Rational Design of Covalent Organic Frameworks for Enhanced Reticular Electrochemiluminescence and Biosensing Applications
Abstract
1. Introduction
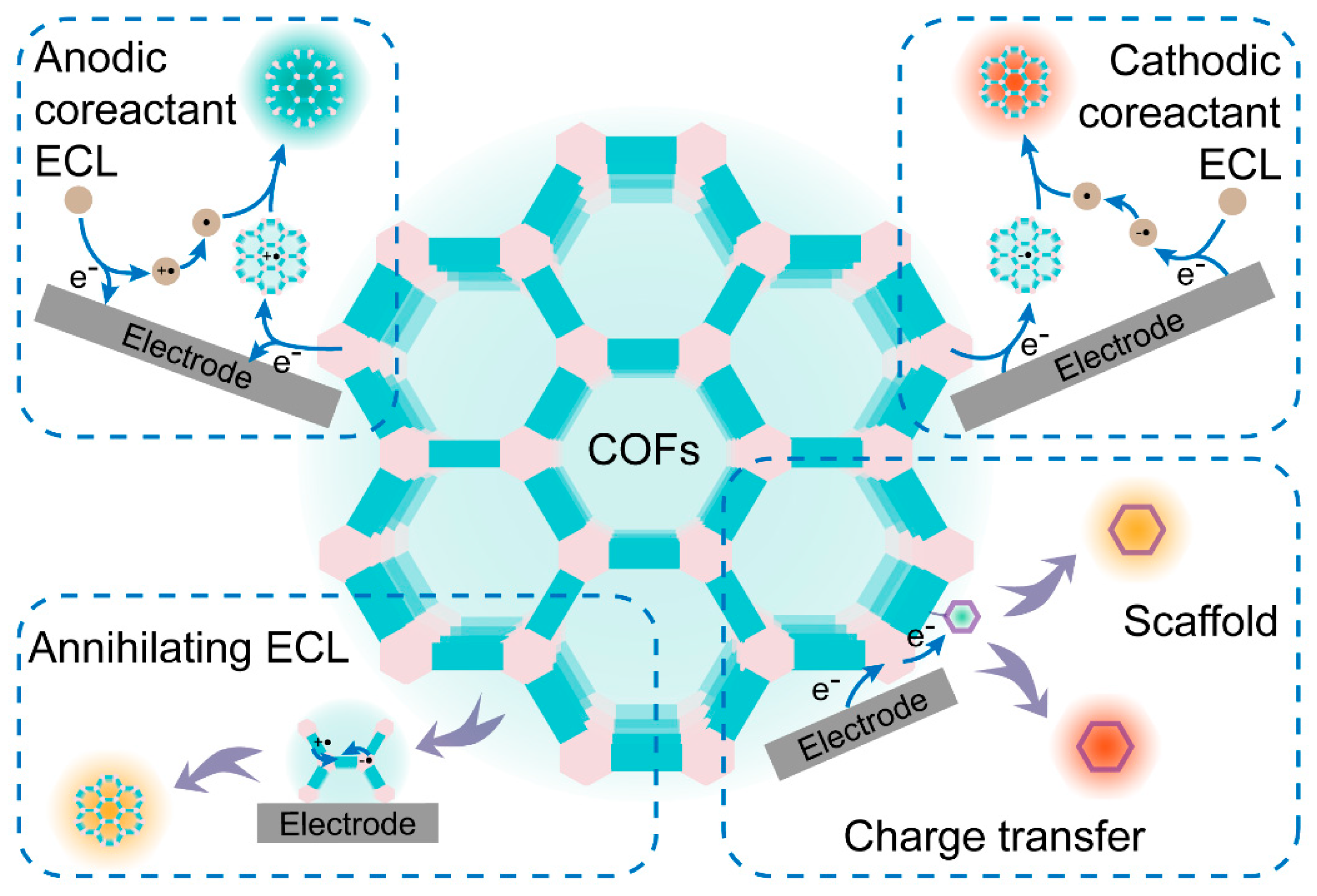
2. Rational Design of COFs for Enhanced ECL
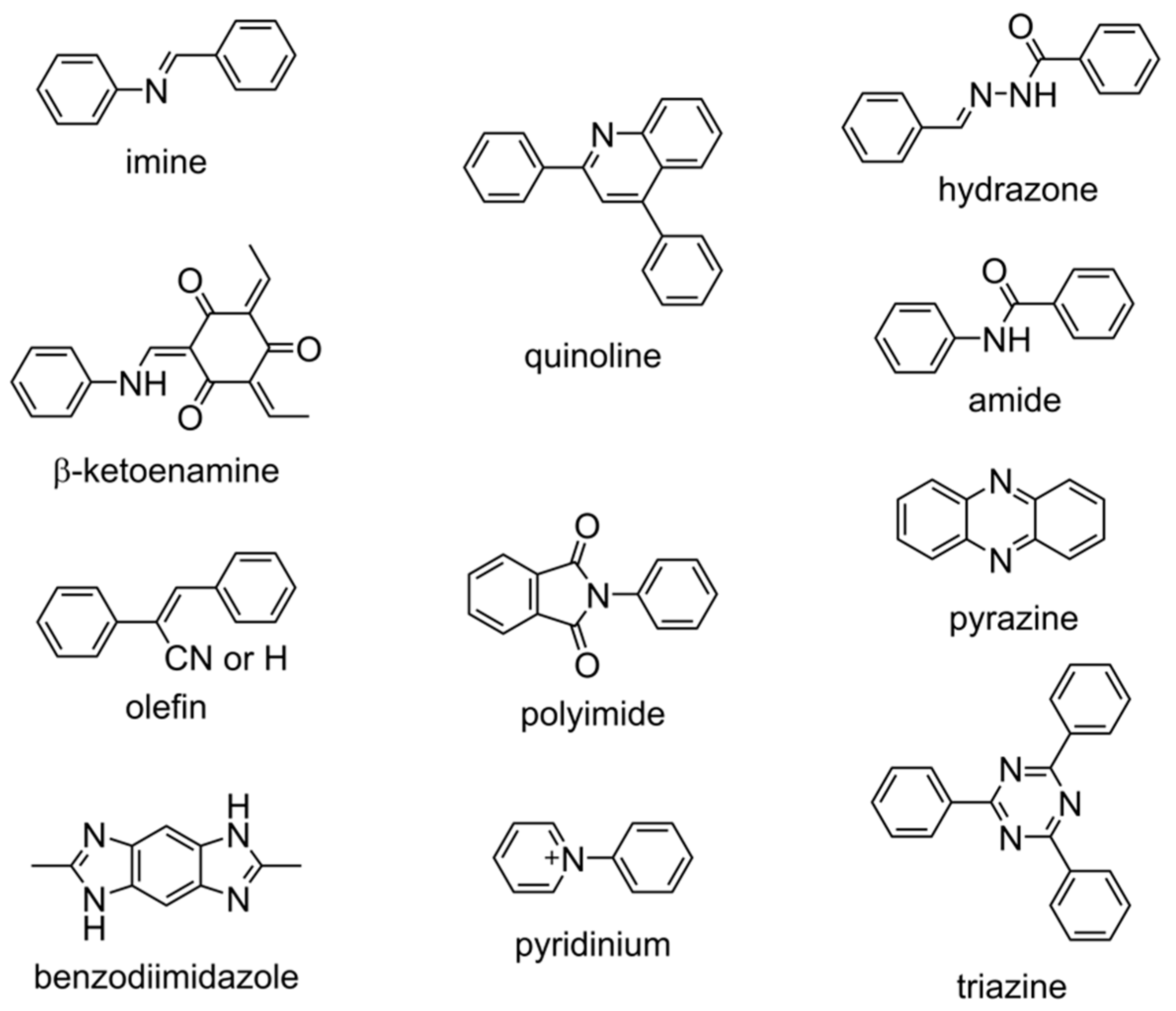
2.1. Linkage Chemistry
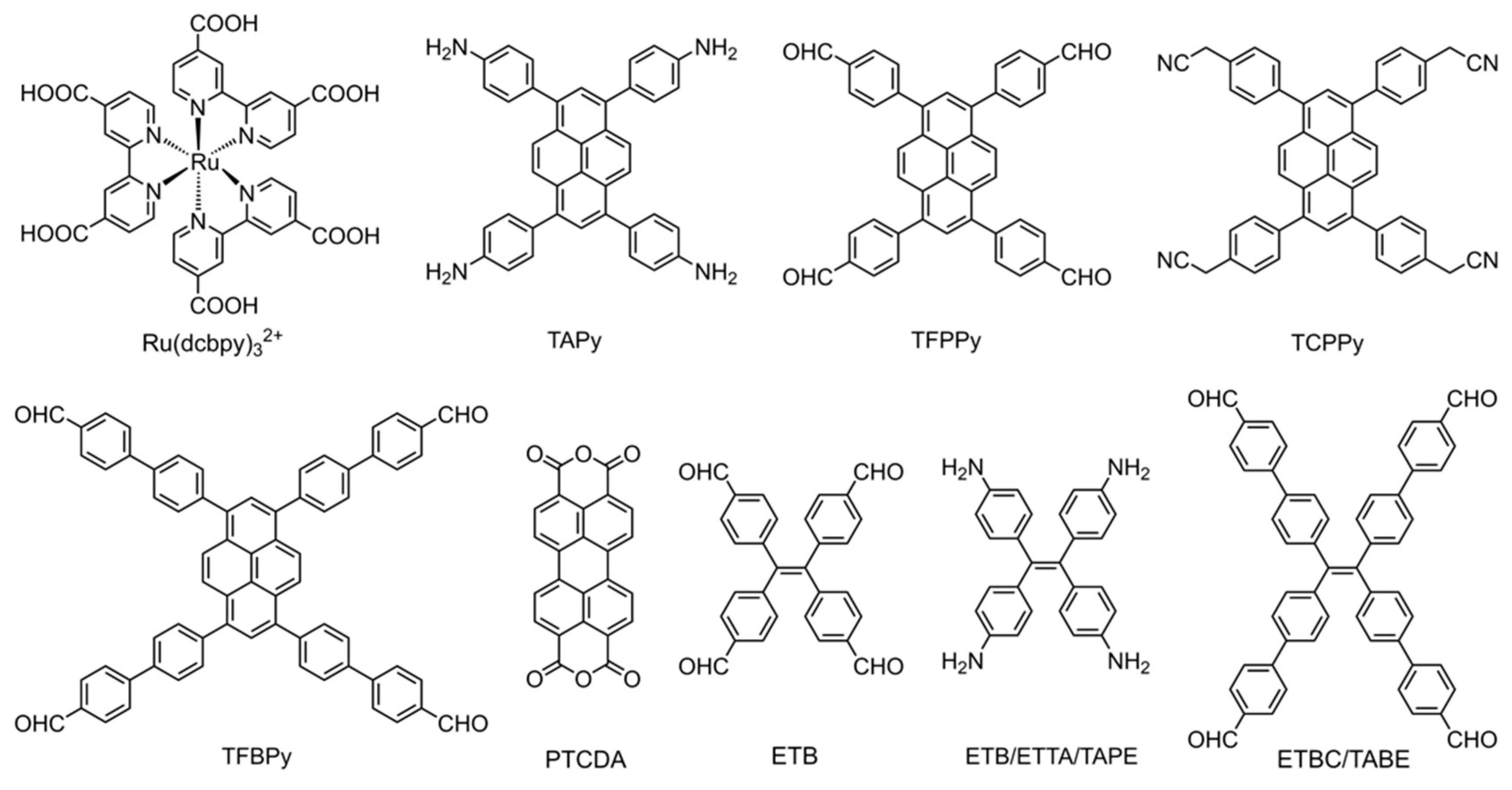
2.2. Luminophore Monomers
2.3. Precise Regulation of ECL-Active COFs
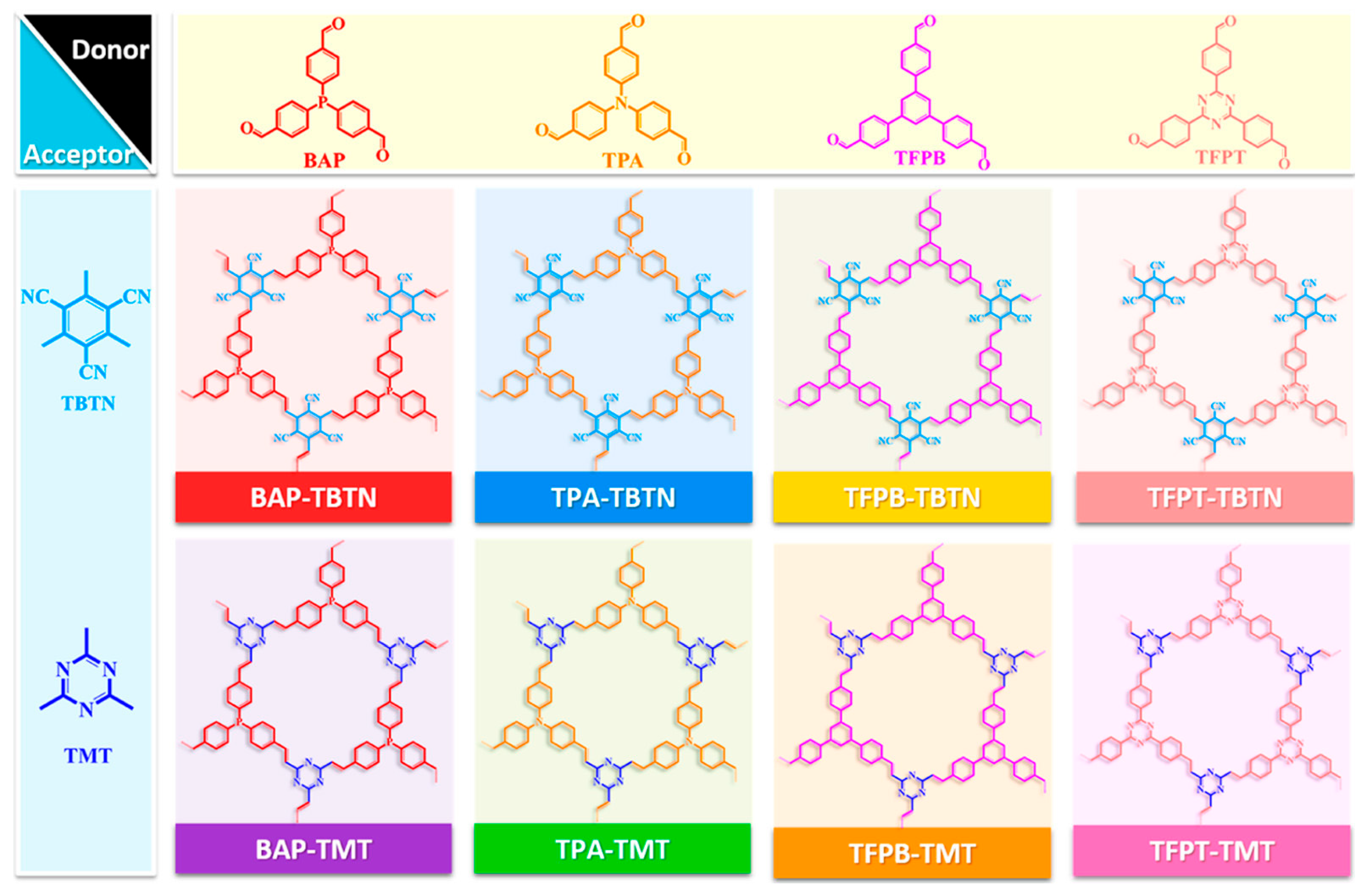
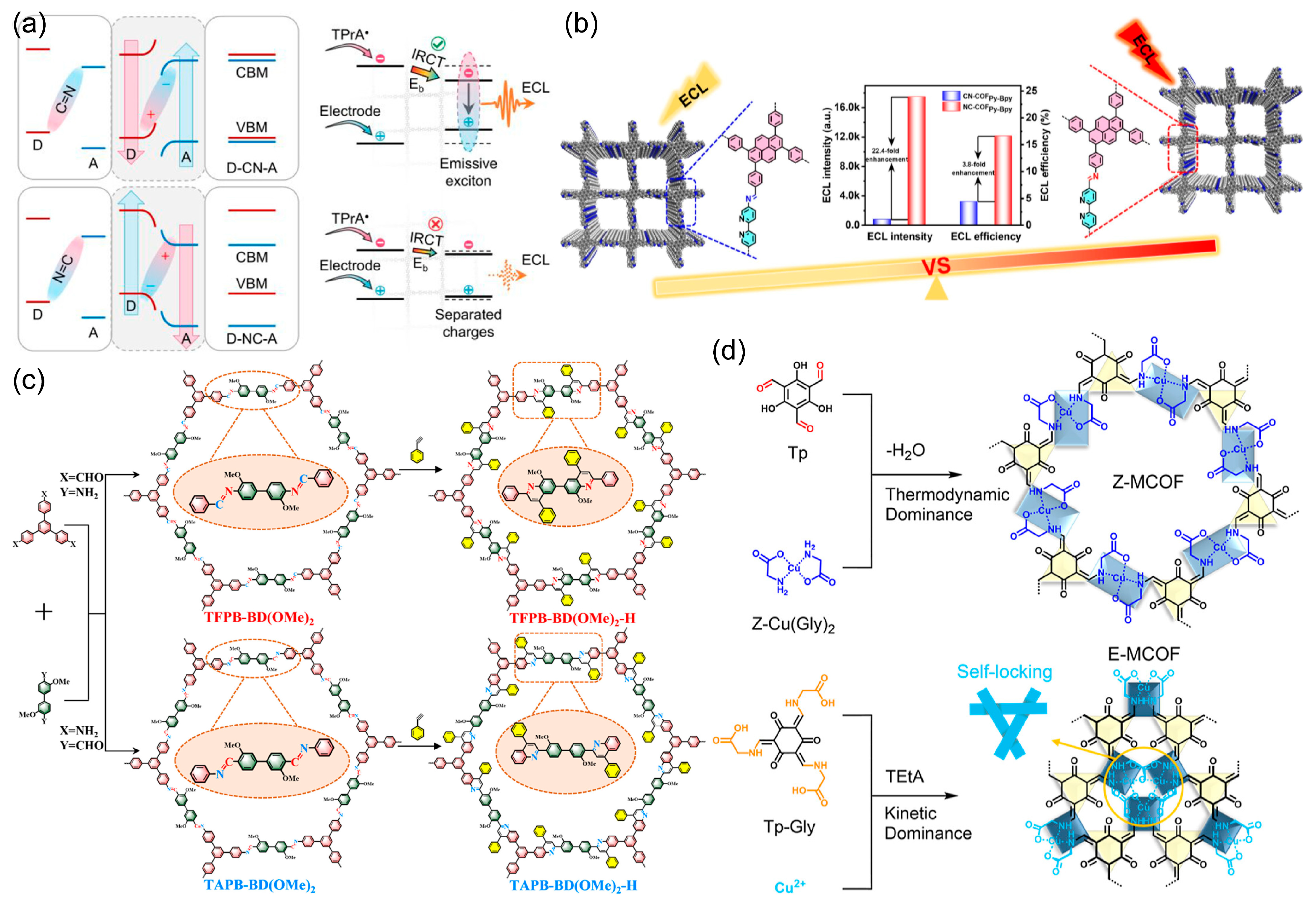
2.3.1. Donor–Acceptor π-Conjugation and Bandgap Regulation
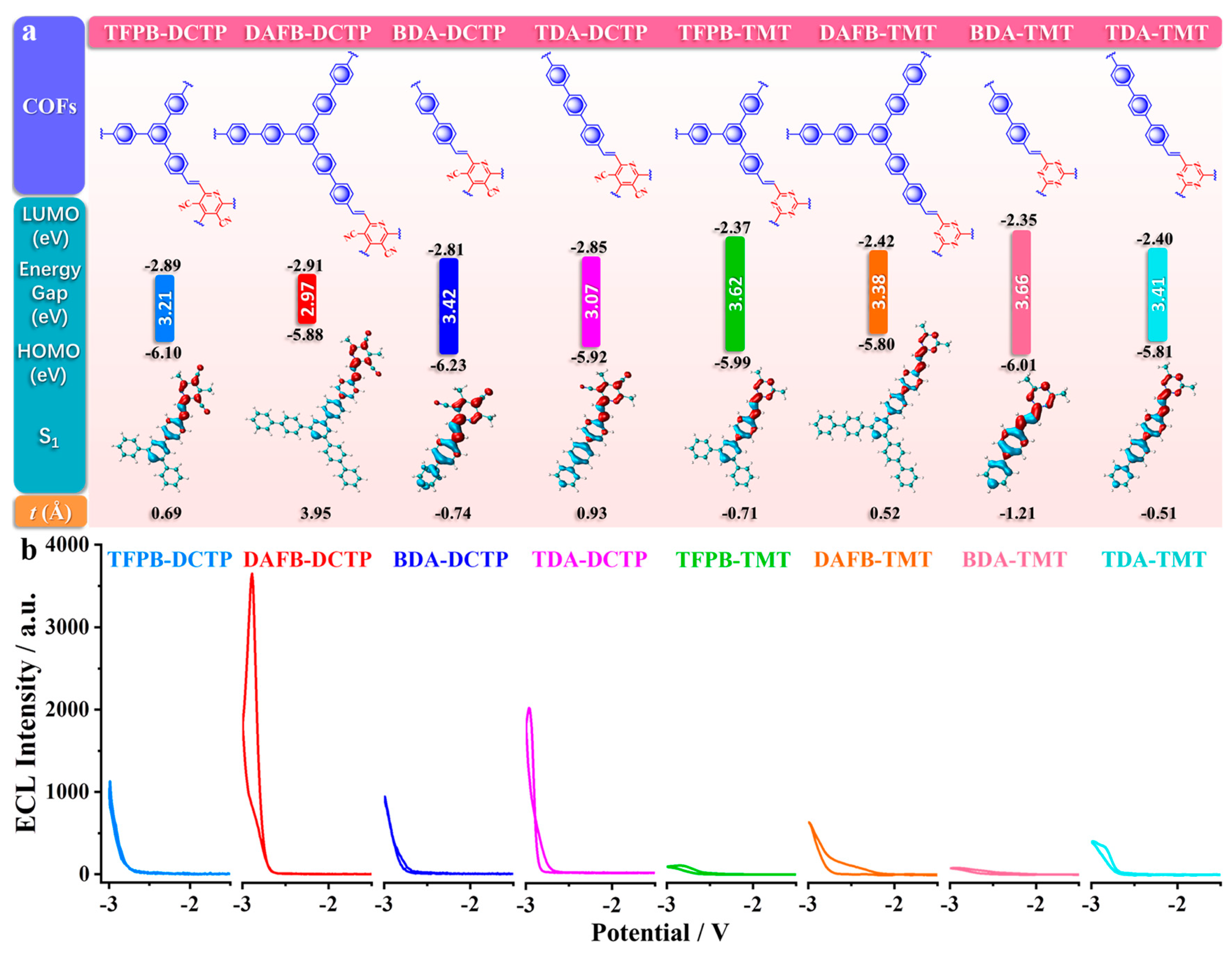
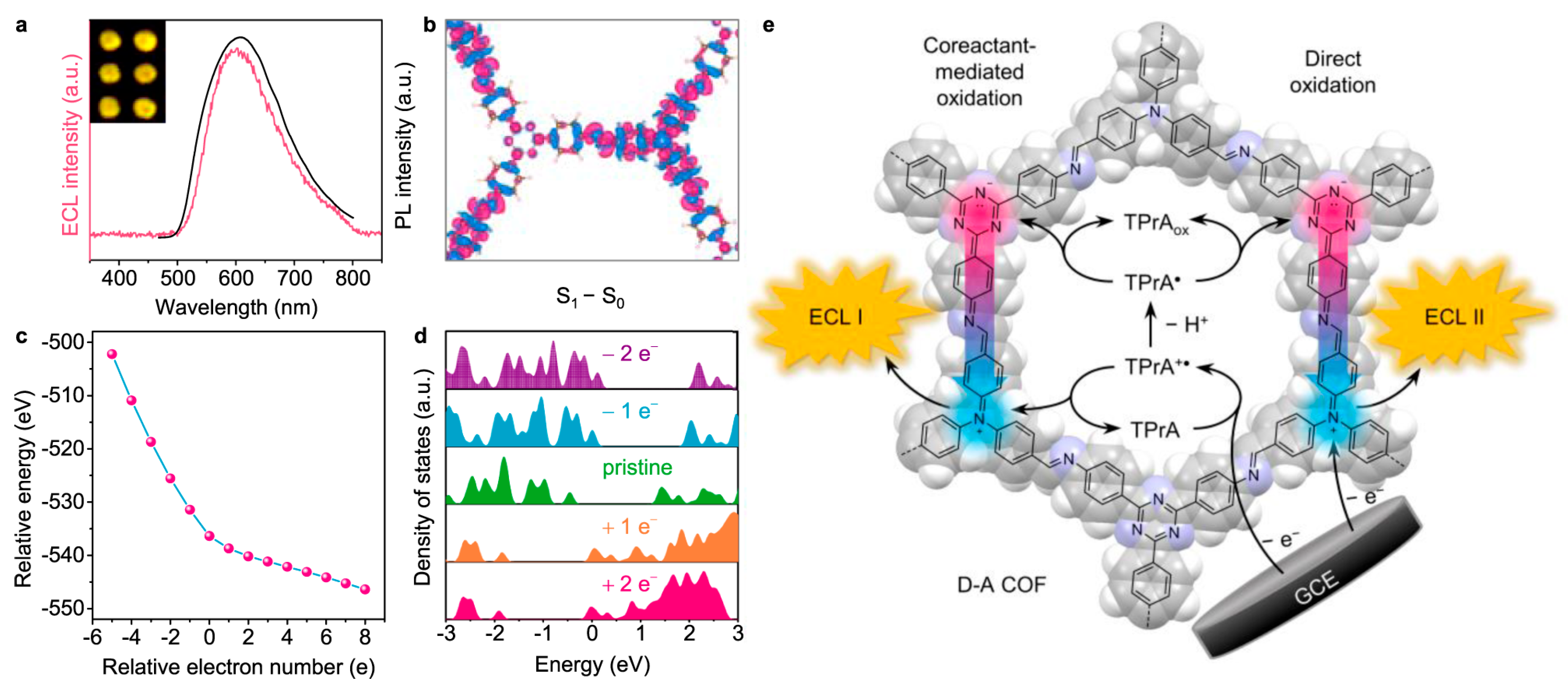
2.3.2. Heteroatom Content Modulation
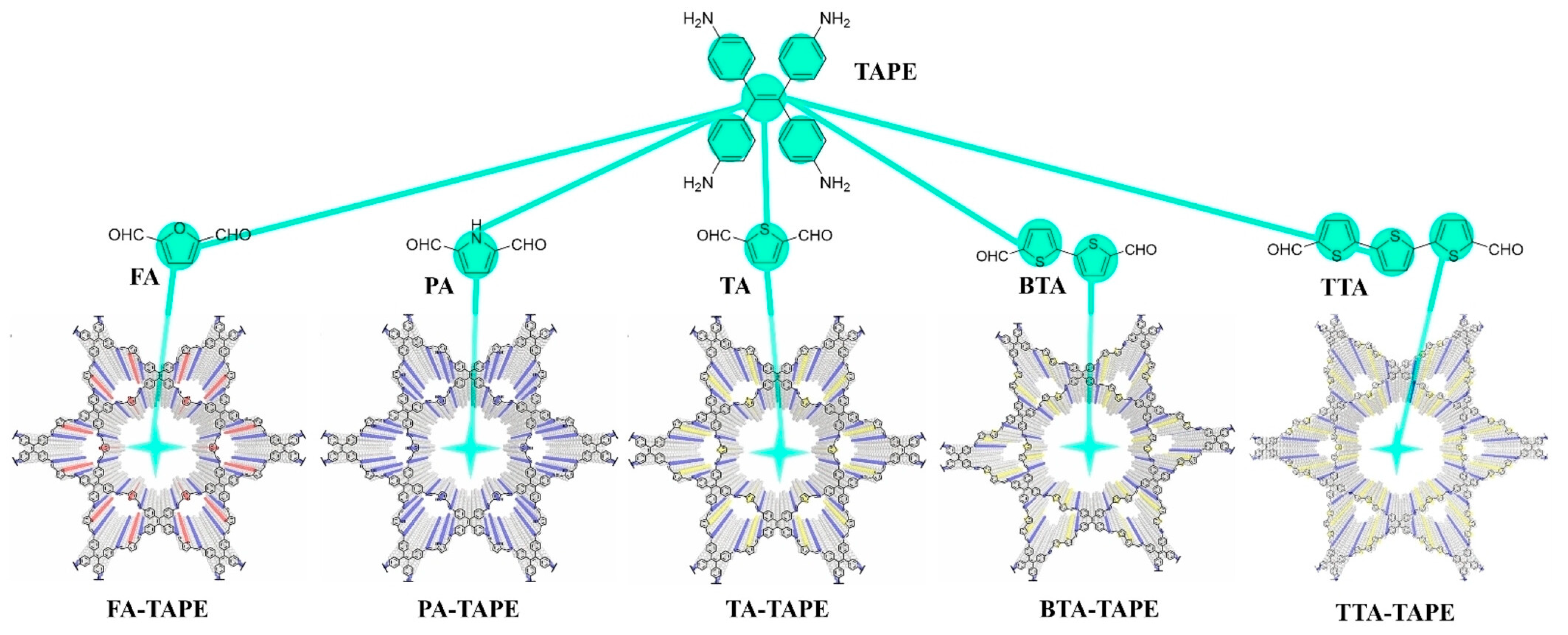
2.3.3. Structural Isomerism
2.3.4. Substituted Groups
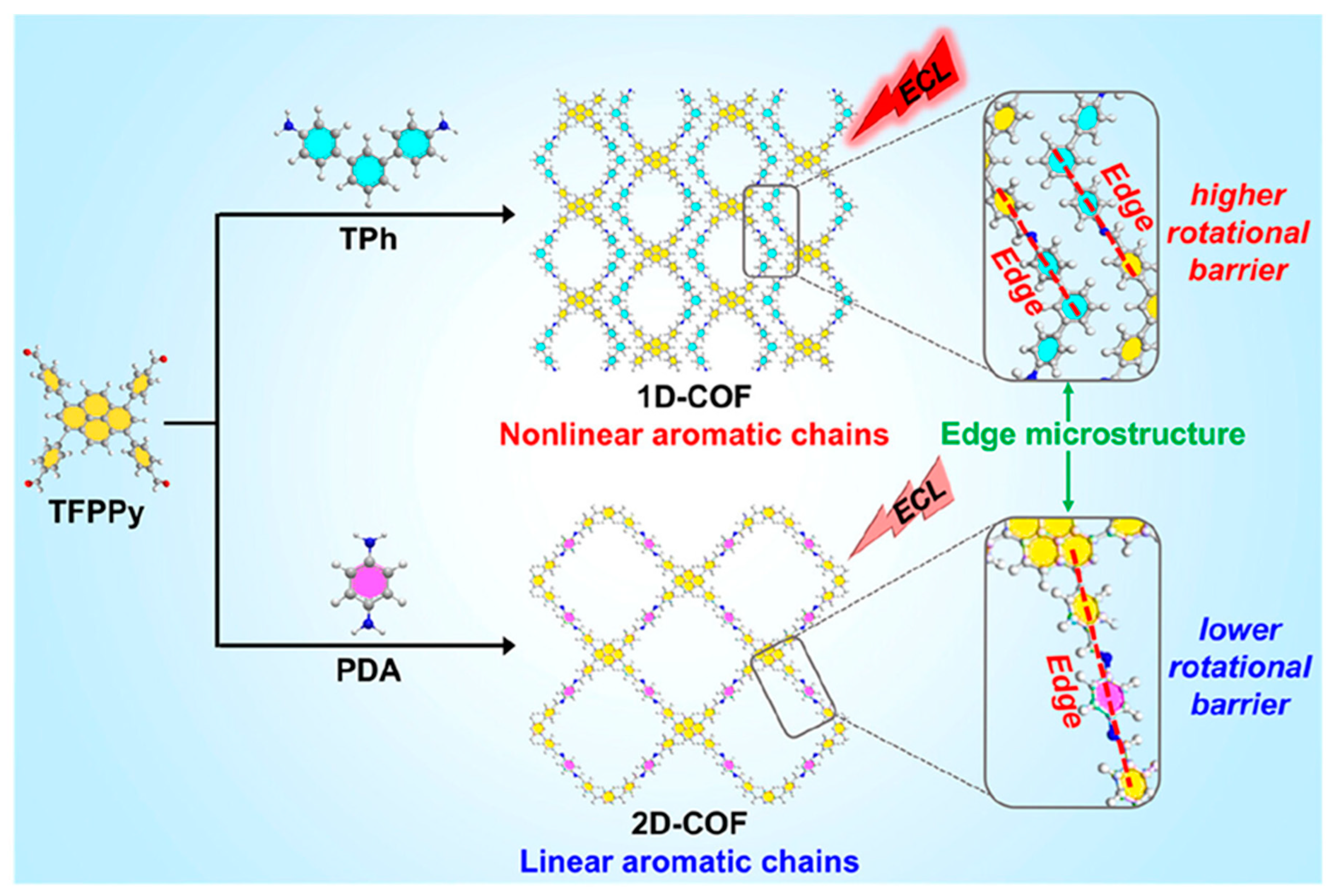
2.3.5. Dimensional Regulation
2.4. Post-Synthesis and Functionalization
2.5. COF-Based Composites for ECL
2.6. Other ECL Enhancement Strategies
3. Applications in Biosensing and Monitoring
3.1. Biosensing
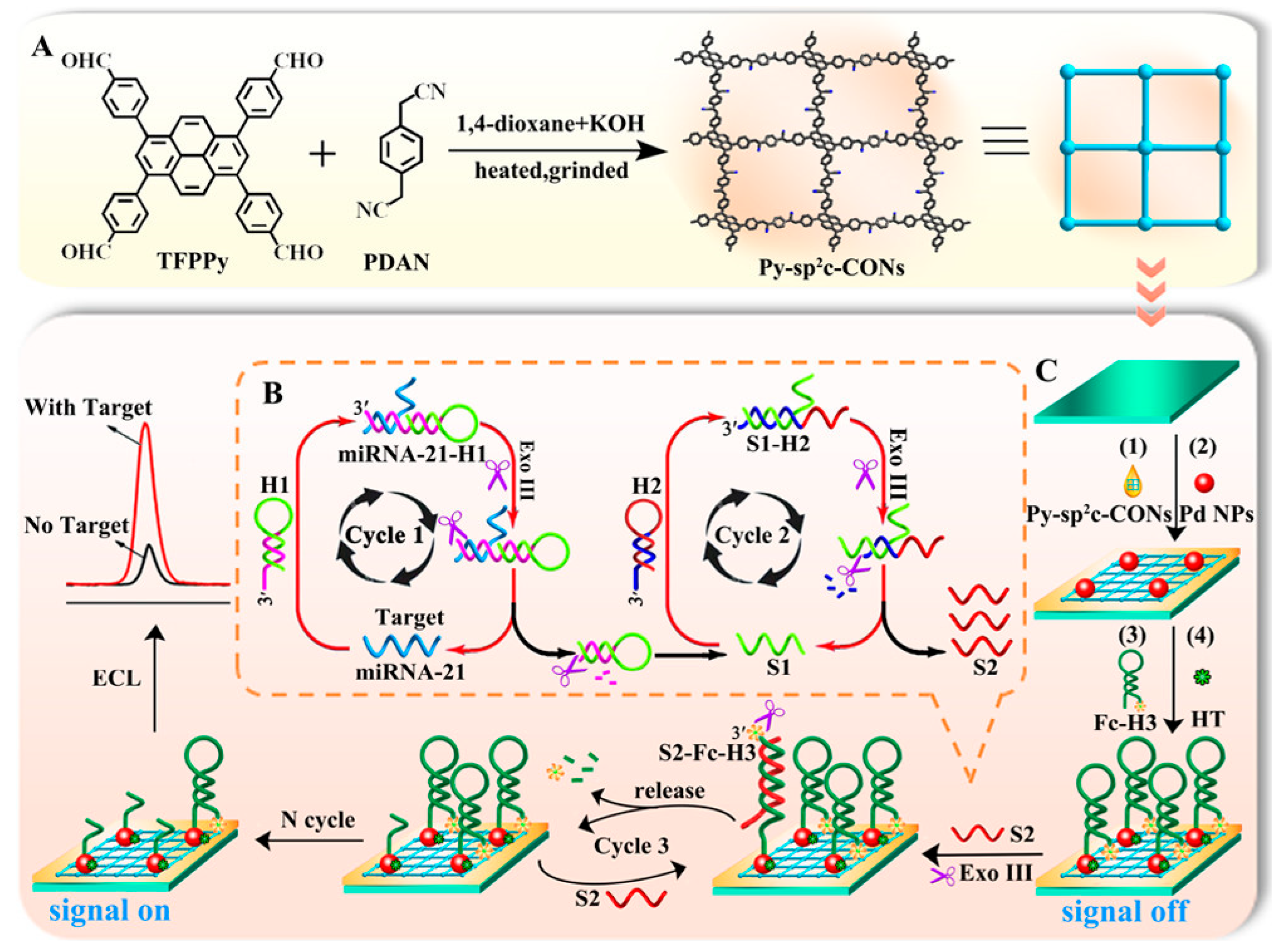
3.1.1. Nucleic-Acid Disease Signatures
3.1.2. Cellular Cancer Entities
3.1.3. Protein Biomarkers
3.1.4. Bioactive and Signaling Molecules
3.1.5. Psychiatric Small-Molecule Drugs
3.2. Food Safety Assay
3.3. Environmental Monitoring
3.4. Enantioselective Sensing
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-CEC | cathinones 4-chloroethcathinone |
| AAV8 | adeno-associated virus serotype 8 |
| ABEI | N-(4-aminobutyl)-N-ethylisoluminol |
| AChE | acetylcholinesterase |
| ACQ | aggregation-caused quenching |
| AD | Alzheimer’s disease |
| AFM1 | aflatoxin M1 |
| AIE | aggregation-induced emission |
| AIECL | aggregation-induced electrochemiluminescence |
| ALP | alkaline phosphatase |
| Aβ | amyloid-β |
| BAP | benzaldehyde-4,4′4″-phosphinidynetris |
| BAP | benzaldehyde,4,4′4″-phosphinidynetris |
| BaP | benzo(a)pyrene |
| BCBA | 4-[4-[4-(4formylphenyl)-N-[4-(4-formylphenyl)phenyl]anilino]phenyl]benzaldehyde |
| BTA | benzene-1,2,4,5-tetramine |
| BTT | 1,3,5-tris(4-formylphenyl)benzothiadiazole |
| C3G | cyanidin-3-O-glucoside |
| CAP | chloramphenicol |
| CB | conductance bands |
| CEA | carcinoembryonic antigen |
| CFX | ciprofloxacin |
| COF | covalent organic framework |
| CRT-ECL | covalent rigidification-triggered Electrochemiluminescence |
| CTCs | Rare circulating tumor cells |
| CTF | covalent triazine framework |
| cTnl | cardiac troponin I |
| cyt c | cytochrome c |
| DA | dopamine |
| D-A | donor–acceptor |
| DAFB | 4-[4-[3,5-bis[4-(4-formylphenyl)phenyl]phenyl]phenyl]benzoic acid |
| D-Arg | D-arginine |
| DBAE | N,N’-dibutyl-2-hydroxyethylamine |
| DCTP | 2,4,6-trimethylpyridine-3,5-dicarbonitrile |
| DEDA | N,N’-diethyl ethylenediamine |
| DFT | density function theory |
| DMeTHz | 2,5-dimethoxyterephthalohydrazide |
| DMTP | 2,5-dimethoxyterephthalaldehyde |
| DVA | 2,5-divinylterephthalaldehyde |
| ECL | electrochemiluminescence |
| ESP | electrostatic-potential polarity |
| ET | electron transfer |
| ETB | 4,4′,4″,4‴-(ethene-1,1,2,2-tetrayl)tetrabenzaldehyde |
| FIECL | framework-induced electrochemiluminescence |
| GCE | glassy carbon electrode |
| HATP | 2,3,6,7,10,11-hexaaminotriphenylene |
| HCR | hybridization chain reaction |
| HHTP | 2,3,6,7,10,11-hexahydroxytriphenylene |
| HOMOs | highest occupied molecular orbitals |
| iBF | isobutyryl fentanyl |
| ICT | intramolecular charge transfer |
| IRCT | intramolecular charge transfer |
| LOD | limits of detection |
| L-PA | L-penicillamine |
| L-Phe | L-phenylalanine |
| LUMOs | lowest unoccupied molecular orbitals |
| MA | melamine |
| Mal | Malathion |
| MCOF | metal covalent organic framework |
| MOF | metal–organic framework |
| NDMA | N-nitrosodimethylamine |
| OTA | ochratoxin A |
| PDA | terephthalaldehyde |
| PET | photoinduced electron |
| PTCDA | perylene-3,4,9,10-tetracarboxylic dianhydride |
| PZ | piperazine |
| RET | resonance energy transfer |
| Ru(dcbpy)32+ | tris(4,4′-dicarboxylicacid-2,2′-bipyridyl)ruthenium(II) |
| TABE | tetra-(4-aldehyde-(1,1-biphenyl))ethylene |
| TAPB | 1,3,5-tris(4- aminophenyl)benzene |
| TB | thrombin |
| TBDA | 2,5-di(thiophen-2-yl)benzene-1,4-diamine |
| TBTN | 2,4,6-trimethylbenzene-1,3,5-tricarbonitrile |
| Tc | tetracycline |
| TCNQ | tetracyanoquinodimethane |
| TEA | triethylamine |
| TFPB | 1,3,5-tri(4-formylphenyl)benzene |
| TFPD | 4,40,40′-(pyrimidine-2,4,6-triyl) tribenzaldehyde |
| TFPP | 4,40,40′-(pyridine-2,4,6-triyl) tribenzaldehyde |
| TFPPy | 1,3,6,8-tetrakis(4-formylphenyl)pyrene |
| TFPT | 4,40,40′-(1,3,5-triazine-2,4,6-triyl) tribenzaldehyde |
| TMT | trimethyltriazine/2,4,6-trimethyl-1,3,5-triazine |
| Tp | 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde |
| TPA | tris(4-formylphenyl)amine |
| TPh | (1,1′:3′,1″-terphenyl)-4,4″-diamine |
| TPrA | Tri-n-propylamine |
| TSLP | thymic stromal lymphopoietin |
| VB | valence bands |
| XEN | zearalenone |
References
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, W.; Xu, G. Recent Advances in Electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef]
- Ma, X.; Gao, W.; Du, F.; Yuan, F.; Yu, J.; Guan, Y.; Sojic, N.; Xu, G. Rational Design of Electrochemiluminescent Devices. Acc. Chem. Res. 2021, 54, 2936–2945. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Tan, L.; Tan, Y.; Wang, Y.; Ye, Z.; Hou, C.; Xu, Y.; Liu, S.; Wang, G. Frontier and Hot Topics in Electrochemiluminescence Sensing Technology Based on CiteSpace Bibliometric Analysis. Biosens. Bioelectron. 2022, 201, 113932. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, N.; Ju, H. Electrochemiluminescence Biosensing and Bioimaging with Nanomaterials as Emitters. Sci. China Chem. 2022, 65, 2417–2436. [Google Scholar] [CrossRef]
- Giagu, G.; Fracassa, A.; Fiorani, A.; Villani, E.; Paolucci, F.; Valenti, G.; Zanut, A. From Theory to Practice: Understanding the Challenges in the Implementation of Electrogenerated Chemiluminescence for Analytical Applications. Microchim. Acta 2024, 191, 359. [Google Scholar] [CrossRef]
- Dong, Z.; Du, F.; Zhang, W.; Tian, Y.; Xu, G. Recent Advances in Tetraphenylethylene-Based Aggregation-Induced Electrochemiluminescence for Biosensing Applications. Curr. Opin. Electrochem. 2025, 49, 101627. [Google Scholar] [CrossRef]
- Sun, Q.; Ning, Z.; Yang, E.; Yin, F.; Wu, G.; Zhang, Y.; Shen, Y. Ligand—Induced Assembly of Copper Nanoclusters with Enhanced Electrochemical Excitation and Radiative Transition for Electrochemiluminescence. Angew. Chem. Int. Ed. 2023, 62, e202312053. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Shi, H.; Pan, M.; Liu, B.; Fang, G.; Wang, S. Electrochemiluminescence Sensor Based on Upconversion Nanoparticles and Oligoaniline-Crosslinked Gold Nanoparticles Imprinting Recognition Sites for the Determination of Dopamine. Biosens. Bioelectron. 2019, 128, 129–136. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, W.; Li, L.; Zhang, Z.; Chen, Z.; Lin, Y.; Zhu, J.-J. Size-Selected and Surface-Passivated CsPbBr3 Perovskite Nanocrystals for Self-Enhanced Electrochemiluminescence in Aqueous Media. Nanoscale 2020, 12, 7321–7329. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Yang, Q.; Li, Q.; Li, H.; Li, F. Quaternary Ammonium Salt-Functionalized Tetraphenylethene Derivative Boosts Electrochemiluminescence for Highly Sensitive Aqueous-Phase Biosensing. Anal. Chem. 2020, 92, 11747–11754. [Google Scholar] [CrossRef]
- Han, T.; Cao, Y.; Chen, H.-Y.; Zhu, J.-J. Versatile Porous Nanomaterials for Electrochemiluminescence Biosensing: Recent Advances and Future Perspective. J. Electroanal. Chem. 2021, 902, 115821. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Xu, R.; Wang, H.; Zhang, Y.; Wei, Q. Progress and Prospects of Electrochemiluminescence Biosensors Based on Porous Nanomaterials. Biosensors 2022, 12, 508. [Google Scholar] [CrossRef]
- Fu, H.; Xu, Z.; Hou, H.; Luo, R.; Ju, H.; Lei, J. Framework-Enhanced Electrochemiluminescence in Biosensing. Chemosensors 2023, 11, 422. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Li, J.-J.; Wang, Y.-H.; Gao, F.-H.; Su, J.-L.; Liu, Y.; Wang, H.-S.; Ding, Y. Metal/Covalent-Organic Framework-Based Biosensors for Nucleic Acid Detection. Coord. Chem. Rev. 2023, 491, 215249. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, R.; Gao, Y.-Y.; Zhou, Y.; Zhu, J.-J. Advances of Electrochemical and Electrochemiluminescent Sensors Based on Covalent Organic Frameworks. Nano-Micro Lett. 2024, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Cui, W.-R.; Jiang, Q.-Q.; Wu, Q.; Liang, R.-P.; Luo, Q.-X.; Qiu, J.-D. A General Design Approach toward Covalent Organic Frameworks for Highly Efficient Electrochemiluminescence. Nat. Commun. 2021, 12, 4735. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, X.; Pang, C.; Wang, M.; Yin, G.; Xu, Z.; Li, J.; Luo, J. Novel Chloramphenicol Sensor Based on Aggregation-Induced Electrochemiluminescence and Nanozyme Amplification. Biosens. Bioelectron. 2021, 176, 112944. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ze, H.; Qiu, Z. Reticular Sensing Materials with Aggregation-Induced Emission Characteristics. TrAC Trends Anal. Chem. 2023, 161, 116997. [Google Scholar] [CrossRef]
- Luo, R.; Lv, H.; Liao, Q.; Wang, N.; Yang, J.; Li, Y.; Xi, K.; Wu, X.; Ju, H.; Lei, J. Intrareticular Charge Transfer Regulated Electrochemiluminescence of Donor–Acceptor Covalent Organic Frameworks. Nat. Commun. 2021, 12, 6808. [Google Scholar] [CrossRef]
- Zeng, W.-J.; Wang, K.; Liang, W.-B.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Covalent Organic Frameworks as Micro-Reactors: Confinement-Enhanced Electrochemiluminescence. Chem. Sci. 2020, 11, 5410–5414. [Google Scholar] [CrossRef]
- Ni, Y.; Jiang, D.; An, X.; Wang, W.; Xu, F.; Liu, H.W.; Chen, Z. Low-Triggering-Potential Electrochemiluminescence Based on Mental-Organic Frameworks Encapsulation of Ruthenium for Synthetic Cathinone Detection by Coupling Photonic Crystal Light-Scattering Signal Amplification of Covalent-Organic Frameworks. Anal. Chim. Acta 2024, 1312, 342763. [Google Scholar] [CrossRef]
- Qin, X.; Zhan, Z.; Ding, Z. Progress in Electrochemiluminescence Biosensors Based on Organic Framework Emitters. Curr. Opin. Electrochem. 2023, 39, 101283. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, W.; Tian, Z.; Zhang, X.; Wang, S. Covalent Organic Framework: A State-of-the-Art Review of Electrochemical Sensing Applications. Talanta 2023, 260, 124613. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Guo, H. Functional COFs for Electrochemical Sensing: From Design Principles to Analytical Applications. Chem. Select. 2023, 8, e202301828. [Google Scholar] [CrossRef]
- Fan, J.; Jiang, G.; Li, J.; Qi, J.; Nalumansi, H.S.; Wang, J.; Pi, F. Covalent Organic Frameworks (COFs)-Based Advanced Sensors for Detecting Food Contaminants: Design Strategies and Applications. Microchem. J. 2025, 211, 113096. [Google Scholar] [CrossRef]
- Luo, R.; Zhu, D.; Ju, H.; Lei, J. Reticular Electrochemiluminescence Nanoemitters: Structural Design and Enhancement Mechanism. Acc. Chem. Res. 2023, 56, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J. Recent Advances in Electrochemiluminescence Emitters for Biosensing and Imaging of Protein Biomarkers. Chemosensors 2023, 11, 432. [Google Scholar] [CrossRef]
- Luo, R.; Luo, X.; Xu, H.; Wan, S.; Lv, H.; Zou, B.; Wang, Y.; Liu, T.; Wu, C.; Chen, Q.; et al. Reticular Ratchets for Directing Electrochemiluminescence. J. Am. Chem. Soc. 2024, 146, 16681–16688. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Liu, G.; Gao, H.; Li, H.; Liang, X. Highly Efficient Aggregation-Induced Electrochemiluminescence Performance of Covalent Organic Frameworks with Electron-Rich Conjugated Structures. Chem. Eur. J. 2025, 31, e202403820. [Google Scholar] [CrossRef]
- Zanut, A.; Fiorani, A.; Canola, S.; Saito, T.; Ziebart, N.; Rapino, S.; Rebeccani, S.; Barbon, A.; Irie, T.; Josel, H.-P.; et al. Insights into the Mechanism of Coreactant Electrochemiluminescence Facilitating Enhanced Bioanalytical Performance. Nat. Commun. 2020, 11, 2668. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Z.; Qiu, H.; Zhu, Z.; Li, J.; Hu, Y.; Lai, X.; Yin, S.; Tang, J. Planar β Keto-Enamine-Based Covalent Organic Frameworks as New Emitters for Electrochemiluminescence Sensing of Organophosphate Pesticides. Biosens. Bioelectron. 2025, 288, 117785. [Google Scholar] [CrossRef]
- Mao, X.-L.; Luo, Q.-X.; Cai, Y.-J.; Liu, X.; Jiang, Q.-Q.; Zhang, C.-R.; Liang, R.-P.; Qiu, J.-D. Structural Isomerism of Covalent Organic Frameworks Causing Different Electrochemiluminescence Effects and Its Application for the Detection of Arsenic. Anal. Chem. 2023, 95, 10803–10811. [Google Scholar] [CrossRef]
- Xu, H.; Luo, R.; Lv, H.; Liu, T.; Liao, Q.; Wang, Y.; Zhong, Z.; Wu, X.; Lei, J.; Xi, K. Deciphering a Volcano-Shaped Relationship between Radical Stability and Reticular Electrochemiluminescence. Nat. Commun. 2025, 16, 1924. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Yao, L.-Y.; Yang, Y.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Conductive Covalent Organic Frameworks with Conductivity- and Pre-Reduction-Enhanced Electrochemiluminescence for Ultrasensitive Biosensor Construction. Anal. Chem. 2022, 94, 3685–3692. [Google Scholar] [CrossRef]
- Song, L.; Gao, W.; Wang, S.; Bi, H.; Deng, S.; Cui, L.; Zhang, C.-Y. Construction of an Aminal-Linked Covalent Organic Framework-Based Electrochemiluminescent Sensor for Enantioselective Sensing Phenylalanine. Sens. Actuators B-Chem. 2022, 373, 132751. [Google Scholar] [CrossRef]
- Bao, J.-Y.; Liu, W.; Chen, C.; Zhu, H.-T.; Wang, A.-J.; Yuan, P.-X.; Feng, J.-J. Automated ECL Aptasensing Platform from an Intrarticular Radical Annihilation Route for Distinguishing Glioma Stages. Anal. Chem. 2024, 96, 16063–16071. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Yu, H.; Wang, W.; Shen, L.-L.; Zhang, G.-R.; Mei, D. Covalent Triazine Framework Encapsulated Ultrafine PdAu Alloy Nanoclusters as Additive-Free Catalysts for Efficient Hydrogen Production from Formic Acid. ACS Catal. 2023, 13, 5135–5146. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, W.; Yuan, S.; Zhao, L.; Wang, L.; Li, J.; Wu, D.; Kong, Y. Ionic Covalent-Organic Frameworks Composed of Anthryl-Extended Viologen as a Kind of Electrochemiluminescence Luminophore. ACS Appl. Mater. Interfaces 2024, 16, 55936–55944. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, H.; Li, J.; Zhang, J.; Gao, S.-Z.; Lu, M.-L.; Zhang, X.-Y.; Liang, W.; Zou, X.; Yuan, R.; et al. Highly Stable Ru-Complex-Based Metal-Covalent Organic Frameworks as Novel Type of Electrochemiluminescence Emitters for Ultrasensitive Biosensing. Mater. Horiz. 2023, 10, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Cui, L.; Wang, S.; Wei, W.; Gao, W.; Zhang, C. Development of an Exogenous Coreactant-Free Electrochemiluminescent Sensor for Sensing Glucose. Analyst 2023, 148, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, Q.; Min, L.; Guo, X.; Gao, W.; Cui, L.; Zhang, C. Electrochemiluminescence Enhanced by Isolating ACQphores in Imine-Linked Covalent Organic Framework for Organophosphorus Pesticide Assay. Talanta 2024, 266, 124964. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Yuan, R.; Zhong, X.; Zhuo, Y. Electrochemiluminescence Covalent Organic Framework Coupling with CRISPR/Cas12a-Mediated Biosensor for Pesticide Residue Detection. Food Chem. 2022, 389, 133049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Yang, Y.; Liang, W.-B.; Yao, L.-Y.; Yuan, R.; Xiao, D.-R. Highly Stable Covalent Organic Framework Nanosheets as a New Generation of Electrochemiluminescence Emitters for Ultrasensitive MicroRNA Detection. Anal. Chem. 2021, 93, 3258–3265. [Google Scholar] [CrossRef]
- Luo, Q.-X.; Cui, W.-R.; Li, Y.-J.; Cai, Y.-J.; Mao, X.-L.; Liang, R.-P.; Qiu, J.-D. Construction of Sp2 Carbon-Conjugated Covalent Organic Frameworks for Framework-Induced Electrochemiluminescence. ACS Appl. Electron. Mater. 2021, 3, 4490–4497. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Wang, T.-T.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Rigidifying AIEgens in Covalent Organic Framework Nanosheets for Electrochemiluminescence Enhancement: TABE-PZ-CON as a Novel Emitter for microRNA-21 Detection. Anal. Chim. Acta 2024, 1295, 342321. [Google Scholar] [CrossRef]
- Cui, W.-R.; Li, Y.-J.; Jiang, Q.-Q.; Wu, Q.; Luo, Q.-X.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Covalent Organic Frameworks as Advanced Uranyl Electrochemiluminescence Monitoring Platforms. Anal. Chem. 2021, 93, 16149–16157. [Google Scholar] [CrossRef]
- Li, Y.-J.; Cui, W.-R.; Jiang, Q.-Q.; Liang, R.-P.; Li, X.-J.; Wu, Q.; Luo, Q.-X.; Liu, J.; Qiu, J.-D. Arousing Electrochemiluminescence Out of Non-Electroluminescent Monomers within Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 47921–47931. [Google Scholar] [CrossRef]
- Cui, W.-R.; Li, Y.-J.; Jiang, Q.-Q.; Wu, Q.; Liang, R.-P.; Luo, Q.-X.; Zhang, L.; Liu, J.; Qiu, J.-D. Tunable Covalent Organic Framework Electrochemiluminescence from Non-Electroluminescent Monomers. Cell Rep. Phys. Sci. 2022, 3, 100630. [Google Scholar] [CrossRef]
- Luo, Q.-X.; Cai, Y.-J.; Mao, X.-L.; Li, Y.-J.; Zhang, C.-R.; Liu, X.; Chen, X.-R.; Liang, R.-P.; Qiu, J.-D. Tuned-Potential Covalent Organic Framework Electrochemiluminescence Platform for Lutetium Analysis. J. Electroanal. Chem. 2022, 923, 116831. [Google Scholar] [CrossRef]
- Cao, X.; Song, L.; Yang, Y.; Chu, W.; Zou, X.; Sun, B.; Yin, H.; Cui, L. Remarkable Increase in Electrochemiluminescence of Isomeric Bipyridine-Based Covalent Organic Frameworks via Regulating the Direction of Imine Linkage for Sensing Application. J. Colloid Interface Sci. 2025, 684, 262–271. [Google Scholar] [CrossRef]
- Liu, T.; Tao, Q.; Wang, Y.; Luo, R.; Ma, J.; Lei, J. Tailored Cis-Trans Isomeric Metal-Covalent Organic Frameworks for Coordination Configuration-Dependent Electrochemiluminescence. J. Am. Chem. Soc. 2024, 146, 18958–18966. [Google Scholar] [CrossRef]
- Hou, H.; Wu, Y.; Wan, J.; Luo, R.; Wu, L.; Zhao, Y.; Wu, X.; Lei, J. P–π Conjugation-Promoted Electrochemiluminescence of Halogenated Covalent Organic Framework Nanoemitters. Angew. Chem. Int. Ed. 2025, 64, e202506309. [Google Scholar] [CrossRef]
- Chu, W.; Cao, X.; Song, L.; Yang, Y.; Gao, W.; Cheng, L.; Ai, S.; He, W.; Cui, L. Improving Electrochemiluminescence with Vinyl-Group-Anchored Covalent-Organic Frameworks for Detection of Iodide Ions and Iodixanol. Sens. Actuators B Chem. 2025, 431, 137459. [Google Scholar] [CrossRef]
- Song, L.; Gao, W.; Jiang, S.; Yang, Y.; Chu, W.; Cao, X.; Sun, B.; Cui, L.; Zhang, C. One-Dimensional Covalent Organic Framework with Improved Charge Transfer for Enhanced Electrochemiluminescence. Nano Lett. 2024, 24, 6312–6319. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, Z.; Feng, X.; Xu, S.; Zhang, Y.; Zhang, Y.; Zeng, L.; Tan, J.; Ren, Y. Novel Pore Confinement Enhanced Europium Functionalized Porphyrin Covalent Organic Framework Electrochemiluminescence Microreactor and Its Application to microRNA-21 Detection. Sens. Actuators B Chem. 2024, 418, 136314. [Google Scholar] [CrossRef]
- Tan, L.; Cai, W.; Wang, F.; Li, J.; Wu, D.; Kong, Y. Postsynthetic Modification Strategy for Constructing Electrochemiluminescence-Active Chiral Covalent Organic Frameworks Performing Efficient Enantioselective Sensing. Anal. Chem. 2024, 96, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zheng, L.; Luo, R.; Kong, W.; Xu, Z.; Dong, P.; Ma, J.; Lei, J. Bimodal Oxidation Electrochemiluminescence Mechanism of Coreactant-Embedded Covalent Organic Frameworks via Postsynthetic Modification. Angew. Chem. Int. Ed. 2024, 63, e202402373. [Google Scholar] [CrossRef]
- Jiang, Q.-Q.; Li, Y.-J.; Wu, Q.; Wang, X.; Luo, Q.-X.; Mao, X.-L.; Cai, Y.-J.; Liu, X.; Liang, R.-P.; Qiu, J.-D. Guest Molecular Assembly Strategy in Covalent Organic Frameworks for Electrochemiluminescence Sensing of Uranyl. Anal. Chem. 2023, 95, 8696–8705. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, W.; Wang, Y.; Liu, X.; Jiang, P.; Luo, L.; Bi, X.; Meng, X.; Niu, Q.; Wu, X.; et al. Gold Nanocluster-Confined Covalent Organic Frameworks as Bifunctional Probes for Electrochemiluminescence and Colorimetric Dual-Response Sensing of Pb2+. J. Hazard. Mater. 2023, 457, 131558. [Google Scholar] [CrossRef]
- Zhen, M.; Wang, Y.; He, Y.; Luo, L.; Ma, G.; Lv, W.; Li, L.; You, T. “Kill Two Birds with One Stone” Role of PTCA-COF: Enhanced Electrochemiluminescence of Au Nanoclusters via Radiative Transitions and Electrochemical Excitation for Sensitive Detection of Cadmium Ions. Sens. Actuators B Chem. 2025, 422, 136690. [Google Scholar] [CrossRef]
- Fan, B.; Jiang, D.; Ni, Y.; Hu, Z.; Sun, Z.; Li, H.; Wang, W.; Chen, Z. Synergistic Coupling of Antenna Effect and Schottky Junction in Tb-Doped Covalent Organic Framework for Enhanced Electrochemiluminescence Sensing of Isobutyryl Fentanyl. Anal. Chem. 2025, 97, 19695–19704. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Jiang, D.; Cao, Q.; Xu, F.; Shiigi, H.; Wang, W.; Chen, Z. Highly Efficient Dual-Color Luminophores for Sensitive and Selective Detection of Diclazepam Based on MOF/COF Bi-Mesoporous Composites. ACS Sens. 2023, 8, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ren, X.; Wu, T.; Lei Li, Y.; Ma, H.; Ru, Z.; Jia, Y.; Feng Gao, Z.; Du, Y.; Wu, D.; et al. Effective Enrichment of Free Radicals through Nanoconfinement Boosts Electrochemiluminescence of Carbon Dots Derived from Luminol. Angew. Chem. Int. Ed. 2025, 64, e202414073. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, M.; Zhang, X.; Jia, D.; Tian, T.; Shi, B.; Ru, Z.; Ma, H.; Wan, Y.; Wei, Q. Nanobody-Based Microfluidic Immunosensor Chip Using Tetraphenylethylene-Derived Covalent Organic Frameworks as Aggregation-Induced Electrochemiluminescence Emitters for the Detection of Thymic Stromal Lymphopoietin. Anal. Chem. 2024, 96, 10116–10120. [Google Scholar] [CrossRef]
- Du, Y.; Li, G.; Yang, Y.; Song, L.; Cui, L.; Wan, Y.; Zhang, C. High-Performance Electrochemiluminescence Imaging Immunosensor for Adeno-Associated Virus Serotype 8 Detection Based on Nanobody-Functionalized Covalent Organic Frameworks and Au@Rh Catalytic Amplification. ACS Sens. 2025, 10, 6065–6073. [Google Scholar] [CrossRef]
- Li, W.; Liang, Z.; Wang, P.; Li, Z.; Ma, Q. CuS@Ag Heterostructure-Based Surface Plasmonic Coupling Electrochemiluminescence Sensor for Glioma miRNA-124-3p Detection. Biosens. Bioelectron. 2025, 274, 117202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Wang, Y.; Guo, C.; Guo, R.; Guo, Y.; Zhang, Z. Development of an Electrochemiluminescence Aptasensor Combining Covalent-Triazine Framework Emitter with Exonuclease III-Driven DNA Walker for Sensitive CEA Detection. Microchem. J. 2025, 215, 114388. [Google Scholar] [CrossRef]
- Cui, L.; Zhu, C.; Hu, J.; Meng, X.; Jiang, M.; Gao, W.; Wang, X.; Zhang, C. Construction of a Dual-Mode Biosensor for Electrochemiluminescent and Electrochemical Sensing of Alkaline Phosphatase. Sens. Actuators B Chem. 2023, 374, 132779. [Google Scholar] [CrossRef]
- Tang, S.-H.; Qin, L.; Yang, W.-G.; Yuan, R.; Yang, J.; Li, Y.; Hu, S.-S. Electrochemiluminescence Immunoassay of cTnI with Ruthenium-Based Metal Covalent Organic Framework and Dual DNAzymes Cascade Amplification Strategy. Chem. Eur. J. 2025, 31, e202404053. [Google Scholar] [CrossRef]
- An, X.; Jiang, D.; Ni, Y.; Wang, W.; Zhu, Q.; Xu, F.; Shiigi, H.; Chen, Z. Synergistic Multieffect Catalytic Amplified Cathodic Electrochemiluminescence Biosensor via Target Binding-Induced Aptamer Conformational Changes for the Ultrasensitive Detection of Synthetic Cathinone. ACS Appl. Mater. Interfaces 2023, 15, 55369–55378. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Ji, F.; Wang, P.; Ma, Q. Natural Supramolecular Hydrogel/N-dots@COF Nanocomposite-Based ECL Sensor for Exosomal miRNA-381 Detection. Chem. Eng. J. 2025, 520, 165871. [Google Scholar] [CrossRef]
- Ren, X.; Shao, M.; Li, X.; Xie, Z.; Zhao, J.; Wang, H.; Gao, M.; Wu, D.; Ju, H.; Wei, Q. Confinement-Enhanced Electrochemiluminescence by Ru(dcbpy)32+-Functionalized γ-CD-MOF@COF-LZU1 Porous Hybrid Material as Micro-Reactor for CYFRA 21-1 Detection. Talanta 2024, 273, 125959. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Shen, B.; He, W.; Li, X.; Li, X.; Li, M.; Hu, R.; Zhang, M.; Yan, Y. A Novel Electrochemiluminescent Cytosensor Using 3D Multivalent Aptamer Recognition and Covalent Organic Frameworks-Associated DNA Walker for Highly Efficient Capture and Detection of Rare CTCs. Chem. Eng. J. 2024, 481, 148606. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.-W.; Shan, H.-Y.; Chen, J.; Wang, A.-J.; Liu, W.; Yuan, P.-X.; Feng, J.-J. Covalent Organic Framework Linked with Amination Luminol Derivative as Enhanced ECL Luminophore for Ultrasensitive Analysis of Cytochrome c. Anal. Methods 2022, 14, 4767–4774. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Xu, C.-H.; Li, X.-Q.; Chen, H.-Y.; Xu, J.-J. Visual Analysis of Alzheimer Disease Biomarker via Low-Potential Driven Bipolar Electrode. Anal. Chim. Acta 2023, 1251, 340980. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Ding, H.; Dong, Y. Detection of Acetylcholinesterase Based on ECL Resonance Energy Transfer between Luminol and Gold Nanoparticle Decorated Covalent Organic Framework. Microchem. J. 2025, 209, 112774. [Google Scholar] [CrossRef]
- Pan, J.-J.; Zhu, H.-T.; Chen, J.; Ma, X.-Q.; Wang, A.-J.; Yuan, P.-X.; Feng, J.-J. The Dual ECL Signal Enhancement Strategy of Pd Nanoparticles Attached Covalent Organic Frameworks and Exonuclease Cycling Reaction for the Ultrasensitive Detection of Progesterone. Talanta 2024, 274, 125934. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pang, C.; Ma, X.; Wu, Y.; Wang, M.; Xu, Z.; Luo, J. Aggregation-Induced Electrochemiluminescence and Molecularly Imprinted Polymer Based Sensor with Fe3O4@Pt Nanoparticle Amplification for Ultrasensitive Ciprofloxacin Detection. Microchem. J. 2022, 178, 107345. [Google Scholar] [CrossRef]
- Liu, C.; Cai, L.; Wang, X.; Guo, Y.; Fang, G.; Wang, S. Construction of Molecularly Imprinted Sensor Based on Covalent Organic Frameworks DAFB-DCTP-Doped Carbon Nitride Nanosheets with High Electrochemiluminescence Activity for Sensitive Detection of Carbaryl. Microchem. J. 2022, 178, 107416. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Gabrielli, S.; Cimarelli, C.; Guo, C.; Du, M.; Pellei, M.; Zhang, Z. Donor–Acceptor Conjugated and Triazine-Containing Covalentorganic Framework: Construction of a Signal “on–off–on” Electrochemiluminescence Immunosensor for Efficiently Detecting Zearalenone. Sens. Actuators B Chem. 2025, 433, 137539. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, C.; Li, K.; Zhang, X.; Tong, M.; Tu, J.; Yang, K.; Yuan, S.; Zhang, H. CRISPR/Cas12a-Assisted Electrochemiluminescent Detection of Ochratoxin A Based on COF@Ru Coupled with a DNA Tetrahedral Scaffold. Anal. Methods 2025, 17, 5298–5307. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Ma, M.; Xu, Y.; Huang, D.; Wang, J.; Lin, C.; Lin, Z.; Luo, F. Nanoconfined-Structured Ru@COF-LZU1 Microenvironment Facilitated Homogeneous Electrochemiluminescence Biosensor for Ultrasensitive N-Nitrosodimethylamine Detection. Sens. Actuators B Chem. 2025, 444, 138456. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Luo, F.; Lin, Z.; Wang, J.; Li, R.; Qiu, B. Stable Halide Perovskite CsPbBr3 Nanocrystals Assisted by Covalent-Organic Frameworks for Electrochemiluminescence Analysis in an Aqueous Medium. Anal. Chem. 2024, 96, 16783–16792. [Google Scholar] [CrossRef]
- Chi, H.; Wang, L.; Wang, S.; Liu, G. An Electrochemiluminescence Sensor Based on CsPbBr3 -Zquantum Dots and Poly (3-Thiophene Acetic Acid) Cross-Linked Nanogold Imprinted Layer for the Determination of Benzo(a)Pyrene in Edible Oils. Food Chem. 2023, 426, 136508. [Google Scholar] [CrossRef]
- Ma, X.; Pang, C.; Li, S.; Xiong, Y.; Li, J.; Luo, J.; Yang, Y. Synthesis of Zr-Coordinated Amide Porphyrin-Based Two-Dimensional Covalent Organic Framework at Liquid-Liquid Interface for Electrochemical Sensing of Tetracycline. Biosens. Bioelectron. 2019, 146, 111734. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Fan, Y.; Chen, Y.; Wei, R.; Lv, J.; Yan, M.; Jiang, D.; Liu, Z. Molecular Imprinting-Based Ru@SiO2-Embedded Covalent Organic Frameworks Composite for Electrochemiluminescence Detection of Cyanidin-3-O-Glucoside. Talanta 2024, 274, 125997. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yin, Z.; Cui, H.; Xiong, W.; Yu, F.; Zhang, J.; Liao, F.; Wei, G.; Yang, L.; Zhang, J.; et al. A Novel Dual-Detection Electrochemiluminescence Sensor for the Selective Detection of Hg2+ and Zn2+: Signal Suppression and Activation Mechanisms. Anal. Chim. Acta 2024, 1330, 343283. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, R.; Zhou, Y.; Jiang, D.; Zhu, W. A Bioinspired Photocatalysis and Electrochemiluminescence Scaffold for Simultaneous Degradation and In Situ Evaluation. Adv. Funct. Mater. 2022, 32, 2203005. [Google Scholar] [CrossRef]
- Jiang, K.-B.; Wang, L.; Wei, Y.-P.; Chen, J.-S.; Liu, X.-P.; Mao, C.-J.; Jin, B.-K. Electrochemiluminescent Aptasensor Based on Covalent Organic Framework and ZnCdS Composite for Sensitive Detection of Oxytetracycline. Sens. Actuators B Chem. 2025, 439, 137858. [Google Scholar] [CrossRef]
- Ma, R.; Jiang, J.; Ya, Y.; Lin, Y.; Zhou, Y.; Wu, Y.; Tan, X.; Huang, K.; Du, F.; Xu, J. A Carbon Dot-Based Nanoscale Covalent Organic Framework as a New Emitter Combined with a CRISPR/Cas12a-Mediated Electrochemiluminescence Biosensor for Ultrasensitive Detection of Bisphenol A. Analyst 2023, 148, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Kuang, X.; Zheng, X.; Sun, X.; Kuang, R. Bidirectional ECL Response of Chiral COFs for the Discrimination of Penicillamine Enantiomers. Talanta 2025, 293, 128081. [Google Scholar] [CrossRef]
- Yuan, S.; Cai, W.; Zhao, L.; Wang, L.; Zhang, R.; Li, J.; Wu, D.; Kong, Y. Strong Electrochemiluminescence Response Derived from Ionic Chiral Covalent Organic Frameworks for Enantioselective Discrimination of Amino Acid Enantiomers via an Electrostatic Attraction Effect. ACS Appl. Mater. Interfaces 2024, 16, 65340–65347. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Tan, L.; Zhao, L.; Wang, F.; Cai, W.; Li, J.; Wu, D.; Kong, Y. Chiral Ru-Based Covalent Organic Frameworks as An Electrochemiluminescence-Active Platform for the Enantioselective Sensing of Amino Acids. ACS Appl. Mater. Interfaces 2024, 16, 13161–13169. [Google Scholar] [CrossRef]
- Zhao, L.; Cai, W.; Yuan, S.; Wang, L.; Zhang, R.; Li, J.; Wu, D.; Kong, Y. Optically Pure Co(III) Complex Absorbed by Electrochemiluminescence-Active Covalent Organic Framework as an Enantioselective Recognition Platform to Give Opposite Responses Toward Amino Alcohol Enantiomers. ACS Appl. Mater. Interfaces 2024, 16, 57649–57658. [Google Scholar] [CrossRef]
- Hu, C.; Xie, W.; Liu, J.; Zhang, Y.; Sun, Y.; Cai, Z.; Lin, Z. Bioinspired Iron Porphyrin Covalent Organic Frameworks-Based Nanozymes Sensor Array: Machine Learning-Assisted Identification and Detection of Thiols. ACS Appl. Mater. Interfaces 2024, 16, 71048–71059. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, H.; Lai, X.; Wang, K.; Yang, Q.; Yu, D. Accelerating the Selection of Covalent Organic Frameworks with Automated Machine Learning. ACS Omega 2021, 6, 17149–17161. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Xuan, X.; Wang, K.; Yao, Y.; Pan, L. Machine Learning Accelerated Discovery of Covalent Organic Frameworks for Environmental and Energy Applications. Environ. Sci. Technol. 2025, 59, 6361–6378. [Google Scholar] [CrossRef]
- Wang, D.; Lv, H.; Wan, Y.; Wu, X.; Yang, J. Band-Edge Prediction of 2D Covalent Organic Frameworks from Molecular Precursor via Machine Learning. J. Phys. Chem. Lett. 2023, 14, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
| App. | COF | ECL System | Co-Reactant | Sensing Mechanism | Analytes | Linear Range | LOD | Samples | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| COF-based ECL lumiphores | Py-sp2 C-CON | Py-sp2 C-CON/S2O82−/Bu4NPF6 | K2S2O8 with Bu4NPF6 as accelerator | DNA/RNA recognition | microRNA-21 | 100 aM~1 nM | 46 aM | extracts of cancer cells (HeLa, MCF-7) | [44] |
| TABE-PZ-CON | Pd NPs/TABE-PZ-CON/GCE | TEA | DNA/RNA recognition | microRNA-21 | 100 aM~1 nM | 17.9 aM | extracts of cancer cells (HeLa, MCF-7) | [46] | |
| Ru-MCOF | HT/H2-Fc/AuNPs/Ru-MCOF/GCE | TPrA | DNA/RNA recognition | microRNA-155 | 10 aM~1 nM | 3.02 aM | cancer cells (MCF-7 and HeLa) | [40] | |
| ET-COF-COOH | AIE-COF/CuS@Ag SC | DEDA | Catalyzed hairpin assembly strategy | miRNA-124-3p | 1 fM~10 nM | 0.49 fM | glioma tumor | [67] | |
| CTF | rHp/CuxMn3-x(HITP)2/ssDNA/CTF/AE | K2S2O8 | Aptamer recognition | carcinoembryonic antigen (CEA) | 1 pg/mL~50 ng/mL | 2.91 fg/mL | human serum | [68] | |
| HHTP-HATP-COF | S2O82–/HHTP-HATP-COF/GCE | K2S2O8 | Pre-reduction+ aptamer recognition | thrombin (TB) | 100 aM~1 nM | 62.1 aM | diluted human serum | [35] | |
| TCPB-DMTA-COF | CoOOH/TCPB-DMTA-COF/GCE | K2S2O8 | Enzyme/nano-enzyme catalysis | alkaline phosphatase (ALP) | 0.01~100 U/L | 6.0 × 10−3 U/L | human serums | [69] | |
| Ru-MCOFs | Ru-MCOFs/GCE | TEA | DNAzymes catalysis | cardiac troponin I (cTnI) | 1 fg/mL~10 ng/mL | 0.42 fg/mL | human serum | [70] | |
| TFPPy-DMeTHz-COF | TFPPy-DMeTHz-COF/GCE | / | catalytically decreased pH | glucose | 0.1~500 μM | 0.031 μM | human serum | [41] | |
| 1D-COF | 1D-COF/GCE | TPrA | Electro-oxidization and ECL RET | dopamine (DA) | 0.1~1000 nM | 4.05 pM | 10% normal human serum | [55] | |
| T-COF | T-COF/Electrode | TEA with Ag+ as co-reactant accelerator | Immune recognition reaction | thymic stromal lymphopoietin (TSLP) | 1.00 pg/mL~4.00 ng/mL | 2.72 pg/mL | serum | [65] | |
| TC-COF | TC-COF/GCE | TEA with Au@Rh as co-reactant accelerator | Immune recognition reaction | adeno-associated virus serotype 8 (AAV8) | 108~5 × 1011 vg/mL | 107.15 vg/mL | serum | [66] | |
| PTCA-COF | PTCA-COF/Cu-HHTP/GCE | K2S2O8 | Specific adsorption | diclazepam | 0.1 pg/L~10 ng/L | 26 fg/L | bear | [63] | |
| PTCA-COF | Fc-aptamer/Ag@CuCo2O4/PTCA-COF/GCE | K2S2O8 | Aptamer recognition | cathinones 4-chloroethcathinone (4-CEC) | 1 pg/L~1 μg/L | 0.25 pg/L | e-Cigarette | [71] | |
| A-COFs | Tb@A-COF/Ag NWs | TPrA | Aptamer recognition | isobutyryl fentanyl (iBF) | 1 fg/L~100 ng/L | 0.897 fg/L | beer beverages | [62] | |
| COF-supported ECL systems | cCTF | asDNA/MBs-Py-Ru-cCTFs/GCE | TPrA | DNA/RNA recognition | miRNA-182 | 1~100 fM | 0.28 fM | human serum | [37] |
| TAPP-COF | PtNPs/Eu@TAPP-COF/GCE | K2S2O8 | DNA/RNA recognition | miRNA-21 | 100 aM~100 pM | 21 aM | human serum | [56] | |
| TBA-TAPB | WS2@COF Lu CDs/GCE | H2O2 | DNA/RNA recognition | miR-126 | / | 30.61 aM | / | [64] | |
| Tp-TAPB COF | N-dots@COF | H2O2 | DNA/RNA recognition | miRNA-381 | 1 fM~10 nM | 0.13 fM | ascites | [72] | |
| COF-LZU1 | Ru@COF-LZU1@γ-CD-MOF-Au-Ab2 | DBAE | Immune recognition reaction | CYFRA 21-1 | 10 fg/mL~50 ng/mL | 5.5 fg/mL | human serum | [73] | |
| COF-LZU1 | Au@COF-LZU1@Ru/GCE | TPrA | Aptamer recognition | Rare circulating tumor cells (CTCs) | 8~100,000 cells/mL | 2 cells/mL | human peripheral blood | [74] | |
| COF-LZU1 | ABEI-COFs/GCE | TPrA | Aptamer recognition | cytochrome c | 1 fg/mL~0.1 ng/mL | 0.73 fg mL−1 | human serum | [75] | |
| TPB-DVA COF | Ir(ppy)3 and Ru(bpy)32+/TPB-DVA COF | TPrA | Immune recognition reaction | amyloid-β (Aβ) | 0~50 pM | 1 pM | 100-fold diluted serum | [76] | |
| Py-PB-COF | Au@COF/Fc/GCE | O2 | Aptamer recognition | acetylcholinesterase (AChE) | 0.5 nM~1 μM | 0.17 nM | serum | [77] | |
| CTpBD | MIP/UCNPs/CTpBD-Au/GCE | K2S2O8 | Molecularly imprinting recognition | dopamine | 0.01 pM~1 μM | 2 fM | rat blood serum | [9] | |
| NH2-COFs | RuP/Pd NPs@COFs/GCE | TPrA | Aptamer recognition | progesterone (P4) | 0.80 pM~7.95 μM | 0.45 pM | human serum | [78] |
| App. | COF | ECL System | Co-Reactant | Sensing Mechanism | Analytes | Linear Range | LOD | Samples | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| COF-based ECL lumiphores | COF-AI-ECL | CAP-MIP/COF-AI-ECL/Co3O4/Au | H2O2 | Molecularly imprinting recognition | chloramphenicol (CAP) | 0.5~400 pM | 0.118 pM | honey, milk, chicken | [18] |
| COF-AIECL | MIP/COF-AIECL/Fe3O4@Pt NPs/GCE | H2O2 | Molecularly imprinting recognition | ciprofloxacin (CFX) | 2 pM~3 nM | 0.598 pM | milk | [79] | |
| TP-ML COF | TP-ML COF/GCE | K2S2O8 | Aptamer recognition | Malathion (Mal) | 0.001~100 ng/mL | 19.03 fg/mL | / | [32] | |
| TFPPy-TPh-COF | ZIF-8/TFPPy-TPh COF/GCE | TPrA | Specific recognition of AChE enzyme | Malathion (Mal) | 0.01~1000 ng/mL | 2.44 pg/mL | apple, lettuce, pak choi | [42] | |
| PTCA-COF | Fc-DNA/Au NPs/PTCA-COF/GCE | K2S2O8 | DNA/RNA recognition | acetamiprid | 0.1 nM~0.1 mM | 2.7 pM | lettuce sample solutions | [43] | |
| DAFB-DCTP | MIPs/DAFB-DCTP @CNNs/GCE | K2S2O8 | Molecularly imprinting recognition | carbaryl | 0.1 nM~0.5 mM | 46.7 pM | milk powder, fruit wine | [80] | |
| TFPT-TAPB-COF | TFPT-TAPB-COF/GCE | K2S2O8 | Immune recognition reaction | zearalenone (XEN) | 10 fg/mL~100 ng/mL | 7.9 fg/mL | corn, wheat flour, tea | [81] | |
| COF-supported ECL systems | COF-LZU1 | Ru@COF-LZU1/GCE | TPrA | Aptamer recognition | aflatoxin M1 (AFM1) | 0.03 pg/mL~0.3 mg/mL | 0.009 pg/mL | defatted milk | [21] |
| COF-LZU1 | DTS/Au/COF@Ru/GCE | TPrA | Aptamer recognition | ochratoxin A (OTA) | 10 fg/mL~100 ng/mL | 3.5 fg/mL | peanut, corn and wine | [82] | |
| COF-LZU1 | Ru@COF-LZU1-MBs | TPrA | Aptamer recognition | N-nitrosodimethylamine (NDMA) | 100 fg/mL~100 ng/mL | 9.11 fg/mL | seafood | [83] | |
| COF-LZU1 | COF/CsPbBr3/ITO | ascorbic acid | Aptamer recognition | T-2 toxin | 10 fg/mL~100 ng/mL | 3.56 fg/mL | maize | [84] | |
| COF-300 | MIPs/CsPbBr3/COF-300-Au/GCE | K2S2O8 | Molecularly imprinting recognition | benzo(a)pyrene (BaP) | 10 fM~10 μM | 4.1 fM | edible oils | [85] | |
| Zr-amide-Por-based 2D COF | Tc-MIP/COF/GCE | luminol-H2O2 | Molecularly imprinting recognition | tetracycline (Tc) | 5~60 pM | 2.3 pM | milk | [86] | |
| TAPB-DMTP | Ru@SiO2-CMIPs/GCE | TPrA | Molecularly imprinting recognition | cyanidin-3-O-glucoside (C3G) | 0.0025~50 ng/mL | 0.15 pg/mL | Blueberry, mulberry | [87] |
| Entry | COF | ECL System | Co-Reactant | Sensing Mechanism | Analytes | Linear Range | LOD | Samples | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TP-TBDA | TP-TBDA@TCNQ/GCE | Na2S2O8 | Charge-transfer | uranyl ions (UO22+) | 10~5000 nM | 3 nM | waste water | [59] |
| 2 | BCBA–TBTN-AO | BCBA–TBTN-AO/GCE | O2 | Chelating and electron transfer | uranyl ions (UO22+) | 0.001~1000 nM | 0.36 pM | / | [47] |
| 3 | BTT-TBTN | BTT-TBTN-AO/GCE | O2 | Molecularly imprinting recognition | uranyl ions (UO22+) | 0~5 μM | 3.5 pM | Seawater/freshwater | [49] |
| 4 | TBTN-TFPT | TBTN-TFPT/GCE | K2S2O8 | Stronger affinity | lutetium ion (Lu3+) | 0.005~20 μM | 1.6 nM | / | [50] |
| 5 | DVA-COF | DVA-COF/GCE | TPrA | Charge transfer | I− and iodixanol | 0.01~500 μM | 39 pM for I−; 1.15 nM for iodixanol | lake water | [54] |
| 6 | TFPB-BD(OMe)2-H | TFPB-BD(OMe)2-H/GCE | K2S2O8 | Strong oxidation and electron affinity | As(V) ions | 0.001~5 μM | 0.33 nM | lake water | [33] |
| 7 | RuCOF | RuCOF/Au electrode | K2S2O8 | Selective N, N′-chelating sites | Hg2+ and Zn2+ | 1 nM~1μM | 4.71 nM for Hg2+; 6.57 nM for Zn2+ | real water | [88] |
| 8 | TAPB-DVA COF | AuNCs@COFs/GCE | TEA | Aptamer recognition | Pb2+ | 10 pM~5 μM | 7.9 pM | soil | [60] |
| 9 | PTCA-COF | AuNCs/PTCA-COF/GCE | TEA | Aptamer recognition | Cd2+ | 1 pM~5 nM | 0.66 pM | river water | [61] |
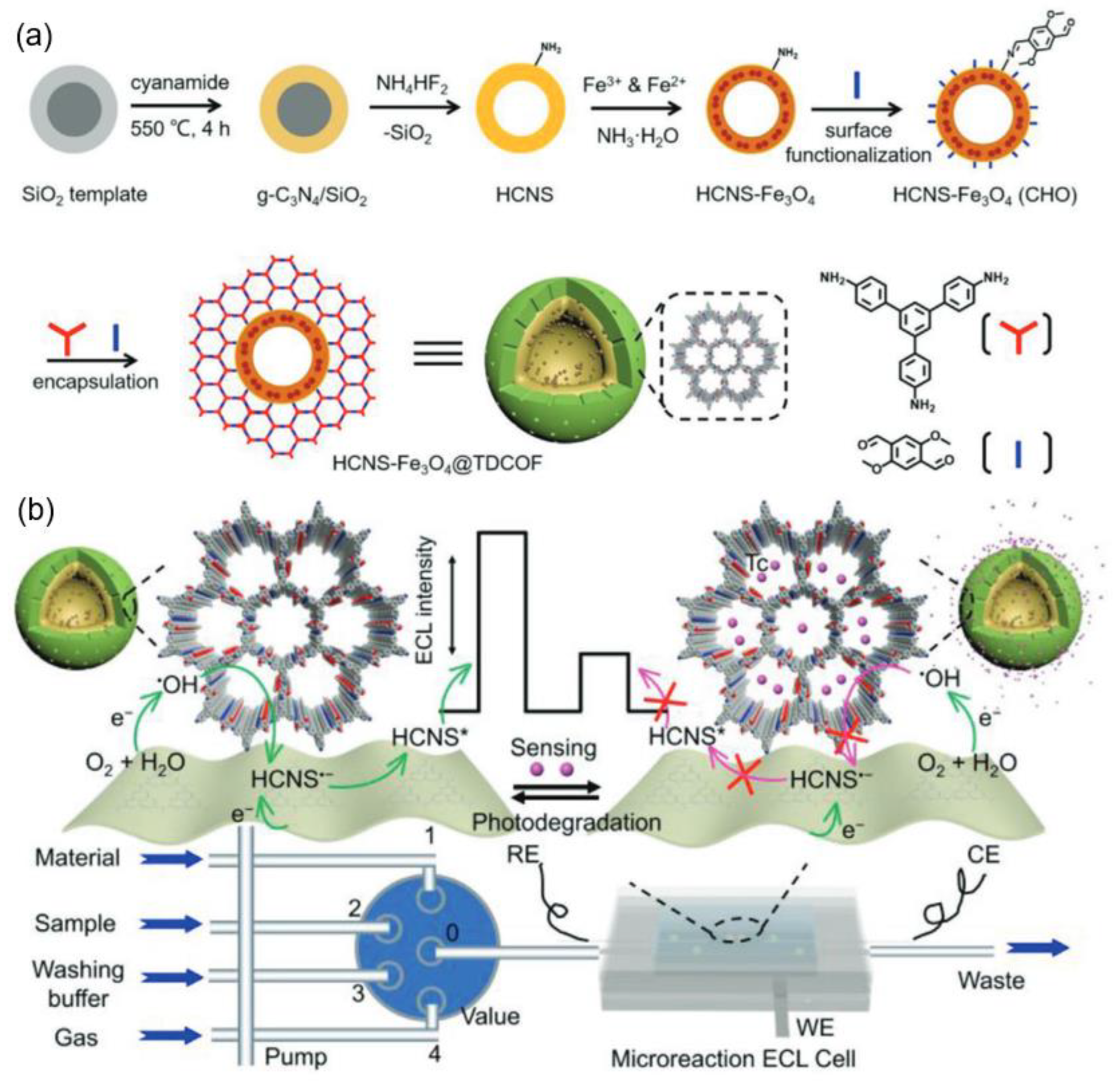
| 10 | TDCOF | HCNSFe3O4@ TDCOF | O2 | Tc suppressed the charge transfer | tetracycline (Tc) | 0.10~10 μg/L | 0.031 μg/L | / | [89] |
| 11 | TAPB-DMTP-COF | ZnCdS@COF/GCE | K2S2O8 | Aptamer recognition | oxytetracycline (OTC) | 1 pg/mL~1 μg/mL | 0.287 pg/mL | lake water | [90] |
| 12 | NC-COFPy-Bpy | NC-COFPy-Bpy/GCE | TPrA | ECL resonance energy transfer | doxorubicin (DOX) | 0.01~100 μM | 0.24 nM | / | [51] |
| 13 | CD-COF | CD-COF/S2O82−/Bu4N+ | K2S2O8 with Bu4NPF6 as co-reactant accelerator | Aptamer recognition | bisphenol A (BPA) | 0.01 pM~10 μM | 2.21 fM | river water, bottled water, tap water | [91] |
| Entry | COF | ECL System | Co-Reactant | Sensing Mechanism | Analytes | Linear Range | LOD | Samples | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | (R)-PTCDA-RMP | (R)-PTCDA-RMP/GCE | K2S2O8 | Chiral ECL-active unit | L-penicillamine (L-PA) | 50 μM~1 mM, | 9.74 μM | human urine | [92] |
| 2 | triPhPy+-(S)-CHA | triPhPy+-(S)-CHA/GCE | K2S2O8 | Chiral ECL-active unit | D-Arg | / | / | / | [93] |
| 3 | aminal-linked COF | β-CD/aminal-linked COF/GCE | TPrA | Competitive host-guest interaction of β-CD | L-phenylalanine (L-Phe) | 0.05~100 μM | 0.045 μM | human serums | [36] |
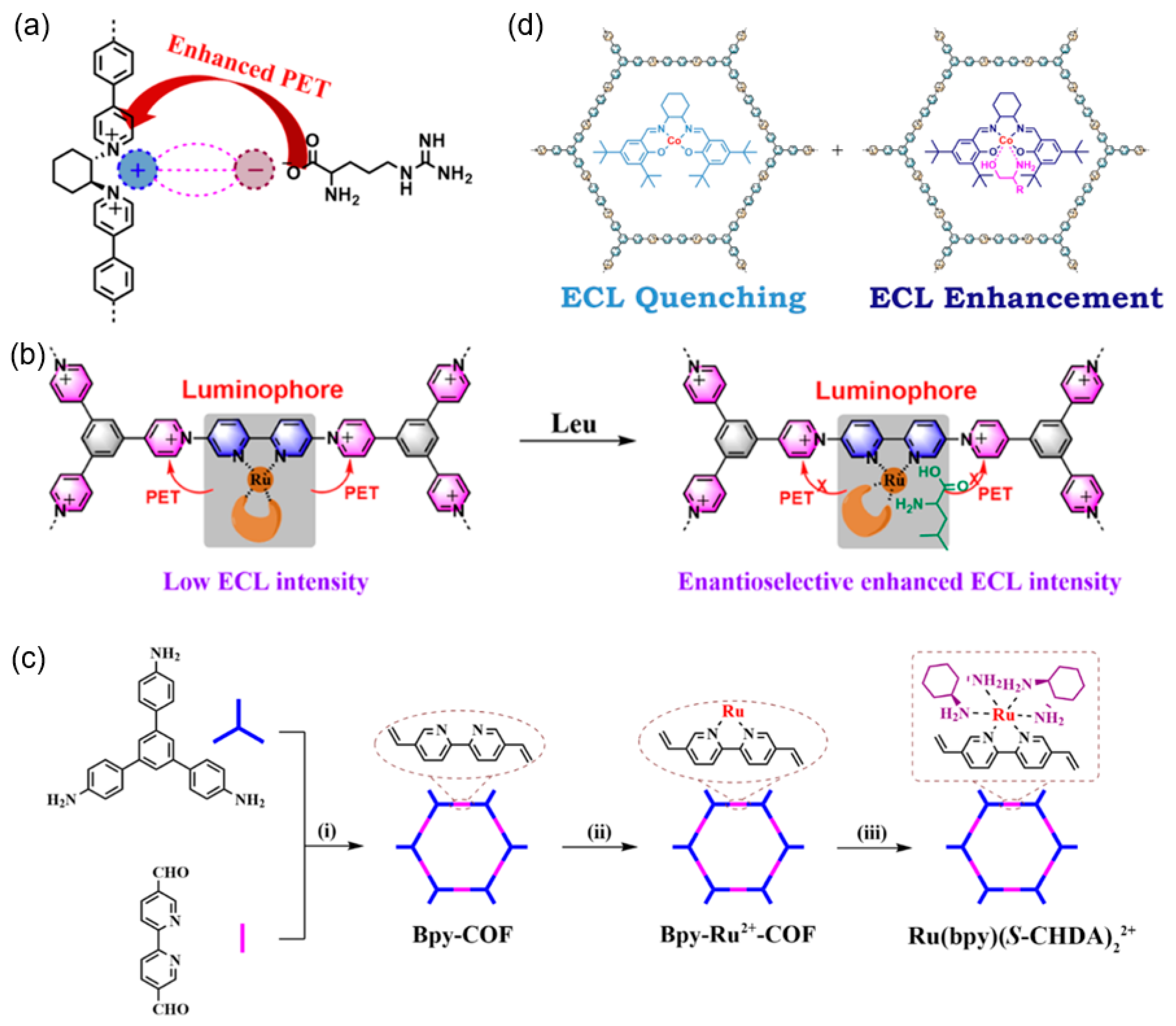
| 4 | Bpy-COF | Ru(bpy)(S-CHDA)22+/GCE | K2S2O8 | Chiral ECL-active unit | L-Trp | 10 μM~0.1 mM | / | / | [94] |
| 5 | Ph-triPy+-(R)-Ru(II) | Ph-triPy+-(R)-Ru(II)/GCE | Na2S2O8 | Chiral ECL-active unit | D-Leu/L-Leu-OH | / | / | / | [57] |
| 6 | TriPhPy+-BiPh | TriPhPy+@(R/S)-CHA | K2S2O8 | Chiral ECL-active unit | (R)/(S)-amino alcohol | / | / | / | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Cui, L. Rational Design of Covalent Organic Frameworks for Enhanced Reticular Electrochemiluminescence and Biosensing Applications. Biosensors 2025, 15, 760. https://doi.org/10.3390/bios15110760
Sun B, Cui L. Rational Design of Covalent Organic Frameworks for Enhanced Reticular Electrochemiluminescence and Biosensing Applications. Biosensors. 2025; 15(11):760. https://doi.org/10.3390/bios15110760
Chicago/Turabian StyleSun, Bing, and Lin Cui. 2025. "Rational Design of Covalent Organic Frameworks for Enhanced Reticular Electrochemiluminescence and Biosensing Applications" Biosensors 15, no. 11: 760. https://doi.org/10.3390/bios15110760
APA StyleSun, B., & Cui, L. (2025). Rational Design of Covalent Organic Frameworks for Enhanced Reticular Electrochemiluminescence and Biosensing Applications. Biosensors, 15(11), 760. https://doi.org/10.3390/bios15110760






