Europium Complex-Loaded Albumin Nanoparticles as Probes for Time-Resolved Luminescent Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
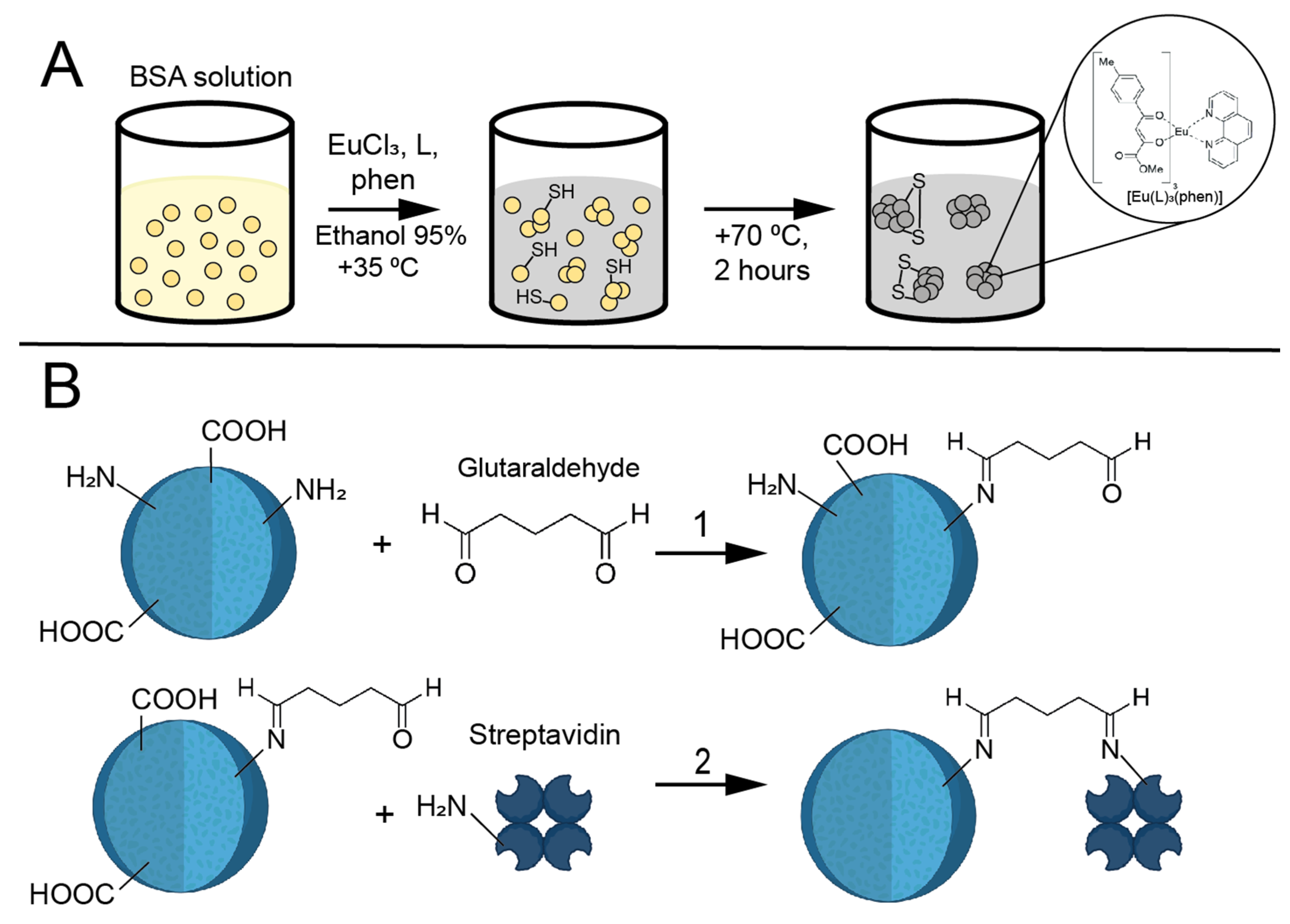
2.3. Synthesis of Eu@BSA
2.4. Synthesis of Eu@BSA/Str
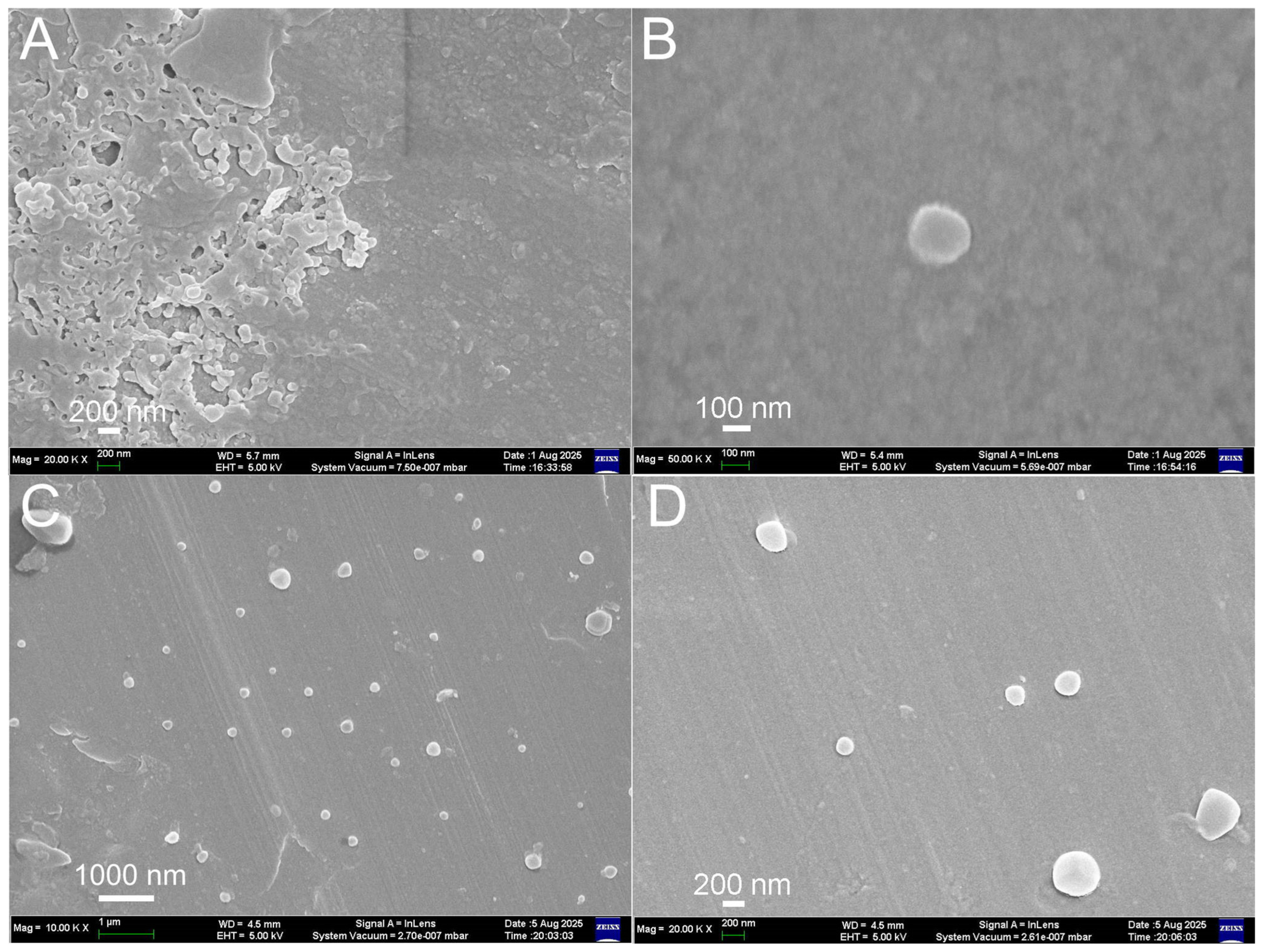
2.5. Characterization of Nanoparticles
2.6. Standard of Human IgG
2.7. Preparation of Human Sera Samples
2.8. Biotinylation of Mouse Monoclonal Antibodies
2.9. IgG Depletion from Human Serum
2.10. SDS-PAGE Analysis
2.11. In-House Human IgG ELISA
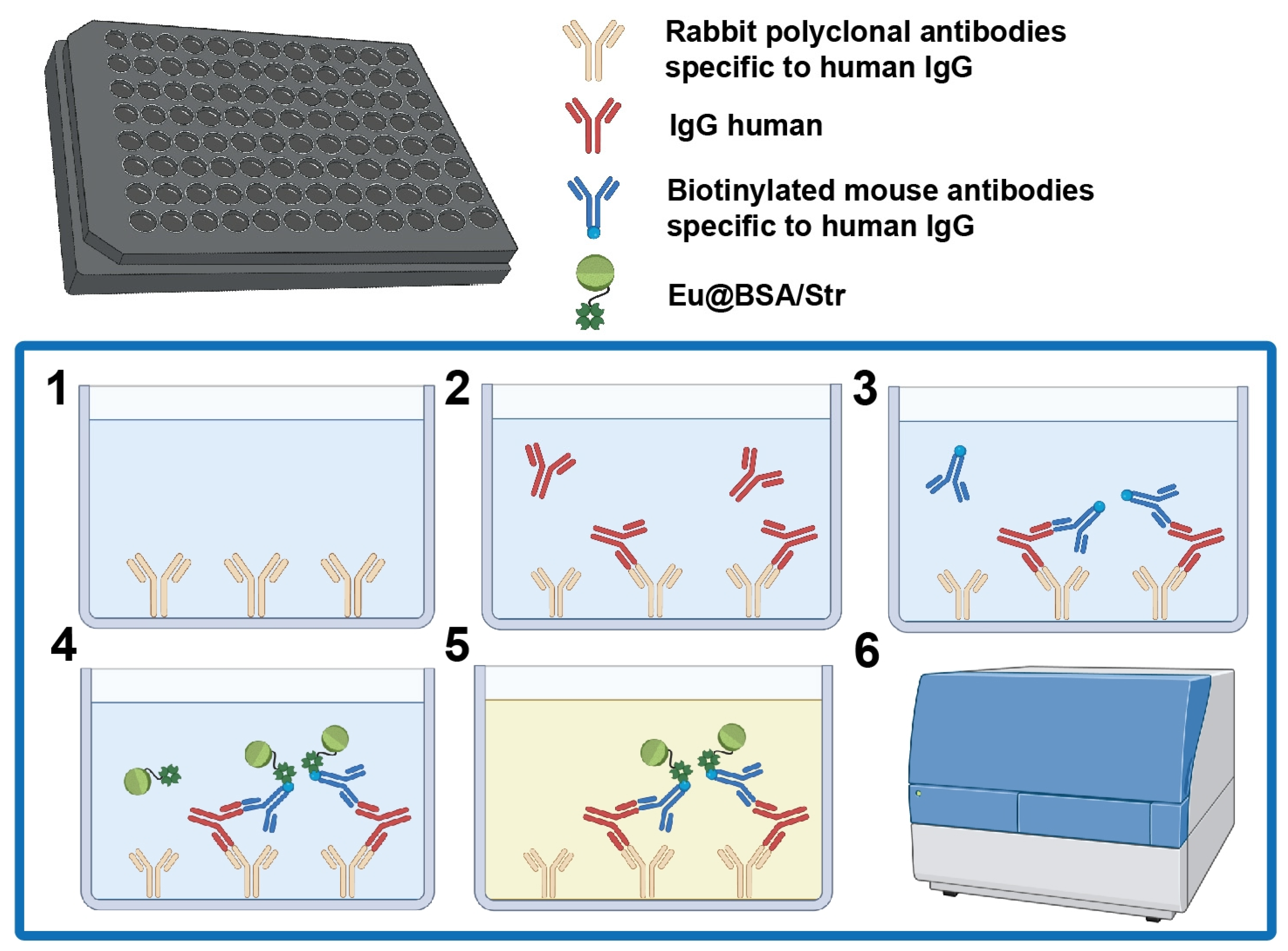
2.12. Time-Resolved Luminescent Immunoassay for Human IgG
2.13. Data Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Nanoparticles
3.2. Development of TRLI for Human IgG
3.2.1. Principle of the Assay
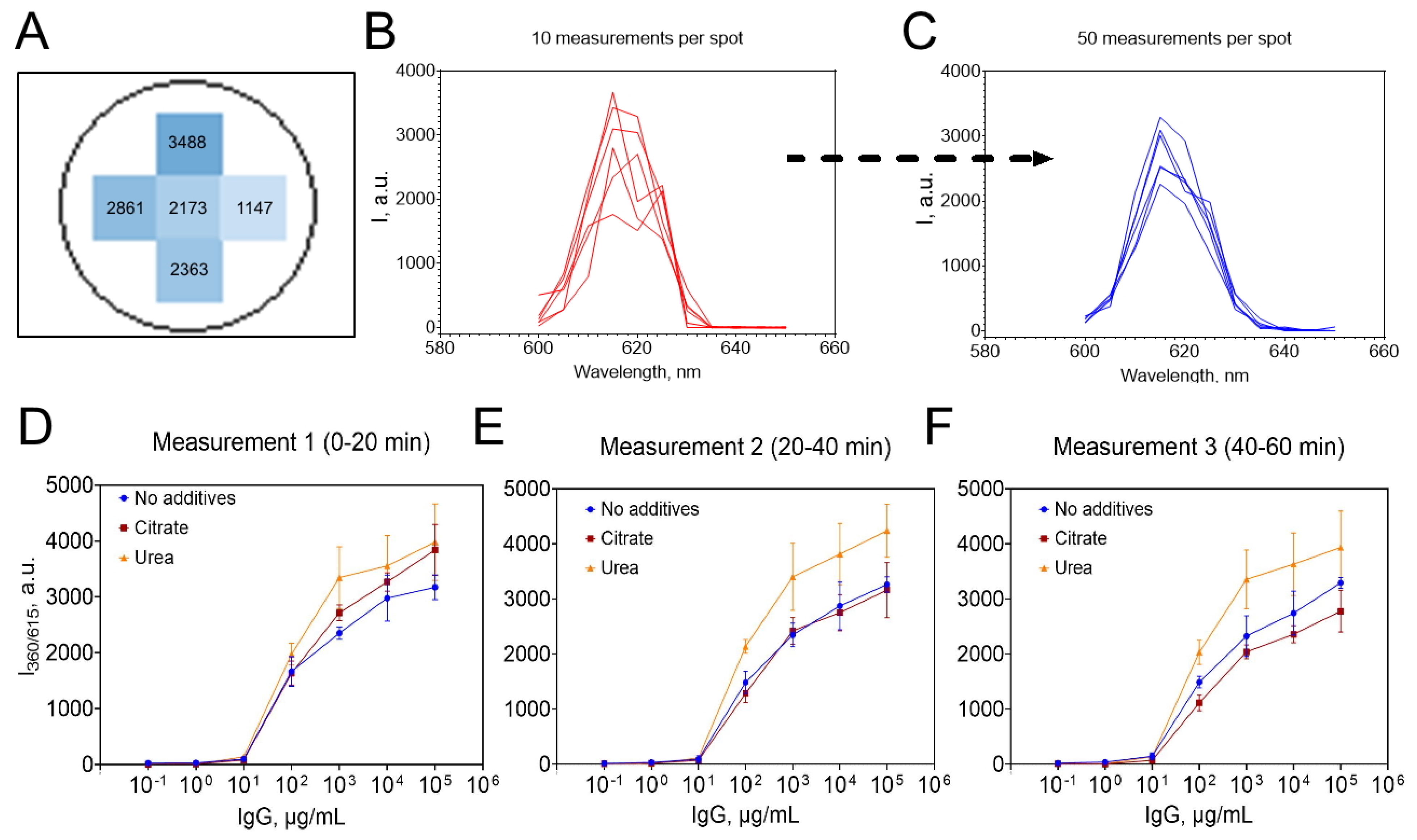
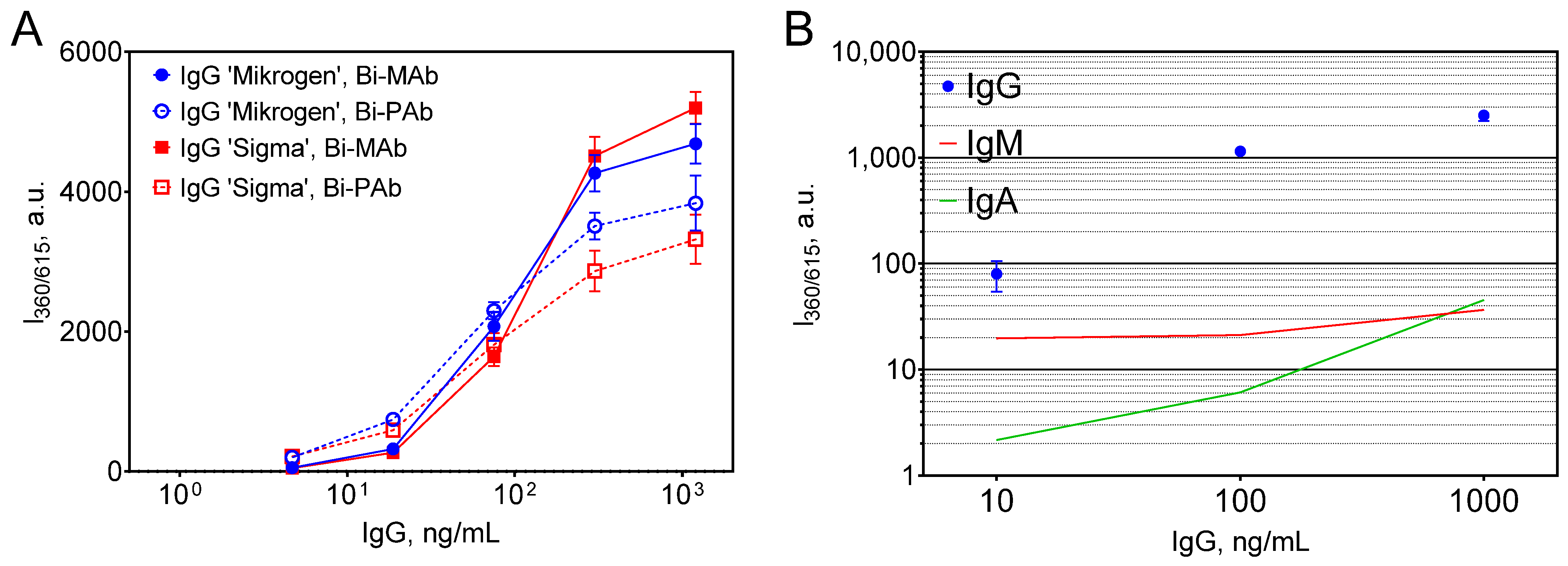
3.2.2. Challenges of TRLI
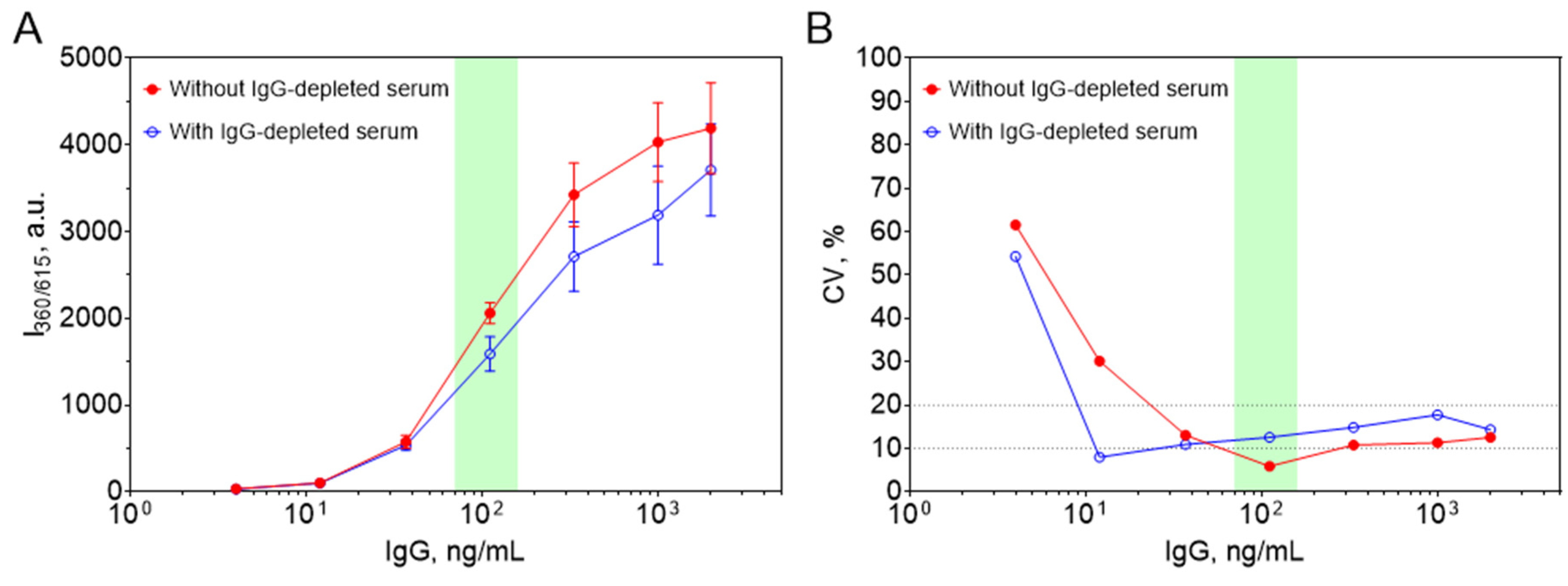
3.2.3. Optimization of TRLI
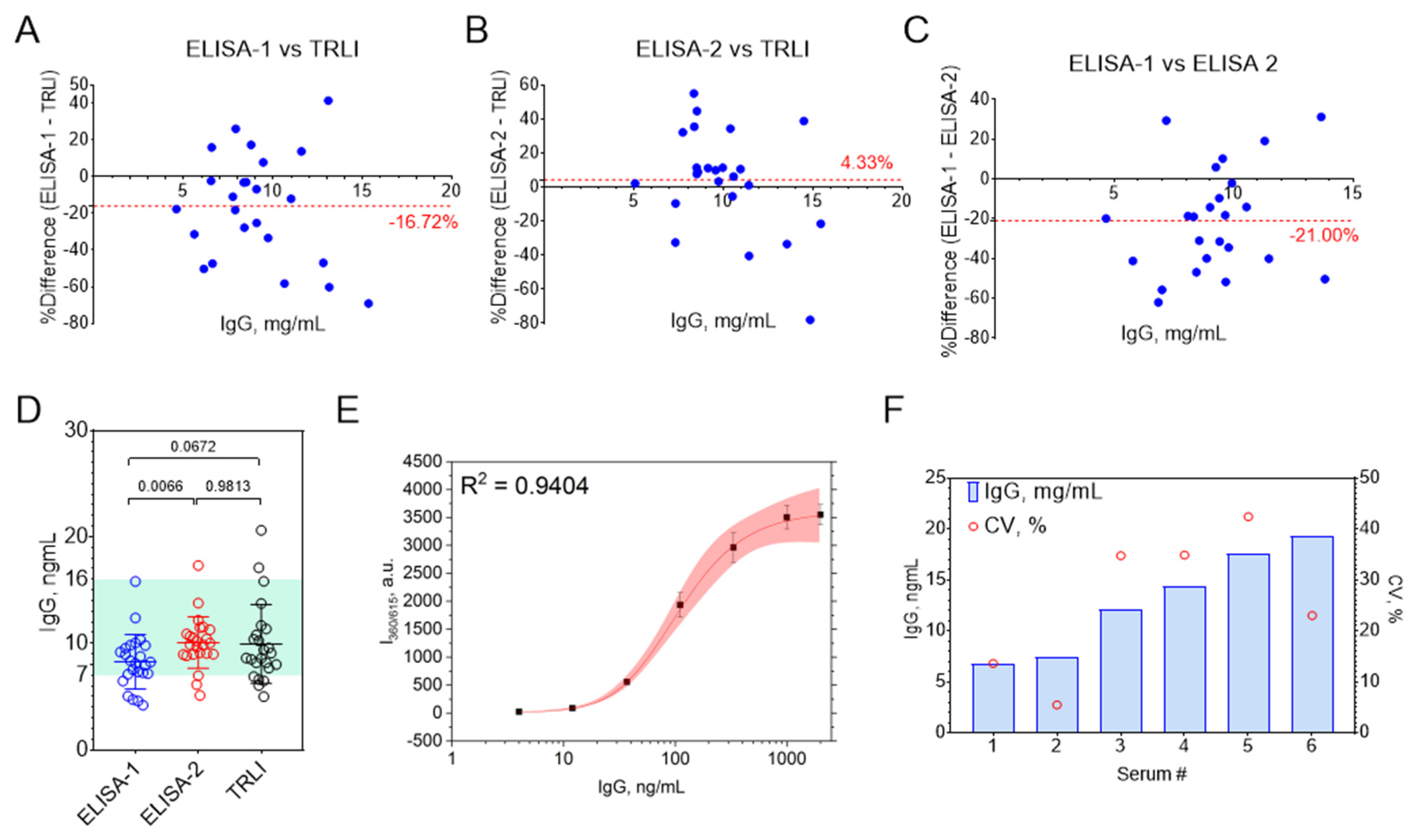
3.3. Comparison with Commercial ELISAs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.-Y.; Gupta, R.; et al. Photoluminescent Nanoparticles for Chemical and Biological Analysis and Imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef]
- Farka, Z.; Brandmeier, J.C.; Mickert, M.J.; Pastucha, M.; Lacina, K.; Skládal, P.; Soukka, T.; Gorris, H.H. Nanoparticle-Based Bioaffinity Assays: From the Research Laboratory to the Market. Adv. Mater. 2023, 36, e2307653. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Runser, A.; Bartenlian, H.; Nonat, A.M.; Charbonnière, L.J.; Klymchenko, A.S.; Hildebrandt, N.; Reisch, A. Lanthanide-Complex-Loaded Polymer Nanoparticles for Background-Free Single-Particle and Live-Cell Imaging. Chem. Mater. 2019, 31, 4034–4041. [Google Scholar] [CrossRef]
- Robin, M.P.; O'REilly, R.K. Strategies for preparing fluorescently labelled polymer nanoparticles. Polym. Int. 2014, 64, 174–182. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin Nanoparticle-Based Drug Delivery Systems. Int. J. Nanomed. 2024, ume 19, 6945–6980. [Google Scholar] [CrossRef]
- Khandelia, R.; Hodgkinson, T.; Crean, D.; Brougham, D.F.; Scholz, D.; Ibrahim, H.; Quinn, S.J.; Rodriguez, B.J.; Kennedy, O.D.; O’bYrne, J.M.; et al. Reproducible Synthesis of Biocompatible Albumin Nanoparticles Designed for Intra-articular Administration of Celecoxib to Treat Osteoarthritis. ACS Appl. Mater. Interfaces 2024, 16, 14633–14644. [Google Scholar] [CrossRef] [PubMed]
- Yedomon, B.; Fessi, H.; Charcosset, C. Preparation of Bovine Serum Albumin (BSA) nanoparticles by desolvation using a membrane contactor: A new tool for large scale production. Eur. J. Pharm. Biopharm. 2013, 85, 398–405. [Google Scholar] [CrossRef]
- Murphy, G.; Brayden, D.J.; Cheung, D.L.; Liew, A.; Fitzgerald, M.; Pandit, A. Albumin-based delivery systems: Recent advances, challenges, and opportunities. J. Control. Release 2025, 380, 375–395. [Google Scholar] [CrossRef]
- Borlan, R.; Stoia, D.; Gaina, L.; Campu, A.; Marc, G.; Perde-Schrepler, M.; Silion, M.; Maniu, D.; Focsan, M.; Astilean, S. Fluorescent Phthalocyanine-Encapsulated Bovine Serum Albumin Nanoparticles: Their Deployment as Therapeutic Agents in the NIR Region. Molecules 2021, 26, 4679. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Sun, Q.; Peng, Y.; Wang, R.; Zhou, Y.; Wang, Y.; Zhang, C.; Qi, X. Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. ACS Nano 2020, 14, 6191–6212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, X.; Kim, B.; Yao, S.; Bondar, M.V.; Belfield, K.D. Bovine Serum Albumin Nanoparticles with Fluorogenic Near-IR-Emitting Squaraine Dyes. ACS Appl. Mater. Interfaces 2013, 5, 8710–8717. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Liu, R.; Qu, C.; Qian, K.; Suo, Y.; Wu, F.; Chen, H.; Li, X.; Li, Y.; Liu, H.; et al. J-aggregates albumin-based NIR-II fluorescent dye nanoparticles for cancer phototheranostics. Mater. Today Bio 2022, 16, 100366. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, X.; Niu, S.; Zhang, W.; Gong, J.; Luo, Z.; Li, M.; Yi, C. AIEgen-Based and Smartphone-Assisted On-Site Quantitation of Cystatin C for Monitoring of Chronic Kidney Disease. Aggregate 2025, 6, e70127. [Google Scholar] [CrossRef]
- Xing, H.; Wei, T.; Lin, X.; Dai, Z. Near-infrared MnCuInS/ZnS@BSA and urchin-like Au nanoparticle as a novel donor-acceptor pair for enhanced FRET biosensing. Anal. Chim. Acta 2018, 1042, 71–78. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Prilepskii, A.; Lomova, M.; Khlebtsov, N. Au-nanocluster-loaded human serum albumin nanoparticles with enhanced cellular uptake for fluorescent imaging. J. Innov. Opt. Heal. Sci. 2016, 09, 1650004. [Google Scholar] [CrossRef]
- Xia, N.; Li, Y.; He, C.; Deng, D. Nanolabels Prepared by the Entrapment or Self-Assembly of Signaling Molecules for Colorimetric and Fluorescent Immunoassays. Biosensors 2024, 14, 597. [Google Scholar] [CrossRef]
- Khramtsov, P.; Galaeva, Z.; Khramtsova, E.; Zairov, R.; Dovzhenko, A.; Vasilyev, V.; Minin, A.; Bochkova, M.; Rayev, M. Paving a pathway for bright red-emitting Eu-loaded albumin nanoparticles fabrication. Colloids Surfaces B Biointerfaces 2025, 257, 115140. [Google Scholar] [CrossRef]
- Alexander, C.; Guo, Z.; Glover, P.B.; Faulkner, S.; Pikramenou, Z. Luminescent Lanthanides in Biorelated Applications: From Molecules to Nanoparticles and Diagnostic Probes to Therapeutics. Chem. Rev. 2025, 125, 2269–2370. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Ye, Q.; Qin, S.; Li, L.; Teng, M.; Wong, W.-Y. Progress in Luminescent Materials Based on Europium(III) Complexes of β-Diketones and Organic Carboxylic Acids. Molecules 2025, 30, 1342. [Google Scholar] [CrossRef]
- Hagan, A.K.; Zuchner, T. Lanthanide-based time-resolved luminescence immunoassays. Anal. Bioanal. Chem. 2011, 400, 2847–2864. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, X.; Liang, K.; Gao, M.; Kong, B. Frontier luminous strategy of functional silica nanohybrids in sensing and bioimaging: From ACQ to AIE. Aggregate 2021, 3, e121. [Google Scholar] [CrossRef]
- Gao, F.; Ye, S.; Huang, L.; Gu, Z. A nanoparticle-assisted signal-enhancement technique for lateral flow immunoassays. J. Mater. Chem. B 2024, 12, 6735–6756. [Google Scholar] [CrossRef]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Frey, A.; Meckelein, B.; Externest, D.; Schmidt, M. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 2000, 233, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yonath, A.; Sielecki, A.; Moult, J.; Podjarny, A.; Traub, W. Crystallographic studies of protein denaturation of renaturation. 1. Effects of denaturants on volume and x-ray pattern of crosslinked triclinic lysozyme crystals. Biochemistry 1977, 16, 1413–1417. [Google Scholar] [CrossRef]
- Kuzuya, A.; Numajiri, K.; Kimura, M.; Komiyama, M. Single-Molecule Accommodation of Streptavidin in Nanometer-Scale Wells Formed in DNA Nanostructures. Nucleic Acids Symp. Ser. 2008, 52, 681–682. [Google Scholar] [CrossRef]
- Salanov, A.; Serkova, A.; Zhirnova, A.; Perminova, L.; Kovalenko, G. Supramolecular Aggregation of Nanoparticles on Aluminum and Gold Surfaces Occurring in Bovine Serum Albumin Solutions. Micro 2022, 2, 334–341. [Google Scholar] [CrossRef]
- Regev, O.; Khalfin, R.; Zussman, E.; Cohen, Y. About the albumin structure in solution and related electro-spinnability issues. Int. J. Biol. Macromol. 2010, 47, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Hovinen, J.; Guy, P.M. Bioconjugation with Stable Luminescent Lanthanide(III) Chelates Comprising Pyridine Subunits. Bioconjugate Chem. 2008, 20, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, E.P.; Bhayana, V.; Conway, K.; Reichstein, E.; Papanastasiou-Diamandis, A. Time-resolved fluoroimmunoassay of cortisol in serum with a europium chelate as label. Clin. Biochem. 1988, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos-Franco, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Rossi, C.A.; Kijek, T.M.; Henchal, E.A.; Ludwig, G.V. Comparison of Dissociation-Enhanced Lanthanide Fluorescent Immunoassays to Enzyme-Linked Immunosorbent Assays for Detection of Staphylococcal Enterotoxin B, Yersinia pestis -Specific F1 Antigen, and Venezuelan Equine Encephalitis Virus. Clin. Diagn. Lab. Immunol. 2001, 8, 1070–1075. [Google Scholar] [CrossRef]
- E Van Eeden, P.; Wiese, M.D.; Aulfrey, S.; Hales, B.J.; Stone, S.F.; A Brown, S.G. Using Time-Resolved Fluorescence to Measure Serum Venom-Specific IgE and IgG. PLoS ONE 2011, 6, e16741. [Google Scholar] [CrossRef]
- Allicotti, G.; Borras, E.; Pinilla, C. A Time-Resolved Fluorescence Immunoassay (DELFIA) Increases the Sensitivity of Antigen-Driven Cytokine Detection. J. Immunoass. Immunochem. 2003, 24, 345–358. [Google Scholar] [CrossRef]
- Cicalini, I.; Del Boccio, P.; Zucchelli, M.; Rossi, C.; Natale, L.; Demattia, G.; De Bellis, D.; Damiani, V.; Tommolini, M.L.; Pizzinato, E.; et al. Validation of the GSP®/DELFIA® Anti-SARS-CoV-2 IgG Kit Using Dried Blood Samples for High-Throughput Serosurveillance and Standardized Quantitative Measurement of Anti-Spike S1 IgG Antibody Responses Post-Vaccination. Vaccines 2022, 10, 514. [Google Scholar] [CrossRef]
- Dufrusine, B.; Natale, L.; Sallese, M.; Mozzillo, E.; Di Candia, F.; Cuccurullo, I.; Iafusco, D.; Zanfardino, A.; Passariello, L.; Iannilli, A.; et al. Development and validation of a novel method for evaluation of multiple islet autoantibodies in dried blood spot using dissociation-enhanced lanthanide fluorescent immunoassays technology, specific and suitable for paediatric screening programmes. Diabetes Obes. Metab. 2024, 27, 414–418. [Google Scholar] [CrossRef]
| Sample | Dh, nm | PdI | Zeta potential, mV | Luminescence Intensity (360/615, 1 μg/mL), a.u. |
|---|---|---|---|---|
| Eu@BSA | 244 ± 16 | 0.139 ± 0.09 | −19.5 ± 0.4 | 14,200 ± 2193 |
| Glutaraldehyde-treated Eu@BSA | 253 ± 23 | 0.102 ± 0.08 | −26.2 ± 0.7 | 6484 ± 1446 |
| Eu@BSA/Str | 263 ± 5 | 0.051 ± 0.03 | −26.1 ± 1.1 | 3277 ± 247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galaeva, Z.; Bochkova, M.; Rayev, M.; Khramtsov, P. Europium Complex-Loaded Albumin Nanoparticles as Probes for Time-Resolved Luminescent Immunoassay. Biosensors 2025, 15, 761. https://doi.org/10.3390/bios15110761
Galaeva Z, Bochkova M, Rayev M, Khramtsov P. Europium Complex-Loaded Albumin Nanoparticles as Probes for Time-Resolved Luminescent Immunoassay. Biosensors. 2025; 15(11):761. https://doi.org/10.3390/bios15110761
Chicago/Turabian StyleGalaeva, Zarina, Maria Bochkova, Mikhail Rayev, and Pavel Khramtsov. 2025. "Europium Complex-Loaded Albumin Nanoparticles as Probes for Time-Resolved Luminescent Immunoassay" Biosensors 15, no. 11: 761. https://doi.org/10.3390/bios15110761
APA StyleGalaeva, Z., Bochkova, M., Rayev, M., & Khramtsov, P. (2025). Europium Complex-Loaded Albumin Nanoparticles as Probes for Time-Resolved Luminescent Immunoassay. Biosensors, 15(11), 761. https://doi.org/10.3390/bios15110761






