Double Lateral Flow Test System for Simultaneous Immunodetection of Enantiomeric Forms of Antibiotics: An Ofloxacin Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of OFL–Protein Conjugates as an Immunogen and Coating Antigens
2.3. Production of PAb
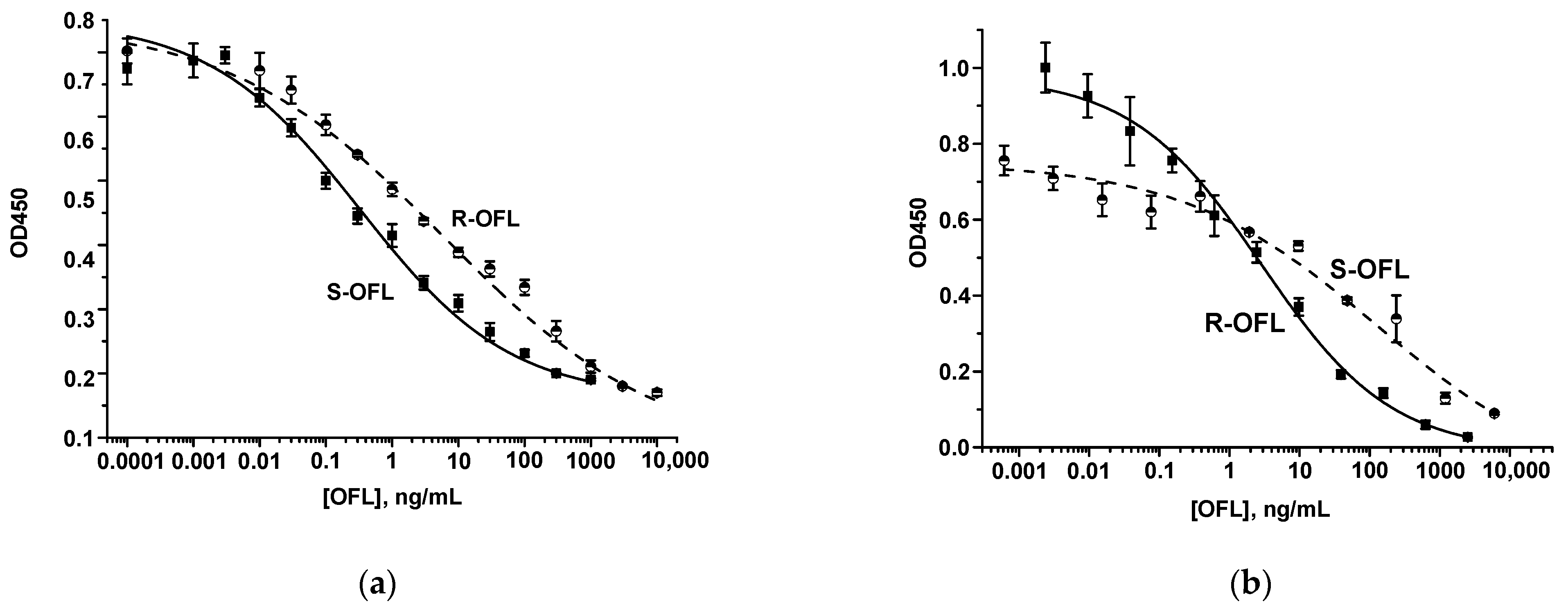
2.4. Antisera Testing by ELISA
2.5. Synthesis of Gold Label and Its Conjugation with Antibodies
2.6. Manufacturing of Test Strips
2.7. Sample Preparation Before LFIA and Obtaining Spiked Samples
2.8. Individual LFIAs of S-OFL and R-OFL
2.9. Double LFIA
2.10. Evaluation of the Immunoassay Results and Statistics
3. Results and Discussion
3.1. Obtaining Key LFIA Components and Their Characterization
3.1.1. Production and Testing of PAb
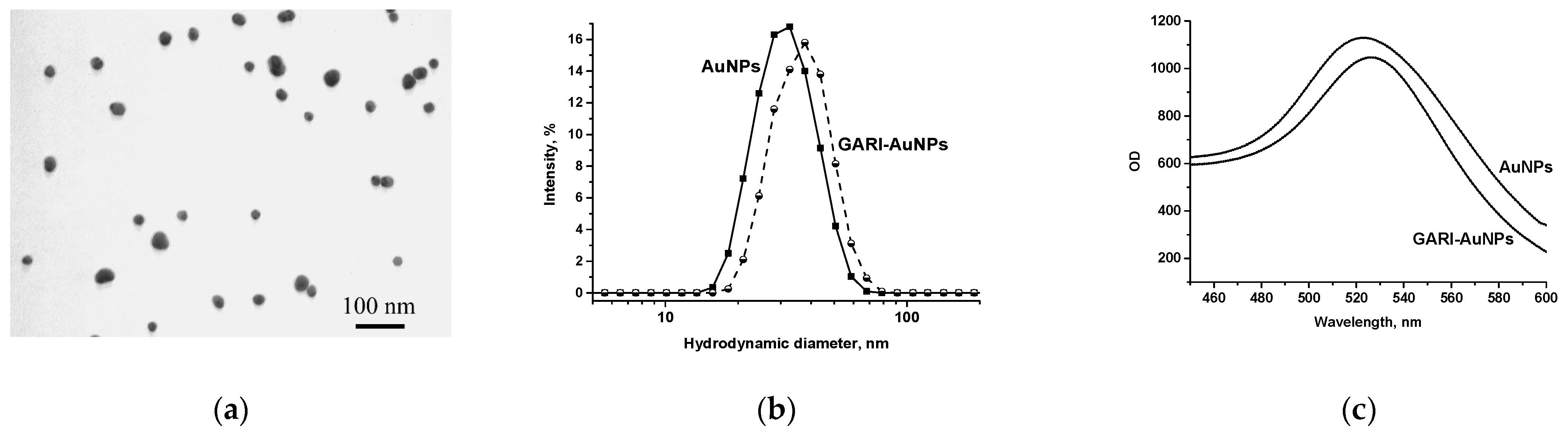
3.1.2. Obtaining, Characterization, and Conjugation of AuNPs
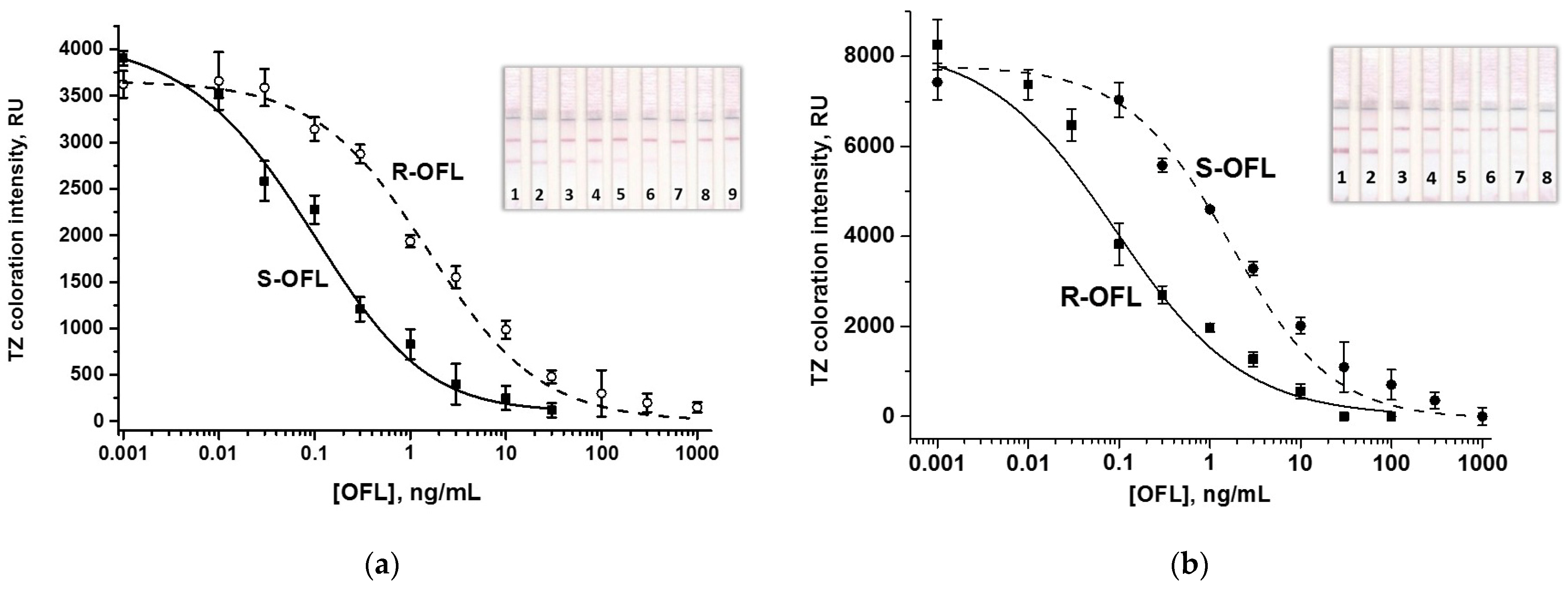
3.2. Individual LFIAs OFL Enantiomers
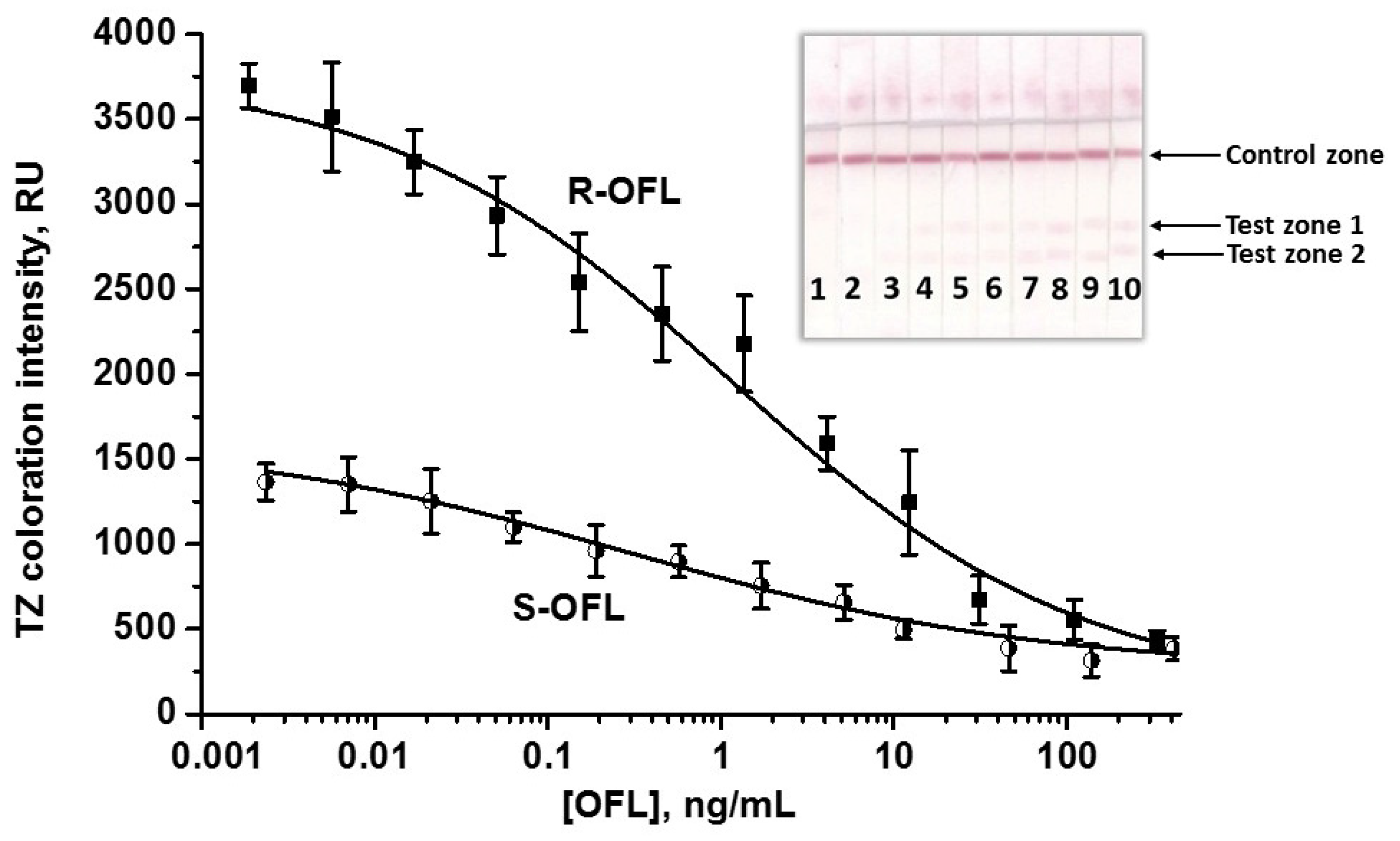
3.3. Double LFIA for Simultaneous Detection of S-OFL and R-OFL
3.4. Application of the Double LFIA for OFL Detection in Milk
3.5. Novelty and Originality of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| AS | Antiserum |
| BSA | Bovine serum albumin |
| CR | cross-reactivity |
| CZ | Control zone |
| DAGI | Donkey anti-goat immunoglobulins |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DLS | Dynamic light scattering |
| DMF | Dimethylformamide |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| ELISA | Enzyme-linked immunosorbent assay |
| FQ | Fluoroquinolone |
| gaba | Gamma-aminobutyric acid |
| GARI | Goat anti-rabbit immunoglobulins |
| HRP | Horseradish peroxidase |
| icELISA | Indirect competitive ELISA |
| LFIA | Lateral flow immunoassay |
| LOD | Limit of detection |
| MAb | Monoclonal antibody |
| NHS | N-hydroxysuccinimide |
| OD | Optical density |
| OFL | Ofloxacin |
| OVA | Ovalbumin |
| PAb | Polyclonal antibody |
| PBS | 50 mM phosphate-buffered saline containing 100 mM NaCl |
| PBST | PBS containing 0.05% Triton X-100 |
| PBSTW1 | PBS containing 1% Tween-20 |
| Pdi | Polydispersity index |
| rac | Racemic mixture of isomers |
| RT | Room temperature |
| RU | Relative units |
| SAL | Salbutamol |
| STI | Soybean trypsin inhibitor |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| TEM | Transmission electron microscopy |
| TZ | Test zone |
| WR | Working range of detectable concentrations |
References
- Boahen, E.; Owusu, L.; Adjei-Anim, S.O. A comprehensive review of emerging environmental contaminants of global concern. Discov. Environ. 2025, 3, 144. [Google Scholar] [CrossRef]
- Garvey, M. Food pollution: A comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutrire 2019, 44, 1. [Google Scholar] [CrossRef]
- Krishnan, A.; Devarajan, Y. Comprehensive assessment of food safety risks in agriculture and dairy processing. Nutrire 2025, 50, 43. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Mitiku, B.A.; Tamrat Admasu, H. Veterinary drug residues in food products of animal origin and their public health consequences: A review. Vet. Med. Sci. 2024, 10, e70049. [Google Scholar] [CrossRef] [PubMed]
- Lathakumari, R.H.; Vajravelu, L.K.; Satheesan, A.; Ravi, S.; Thulukanam, J. Antibiotics and the gut microbiome: Understanding the impact on human health. Med. Microecol. 2024, 20, 100106. [Google Scholar] [CrossRef]
- Hussen, N.H.A.; Qadir, S.H.; Rahman, H.S.; Hamalaw, Y.Y.; Kareem, P.S.S.; Hamza, B.A. Long-term toxicity of fluoroquinolones: A comprehensive review. Drug Chem. Toxicol. 2024, 47, 795–806. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, lethality and their contributions to antibiotic resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Chatterjee, S. Fluoroquinolone antibiotics: Occurrence, mode of action, resistance, environmental detection, and remediation–A comprehensive review. Environ. Pollut. 2022, 315, 120440. [Google Scholar] [CrossRef]
- Du, J.; Liu, Q.; Pan, Y.; Xu, S.; Li, H.; Tang, J. The research status, potential hazards and toxicological mechanisms of fluoroquinolone antibiotics in the environment. Antibiotics 2023, 12, 1058. [Google Scholar] [CrossRef]
- Bertino, J., Jr.; Fish, D. The safety profile of the fluoroquinolones. Clin. Ther. 2000, 22, 798–817. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, L.; Wang, H.; Lv, X.; Ding, K. The molecular recognition paradigm of environmental chemicals with biomacromolecule. Curr. Prot. Pept. Sci. 2017, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.C.; Armstrong, D.W. Chiral surfaces: The many faces of chiral recognition. Curr. Opin. Colloid Interface Sci. 2017, 32, 94–107. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Recognition in the domain of molecular chirality: From noncovalent interactions to separation of enantiomers. Chem. Rev. 2022, 122, 13235–13400. [Google Scholar] [CrossRef]
- Laldinchhana, L.J.; Komu, L.T.; Roy, P.K. Enantioselectivity in pharmacokinetics: A mini review. Int. J. Pharm. Sci. Nanotechnol. 2024, 17, 7736–7745. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in drug pharmacokinetics and toxicity: Pharmacological relevance and analytical methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Ahluwalia, V.K. Stereochemistry of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Morrissey, I.; Hoshino, K.; Sato, K.; Yoshida, A.; Hayakawa, I.; Bures, M.G.; Shen, L.L. Mechanism of differential activities of ofloxacin enantiomers. Antimicrob. Agents Chemother. 1996, 40, 1775–1784. [Google Scholar] [CrossRef]
- Gandhi, K.; Shah, U.; Patel, S. Drug stereochemistry: A prodigy for pharmacology and drug development. Curr. Drug Discov. Technol. 2020, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological effects of quinolones: A family of broad-spectrum antimicrobial agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef]
- Kaya, C.; Birgül, K.; Bülbül, B. Fundamentals of chirality, resolution, and enantiopure molecule synthesis methods. Chirality 2023, 35, 4–28. [Google Scholar] [CrossRef]
- Hutt, A.G.; O’Grady, J. Drug chirality: A consideration of the significance of the stereochemistry of antimicrobial agents. J. Antimicrob. Chemother. 1996, 37, 7–32. [Google Scholar] [CrossRef]
- Khan, A.Y.; Preskorn, S.H.; Wimalasena, K. Single enantiomer drugs: Should they be developed? Essent. Psychopharmacol. 2006, 7, 15–23. [Google Scholar]
- Sethi, S.; Bhushan, R. LC enantioseparation of active pharmaceutical ingredients using rationally synthesized CDRs and chiral molecules with high molar absorptivity. Biomed. Chromatogr. 2024, 38, e6022. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Felletti, S.; Franchina, F.A.; Bozza, D.; Compagnin, G.; Nosengo, C.; Pasti, L.; Cavazzini, A.; Catani, M. Recent developments in the high-throughput separation of biologically active chiral compounds via high performance liquid chromatography. J. Pharm. Biomed. Anal. 2024, 238, 115794. [Google Scholar] [CrossRef]
- Řemínek, R.; Foret, F. Capillary electrophoretic methods for quality control analyses of pharmaceuticals: A review. Electrophoresis 2021, 42, 19–37. [Google Scholar] [CrossRef]
- Bai, F.; Bu, T.; Wang, Z.; Shao, B. Integration of a new generation of immunochromatographic assays: Recent advances and future trends. Nano Today 2024, 57, 102403. [Google Scholar] [CrossRef]
- Majdinasab, M.; Kumar Mishra, R.; Tang, X.; Marty, J.L. Detection of antibiotics in food: New achievements in the development of biosensors. TrAC Trends Anal. Chem. 2020, 127, 115883. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, C.; Zhao, S.; Cheng, J.; Li, D.; Liu, Q. Multifunctional nanomaterials combined lateral flow immunoassay for food contaminant detection: A review. Coord. Chem. Rev. 2026, 547, 217125. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, B.; Abd El-Aty, A.M.; Zhang, X.; Chen, L.; Liu, G.; Xu, X.; Wang, J.; Jin, M.; Wang, Q.; et al. The high-throughput and high-sensitivity strategies of multiplex lateral flow immunoassays for agricultural product contaminants. TrAC Trends Anal. Chem. 2025, 193, 8455. [Google Scholar] [CrossRef]
- Byzova, N.A.; Smirnova, N.I.; Zherdev, A.V.; Eremin, S.A.; Shanin, I.A.; Lei, H.-T.; Sun, Y.; Dzantiev, B.B. Rapid immunochromatographic assay for ofloxacin in animal original foodstuffs using native antisera labeled by colloidal gold. Talanta 2014, 119, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nan, L.; Luo, L.; Li, Z.; Zheng, S.; Xia, X.; Xing, C.; Wang, Z.; Pan, Y.; Wen, K. Au@PdPt nanoparticles-based colorimetric and photothermal dual-mode lateral flow immunoassay for the sensitive detection of ofloxacin. Mikrochim. Acta 2025, 192, 503. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cui, S.; Zhang, J.; Zhao, Y.; Peng, X.; Sun, F. Sensitive detection of ofloxacin by lateral flow immunoassay based on Prussian blue nanoparticles and colloidal gold. J. Food Compos. Anal. 2024, 131, 106262. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, H.; Zhu, J.; Zhang, S.; Xu, N.; Wang, Y. A gold-platinum nanozyme-based immunochromatographic strip for rapid detection of ofloxacin in chicken and fish. J. Food Compos. Anal. 2025, 146, 107888. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Li, N.; Salah, M.; Guan, S.; Pan, W.; Wang, Z.; Zhou, X.; Wang, Y. Development of a colloidal gold immunochromatographic assay strip using a monoclonal antibody for the rapid detection of ofloxacin. Foods 2024, 13, 4137. [Google Scholar] [CrossRef]
- Shanin, I.A.; Zvereva, E.A.; Eremin, S.A.; Sviridov, O.V.; Zherdev, A.V.; Dzantiev, B.B. Development of an immunoenzyme assay to control the total content of antibiotics of the fluoroquinolone group in milk. Appl. Biochem. Microbiol. 2019, 55, 563–569. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Feng, L.; Cao, G.; Tao, Y.; Liu, Z.; Yuan, Z. Preparation of broadly specific monoclonal antibodies for simultaneous determination of fluoroquinolone residues in eggs. Food Anal. Methods 2016, 9, 3520–3531. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Fedyunina, N.S.; Martianov, A.A.; Zherdev, A.V.; Dzantiev, B.B. Production of anti-fullerene C60 polyclonal antibodies and study of their interaction with a conjugated form of fullerene. J. Nanopart. Res. 2011, 13, 3713–3719. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar]
- Sultana, E.; Shamim Al Mamun, M. Golden eyes on pollutants: Colorimetric detection of emerging contaminants with AuNPs. RSC Adv. 2025, 15, 32833–32870. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Darwish, I.A.; Wang, J.; Zhang, D. Gold nanomaterials-derived colorimetric signaling strategies for immunosensing and food safety. TrAC Trends Anal. Chem. 2025, 188, 118238. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Retention of activity by antibodies immobilized on gold nanoparticles of different sizes: Fluorometric method of determination and comparative evaluation. Nanomaterials 2021, 11, 3117. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Byzova, N.A.; Dzantiev, B.B.; Zherdev, A.V. Prussian-blue-nanozyme-enhanced simultaneous immunochromatographic control of two relevant bacterial pathogens in milk. Foods 2024, 13, 3032. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Byzova, N.A.; Panferov, V.G.; Zvereva, E.A.; Xing, S.; Zherdev, A.V.; Liu, J.; Lei, H.; Dzantiev, B.B. Ultrasensitive lateral flow immunoassay of fluoroquinolone antibiotic gatifloxacin using Au@Ag nanoparticles as a signal-enhancing label. Biosensors 2024, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, A.V.; Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Handling detection limits of multiplex lateral flow immunoassay by choosing the order of binding zones. Micromachines 2023, 14, 333. [Google Scholar] [CrossRef]
- Bartosh, A.V.; Sotnikov, D.V.; Hendrickson, O.D.; Zherdev, A.V.; Dzantiev, B.B. Design of multiplex lateral flow tests: A case study for simultaneous detection of three antibiotics. Biosensors 2020, 10, 17. [Google Scholar] [CrossRef]
- Suryoprabowo, S.; Nasyiruddin, R.L.; Wang, Z.; Hendriko, A.; Tristanto, N.A. A comprehensive review on the pretreatment and detection methods of fluoroquinolones in food and environment. J. Food Compos. Anal. 2025, 140, 107179. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. J. Food Sci. Technol. 2021, 58, 292–301. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Z.; Xie, H.; Fang, Y.; Quan, Q.; Shen, X.; Lei, H.; Xu, Z.; Li, X. Ultrasensitive magnetic assisted lateral flow immunoassay based on chiral monoclonal antibody against R-(−)-salbutamol of broad-specificity for 38 β-agonists detection in swine urine and pork. J. Agric. Food Chem. 2022, 70, 4112–4122. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Xu, J.; Yu, X.; Bai, W.; Huang, X.; Lei, H. Central chirality and axial chirality recognition of the enantioselective antibodies to herbicide metolachlor. J. Agric. Food Chem. 2024, 72, 10055–10064. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, G.; Zhao, G.; Zhou, T.; Yu, J.; Zhang, X.; Gai, Z.; Xu, Z.; Lei, H.; Shen, X. Enantioselectivity regulation of antibody against chiral herbicide metolachlor based on interaction at chiral center. Int. J. Biol. Macromol. 2024, 270, 132471. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Li, M.; Yan, X.; Wan, M. Stereoselectivity of an enzyme-linked, immunosorbent assay for S-bioallethrin. Anal. Methods 2012, 4, 534–538. [Google Scholar] [CrossRef]
- Wang, L.; Xie, W.; Jiao, W.; Zhang, C.; Li, X.; Xu, Z.; Huang, X.A.; Lei, H.; Shen, X. Conformational adaptability determining antibody recognition to distomer: Structure analysis of enantioselective antibody against chiral drug gatifloxacin. RSC Adv. 2021, 11, 39534–39544. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, G.G.; Huth, T.; Harms, D. Screening of stereoisomeric chloramphenicol residues in honey by ELISA and CHARM ® II test—The potential risk of systematically false-compliant (false negative) results. Food Addit. Contam. Part A 2020, 37, 94–103. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, F.; Zeng, S.; Tian, Y.; Chai, X.; Gee, S.; Hammock, B.D.; Zheng, J. Development of enantioselective polyclonal antibodies to detect styrene oxide protein adducts. Anal. Chem. 2009, 81, 2668–2677. [Google Scholar] [CrossRef]
- Morita, I.; Oyama, H.; Kanda, Y.; Yasuo, M.; Ito, A.; Toyota, M.; Hayashi, Y.; Yokoyama, T.; Kobayashi, N. Enantioselective monoclonal antibodies for detecting ketamine to crack down on illicit use. Biol. Pharmaceut. Bull. 2018, 41, 123–131. [Google Scholar] [CrossRef]
- Kupiec, T.C.; Chaturvedi, A.K. Stereochemical determination of selegiline metabolites in postmortem biological specimens. J. Forensic. Sci. 1999, 44, 222–226. [Google Scholar] [CrossRef]
- Chouchane, L.; Strosberg, A.D.; Hoebeke, J. Stereospecific immuno-recognition of the tetracyclic anti-depressant oxaprotiline. Mol. Immunol. 1988, 25, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Wang, B.; Xu, Z.; Sun, Y.; Huang, X.; Shen, Y.; Eremin, S.A.; Zherdev, A.V.; Dzantiev, B.B.; Lei, H. Stereospecific recognition and quantitative structure-activity relationship between antibodies and enantiomers: Ofloxacin as a model hapten. Analyst 2015, 140, 1037–1045. [Google Scholar] [CrossRef]
- Mu, H.; Lei, H.; Wang, B.; Xu, Z.; Zhang, C.; Ling, L.; Tian, Y.; Hu, J.; Sun, Y. Molecular modeling application on hapten epitope prediction: An enantioselective immunoassay for ofloxacin optical isomers. J. Agric. Food Chem. 2014, 62, 7804–7812. [Google Scholar] [CrossRef] [PubMed]

| Detected Analyte | LOD, ng/mL | WR, ng/mL | CR with the Second Enantiomer, % |
|---|---|---|---|
| S-OFL | 0.001 | 0.009–12 | 5.2 |
| R-OFL | 0.03 | 0.14–55 | 2.1 |
| Immunoassay Mode | ||||||||
|---|---|---|---|---|---|---|---|---|
| Double LFIA | icELISA | |||||||
| OFL | S/R | S/R | S/R | S/R | S | S | R | R |
| Added, ng/g | 0.56/12.3 | 1.7/12.3 | 0.56/4.1 | 1.7/4.1 | 0.56 | 1.7 | 4.1 | 12.3 |
| Revealed, ng/g | 0.52 ± 0.04/ 11.0 ± 0.81 | 1.54 ± 0.2/ 11.1 ± 1.2 | 0.49 ± 0.06/ 3.6 ± 0.4 | 1.61 ± 0.13/ 3.5 ± 0.4 | 0.60 ± 0.02 | 1.85 ± 0.04 | 3.9 ± 0.1 | 12.5 ± 0.1 |
| Recovery ± SD *, % | 92.2 ± 7.5/ 89.8 ± 6.6 | 90.7 ± 10.9/ 90.3 ± 10.3 | 87.2 ± 3.7/ 87.0 ± 10.0 | 94.9 ± 8.2/ 84.7 ± 8.7 | 107.1 ± 2.9 | 104.8 ±1.9 | 95.1 ± 2.6 | 105.6 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickson, O.D.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Double Lateral Flow Test System for Simultaneous Immunodetection of Enantiomeric Forms of Antibiotics: An Ofloxacin Case Study. Biosensors 2025, 15, 765. https://doi.org/10.3390/bios15120765
Hendrickson OD, Byzova NA, Zherdev AV, Dzantiev BB. Double Lateral Flow Test System for Simultaneous Immunodetection of Enantiomeric Forms of Antibiotics: An Ofloxacin Case Study. Biosensors. 2025; 15(12):765. https://doi.org/10.3390/bios15120765
Chicago/Turabian StyleHendrickson, Olga D., Nadezhda A. Byzova, Anatoly V. Zherdev, and Boris B. Dzantiev. 2025. "Double Lateral Flow Test System for Simultaneous Immunodetection of Enantiomeric Forms of Antibiotics: An Ofloxacin Case Study" Biosensors 15, no. 12: 765. https://doi.org/10.3390/bios15120765
APA StyleHendrickson, O. D., Byzova, N. A., Zherdev, A. V., & Dzantiev, B. B. (2025). Double Lateral Flow Test System for Simultaneous Immunodetection of Enantiomeric Forms of Antibiotics: An Ofloxacin Case Study. Biosensors, 15(12), 765. https://doi.org/10.3390/bios15120765







