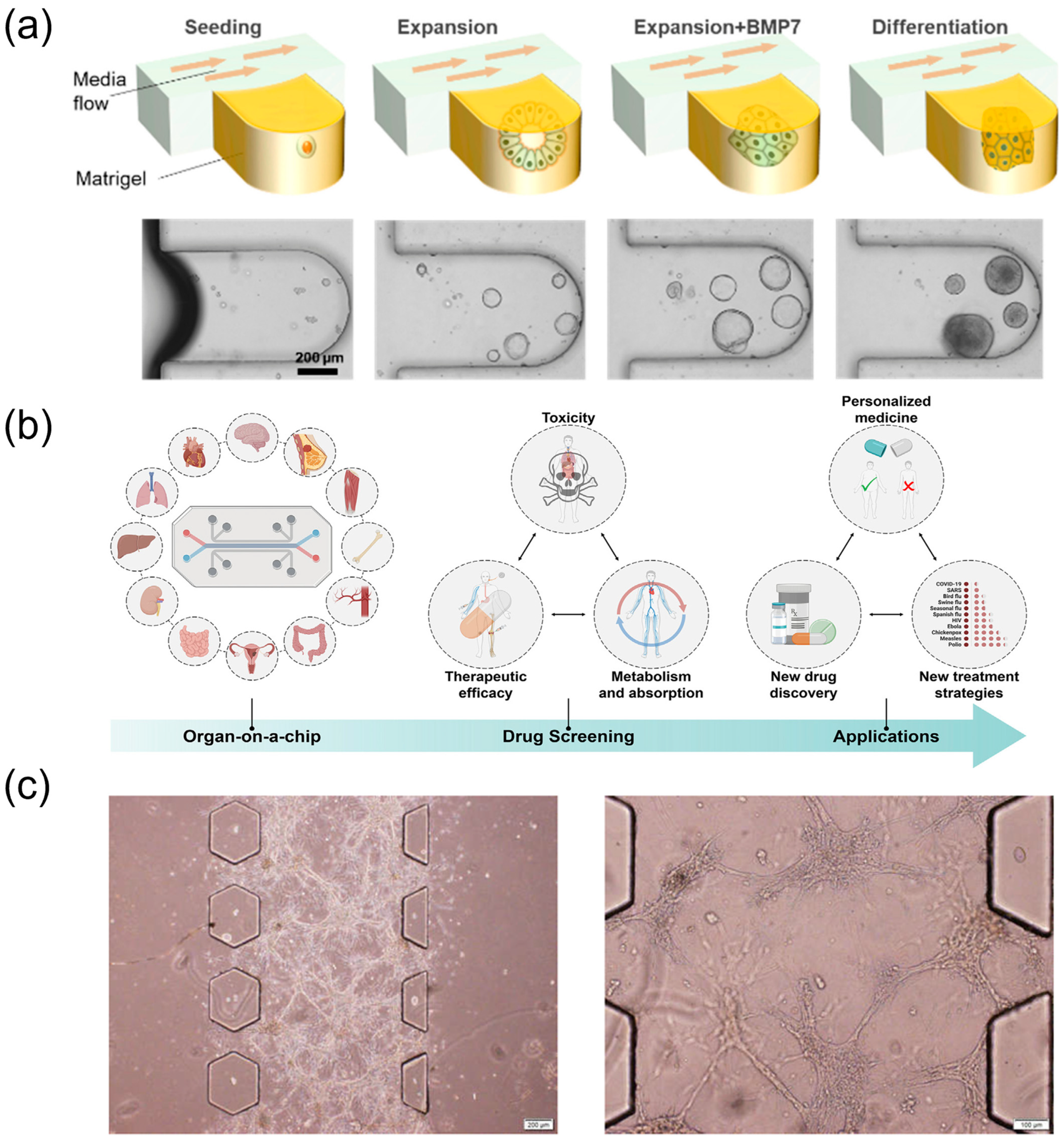
2.2.2. Pharmacological and Bioactivity Assessment Using Microfluidic Organ-on-a-Chip Models
2.3. Drug Synthesis
2.3.1. Microfluidic Reactors for Drug Synthesis
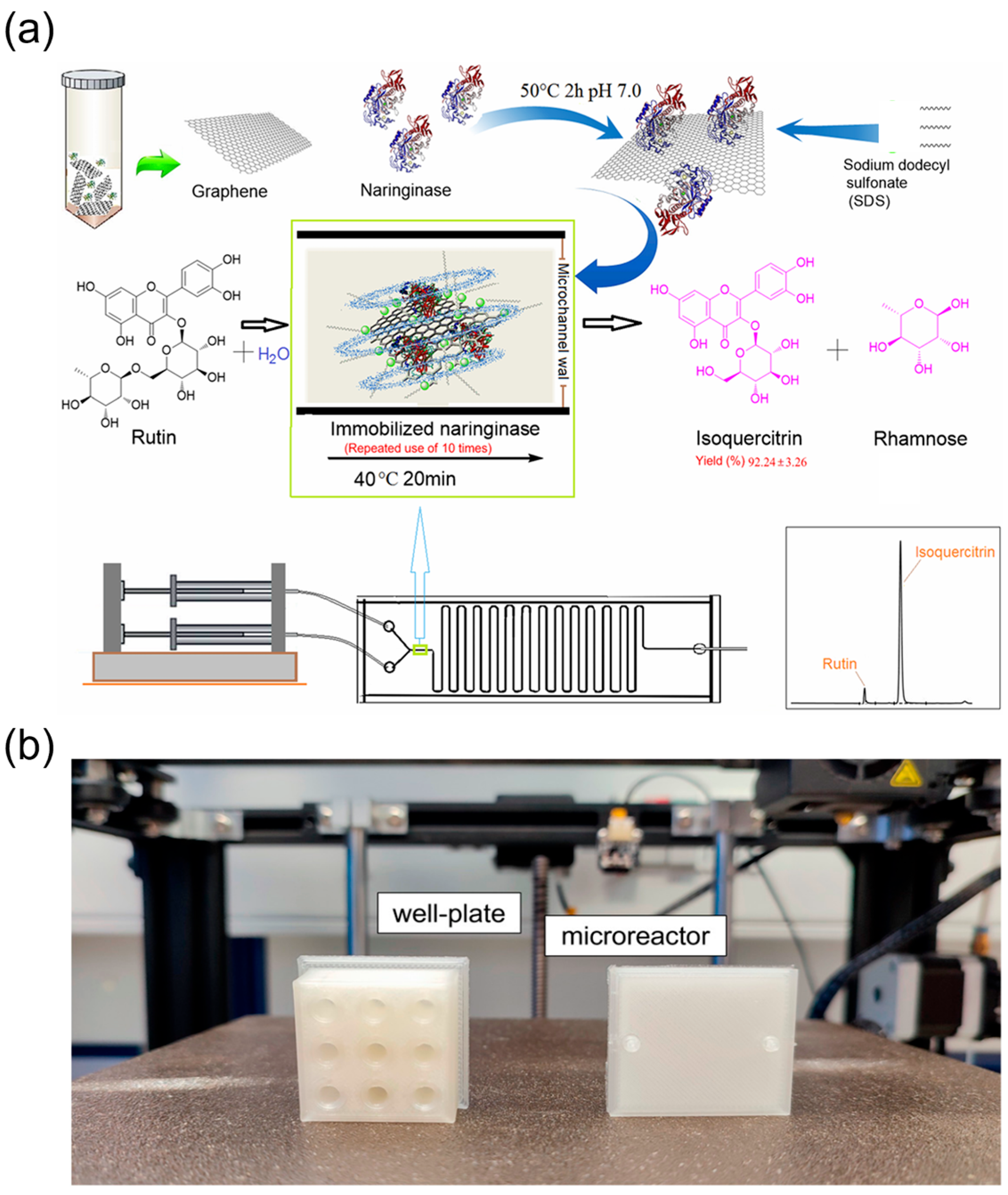
2.3.2. Microdroplet Reactors for Drug Synthesis
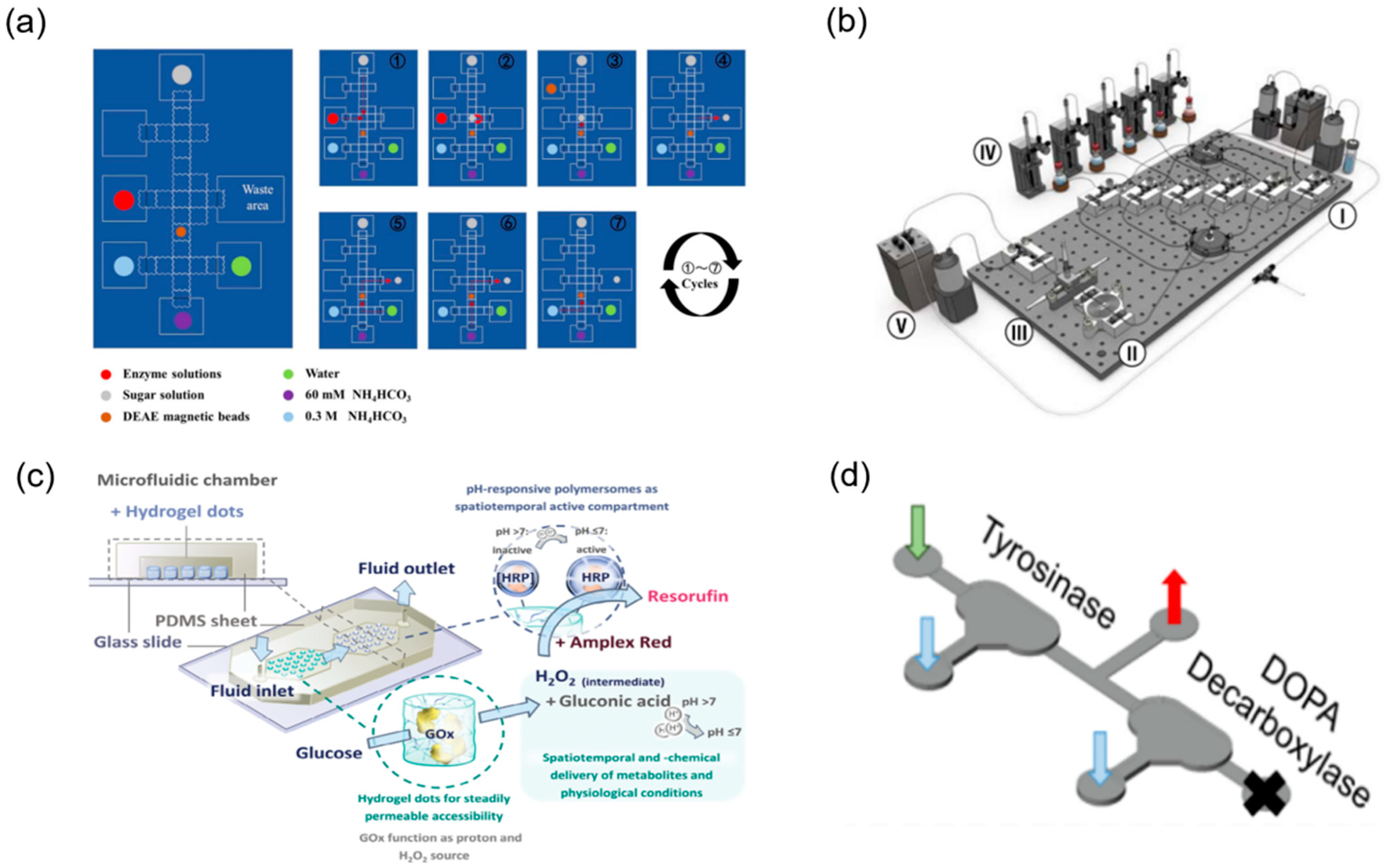
2.3.3. Integration of Multi-Step Reactions and Enzymatic Synthesis in Microfluidics
2.4. Drug Screening
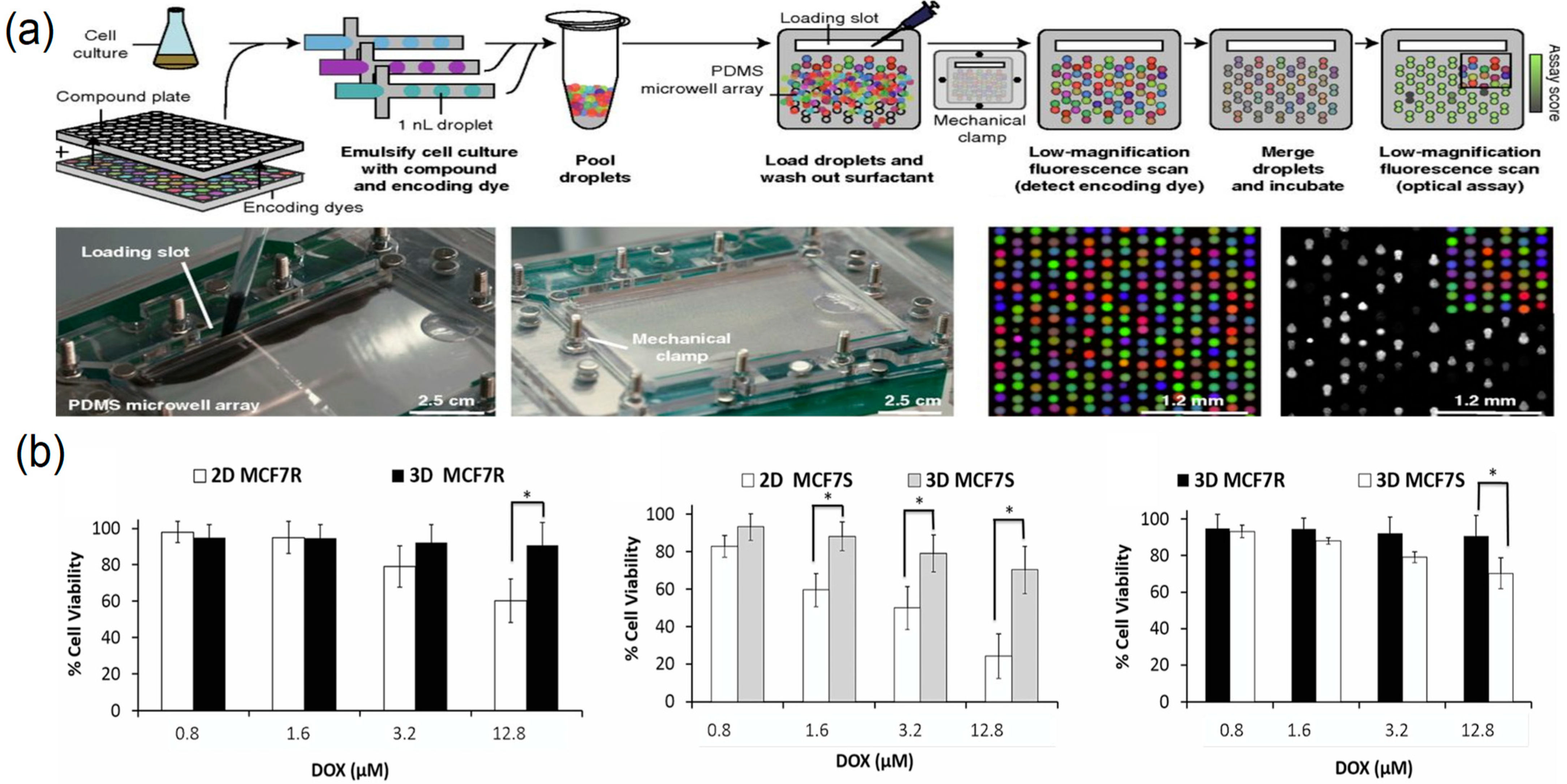
2.4.1. Droplet-Based High-Throughput Drug Screening
2.4.2. Microfluidic Array Chip-Based Parallel Drug Screening
2.4.3. Microfluidic Screening for Multicomponent Synergistic Effects in TCM
2.5. Drug Delivery and Release
2.5.1. Microfluidic Preparation of Nano-/Micro-Drug-Loaded Particles
2.5.2. Microfluidic Controlled Release and Targeted Delivery Technology
3. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- You, Z.; Lu, J.; Xu, Y.; Wang, Y.; Hao, Y. A Review of Traditional Chinese Medicine Syndrome Research on Type 2 Diabetes Mellitus and its Common Chronic Complications. Pharmacogn. Mag. 2024, 20, 712–720. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, M. Holistic View of TCM on Cancer Integrative Therapy. Future Integr. Med. 2023, 2, 159–167. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, W.; Huang, L. Theory and scientificity of traditional Chinese medicine. Sci. Tradit. Chin. Med. 2023, 1, 26–34. [Google Scholar] [CrossRef]
- Chen, A.; Sun, L.; Yuan, H.; Wu, A.; Lu, J.; Ma, S. A holistic strategy for quality and safety control of traditional Chinese medicines by the “iVarious” standard system. J. Pharm. Anal. 2017, 7, 271–279. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Li, G.L. Current Status of Standardization of Traditional Chinese Medicine in China. Evid. Based Complement. Altern. Med. 2016, 2016, 9123103. [Google Scholar] [CrossRef]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, C.G.; Chang, D.; Bensoussan, A. Current Status and Major Challenges to the Safety and Efficacy Presented by Chinese Herbal Medicine. Medicines 2019, 6, 14. [Google Scholar] [CrossRef]
- Ma, J.; Li, K.; Shi, S.; Li, J.; Tang, S.; Liu, L. The Application of UHPLC-HRMS for Quality Control of Traditional Chinese Medicine. Front. Pharmacol. 2022, 13, 922488. [Google Scholar] [CrossRef]
- Kong, W.J.; Zhang, S.S.; Zhao, Y.L.; Wu, M.Q.; Chen, P.; Wu, X.R.; Ma, X.P.; Guo, W.Y.; Yang, M.H. Combination of chemical fingerprint and bioactivity evaluation to explore the antibacterial components of Salvia miltiorrhizae. Sci. Rep. 2017, 7, 8112. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Liu, L.; Zhao, W.; Gu, L.; Xiong, A.; Wang, Z.; Yang, L. Development of a microarray microfluidic chip mass spectrometry platform based on UV curable 3D hepatocellular sphere bio-ink for rapid screening inhibitors of advanced glycosylation end products from natural compounds. Biosens. Bioelectron. 2025, 284, 117499. [Google Scholar] [CrossRef]
- Krauss, S.W.; Weiss, M. Controlling phase separations and reactions in trapped microfluidic droplets. Sci. Rep. 2024, 14, 20998. [Google Scholar] [CrossRef]
- Tazikeh Lemeski, A.; Seyyedi, S.M.; Hashemi-Tilehnoee, M.; Naeimi, A.S. Influence of triangular obstacles on droplet breakup dynamics in microfluidic systems. Sci. Rep. 2024, 14, 13324. [Google Scholar] [CrossRef] [PubMed]
- Fauquet, J.; Carette, J.; Duez, P.; Zhang, J.; Nachtergael, A. Microfluidic Diffusion Sizing Applied to the Study of Natural Products and Extracts That Modulate the SARS-CoV-2 Spike RBD/ACE2 Interaction. Molecules 2023, 28, 8072. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, D.; Li, T.; Yan, J.; Zhu, J.; He, T.; Hu, R.; Li, Y.; Yang, Y.; Liu, M. Microfluidic space coding for multiplexed nucleic acid detection via CRISPR-Cas12a and recombinase polymerase amplification. Nat. Commun. 2022, 13, 6480. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.; Alves e Sousa, J.; Saraiva, F.; Lopes, A.; Silverio, V.; Martins, R.F.; Martins, L. The Importance of Dimensional Traceability in Microfluidic Systems. Metrology 2024, 4, 240–253. [Google Scholar] [CrossRef]
- Neto, J.P.; Mota, A.; Lopes, G.; Coelho, B.J.; Frazao, J.; Moura, A.T.; Oliveira, B.; Sieira, B.; Fernandes, J.; Fortunato, E.; et al. Open-source tool for real-time and automated analysis of droplet-based microfluidic. Lab Chip 2023, 23, 3238–3244. [Google Scholar] [CrossRef]
- Pinheiro, T.; Marques, A.C.; Carvalho, P.; Martins, R.; Fortunato, E. Paper Microfluidics and Tailored Gold Nanoparticles for Nonenzymatic, Colorimetric Multiplex Biomarker Detection. ACS Appl. Mater. Interfaces 2021, 13, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, B.; Zuilhof, H.; van Beek, T.A. Microfluidic Chip-Based Induced Phase Separation Extraction as a Fast and Efficient Miniaturized Sample Preparation Method. Molecules 2020, 26, 38. [Google Scholar] [CrossRef]
- Li, Z.H.; Ai, N.; Yu, L.X.; Qian, Z.Z.; Cheng, Y.Y. A multiple biomarker assay for quality assessment of botanical drugs using a versatile microfluidic chip. Sci. Rep. 2017, 7, 12243. [Google Scholar] [CrossRef]
- Guo, G.; Wu, X.; Liu, D.; Liao, L.; Zhang, D.; Zhang, Y.; Mao, T.; He, Y.; Huang, P.; Wang, W.; et al. A Self-Regulated Microfluidic Device with Thermal Bubble Micropumps. Micromachines 2022, 13, 1620. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, B.; Yin, D.; Li, H.; Li, S.-H.; Zheng, Y.-H.; Han, X.-G.; Wang, Y.-G.; Fan, Q.-M.; Shih-Mo, Y.; et al. Kinsenoside screening with a microfluidic chip attenuates gouty arthritis through inactivating NF-κB signaling in macrophages and protecting endothelial cells. Cell Death Dis. 2016, 7, e2350. [Google Scholar]
- Ma, X.; Zhang, H.; Luan, J.; Tian, M.; Zhang, X.; Sohail, A.; Liang, D.; Liu, J.; Tao, F.; Wang, Z.; et al. Study of the Stability and Anti-Inflammatory Activity of Paeonol–Oleanolic Acid Liposomes by Microfluidic Technology. Foods 2025, 14, 2030. [Google Scholar] [CrossRef] [PubMed]
- Givarian, M.; Moztarzadeh, F.; Ghaffari, M.; Bahmanpour, A.; Mollazadeh-Bajestani, M.; Mokhtari-Dizaji, M.; Mehradnia, F. Dual-trigger release of berberine chloride from the gelatin/perfluorohexane core–shell structure. Bull. Natl. Res. Cent. 2024, 48, 65. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zeng, J.; Lu, Q.; Li, P.C. Microchip electrophoretic separation and fluorescence detection of chelerythrine and sanguinarine in medicinal plants. Talanta 2015, 142, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahman, N.; Ibrahim, F.; Aeinehvand, M.M.; Yusof, R.; Madou, M. A Microfluidic Lab-on-a-Disc (LOD) for Antioxidant Activities of Plant Extracts. Micromachines 2018, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yin, X.; Zhang, W.; Chen, M.; Feng, D.; Zhao, Y.; Zhu, Y. Simultaneous and Sensitive Detection of Three Pesticides Using a Functional Poly(Sulfobetaine Methacrylate)-Coated Paper-Based Colorimetric Sensor. Biosensors 2023, 13, 309. [Google Scholar] [CrossRef]
- Manmana, Y.; Macka, M.; Nuchtavorn, N. Distance-based paper microfluidic devices for rapid visual quantification of heavy metals in herbal supplements and cosmetics. RSC Adv. 2024, 14, 36142–36151. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Ma, J.; Zhu, S.; Zhao, X.; Mou, H.; Ke, X.; Wu, Z.; Wang, Y.; Lin, S.; et al. A Microfluidic Biosensor for Quantitative Detection of Salmonella in Traditional Chinese Medicine. Biosensors 2025, 15, 10. [Google Scholar] [CrossRef]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.J.; Joshipura, S.R.; et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun. Med. 2022, 2, 154. [Google Scholar] [CrossRef]
- Bircsak, K.M.; DeBiasio, R.; Miedel, M.; Alsebahi, A.; Reddinger, R.; Saleh, A.; Shun, T.; Vernetti, L.A.; Gough, A. A 3D microfluidic liver model for high throughput compound toxicity screening in the OrganoPlate(R). Toxicology 2021, 450, 152667. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Fan, F.; Xu, N.; Meng, X.L.; Zhang, Y.; Lin, J.M. Neurotoxicity mechanism of aconitine in HT22 cells studied by microfluidic chip-mass spectrometry. J. Pharm. Anal. 2023, 13, 88–98. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Sun, M.; Men, L.; Wang, E.; Zhao, Y.; Li, K.; Gong, X. Analysis of metabolites and metabolism-mediated biological activity assessment of ginsenosides on microfluidic co-culture system. Front. Pharmacol. 2023, 14, 1046722. [Google Scholar] [CrossRef]
- Cho, A.N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.Y.; Koo, D.J.; Yu, W.; et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef]
- Byeon, J.H.; Jung, D.J.; Han, H.J.; Son, W.C.; Jeong, G.S. Fast formation and maturation enhancement of human liver organoids using a liver-organoid-on-a-chip. Front. Cell Dev. Biol. 2024, 12, 1452485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Meng, Y.; Gao, W.; Yang, S.; Zhu, W.; Ji, X.; Zhai, X.; Liu, W.Q.; Luo, Y.; Ling, S.; et al. AI-driven high-throughput droplet screening of cell-free gene expression. Nat. Commun. 2025, 16, 2720. [Google Scholar] [CrossRef]
- Sarkar, S.; Cohen, N.; Sabhachandani, P.; Konry, T. Phenotypic drug profiling in droplet microfluidics for better targeting of drug-resistant tumors. Lab Chip 2015, 15, 4441–4450. [Google Scholar] [CrossRef]
- Dorrigiv, D.; Goyette, P.A.; St-Georges-Robillard, A.; Mes-Masson, A.M.; Gervais, T. Pixelated Microfluidics for Drug Screening on Tumour Spheroids and Ex Vivo Microdissected Tumour Explants. Cancers 2023, 15, 1060. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Lei, P.; Luo, L.; Yang, Q.; Yang, Q.; Tian, Y.; Shi, W.; Liu, Y.; Zeng, R.; Li, Y.; et al. Microfluidic-engineered Chinese herbal nanocomposite hydrogel microspheres for diabetic wound tissue regeneration. J. Nanobiotechnol. 2024, 22, 724. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299. [Google Scholar] [CrossRef]
- Gu, Y.; Jin, L.; Wang, L.; Ma, X.; Tian, M.; Sohail, A.; Wang, J.; Wang, D. Preparation of Baicalin Liposomes Using Microfluidic Technology and Evaluation of Their Antitumor Activity by a Zebrafish Model. ACS Omega 2024, 9, 41289–41300. [Google Scholar] [CrossRef]
- Cai, Y.; Li, X.; Li, M.; Chen, X.; Hu, H.; Ni, J.; Wang, Y. Traceability and Quality Control in Traditional Chinese Medicine: From Chemical Fingerprint to Two-Dimensional Barcode. Evid. Based Complement. Altern. Med. 2015, 2015, 251304. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, C.; Guo, D.A. Insight into chemical basis of traditional Chinese medicine based on the state-of-the-art techniques of liquid chromatography-mass spectrometry. Acta Pharm. Sin. B 2021, 11, 1469–1492. [Google Scholar] [CrossRef]
- Cai, M.; Wang, S.; Jiang, J.; Gao, K.; Sun, G. Comprehensive analysis strategy of quality for traditional Chinese medicine compound based on fingerprint technology and quantitative prediction of spectra. Talanta 2025, 287, 127661. [Google Scholar] [CrossRef]
- Lan, X.; Lin, L.; Yang, L.; Wang, Y.; Ke, X.; Yang, R.; Li, H.; Yang, B. Comprehensive quality evaluation of Magnolia officinalis leaves based on wavelength fusion fingerprint, content determination, and antioxidant activity analysis. Sci. Tradit. Chin. Med. 2024, 2, 48–56. [Google Scholar] [CrossRef]
- Taiedinejad, E.; Bausch, C.; Wittek, J.; Gul, G.; Erfle, P.; Schwarz, N.; Mozafari, M.; Bassler, M.; Dietzel, A. Diffusive micromixing combined with dynamic in situ laser scattering allows shedding light on lipid nanoparticle precipitation. Sci. Rep. 2024, 14, 24356. [Google Scholar] [CrossRef]
- Shih, T.T.; Lee, H.L.; Chen, S.C.; Kang, C.Y.; Shen, R.S.; Su, Y.A. Rapid analysis of traditional Chinese medicine Pinellia ternata by microchip electrophoresis with electrochemical detection. J. Sep. Sci. 2018, 41, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; He, Y.; Xiao, J.; Liang, S.; Wang, S.; Li, P.C.H.; Sun, Y. A successive laminar flow extraction for plant medicine preparation by microfluidic chip. Microfluid. Nanofluidics 2019, 23, 61. [Google Scholar] [CrossRef]
- Yang, J.; Deng, Y.; Zhang, M.; Feng, S.; Peng, S.; Yang, S.; Liu, P.; Cai, G.; Ge, G. Construction and Manipulation of Serial Gradient Dilution Array on a Microfluidic Slipchip for Screening and Characterizing Inhibitors against Human Pancreatic Lipase. Biosensors 2023, 13, 274. [Google Scholar] [CrossRef]
- Xie, J.; Pang, H.; Sun, R.; Wang, T.; Meng, X.; Zhou, Z. Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip. Micromachines 2021, 12, 290. [Google Scholar] [CrossRef]
- Kavruk, M.; Ozalp, V.C. Paper-Based Aptasensor Assay for Detection of Food Adulterant Sildenafil. Biosensors 2024, 14, 620. [Google Scholar] [CrossRef]
- Yin, B.; Zeng, S.; Liu, J.; Muhammad, R.; Jiang, Z.; Tan, G.; Yang, Q. Dual-Mode Microfluidic Workstation for Rapid Detection of Multiple Mycotoxins on Chip. Foods 2025, 14, 1928. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhuang, Y.; Guo, S.; Sohan, A.; Yin, B. Advances in Microfluidics Techniques for Rapid Detection of Pesticide Residues in Food. Foods 2023, 12, 2868. [Google Scholar] [CrossRef]
- Najjar, D.; Rainbow, J.; Sharma Timilsina, S.; Jolly, P.; de Puig, H.; Yafia, M.; Durr, N.; Sallum, H.; Alter, G.; Li, J.Z.; et al. A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nat. Biomed. Eng. 2022, 6, 968–978. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Jing, B.; Yan, L.; Li, J.; Luo, P.; Ai, X.; Tu, P. Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip. Biosensors 2022, 12, 718. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.-H.; Zhao, X.-M.; Liu, X.; Hu, Y.-W.; Deng, D.-W. Perspectives on organ-on-a-chip technology for natural products evaluation. Food Med. Homol. 2024, 1, 9420013. [Google Scholar] [CrossRef]
- Fan, F.; Xu, N.; Sun, Y.; Li, X.; Gao, X.; Yi, X.; Zhang, Y.; Meng, X.; Lin, J.M. Uncovering the Metabolic Mechanism of Salidroside Alleviating Microglial Hypoxia Inflammation Based on Microfluidic Chip-Mass Spectrometry. J. Proteome Res. 2022, 21, 921–929. [Google Scholar] [CrossRef]
- Sinha, N.; Yang, H.; Janse, D.; Hendriks, L.; Rand, U.; Hauser, H.; Köster, M.; van de Vosse, F.N.; de Greef, T.F.A.; Tel, J. Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics. Commun. Eng. 2022, 1, 18. [Google Scholar] [CrossRef]
- Guzzi, F.; Candeloro, P.; Coluccio, M.L.; Cristiani, C.M.; Parrotta, E.I.; Scaramuzzino, L.; Scalise, S.; Dattola, E.; D’Attimo, M.A.; Cuda, G.; et al. A Disposable Passive Microfluidic Device for Cell Culturing. Biosensors 2020, 10, 18. [Google Scholar] [CrossRef]
- Khot, M.I.; Levenstein, M.A.; de Boer, G.N.; Armstrong, G.; Maisey, T.; Svavarsdottir, H.S.; Andrew, H.; Perry, S.L.; Kapur, N.; Jayne, D.G. Characterising a PDMS based 3D cell culturing microfluidic platform for screening chemotherapeutic drug cytotoxic activity. Sci. Rep. 2020, 10, 15915. [Google Scholar] [CrossRef]
- Quintard, C.; Tubbs, E.; Jonsson, G.; Jiao, J.; Wang, J.; Werschler, N.; Laporte, C.; Pitaval, A.; Bah, T.S.; Pomeranz, G.; et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat. Commun. 2024, 15, 1452. [Google Scholar] [CrossRef]
- Beaurivage, C.; Naumovska, E.; Chang, Y.X.; Elstak, E.D.; Nicolas, A.; Wouters, H.; van Moolenbroek, G.; Lanz, H.L.; Trietsch, S.J.; Joore, J.; et al. Development of a Gut-On-A-Chip Model for High Throughput Disease Modeling and Drug Discovery. Int. J. Mol. Sci. 2019, 20, 5661. [Google Scholar] [CrossRef]
- Deng, B.; Wang, H.; Tan, Z.; Quan, Y. Microfluidic Cell Trapping for Single-Cell Analysis. Micromachines 2019, 10, 409. [Google Scholar] [CrossRef]
- Pang, L.; Liu, W.; Tian, C.; Xu, J.; Li, T.; Chen, S.-W.; Wang, J. Construction of single-cell arrays and assay of cell drug-resistance in an integrated microfluidics. Lab Chip 2016, 16, 4612–4620. [Google Scholar] [CrossRef]
- Du, G.; Fang, Q.; den Toonder, J.M. Microfluidics for cell-based high throughput screening platforms—A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef]
- Konishi, N.; Shirahata, T.; Yoshida, Y.; Sato, N.; Kaji, E.; Kobayashi, Y. Efficient synthesis of diverse C-3 monodesmosidic saponins by a continuous microfluidic glycosylation/batch deprotection method. Carbohydr. Res. 2021, 510, 108437. [Google Scholar] [CrossRef]
- Xiao, R.R.; Jin, L.; Xie, N.; Luo, P.; Gao, W.; Tu, P.; Ai, X. Establishment and large-scale validation of a three-dimensional tumor model on an array chip for anticancer drug evaluation. Front. Pharmacol. 2022, 13, 1032975. [Google Scholar] [CrossRef]
- Jabali, A.; Hoffrichter, A.; Uzquiano, A.; Marsoner, F.; Wilkens, R.; Siekmann, M.; Bohl, B.; Rossetti, A.C.; Horschitz, S.; Koch, P.; et al. Human cerebral organoids reveal progenitor pathology in EML1-linked cortical malformation. EMBO Rep. 2022, 23, e54027. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Li, Z.; Shi, L.; Liu, J.; Luo, H.; Wang, L.; Du, X.; Chen, W.; Guo, J.; et al. Application of Microfluidics in Drug Development from Traditional Medicine. Biosensors 2022, 12, 870. [Google Scholar] [CrossRef]
- Elvira, K.S. Microfluidic technologies for drug discovery and development: Friend or foe? Trends Pharmacol. Sci. 2021, 42, 518–526. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hashizume, H.; Takiguchi, S.; Ji, J.; Kawano, R.; Koiwai, K.; Yamamoto, H.; Elbadawy, M.; Omatsu, T.; Abugomaa, A.; et al. A microfluidics platform for simultaneous evaluation of sensitivity and side effects of anti-cancer drugs using a three-dimensional culture method. Sci. Rep. 2025, 15, 39. [Google Scholar] [CrossRef]
- Han, C.H.; Ma, J.Y.; Zou, W.; Qu, J.L.; Du, Y.; Li, N.; Liu, Y.; Jin, G.; Leng, A.J.; Liu, J. 3D Microfluidic System for Evaluating Inhibitory Effect of Chinese Herbal Medicine Oldenlandia diffusa on Human Malignant Glioma Invasion Combined with Network Pharmacology Analysis. Chin. J. Integr. Med. 2023, 29, 52–60. [Google Scholar] [CrossRef]
- Papamichail, L.; Koch, L.S.; Veerman, D.; Broersen, K.; van der Meer, A.D. Organoids-on-a-chip: Microfluidic technology enables culture of organoids with enhanced tissue function and potential for disease modeling. Front. Bioeng. Biotechnol. 2025, 13, 1515340. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Pan, Y.; Zhou, D.; Liu, Y.; Yin, Y.; Yang, J.; Wang, Y.; Song, Y. Emerging trends in organ-on-a-chip systems for drug screening. Acta Pharm. Sin. B 2023, 13, 2483–2509. [Google Scholar] [CrossRef]
- Shi, Y.; He, X.; Wang, H.; Dai, J.; Fang, J.; He, Y.; Chen, X.; Hong, Z.; Chai, Y. Construction of a novel blood brain barrier-glioma microfluidic chip model: Applications in the evaluation of permeability and anti-glioma activity of traditional Chinese medicine components. Talanta 2023, 253, 123971. [Google Scholar] [CrossRef]
- Myachin, I.V.; Kononov, L.O. Phase-Transfer Catalyzed Microfluidic Glycosylation: A Small Change in Concentration Results in a Dramatic Increase in Stereoselectivity. Catalysts 2023, 13, 313. [Google Scholar] [CrossRef]
- Jiang, R.-K.; Pan, Y.; Du, L.-H.; Zheng, L.-Y.; Sheng, Z.-K.; Zhang, S.-Y.; Lin, H.; Zhang, A.-Y.; Xie, H.-J.; Yang, Z.-K.; et al. Microfluidics Biocatalysis System Applied for the Synthesis of N-Substituted Benzimidazole Derivatives by Aza-Michael Addition. Catalysts 2022, 12, 1658. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Pei, C.; Peng, X.; Liu, X.; Qian, E.W.; Du, Y.; Li, J.J. Automated chemoenzymatic modular synthesis of human milk oligosaccharides on a digital microfluidic platform. RSC Adv. 2024, 14, 17397–17405. [Google Scholar] [CrossRef]
- Gong, A.; Zhu, C.T.; Xu, Y.; Wang, F.Q.; Tsabing, D.K.; Wu, F.A.; Wang, J. Moving and unsinkable graphene sheets immobilized enzyme for microfluidic biocatalysis. Sci. Rep. 2017, 7, 4309. [Google Scholar] [CrossRef]
- Du, L.-H.; Lin, H.; Fu, G.-N.; Huang, Z.-H.; Chen, Y.-M.; Xie, H.-J.; Yan, B.-L.; Xue, M.-M.; Zhang, A.-Y.; Wang, L.; et al. Two-Step Tandem Synthesis of Coumarin Derivatives Containing Bioamide Skeleton Catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in Sustainable Continuous-Flow Microreactors. Catalysts 2025, 15, 268. [Google Scholar] [CrossRef]
- Bellou, M.G.; Gkantzou, E.; Skonta, A.; Moschovas, D.; Spyrou, K.; Avgeropoulos, A.; Gournis, D.; Stamatis, H. Development of 3D Printed Enzymatic Microreactors for Lipase-Catalyzed Reactions in Deep Eutectic Solvent-Based Media. Micromachines 2022, 13, 1954. [Google Scholar] [CrossRef]
- Orbay, S.; Sanyal, R.; Sanyal, A. Porous Microgels for Delivery of Curcumin: Microfluidics-Based Fabrication and Cytotoxicity Evaluation. Micromachines 2023, 14, 1969. [Google Scholar] [CrossRef]
- Ladeveze, S.; Zurek, P.J.; Kaminski, T.S.; Emond, S.; Hollfelder, F. Versatile Product Detection via Coupled Assays for Ultrahigh-Throughput Screening of Carbohydrate-Active Enzymes in Microfluidic Droplets. ACS Catal. 2023, 13, 10232–10243. [Google Scholar] [CrossRef]
- Li, Q.; Lu, J.; Liu, J.; Li, J.; Zhang, G.; Du, G.; Chen, J. High-throughput droplet microfluidics screening and genome sequencing analysis for improved amylase-producing Aspergillus oryzae. Biotechnol. Biofuels Bioprod. 2023, 16, 185. [Google Scholar] [CrossRef]
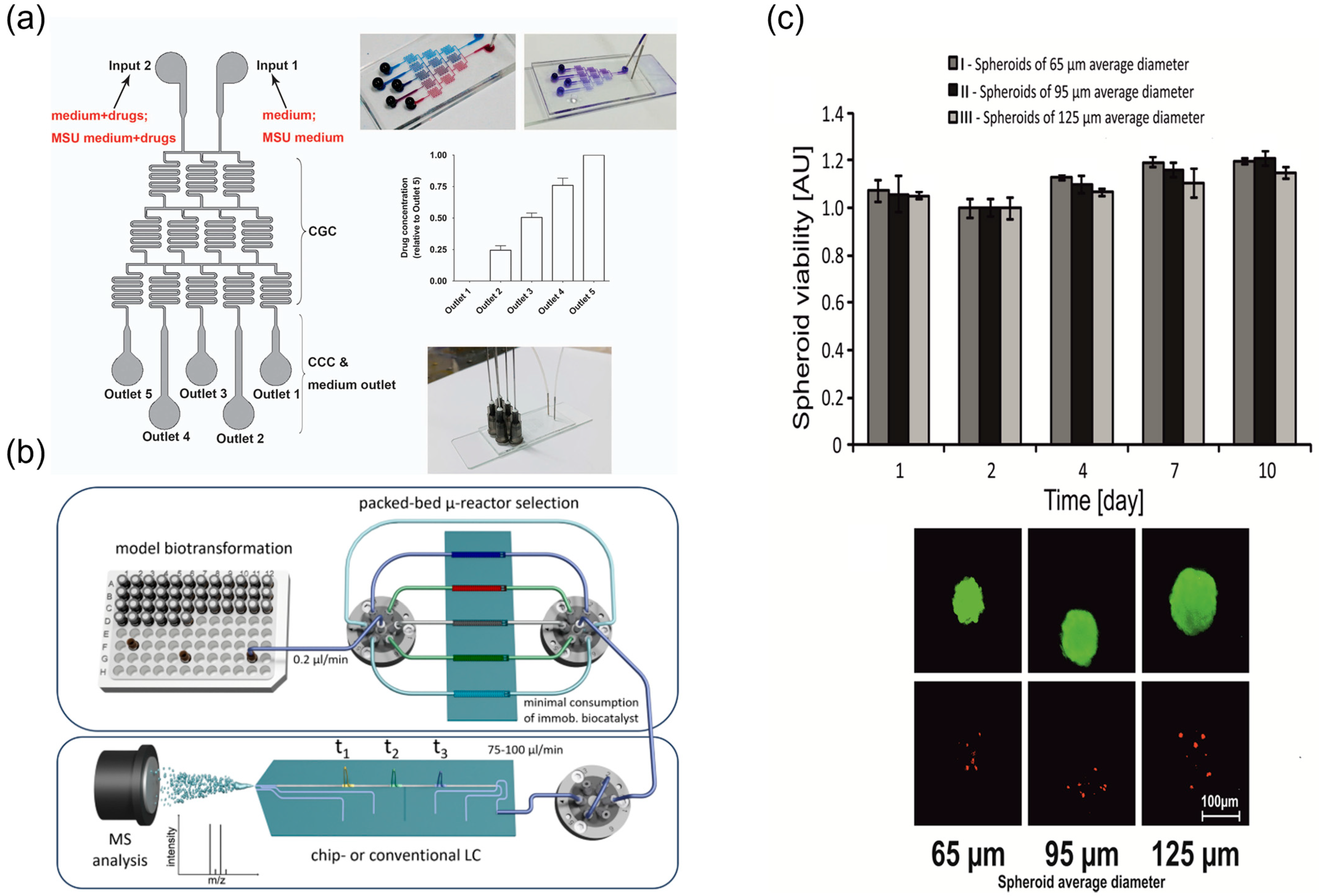
- Bras, E.J.S.; Domingues, C.; Chu, V.; Fernandes, P.; Conde, J.P. Microfluidic bioreactors for enzymatic synthesis in packed-bed reactors-Multi-step reactions and upscaling. J. Biotechnol. 2020, 323, 24–32. [Google Scholar] [CrossRef]
- Wang, J.; Liang, F.; Dong, Z.; Huang, J.; Zhu, Y.; You, H.; Chen, F.E. Four-step continuous-flow total synthesis of (-)-debromoflustramine B using a chiral heterogeneous Pd NP catalyst. Chem. Sci. 2024, 15, 16205–16209. [Google Scholar] [CrossRef]
- Koball, A.; Obst, F.; Gaitzsch, J.; Voit, B.; Appelhans, D. Boosting Microfluidic Enzymatic Cascade Reactions with pH-Responsive Polymersomes by Spatio-Chemical Activity Control. Small Methods 2024, 8, e2400282. [Google Scholar] [CrossRef]
- Volk, A.A.; Epps, R.W.; Yonemoto, D.T.; Masters, B.S.; Castellano, F.N.; Reyes, K.G.; Abolhasani, M. AlphaFlow: Autonomous discovery and optimization of multi-step chemistry using a self-driven fluidic lab guided by reinforcement learning. Nat. Commun. 2023, 14, 1403. [Google Scholar] [CrossRef]
- Fukuda, R.; Cira, N.J. High-throughput, combinatorial droplet generation by sequential spraying. Lab Chip 2025, 25, 1502–1511. [Google Scholar] [CrossRef]
- Westphal, H.; Schmidt, S.; Lama, S.; Polack, M.; Weise, C.; Oestereich, T.; Warias, R.; Gulder, T.; Belder, D. Development of an automated platform for monitoring microfluidic reactors through multi-reactor integration and online (chip-)LC/MS-detection. React. Chem. Eng. 2024, 9, 1739–1750. [Google Scholar] [CrossRef]
- Mathur, L.; Szalai, B.; Du, N.H.; Utharala, R.; Ballinger, M.; Landry, J.J.M.; Ryckelynck, M.; Benes, V.; Saez-Rodriguez, J.; Merten, C.A. Combi-seq for multiplexed transcriptome-based profiling of drug combinations using deterministic barcoding in single-cell droplets. Nat. Commun. 2022, 13, 4450. [Google Scholar] [CrossRef]
- Tan, Z.; Yan, Y.; Liao, J.; Shi, H.; Zheng, Y.; Xu, W.; Yi, C.; Dai, Z.; Xu, C. Droplet array with microfluidic concentration gradient (DA-MCG) for 2-dimensional reaction condition screening. Chem. Eng. Sci. 2024, 298, 120432. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, H.; Li, L.; Wang, F.; Meng, J.; Huang, H.; Wang, S.; Li, P.C.H.; Sun, Y. Screening of high-efficiency and low-toxicity antitumor active components in Macleaya cordata seeds based on the competitive effect of drugs on double targets by a new laminar flow chip. Analyst 2021, 146, 4934–4944. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef] [PubMed]
- Brouzes, E.; Medkova, M.; Savenelli, N.; Marran, D.; Twardowski, M.; Hutchison, J.B.; Rothberg, J.M.; Link, D.R.; Perrimon, N.; Samuels, M.L. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. USA 2009, 106, 14195–14200. [Google Scholar] [CrossRef]
- Kulesa, A.; Kehe, J.; Hurtado, J.E.; Tawde, P.; Blainey, P.C. Combinatorial drug discovery in nanoliter droplets. Proc. Natl. Acad. Sci. USA 2018, 115, 6685–6690. [Google Scholar] [CrossRef] [PubMed]
- Sabhachandani, P.; Motwani, V.; Cohen, N.; Sarkar, S.; Torchilin, V.; Konry, T. Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 2016, 16, 497–505. [Google Scholar] [CrossRef]
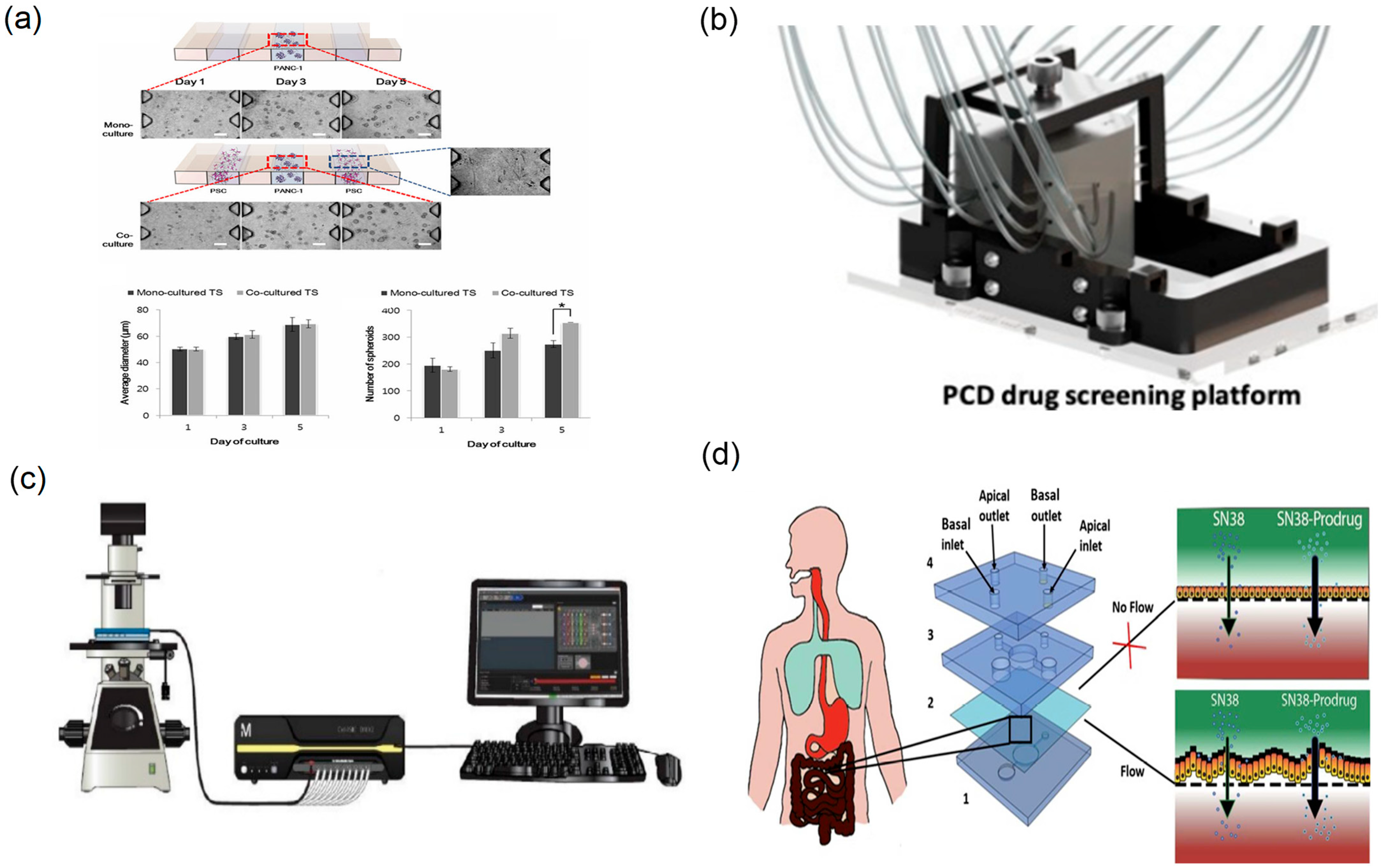
- Lee, J.H.; Kim, S.K.; Khawar, I.A.; Jeong, S.Y.; Chung, S.; Kuh, H.J. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 4. [Google Scholar] [CrossRef]
- Qin, W.; Yang, Z.; Yin, J.; Chen, D.; Huo, J.; Wang, J.; Wang, L.; Zhuo, Q. Effect Assessment of Aurantio-Obtusin on Novel Human Renal Glomerular Endothelial Cells Model Using a Microfluidic Chip. Nutrients 2022, 14, 4615. [Google Scholar] [CrossRef]
- Wu, J.; Wheeldon, I.; Guo, Y.; Lu, T.; Du, Y.; Wang, B.; He, J.; Hu, Y.; Khademhosseini, A. A sandwiched microarray platform for benchtop cell-based high throughput screening. Biomaterials 2011, 32, 841–848. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, W.; Chen, D.; Huo, J.; Wang, J.; Wang, L.; Zhuo, Q.; Yin, J. In vitro study of emodin-induced nephrotoxicity in human renal glomerular endothelial cells on a microfluidic chip. Biocell 2023, 47, 125–131. [Google Scholar] [CrossRef]
- Lucchetti, M.; Aina, K.O.; Grandmougin, L.; Jager, C.; Perez Escriva, P.; Letellier, E.; Mosig, A.S.; Wilmes, P. An Organ-on-Chip Platform for Simulating Drug Metabolism Along the Gut-Liver Axis. Adv. Healthc. Mater. 2024, 13, e2303943. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Bao, Y.; Wang, S.; Meng, X. A multifunctional integrated simultaneously online screening microfluidic biochip for the examination of “efficacy-toxicity” and compatibility of medicine. Chin. Chem. Lett. 2019, 30, 403–405. [Google Scholar] [CrossRef]
- Zuchowska, A.; Kwapiszewska, K.; Chudy, M.; Dybko, A.; Brzozka, Z. Studies of anticancer drug cytotoxicity based on long-term HepG2 spheroid culture in a microfluidic system. Electrophoresis 2017, 38, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Luo, L.; Guo, P.; Yang, Q.; Shi, W.; Yang, Q.; Tian, Y.; Liu, Y.; Zeng, R.; Li, Y.; et al. Microfluidic design and preparation of hydrogel microcapsules of Mesona chinensis polysaccharide: Characterization, pH-responsive behavior and gastrointestinal protection for Lactobacillus plantarum. Int. J. Biol. Macromol. 2025, 301, 140446. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Han, M.; Gao, S.; Shao, X.; Wang, X.; Sun, L.; Fu, X.; Wei, Q. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J. Mater. Chem. B 2020, 8, 6333–6351. [Google Scholar] [CrossRef]
- Kang, C.; Wang, J.; Li, R.; Gong, J.; Wang, K.; Wang, Y.; Wang, Z.; He, R.; Li, F. Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine. Molecules 2023, 28, 5955. [Google Scholar] [CrossRef]
- He, C.; Fang, Z.; Wu, H.; Li, X.; Cheng, L.; Wen, Y.; Lin, J. A flexible and dissolving traditional Chinese medicine microneedle patch for sleep-aid intervention. Heliyon 2024, 10, e33025. [Google Scholar] [CrossRef]
- Arduino, I.; Liu, Z.; Iacobazzi, R.M.; Lopedota, A.A.; Lopalco, A.; Cutrignelli, A.; Laquintana, V.; Porcelli, L.; Azzariti, A.; Franco, M.; et al. Microfluidic preparation and in vitro evaluation of iRGD-functionalized solid lipid nanoparticles for targeted delivery of paclitaxel to tumor cells. Int. J. Pharm. 2021, 610, 121246. [Google Scholar] [CrossRef]
- Ernits, M.; Reinsalu, O.; Yandrapalli, N.; Kopanchuk, S.; Moradpur-Tari, E.; Sanka, I.; Scheler, O.; Rinken, A.; Kurg, R.; Kyritsakis, A.; et al. Microfluidic production, stability and loading of synthetic giant unilamellar vesicles. Sci. Rep. 2024, 14, 14071. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.; Kim, J.S.; Lee, H.; Ju, I.G.; Yoo, N.Y.; La, S.; Jeong, D.H.; Na, C.; Park, H.J.; et al. Stimulation of microneedles alleviates pathology of Parkinson’s disease in mice by regulating the CD4+/CD8+ cells from the periphery to the brain. Front. Immunol. 2024, 15, 1454102. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ke, R.; Zeng, S.; Liu, Q. Flow Control Based on Constant Pressure Microfluidic Pump System. In Proceedings of the International Conference on Chemical, Material, and Food Engineering 2015 (CMFE 2015), Kunming, China, 25–26 July 2015; pp. 883–886. [Google Scholar]
- Ma, Z.; Li, B.; Peng, J.; Gao, D. Recent Development of Drug Delivery Systems through Microfluidics: From Synthesis to Evaluation. Pharmaceutics 2022, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, M.; Leite, S.B.; Corvi, R.; Whelan, M. Standardisation needs for organ on chip devices. Lab Chip 2021, 21, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- ISO 22916:2022; Microfluidic Devices—Interoperability Requirements for Dimensions, Connections and Initial Device Classification. International Organization for Standardization: Geneva, Switzerland, 2022.
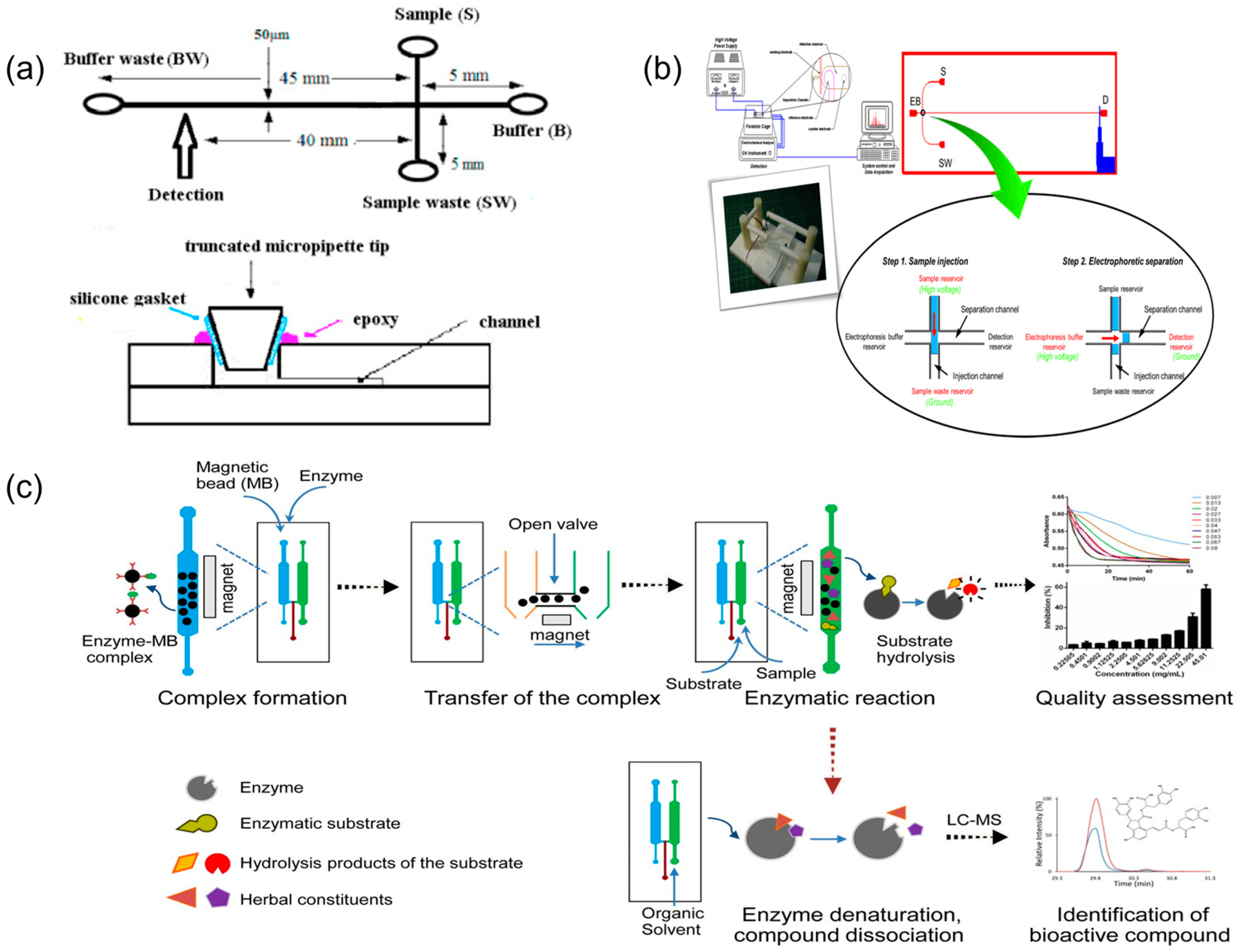
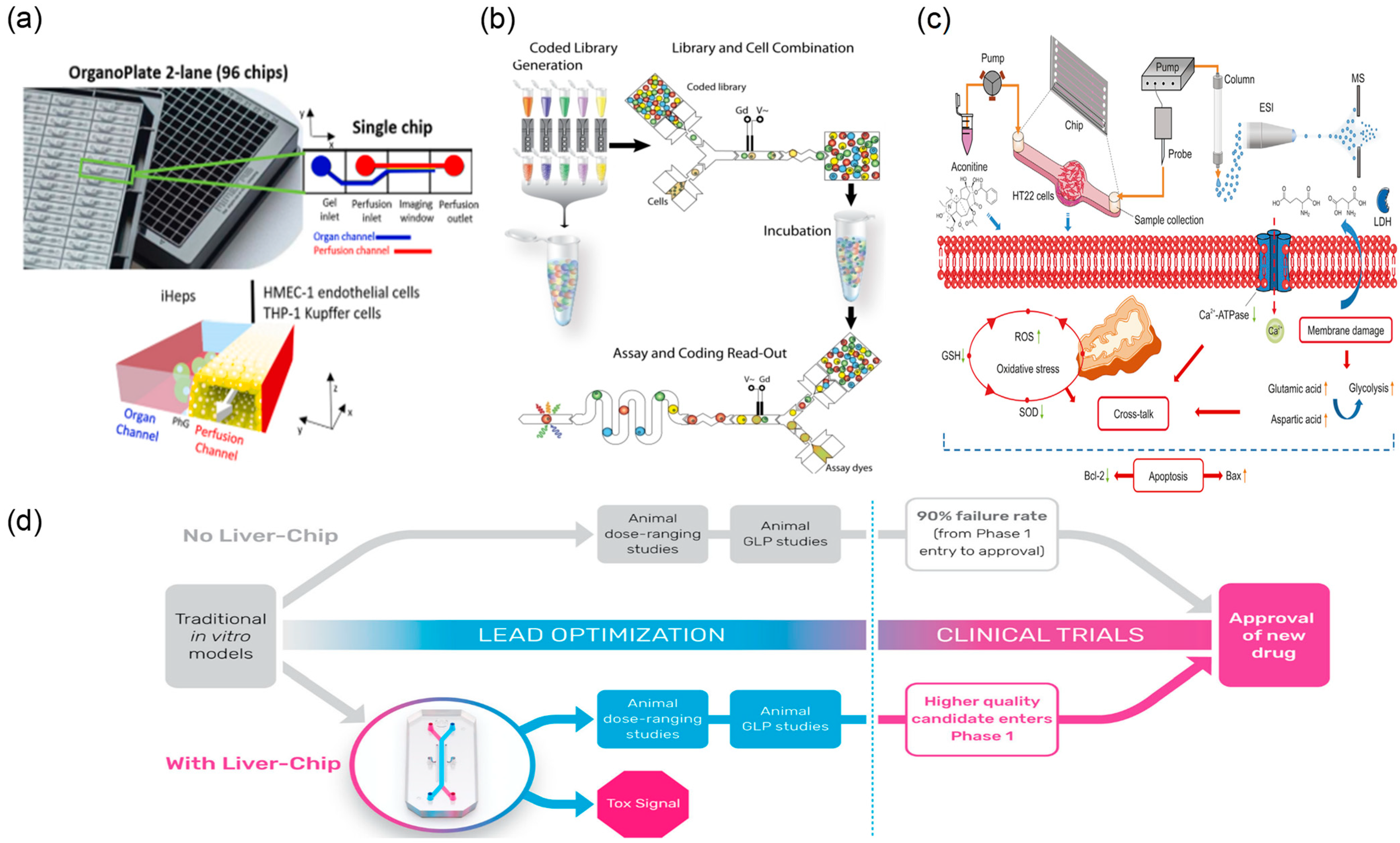
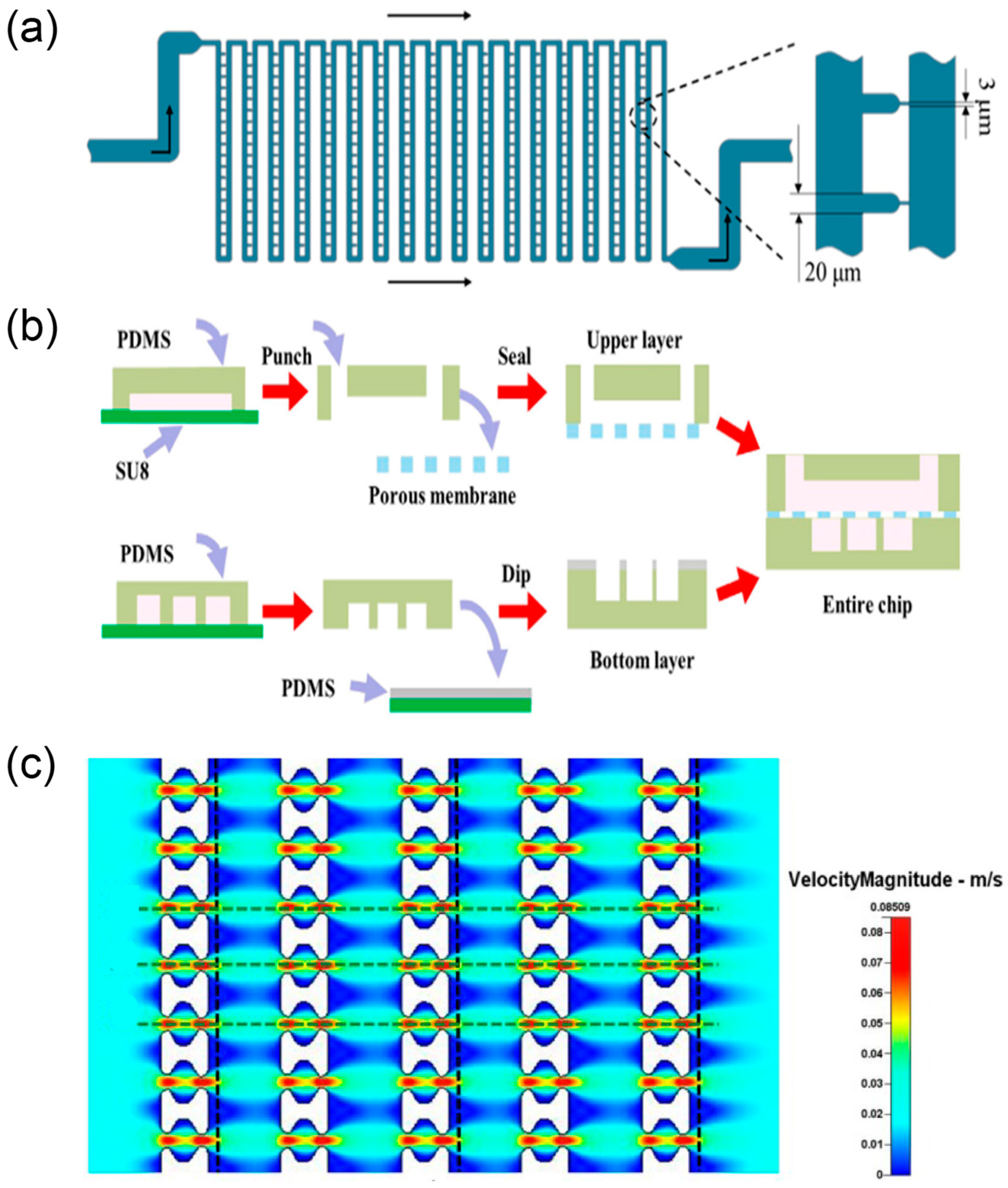
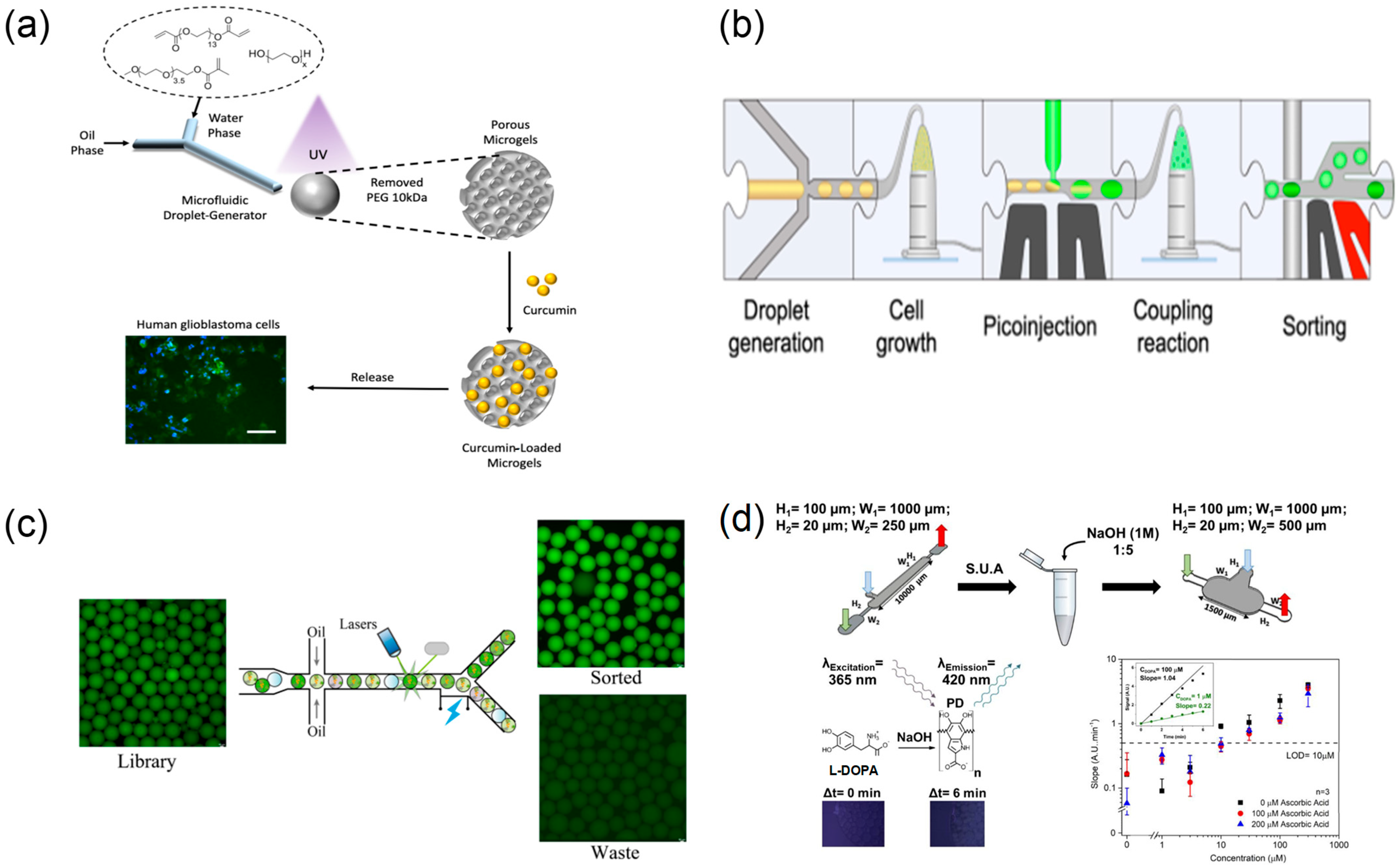
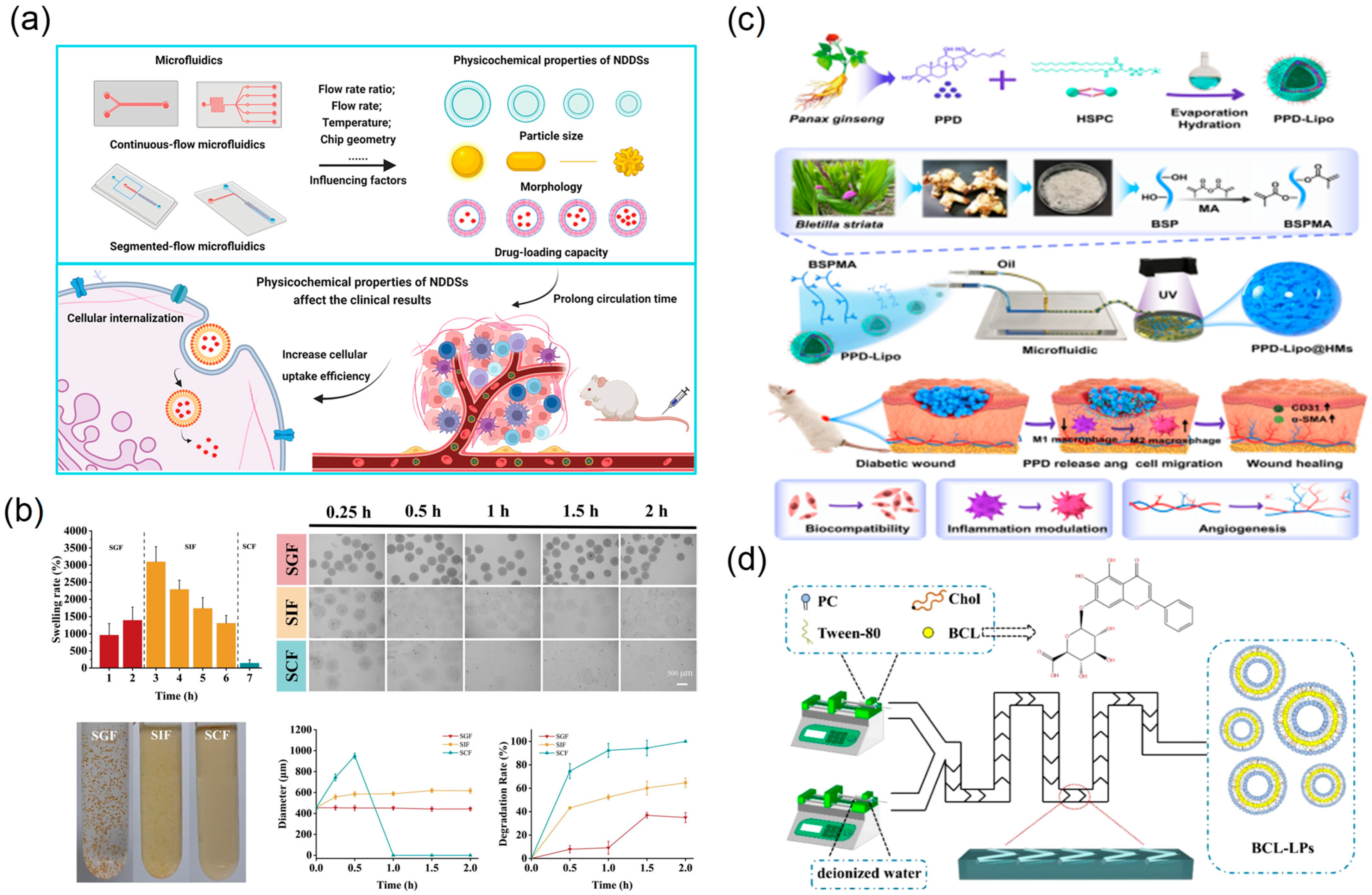
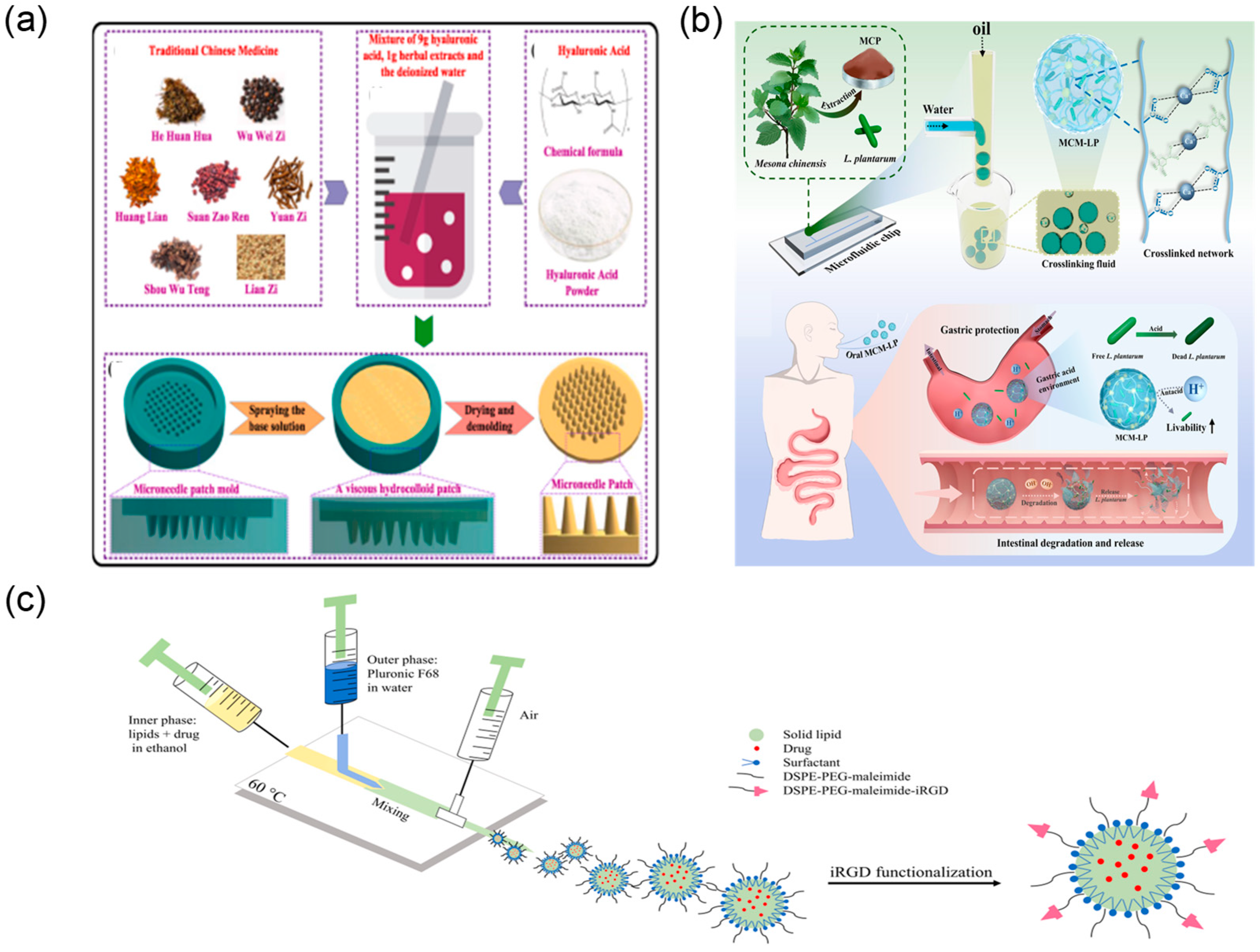
| Application Area | Representative Microfluidic Architecture | Notes on Reliability and Sustainability | Representative Refs. |
|---|---|---|---|
| Quality analysis—chemical composition and fingerprinting | Microchip electrophoresis; laminar flow extraction; SlipChip serial dilution; lab-on-a-disc | Fixed geometry and steady-state flow facilitate reproducibility; significantly reduces sample/reagent consumption compared to benchtop HPLC. | [19,24,25] |
| Safety evaluation—pathogens, heavy metals, additives | Optical immunosensor-on-chip; paper-based distance-readout (µPAD); enzyme inhibition microchips | Portable with fewer machines, enabling on-site screening; low reagent and waste levels facilitate large-scale monitoring. | [26,27,28] |
| Toxicity evaluation | Perfused liver-on-a-chip; droplet single-cell cytotoxicity; neuronal/cardiac tissue chips | Continuous perfusion enhances human relevance; single-cell format reveals rare toxic phenotypes, enhancing predictive value. | [29,30,31] |
| Pharmacological evaluation | Organoid/organ-on-a-chip; co-culture under flow | Stable nutrition and metabolic clearance reduce cross-repetition variability and improve the accuracy of effect size estimation. | [32,33,34] |
| High-throughput screening | Droplet microfluidics (merging/encoding) array chip platforms | Conditional barcoding and arraying facilitate reproducibility; unit condition cost is significantly reduced. | [35,36,37] |
| Drug delivery and release | Microfluidic emulsification; liposomes; core–shell hydrogel microcapsules; nanocomposite microspheres | Chip-based fabrication improves batch-to-batch consistency; reduces solvent and energy consumption. | [38,39,40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Ke, X.; Li, Y.; Shu, Z.; Zheng, J.; Xue, Z.; Qi, W.; Xu, B. The Application of Microfluidics in Traditional Chinese Medicine Research. Biosensors 2025, 15, 770. https://doi.org/10.3390/bios15120770
Zhu S, Ke X, Li Y, Shu Z, Zheng J, Xue Z, Qi W, Xu B. The Application of Microfluidics in Traditional Chinese Medicine Research. Biosensors. 2025; 15(12):770. https://doi.org/10.3390/bios15120770
Chicago/Turabian StyleZhu, Shanxi, Xuanqi Ke, Yayuan Li, Zixuan Shu, Jiale Zheng, Zihan Xue, Wuzhen Qi, and Bing Xu. 2025. "The Application of Microfluidics in Traditional Chinese Medicine Research" Biosensors 15, no. 12: 770. https://doi.org/10.3390/bios15120770
APA StyleZhu, S., Ke, X., Li, Y., Shu, Z., Zheng, J., Xue, Z., Qi, W., & Xu, B. (2025). The Application of Microfluidics in Traditional Chinese Medicine Research. Biosensors, 15(12), 770. https://doi.org/10.3390/bios15120770






