Nanogold-Lateral Flow Assay for Ginseng DNA Differentiation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Extraction of Genomic Ginseng DNAs and Their Amplification by PCR
2.3. Design of DNA-LFA Test Strip
2.4. Immobilization of Capture Probe
2.5. Preparation of DNA–AuNP Conjugate
2.6. Image Analysis and Signal Quantification
3. Results and Discussion
3.1. DNA-Lateral Flow Assay Design
3.2. Determination of Successful DNA–AuNP Conjugate Formation
| Solution | NaCl Concentration and Colour of Colloids | |||||
|---|---|---|---|---|---|---|
| 50 mM | 80 mM | 100 mM | 130 mM | 160 mM | 200 mM | |
| AuNP only | Red | Red | Red | Pale blue | Pale blue | Pale blue |
| DNA–AuNP conjugate | Red | Red | Red | Red | Red | Red |
| DNA/AuNP mixture | Red | Red | Pale blue | Pale blue | Pale blue | Pale blue |
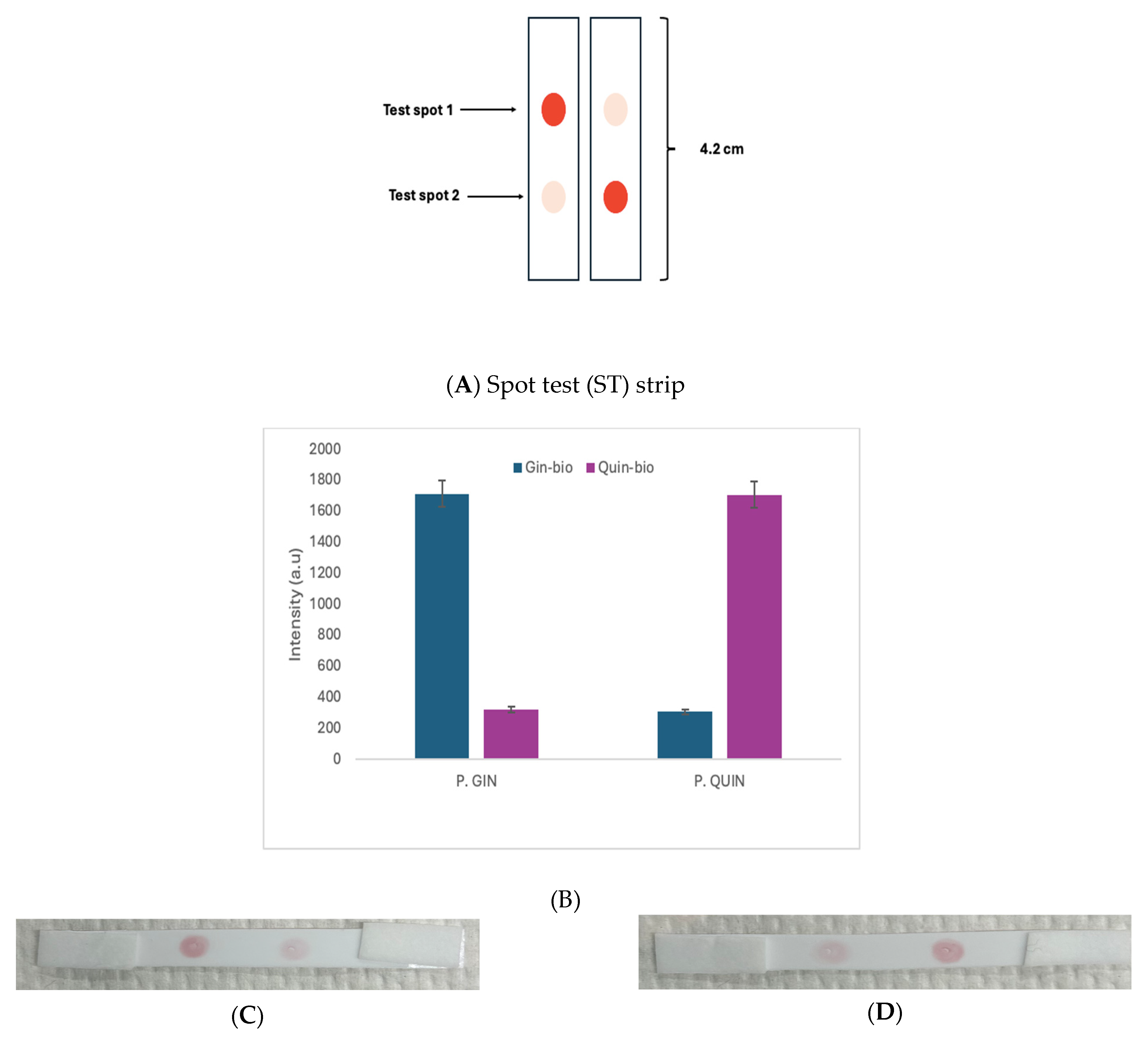
3.3. Spot Test Differentiation of P. Gin and P. Quin DNA
3.4. DNA-Lateral Flow Assay Detection of P. gin and P. quin DNA
3.5. DNA-Lateral Flow Assay When the Target DNA Is Separated from the DNA–AuNP Conjugate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. A Review of Ginseng Species in Different Regions as a Multipurpose Herb in Traditional Chinese Medicine, Modern Herbology and Pharmacological Science. J. Med. Plants Res. 2019, 13, 213–226. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, P.; Shin, C.Y. A Comprehensive Review of the Therapeutic and Pharmacological Effects of Ginseng and Ginsenosides in Central Nervous System. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef]
- Xu, W.; Choi, H.-K.; Huang, L. State of Panax Ginseng Research: A Global Analysis. Molecules 2017, 22, 1518. [Google Scholar] [CrossRef]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American Ginseng (Panax Quinquefolium L.) as a Source of Bioactive Phytochemicals with Pro-Health Properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef]
- Irfan, M.; Kwak, Y.-S.; Han, C.-K.; Hyun, S.H.; Rhee, M.H. Adaptogenic Effects of Panax Ginseng on Modulation of Cardiovascular Functions. J. Ginseng Res. 2020, 44, 538–543. [Google Scholar] [CrossRef]
- Kang, H.H.; Kim, S.-K. Adaptogenic Theory and Korean Ginseng (Panax Ginseng C.A. Meyer). Food Suppl. Biomater. Health 2023, 3, e11. [Google Scholar] [CrossRef]
- Choi, Y.D.; Park, C.W.; Jang, J.; Kim, S.H.; Jeon, H.Y.; Kim, W.G.; Lee, S.J.; Chung, W.S. Effects of Korean Ginseng Berry Extract on Sexual Function in Men with Erectile Dysfunction: A Multicenter, Placebo-Controlled, Double-Blind Clinical Study. Int. J. Impot. Res. 2013, 25, 45–50. [Google Scholar] [CrossRef]
- Hyun, S.H.; Bhilare, K.D.; In, G.; Park, C.-K.; Kim, J.-H. Effects of Panax Ginseng and Ginsenosides on Oxidative Stress and Cardiovascular Diseases: Pharmacological and Therapeutic Roles. J. Ginseng Res. 2022, 46, 33–38. [Google Scholar] [CrossRef]
- Yi, Y.-S. Roles of Ginsenosides in Inflammasome Activation. J. Ginseng Res. 2019, 43, 172–178. [Google Scholar] [CrossRef]
- Oliynyk, S.; Oh, S. Actoprotective Effect of Ginseng: Improving Mental and Physical Performance. J. Ginseng Res. 2013, 37, 144–166. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng Pharmacology: Multiple Constituents and Multiple Actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Sojoudi, P.; Oberc, C.; Tiffere, A.-H.; Li, P.C.H. Development of a Spot Test (ST) Based on the Nucleic Acid Amplification Test (NAAT) for Ginseng Species Authentication. Anal. Lett. 2025, 58, 2439–2452. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.-H. A Review on the Medicinal Potentials of Ginseng and Ginsenosides on Cardiovascular Diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Kim, J.-H. Pharmacological and Medical Applications of Panax Ginseng and Ginsenosides: A Review for Use in Cardiovascular Diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Christensen, L.P.; Jensen, M.; Kidmose, U. Simultaneous Determination of Ginsenosides and Polyacetylenes in American Ginseng Root (Panax quinquefolium L.) by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 8995–9003. [Google Scholar] [CrossRef]
- Xiu, Y.; Li, X.; Sun, X.; Xiao, D.; Miao, R.; Zhao, H.; Liu, S. Simultaneous Determination and Difference Evaluation of 14 Ginsenosides in Panax Ginseng Roots Cultivated in Different Areas and Ages by High-Performance Liquid Chromatography Coupled with Triple Quadrupole Mass Spectrometer in the Multiple Reaction–Monitoring Mode Combined with Multivariate Statistical Analysis. J. Ginseng Res. 2019, 43, 508–516. [Google Scholar] [CrossRef]
- Jauset-Rubio, M.; Svobodová, M.; Mairal, T.; McNeil, C.; Keegan, N.; Saeed, A.; Abbas, M.N.; El-Shahawi, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; et al. Ultrasensitive, Rapid and Inexpensive Detection of DNA Using Paper Based Lateral Flow Assay. Sci. Rep. 2016, 6, 37732. [Google Scholar] [CrossRef]
- Oberc, C.; Sojoudi, P.; Li, P.C.H. Nucleic Acid Amplification Test (NAAT) Conducted in a Microfluidic Chip to Differentiate between Various Ginseng Species. Analyst 2023, 148, 525–531. [Google Scholar] [CrossRef]
- Yu, J.Y.; Jin, Y.-R.; Lee, J.-J.; Chung, J.-H.; Noh, J.-Y.; You, S.-H.; Kim, K.-N.; Im, J.-H.; Lee, J.-H.; Seo, J.-M.; et al. Antiplatelet and Antithrombotic Activities of Korean Red Ginseng. Arch. Pharm. Res. 2006, 29, 898–903. [Google Scholar] [CrossRef]
- Inyangala, R.M. A Study of the Quality of Ginseng Containing Products in Nairobi, Kenya. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2012. [Google Scholar]
- Wang, H.-P.; Zhang, Y.-B.; Yang, X.-W.; Zhao, D.-Q.; Wang, Y.-P. Rapid Characterization of Ginsenosides in the Roots and Rhizomes of Panax Ginseng by UPLC-DAD-QTOF-MS/MS and Simultaneous Determination of 19 Ginsenosides by HPLC-ESI-MS. J. Ginseng Res. 2016, 40, 382–394. [Google Scholar] [CrossRef]
- Jegal, J.; Jeong, E.J.; Yang, M.H. A Review of the Different Methods Applied in Ginsenoside Extraction From Panax Ginseng and Panax Quinquefolius Roots. Nat. Prod. Commun. 2019, 14, 1934578X19868393. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.-Y.; Xiao, X.-Y.; Lin, R.-C.; Cheng, Y.-Y. Simultaneous Determination of Ginsenosides in Panax Ginseng with Different Growth Ages Using High-Performance Liquid Chromatography–Mass Spectrometry. Phytochem. Anal. 2006, 17, 424–430. [Google Scholar] [CrossRef]
- Chan, T.W.D.; But, P.P.H.; Cheng, S.W.; Kwok, I.M.Y.; Lau, F.W.; Xu, H.X. Differentiation and Authentication of Panax Ginseng, Panax Quinquefolius, and Ginseng Products by Using HPLC/MS. Anal. Chem. 2000, 72, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ji, J.; Ji, F.; Wu, S.; Tian, Y.; Jin, B.; Li, Z. Recombinase Polymerase Amplification Integrated with Microfluidics for Nucleic Acid Testing at Point of Care. Talanta 2022, 240, 123209. [Google Scholar] [CrossRef]
- Hu, W.; Liu, N.; Tian, Y.; Zhang, L. Molecular Cloning, Expression, Purification, and Functional Characterization of Dammarenediol Synthase from Panax Ginseng. BioMed Res. Int. 2013, 2013, 285740. [Google Scholar] [CrossRef] [PubMed]
- Schlag, E.M.; McIntosh, M.S. Ginsenoside Content and Variation among and within American Ginseng (Panax quinquefolius L.) Populations. Phytochemistry 2006, 67, 1510–1519. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Comparison of Ginsenoside Components of Various Tissues of New Zealand Forest-Grown Asian Ginseng (Panax ginseng) and American Ginseng (Panax quinquefolium L.). Biomolecules 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, Y.; Xie, J.; Cheng, Y.; Qi, R.; Wu, Y.; Zhang, S. Rapid Determination of Ginsenoside Rg1, Re and Rb1 in Ginseng Samples by Capillary Electrophoresis. Anal. Methods 2009, 1, 203–207. [Google Scholar] [CrossRef]
- Guo, M.; Shao, S.; Wang, D.; Zhao, D.; Wang, M. Recent Progress in Polysaccharides from Panax Ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef]
- Ghosh, R.; Bryant, D.L.; Farone, A.L. Panax Quinquefolius (North American Ginseng) Polysaccharides as Immunomodulators: Current Research Status and Future Directions. Molecules 2020, 25, 5854. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.-Z.; Zhu, X.-Y.; Wan, J.-Y.; Zhang, J.; Li, W.; Ruan, C.-C.; Yuan, C.-S. Dynamic Changes in Neutral and Acidic Ginsenosides with Different Cultivation Ages and Harvest Seasons: Identification of Chemical Characteristics for Panax Ginseng Quality Control. Molecules 2017, 22, 734. [Google Scholar] [CrossRef]
- Kausar, A.; Mitran, C.J.; Li, Y.; Gibbs-Davis, J.M. Rapid, Isothermal DNA Self-Replication Induced by a Destabilizing Lesion. Angew. Chem. Int. Ed. 2013, 52, 10577–10581. [Google Scholar] [CrossRef]
- Kausar, A.; Osman, E.A.; Gadzikwa, T.; Gibbs-Davis, J.M. The Presence of a 5′-Abasic Lesion Enhances Discrimination of Single Nucleotide Polymorphisms While Inducing an Isothermal Ligase Chain Reaction. Analyst 2016, 141, 4272–4277. [Google Scholar] [CrossRef]
- Alladin-Mustan, B.S.; Liu, Y.; Li, Y.; de Almeida, D.R.; Yuzik, J.; Mendes, C.F.; Gibbs, J.M. Reverse Transcription Lesion-Induced DNA Amplification: An Instrument-Free Isothermal Method to Detect RNA. Anal. Chim. Acta 2021, 1149, 238130. [Google Scholar] [CrossRef] [PubMed]
- Parveen, I.; Gafner, S.; Techen, N.; Murch, S.J.; Khan, I.A. DNA Barcoding for the Identification of Botanicals in Herbal Medicine and Dietary Supplements: Strengths and Limitations. Planta Med. 2016, 82, 1225–1235. [Google Scholar] [CrossRef]
- Osisiogu, E.U.; Singh, B.; Feglo, P.K.; Duedu, K.O. Detection of PhoP-Mediated Colistin Resistance in Gram-Negative Bacteria without Mcr Genes in Human Population in the Ho Municipality, Ghana. Heliyon 2024, 10, e39633. [Google Scholar] [CrossRef]
- Brennan, D.; Glynn, B.; Keegan, G.; McDonagh, C.; Barry, T.; Galvin, P. Incorporating Asymmetric PCR and Microarray Hybridization Protocols onto an Integrated Microfluidic Device, Screening for the Escherichia Coli ssrA Gene. Sens. Actuators B Chem. 2018, 261, 325–334. [Google Scholar] [CrossRef]
- Reid, M.S.; Le, X.C.; Zhang, H. Exponential Isothermal Amplification of Nucleic Acids and Assays for Proteins, Cells, Small Molecules, and Enzyme Activities: An EXPAR Example. Angew. Chem. Int. Ed. 2018, 57, 11856–11866. [Google Scholar] [CrossRef] [PubMed]
- Grazina, L.; Amaral, J.S.; Costa, J.; Mafra, I. Towards authentication of Korean ginseng-containing foods: Differentiation of five Panax species by a novel diagnostic tool. LWT 2021, 151, 112211. [Google Scholar] [CrossRef]
- Pang, Y.; Jiang, Y.; Li, G.; Wang, H. Species identification of Panax ginseng throughout the entire industrial chain: From the ginseng field to highly processed products. Food Control 2024, 167, 110824. [Google Scholar] [CrossRef]
- Ying, Z.; Awais, M.; Akter, R.; Xu, F.; Baik, S.; Jung, D.; Yang, D.; Kwak, G.Y.; Wenying, Y. Discrimination of Panax ginseng from counterfeits using single nucleotide polymorphism: A focused review. Front. Plant Sci. 2022, 13, 903306. [Google Scholar] [CrossRef]
- Kim, D.Y.; Miranda-Romo, D.; Ten Cate, A.R.; Hellberg, R.S. Use of a novel combination of multiplex PCR and DNA barcoding in assessing authenticity of ginseng products. Food Control 2024, 168, 110893. [Google Scholar] [CrossRef]
- Lu, Z.; Handy, S.M.; Zhang, N.; Quan, Z.; Xu, Q.; Ambrose, M.; Giancaspro, G.; Sarma, N.D. Development and Validation of a Species-specific PCR Method for the Identification of Ginseng Species Using Orthogonal Approaches. Planta Med. 2022, 88, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lo, Y.T.; Quan, Z.; He, J.; Chen, Y.; Faller, A.; Chua, T.; Wu, H.Y.; Zhang, Y.; Zou, Q.; et al. Application of a modified tetra-primer ARMS–PCR assay for rapid Panax species identity authentication in ginseng products. Sci. Rep. 2023, 13, 14396. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, A.; Oberc, C.; Whitehall, V.; Li, P.C.H. NanoHDA: A nanoparticle-assisted isothermal amplification technique for genotyping assays. Nano Res. 2017, 10, 12–21. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-Chip: Revising Microfluidic Platforms for Loop-Mediated DNA Amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef]
- Jung, Y.K.; Kim, J.; Mathies, R.A. Microfluidic Hydrogel Arrays for Direct Genotyping of Clinical Samples. Biosens. Bioelectron. 2016, 79, 371–378. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, Z.; Chan, S.D.; Eto, D.; Wu, W.; Zhang, J.P.; Chien, R.-L.; Wada, H.G.; Greenstein, M.; et al. On-Chip Quantitative PCR Using Integrated Real-Time Detection by Capillary Electrophoresis. Electrophoresis 2016, 37, 545–552. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in Paper-Based Point-of-Care Diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Wang, P. Lateral Flow Assay Ruler for Quantitative and Rapid Point-of-Care Testing. Analyst 2019, 144, 3314–3322. [Google Scholar] [CrossRef]
- Magiati, M.; Myridaki, V.M.; Christopoulos, T.K.; Kalogianni, D.P. Lateral Flow Test for Meat Authentication with Visual Detection. Food Chem. 2019, 274, 803–807. [Google Scholar] [CrossRef]
- Mak, W.C.; Beni, V.; Turner, A.P. Lateral-Flow Technology: From Visual to Instrumental. TrAC Trends Anal. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- Oberc, C.; Sedighi, A.; Li, P.C.H. The genetic authentication of Panax ginseng and Panax quinquefolius based on using single nucleotide polymorphism (SNP) conducted in a nucleic acid test chip. Anal. Bioanal. Chem. 2022, 414, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; El-Sayed, M.A. Plasmonic Coupling in Noble Metal Nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Deka, J.; Měch, R.; Ianeselli, L.; Amenitsch, H.; Cacho-Nerin, F.; Parisse, P.; Casalis, L. Surface Passivation Improves the Synthesis of Highly Stable and Specific DNA-Functionalized Gold Nanoparticles with Variable DNA Density. ACS Appl. Mater. Interfaces 2015, 7, 7033–7040. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Luo, G.; Jiao, J. DNA-Functionalized Gold Nanoparticles: Modification, Characterization, and Biomedical Applications. Front. Chem. 2022, 10, 1095488. [Google Scholar] [CrossRef]








| Probe | Sequence (5’-3’) | Modification |
|---|---|---|
| Gin-bio (P. ginseng capture probe) | CTAAAAAAAAAGTATTTTTCATCTAAATTTT GAA | 5’-biotin |
| Quin-bio (P. quinquefolius capture probe) | CTAAAAAAAAAGTATTTCTCATCTAAATTTT GAA | 5’-biotin |
| ss-Gin (P. ginseng sample) | TGA AAA TCA ATT TAA GAC ACT TTC AAA TTC AAA ATT TAG ATG AAA AAT ACT TTT TTT TTA | 5′-phosphate |
| ss-Quin (P. quinquefolius sample) | TGA AAA TCA ATT TAA AAC ACT TTC AAA TTC AAA ATT TAG ATG AGA AAT ACT TTT TTT TTA | 5′-phosphate |
| Detection probe | TTA AAT TGA TTT TCA | 3’ disulfide (S-S) |
| Bio-control | TGA AAA TCA ATT TAA | 5’-biotin |
| P7 forward primer | ATGTTTGT TACTCCCTCC GTT | 5′-phosphate |
| P8′ reverse primer | TACAGTGAT AATTAAATAT TGTAACTATC TAA | 5′-phosphate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiffere, A.-H.; Sojoudi, P.; Oberc, C.; Li, P.C.H. Nanogold-Lateral Flow Assay for Ginseng DNA Differentiation. Biosensors 2025, 15, 757. https://doi.org/10.3390/bios15110757
Tiffere A-H, Sojoudi P, Oberc C, Li PCH. Nanogold-Lateral Flow Assay for Ginseng DNA Differentiation. Biosensors. 2025; 15(11):757. https://doi.org/10.3390/bios15110757
Chicago/Turabian StyleTiffere, Al-Hashim, Parsa Sojoudi, Christopher Oberc, and Paul C. H. Li. 2025. "Nanogold-Lateral Flow Assay for Ginseng DNA Differentiation" Biosensors 15, no. 11: 757. https://doi.org/10.3390/bios15110757
APA StyleTiffere, A.-H., Sojoudi, P., Oberc, C., & Li, P. C. H. (2025). Nanogold-Lateral Flow Assay for Ginseng DNA Differentiation. Biosensors, 15(11), 757. https://doi.org/10.3390/bios15110757





