Artificial Intelligence-Based Wearable Sensing Technologies for the Management of Cancer, Diabetes, and COVID-19
Abstract
1. Introduction
2. Artificial Intelligence-Based Wearable Sensing Techniques
2.1. Artificial Intelligence-Based Wearable Electrochemical Sensing
2.2. Artificial Intelligence-Based Wearable Colorimetric Sensing
2.3. Artificial Intelligence-Based Wearable Chemical Sensing
2.4. Artificial Intelligence-Based Wearable Optical Sensing
2.5. Artificial Intelligence-Based Wearable Pressure/Strain Sensing
3. Artificial Intelligence-Based Wearable Sensing Technology for COVID-19 Management
3.1. Heart Rate and Heart Rate Variability (HRV) Sensors
3.2. Oxygen Saturation (SpO2) Sensors
3.3. Temperature Sensors
3.4. Respiratory Rate Sensors
3.5. Multi-Sensor Devices
| Type of Wearable Sensor | AI/Algorithm Used | Target Analyte (Intended) | Limit of Detection (LOD) | Detection Range | Selectivity | Specificity (Reported) | Pros | Cons | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Smartwatch/fitness tracker | Signal-processing + online detection algorithm; anomaly detection | Pre-symptomatic physiological signature of infection (COVID-19) | N/A | Human HR/activity dynamic range | Detects physiological deviation but not pathogen-specific | 63% pre-symptomatic detection in the cohort; 81% had alterations | Non-invasive, real-time, widely deployed, population scale | Not pathogen-specific; confounded by exercise/stress; device heterogeneity | [47] |
| Smartwatch + smartphone app (DETECT) | Multivariate classifier combining sensor metrics + symptoms (ML/statistical) | Distinguish symptomatic COVID+ vs. symptomatic COVID− | N/A | N/A | Improved discrimination when fusing sensor + symptom modalities | AUC = 0.80 (sensor+symptoms) vs. 0.71 (symptoms only) | Large cohort (30,529); scalable app-based collection | Self-report biases; device/platform heterogeneity | [50] |
| Biosensing wearable network (iPREDICT)—conceptual framework | Anomaly detection; Graph Neural Networks (GNNs); spatiotemporal models; federated learning (proposed) | Early outbreak/pandemic risk indicators | Conceptual; depends on underlying sensors | Population/spatiotemporal scale | Aims to improve selectivity via cross-user correlation and contextual data | Framework-level; specificity depends on model thresholds and context | Proactive outbreak detection: leverages crowd-sensed data and graph models | Requires standardized data, privacy-preserving infra, and broad adoption | [69] |
| Wearable biosensors + ICT (systematic review) | Surveyed ML/DL across studies (SVM, RF, CNN, ensemble, anomaly detection) | Patient deterioration, infection monitoring, remote triage | Varies by device/study; review-level | Device-dependent | Improved with multimodal fusion | Varied across included studies; heterogeneous reporting | Comprehensive mapping of ICT + wearable solutions; identifies effective strategies | Heterogeneous methods and metrics; variable evidence quality | [71] |
| IoT-based smart health monitoring system (prototype) | Rule-based alerts; proposed ML integration | Physiological indicators of infection/severity | Reported relative errors vs. commercial devices: HR 2.89%, Temp 3.03%, SpO2 1.05% | Clinical vital sign ranges | Good for gross physiological changes; limited pathogen specificity | Not explicitly quantified | Low-cost, suitable for rural deployment; cloud storage for longitudinal tracking | Requires connectivity; privacy/security and calibration concerns | [74] |
| Smartwatch + explainable unsupervised learning pipeline | Unsupervised clustering; validated with supervised classifiers; GPT-3 for interpretation | Physiological anomaly clusters indicating infection (COVID-19) | N/A (clustering/classification task) | N/A | Clusters capture anomalies but need clinical labels for disease specificity | Supervised validation: accuracy 0.884 ± 0.005; precision 0.80 ± 0.112; recall 0.817 ± 0.037 | Reduces reliance on labeled data; explainability aids clinician trust | LLM interpretation may introduce noise; cohort-dependent | [75] |
| 24 h Holter ECG (clinical-grade HRV monitoring) | Statistical analysis (no ML reported) | Autonomic dysregulation in Post-COVID-19 Syndrome (PCS) | N/A | 24 h monitoring window | HRV is sensitive to autonomic changes but not disease-specific | PCS patients showed significant HRV alterations vs. controls (after correction) | An objective clinical biomarker for autonomic dysfunction | Requires clinical equipment and interpretation; not a consumer wearable | [76] |
| Non-contact infrared thermometers (NCIT)—screening | ROC analysis and thresholding (no AI) | Fever detection as a proxy for infection | NCIT resolution ~0.1 °C | Skin temperatures ~30–40 °C | The neck site had the highest accuracy among the sites tested | Triple neck detection sensitivity up to 0.998; accuracy reduced at ambient < 18 °C | Fast, contactless, scalable for mass screening | Many infections are afebrile; ambient and site dependence; not pathogen-specific | [84] |
| IoT + Cloud + AI framework review for self-monitoring (5G enabled) | Survey of ML/AI approaches, cloud/edge analytics architectures | Physiological indicators associated with COVID-19/self-diagnosis | Device-dependent (review) | Varies with sensors | Multi-modal fusion is proposed to increase selectivity | Not experimentally quantified (review) | Comprehensive technology stack view; emphasizes low-cost cloud analytics and 5G benefits | Privacy, data security, deployment, and standardization challenges | [85] |
| UAV-mounted thermal camera (aerial thermal imaging) | Computer vision/deep learning classifiers for face detection, mask detection, temperature anomaly detection (hybrid ML/DL) | Fever screening/identify potentially febrile individuals in crowds (COVID-19 triage) | Thermal camera resolution dependent; not reported as concentration LOD (temperature resolution typical ~0.1 °C) | Up to drone operational range; system table: drone payload 2 kg, flight time 30–35 min (per paper) | Detects elevated skin temperature; not pathogen-specific; can include false positives (environmental effects) | Overall average accuracy reported ~99.5% for the proposed pipeline in test scenarios (paper reports high accuracy for detection tasks) | Rapid, contactless mass screening; can cover large crowds; includes mask detection | Skin temp not always reflective of core temp; environmental/ambient effects; cannot confirm infection | [90] |
| Smartphone onboard sensors (conceptual/app) | Deep learning frameworks (CNN/RNN/hybrid DL) for classification; proposed DL pipeline | Preliminary diagnosis/screening for COVID-19 | N/A (classification task); performance metric: reported overall accuracy ~79% using smartphone sensors | User-device range (onboard sensors) | Depending on features used; cough/audio may be confounded with other respiratory illnesses | Not universally reported; overall accuracy 79% reported in this study | Widely available, low-cost, quick-deployable screening without medical tests | Requires labeled data, user compliance, false positives/negatives, and variability across devices | [93] |
| Contact piezoelectric sensor (and ultrasonic non-contact) for respiration | Logistic regression for classification of respiratory disease from collected vitals (simple ML) | Respiratory rate monitoring and screening for respiratory disease | N/A (physiological metric); device accuracy reported: overall device accuracy 96.58% for RR measurement | Respiratory rates in the typical human range (~5–40 bpm)—device validated on patients | Detects abnormal respiratory rate patterns; not disease-specific | Logistic regression classifier achieved 88% accuracy (5-fold CV) for respiratory disease detection | Low cost, accurate RR measurement, suitable for continuous monitoring | Contact sensor required; placement sensitivity (best positions vary by BMI); may be uncomfortable for long-term use | [96] |
| FMCW radar (non-contact) | Stacked ensemble ML models (ensemble of MLR, DT, RF, SVM, XGB, LGBM, CatBoost, MLP) and proposed Neural Stacked Ensemble Model (NSEM) | Classify respiratory behavior/detect abnormal breathing patterns (COVID-19 supervision) | Not concentration-based; radar sensitivity to chest micro-displacement; not reported as LOD | Room-scale; can detect multiple subjects and AoA separation | Can separate multiple objects and breathing characteristics; robust to lighting/privacy issues compared to the camera | The best model (NSEM) achieved 97.1% accuracy in experiments | Non-contact, privacy-preserving, can monitor multiple subjects simultaneously, with high accuracy reported | Requires RF hardware, signal interference, and may need careful calibration and line-of-sight | [99] |
| Wearable biosignal sensors (ECG, PPG, sEMG) for RR prediction | Deep learning: LSTM, Bi-LSTM, Attention LSTM, CNN-LSTM, ConvLSTM; Bi-LSTM with Bahdanau attention best | Accurate respiratory rate prediction from biosignals | N/A (physiological regression task); MAE reported as performance metric (best MAE 0.24 ± 0.03 for PPG + ECG dataset) | Depends on dataset; models evaluated on clinical/public datasets | Model differentiates RR patterns effectively; sensor-dependent noise affects selectivity | Performance reported as MAE; no binary specificity since regression task | High accuracy RR prediction with deep models; works across ECG/PPG/sEMG data | Require quality biosignals; model complexity and computational needs; window length affects performance | [100] |
| Imaging (chest X-ray) based diagnostic tool (hospital imaging) | Deep learning: transfer learning with CNNs (InceptionV3, ResNet50, Xception) and Vision Transformer (ViT) | Automatic COVID-19 detection and classification from CXR images | N/A (imaging classification) | Image-level classification, dependent on dataset quality and radiographic features | ViT showed superior ability to distinguish four classes vs. CNNs | Vision Transformer achieved a test accuracy of 99.3% (reported), outperforming ResNet50 (85.58%) in their experiments | High diagnostic accuracy reported (on their dataset); rapid automated triage potential | Requires clinical imaging equipment; dataset biases and limited generalizability; high accuracy may not generalize to diverse populations | [101] |
| Various wearables + smartphone/camera-based approaches (review) | Survey of ML/DL methods: CNN, RNN, image/signal processing, anomaly detection, explainable AI | Remote monitoring of vital signs for COVID-19 screening and monitoring | Varies by modality; review summarizes methods rather than specific LODs | Device-dependent; many methods are suitable for smartphone deployment | Varies; methods may struggle to be disease-specific, but useful for anomaly detection | Varied across studies; review discusses strengths and limitations (no single specificity value) | Enables remote, low-cost monitoring using ubiquitous devices; discusses practical deployment challenges | Heterogeneous literature; privacy and data-quality concerns; not yet clinical-grade across the board | [102] |
| Wearable IoT sensors for remote patient activity monitoring (multi-sensor wearables) | Proposed CNN-UUGRU deep model (convolution + updated gated recurrent units) for activity recognition | Activity recognition, remote patient monitoring, and detection of confinement breaches for quarantined patients | N/A (activity recognition/vital signs) | Wearable/device dependent; remote cloud connectivity via IoT | High for activity classes (model accuracy reported) | Reported performance: accuracy 97.7%, precision 96.8%, F-measure 97.75% on evaluated datasets | Integrated IoT-stack with high activity classification accuracy; cloud alerts and GPS tracking enable quarantine monitoring | Privacy concerns, connectivity needs, sensor calibration, and battery constraints | [103] |
4. Artificial Intelligence-Based Wearable Sensing Technology for Diabetes Management
4.1. Continuous Glucose Monitoring (CGM) Devices
4.1.1. Electrochemical Sensors
4.1.2. Optical Sensors
4.1.3. Microneedle Sensors
4.2. AI-Driven Insulin Pumps and Closed-Loop Systems
4.3. Non-Invasive Glucose Monitoring Wearables
4.3.1. Smart Contact Lenses
4.3.2. Sweat-Based Sensors
4.3.3. Multi-Parameter Wearables: Smartwatches and Fitness Trackers
| Type of Wearable Sensor | AI/Algorithm Used | Target Analyte | Limit of Detection | Detection Range | Selectivity | Specificity | Pros | Cons | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Noncontact speckle-based optical finger sensor | Machine Learning classifiers; tested DNNs (ML outperformed DNNs) | Plasma glucose levels (classification) | Not reported as LOD; classification accuracy reported | Physiological glucose ranges (capable of classifying hypo/standard/hyper bands) | Improved by magneto-optic modulation + preprocessing | High classification accuracy reported (ML > DNN in this dataset) | Totally noncontact; low-cost hardware; AI improves selectivity and sensitivity | Needs larger, diverse cohorts; environmental and motion sensitivity | [125] |
| Fully integrated microneedle continuous glucose monitor (MN-CGM) | Signal processing + potential ML for calibration discussed (not primary focus) | Glucose in ISF (clinical CGM range) | LOD not explicitly stated; wide linear range demonstrated | 0.25–35 mM (wide clinical range) | High via enzyme specificity and PB mediator | Good correlation with commercial glucose meters in animal studies | Wide linear range, stable, suitable for real-time continuous monitoring | Implantable/microneedle invasiveness (minimally), enzyme stability over long term | [128] |
| Swelling microneedle patch delivering TSA for wound healing (AI-guided) | AI-assisted bioinformatics, molecular docking, and sequencing analysis | HDAC4 and associated inflammatory pathways | N/A (therapeutic focus) | N/A | High target specificity per bioinformatics/docking | Validated in vitro and in vivo models | AI-guided drug repurposing; minimally invasive targeted therapy | Not a continuous wearable sensor; translational work needed | [135] |
| Survey/review of BG prediction methods (not a single device) | Surveyed ML/DL methods: SVM, RF, ANN, LSTM, hybrid models | Predicted blood glucose levels and adverse events | N/A (review) | Depends on underlying sensors (typical CGM ranges) | Varies by model and feature set | Discussed in literature; performance metrics summarized across studies | Comprehensive overview of trends, input features, modeling techniques, and challenges | Not original experimental device data; heterogeneity across studies | [140] |
| AI-based insulin dose optimization (AI-DSS) integrated with insulin pumps and CGM | Proprietary AI-DSS (DreaMed Advisor Pro)—rule-based + ML components | Glucose control and insulin dose settings | CGM device-dependent | CGM operational range (e.g., 40–400 mg/dL) | Effective for individualized insulin titration | Non-inferior to physician adjustments in RCT (safety endpoints) | Reduces clinician workload; safe and effective per multicenter RCT | Requires accurate CGM and adherence; regulatory and integration aspects | [146] |
| Smart, soft contact lens with integrated glucose sensor and display | Signal processing + ML-based filtering for improved readout | Glucose in tear fluid | Reported ~30 µM | Approximately 50–500 µM in tears | High via enzyme specificity | Correlating with blood glucose with time lag consideration | Fully integrated, transparent, wireless, real-time visualization | Tear-blood correlation lag; fabrication complexity; comfort and safety considerations | [148] |
| On-demand sweat glucose EIS sensor with ML reporting (SWEET platform) | Decision Tree Regression for prediction and mapping to glucose | Sweat glucose (mg/dL) | RMSE ~0.1 mg/dL in reported tests | Reported 1–4 mg/dL (physiological sweat glucose range) | High via affinity probe; reduced interferents | R2 = 0.94 in validation datasets | Completely non-invasive, frequent sampling (1–5 min), ML enables robust reporting | Inter-subject variability; dependency on sweat availability; needs broader clinical validation | [149] |
| Systematic review: ML and smart devices for diabetes management | Survey of ML techniques: SVM, RF, ANN, ensemble, DL | Glycemic events, BG prediction, complications detection | Not applicable | Dependent on the specific device/sensor | N/A (review) | Summarized per the study surveyed | Comprehensive synthesis of 89 studies; highlights trends and gaps | Heterogeneity in study designs, limited to 2011–2021 | [161] |
| Remote healthcare monitoring framework using wearables for diabetes prediction | Support Vector Machine (SVM) classifier | Diabetes risk/classification | N/A (classification task) | Binary or risk-score outputs | Accuracy 83.2% (10-fold CV) | 79% specificity; sensitivity 87.2% | Vendor interoperability; remote monitoring potential | Limited dataset generalization; relies on input quality | [163] |
| ARISES: Multi-modal wearable + DL platform for T1D self-management | Deep Learning model (RNN/LSTM-based) for 60 min prediction horizon | Glucose forecasting | RMSE = 35.28 ± 5.77 mg/dL (60 min horizon) | Reported clinically relevant ranges; the model reduces prediction error with wristband data | Improved detection of events (Matthew’s coefficients reported) | Matthew’s coefficients: 0.56 (hypo), 0.70 (hyper), indicating a reasonable specificity/sensitivity balance | Demonstrated reduction in prediction errors when including wearable data; implemented in smartphone app | Small study cohort (12 adults in longitudinal study for model development); needs larger validation | [165] |
5. Artificial Intelligence-Based Wearable Sensing Technology for Cancer Management
5.1. Biosensing Technology-Based Wearables
5.2. Optical Sensor-Based Wearables
5.3. Thermal Imaging Sensors
5.4. Ultrasound-Based Wearable Sensors
| Type of Wearable Sensor | AI/Algorithm Used | Target Analyte | Limit of Detection | Detection Range | Selectivity | Specificity | Pros | Cons | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Sweat biomarker monitoring patch | Machine learning-based pattern recognition for multi-analyte data integration (described conceptually in review) | Sweat metabolites and hormones | Varies by analyte: Lactate ~0.35 µM; Cortisol ~0.1 pg/mL; Glucose ~35 µM | 1 µM–15 mM (depending on analyte) | High with enzyme/MIP-based sensors | Depends on immobilization method (LOx, GOx, Ab-based) | Non-invasive, real-time, multi-analyte detection, compatible with AI data analysis, wireless capability | Biofouling, enzyme degradation, sensor drift, and limited sweat volume in low perspiration conditions | [177] |
| Osmotic hydrogel–microfluidic electrochemical lactate patch | Data-driven modeling for activity recognition and lactate trend prediction (custom AI model used for calibration drift correction) | Lactate | 350 nM | Up to 15 mM | High (enzyme-based) | Specific to lactate oxidase substrate | Zero-power operation; continuous monitoring at rest and exercise; ultra-low power wireless module (0.706 mW) | Enzyme degradation over time; humidity-dependent response; limited long-term stability | [178] |
| OLED-based organic photonic biosensor | AI-assisted signal deconvolution and light intensity correlation model (image pattern recognition) | Ovarian cancer-related bioluminescent markers | 13–29 mA photocurrent at 420–440 nm (relative intensity-based detection) | Optical wavelength 400–500 nm | High spectral specificity | Light wavelength-based signal discrimination ensures selectivity | Flexible, low-cost, biocompatible, lightweight; suitable for optical AI processing | Needs calibration; sensitive to ambient light; lacks multiplexing capability | [188] |
| Flat-panel OLED display-based multiplexed immunosensor | Neural network-based fluorescence signal recognition for multiplexed biomarker classification | HPV-related IgG antibodies | 10 pg/mL (for IgG antibodies) | 10 pg/mL–10 µg/mL | High with antibody–antigen binding | Multiplexed detection reduces false positives via AI pattern discrimination | Compact, disposable, low-cost, high-throughput multiplexing; scalable via display manufacturing | Complex calibration; needs fluorescence reference standardization | [189] |
| AI-enhanced optical polarization sensor for skin cancer detection | Classification and Regression Tree (CART) algorithm | Skin cancer lesions (melanoma, SCC, BCC) | Not applicable (classification-based system) | Optical feature extraction model-based | High (92.6% accuracy in distinguishing cancer types) | Accurate classification of malignant vs. non-malignant samples | Non-invasive, interpretable AI model, real-time diagnosis | Limited sample diversity; optical noise under skin curvature | [193] |
| Vibrational Optical Coherence Tomography (VOCT) Patch | Machine Learning Classification (unspecified model) | Pigmented vs. non-pigmented melanoma lesions | N/A (classification accuracy based) | 80–250 Hz frequency response range | High (78–90% accuracy for lesion differentiation) | Up to 90% | Non-invasive, quantitative, real-time detection of melanoma phenotypes | Requires calibration; limited large-scale clinical validation | [199] |
| Infrared Thermal Imaging System (contactless wearable-assisted imaging) | Deep Learning Image Classification Models (DNN, CNN, SVM) | Breast cancer lesions | Temperature resolution ~0.05 °C | Skin surface temperature 30–40 °C | Distinguishes malignant vs. benign tumors based on thermal variance | Up to 94% with a CNN-based classifier | Non-contact, radiation-free, suitable for early cancer screening | Affected by ambient temperature; requires calibration and a trained dataset | [208] |
| Medical Infrared Thermography (IRT) for skin neoplasms | Pattern recognition and machine learning-based classification | Skin cancer (melanoma, BCC, SCC) | Thermal sensitivity < 0.1 °C | 30–42 °C | High when combined with ML | Improved diagnostic accuracy via dynamic thermal analysis | Contactless, safe, non-ionizing, real-time detection | Sensitive to environment; limited depth resolution | [206] |
| Conformable Ultrasound Breast Patch (cUSBr-Patch) | AI-based image reconstruction and lesion classification (CNN-enhanced) | Breast tissue cysts and lesions | ~0.3 cm cyst | Up to 30 mm tissue depth | High acoustic contrast (3 dB) | Clinical-level accuracy with AI-assisted analysis | Non-invasive, comfortable, real-time deep tissue monitoring | Complex fabrication; limited penetration beyond 30 mm | [218] |
| Fully Integrated Conformal Wearable Ultrasound Patch (CWUS) | AI-based control and optimization for ultrasound power modulation | Cancerous tissue (tumor apoptosis induction) | N/A (therapy-based system) | Deep tissue penetration (>30 mm) | High, focused ultrasound localization | Precise tumor targeting via AI-controlled focusing | Continuous, non-invasive tumor treatment; adaptive control via AI | Requires power management and safety calibration | [218] |
| Hybrid Deep-CNN LungNet System (IoT-integrated) | Hybrid Deep-CNN (LungNet) | Lung cancer detection and stage classification | N/A (classification accuracy-based) | Stage 1A–2B classification | 96.81% classification accuracy | Low false positive rate (3.35%) | The hybrid IoT-CNN model integrates real-time physiological data with imaging | Centralized server required; data privacy considerations | [203] |
| CNN-Transformer Breast Ultrasound Classifier | Hybrid CNN + Vision Transformer (ViT) + MLP-Mixer | Breast tumor segmentation and classification | N/A (accuracy-based segmentation) | Tumor size variability from ultrasound imagery | High (Dice 83.42%) | 86% classification accuracy | High interpretability; captures long-range dependencies; robust tumor segmentation | High computational load; training dataset requirement | [223] |
| Dataset | Biological Fluid or Modality | Cancer Biomarkers or Features | Data Type | Refs. |
|---|---|---|---|---|
| Salivary Biomarker Datasets | Saliva | IL-6, IL-8, TNF-α, MMP-9, CA-125 | Proteomic and cytokine patterns for breast and oral cancer | [226,227] |
| SalivaDB dataset | Saliva | miR-146a | proteins, metabolites, microbes, micro-ribonucleic acid (miRNA), and human genes | [228] |
| Dataset for Oral Cancer Cytology (OSCC) | oral swabs or images of cells | Oral squamous cell carcinoma-related cellular abnormalities | Images of cytology with annotations | [229] |
| Cancer Pain Multimodal Dataset | Physiological indicator | Physiological indicators of pain in cancer | Time-series behavioral and wearable signals | [230] |
| HAM10000 Dataset | Optical dermoscopy images | Skin lesions with pigmentation, such as melanoma | High-resolution dermoscopy images | [231] |
| International Skin Imaging Collaboration (ISIC) Archive | Dermoscopy Images | Skin cancer (BCC, SCC, melanoma) | An extensive public collection of dermoscopy images | [232] |
6. Limitations and Challenges
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef]
- El-Rashidy, N.; El-Sappagh, S.; Riazul Islam, S.M.; El-Bakry, H.M.; Abdelrazek, S. Mobile Health in Remote Patient Monitoring for Chronic Diseases: Principles, Trends, and Challenges. Diagnostics 2021, 11, 607. [Google Scholar] [CrossRef]
- Siller, A.F.; Tosur, M.; Relan, S.; Astudillo, M.; McKay, S.; Dabelea, D.; Redondo, M.J. Challenges in the Diagnosis of Diabetes Type in Pediatrics. Pediatr. Diabetes 2020, 21, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Aadil, K.R.; Bhange, K.; Mishra, G.; Sahu, A.; Sharma, S.; Pandey, N.; Mishra, Y.K.; Kaushik, A.; Kumar, R. Nanotechnology Assisted Strategies to Tackle COVID and Long-COVID. Bionanoscience 2025, 15, 217. [Google Scholar] [CrossRef]
- Alshuhri, M.S.; Al-Musawi, S.G.; Al-Alwany, A.A.; Uinarni, H.; Rasulova, I.; Rodrigues, P.; Alkhafaji, A.T.; Alshanberi, A.M.; Alawadi, A.H.; Abbas, A.H. Artificial Intelligence in Cancer Diagnosis: Opportunities and Challenges. Pathol. Res. Pract. 2024, 253, 154996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, A.; Gupta, V.; Arya, S. Advancements and Future Prospects of Wearable Sensing Technology for Healthcare Applications. Sens. Diagn. 2022, 1, 387–404. [Google Scholar] [CrossRef]
- Kuhar, N.; Kumria, P.; Rani, S. Overview of Applications of Artificial Intelligence (AI) in Diverse Fields. In Application of Artificial Intelligence in Wastewater Treatment; Springer: Cham, Switzerland, 2024; pp. 41–83. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Kour, S.; Biswas, I.; Sheoran, S.; Arora, S.; Sheela, P.; Duppala, S.K.; Murthy, D.K.; Pawar, S.C.; Singh, H.; Kumar, D.; et al. Artificial Intelligence and Nanotechnology for Cervical Cancer Treatment: Current Status and Future Perspectives. J. Drug Deliv. Sci. Technol. 2023, 83, 104392. [Google Scholar] [CrossRef]
- Lv, C.; Guo, W.; Yin, X.; Liu, L.; Huang, X.; Li, S.; Zhang, L. Innovative Applications of Artificial Intelligence during the COVID-19 Pandemic. Infect. Med. 2024, 3, 100095. [Google Scholar] [CrossRef]
- Zhu, P.; Niu, M.; Liang, S.; Yang, W.; Zhang, Y.; Chen, K.; Pan, Z.; Mao, Y. Non-Hand-Worn, Load-Free VR Hand Rehabilitation System Assisted by Deep Learning Based on Ionic Hydrogel. Nano Res. 2025, 18, 94907301. [Google Scholar] [CrossRef]
- Li, Z.; Yu, N.; Hartel, M.C.; Haghniaz, R.; Emaminejad, S.; Zhu, Y. An Ultra-Robust, Highly Compressible Silk/Silver Nanowire Sponge-Based Wearable Pressure Sensor for Health Monitoring. Biosensors 2025, 15, 498. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, G.; Liu, J.; Yang, B. 5G NB-IoT System Integrated with High-Performance Fiber Sensor Inspired by Cirrus and Spider Structures. Adv. Sci. 2024, 11, 2309894. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Yan, Y.; Xiao, K.; Wang, Z.; Zheng, D.; Leal-Junior, A.; Kumar, S.; Ortega, B.; Marques, C.; et al. Wearable Photonic Smart Wristband for Cardiorespiratory Function Assessment and Biometric Identification. Opto-Electron. Adv. 2025, 8, 240254. [Google Scholar] [CrossRef]
- Poongodi, J.; Kavitha, K.; Sathish, S.; Lakshmana Kumar, R. Hybrid AI-Driven Bio-Inspired Wearable Sensors with Aquasense AI Technology for Multimodal Health Monitoring and Rehabilitation in Dynamic Environments. Trans. Emerg. Telecommun. Technol. 2025, 36, e70081. [Google Scholar] [CrossRef]
- Liu, J.H.; Shih, C.Y.; Huang, H.L.; Peng, J.K.; Cheng, S.Y.; Tsai, J.S.; Lai, F. Evaluating the Potential of Machine Learning and Wearable Devices in End-of-Life Care in Predicting 7-Day Death Events Among Patients with Terminal Cancer: Cohort Study. J. Med. Internet Res. 2023, 25, e47366. [Google Scholar] [CrossRef]
- Torrente, M.; Sousa, P.A.; Hernández, R.; Blanco, M.; Calvo, V.; Collazo, A.; Guerreiro, G.R.; Núñez, B.; Pimentao, J.; Sánchez, J.C.; et al. An Artificial Intelligence-Based Tool for Data Analysis and Prognosis in Cancer Patients: Results from the Clarify Study. Cancers 2022, 14, 4041. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Lund, J.; Kepka, D.; Mariotto, A. Economic Burden of Cancer in the United States: Estimates, Projections, and Future Research. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am. Health Drug Benefits 2016, 9, 23–32. [Google Scholar]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar]
- Belić, M.; Bobić, V.; Badža, M.; Šolaja, N.; Đurić-Jovičić, M.; Kostić, V.S. Artificial Intelligence for Assisting Diagnostics and Assessment of Parkinson’s Disease-A Review. Clin. Neurol. Neurosurg. 2019, 184, 105442. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease Detection with Molecular Biomarkers: From Chemistry of Body Fluids to Nature-Inspired Chemical Sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Dutt, S.; Gupta, A.K.; Aadil, K.R.; Bunekar, N.; Mishra, V.K.; Kumar, R.; Gupta, A.; Chaudhary, A.; Kumar, A.; Chawla, M.; et al. Nanomaterials of metal and metal oxides for optical biosensing application. In Metal Oxides for Biomedical and Biosensor Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–352. [Google Scholar] [CrossRef]
- Mondal, K.; Kumar, R.; Isaac, B.; Pawar, G. Metal Oxide Nanofibers and Their Applications for Biosensing. In Metal Oxides for Biomedical and Biosensor Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 113–137. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.I.; Faleiros, M.C.; Ferreira, D.C.; Mani, V.; Salama, K.N. Empowering Electrochemical Biosensors with AI: Overcoming Interference for Precise Dopamine Detection in Complex Samples. Adv. Intell. Syst. 2023, 5, 2300227. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, H.-Y.; Shin, M.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible Electrochemical Biosensors for Healthcare Monitoring. J. Mater. Chem. B 2020, 8, 7303–7318. [Google Scholar] [CrossRef]
- Hosseinzadeh Fakhr, M.; Lopez Carrasco, I.; Belyaev, D.; Kang, J.; Shin, Y.H.; Yeo, J.S.; Koh, W.G.; Ham, J.; Michaelis, A.; Opitz, J.; et al. Recent Advances in Wearable Electrochemical Biosensors towards Technological and Material Aspects. Biosens. Bioelectron. X 2024, 19, 100503. [Google Scholar] [CrossRef]
- Ramachandran, B.; Liao, Y.-C. Microfluidic Wearable Electrochemical Sweat Sensors for Health Monitoring. Biomicrofluidics 2022, 16, 51501. [Google Scholar] [CrossRef]
- Shakil, S.; Yuan, D.; Li, M. Review—Electrochemical Sensors for Acetylcholine Detection. J. Electrochem. Soc. 2024, 171, 67512. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, J.; Li, Y. MXene with Great Adsorption Ability toward Organic Dye: An Excellent Material for Constructing a Ratiometric Electrochemical Sensing Platform. ACS Sens. 2019, 4, 2058–2064. [Google Scholar] [CrossRef]
- Kumar, R.; Sarkar, C.; Bunekar, N.; Mishra, Y.K.; Kaushik, A. State-of-the-Art Transition Metal Dichalcogenides: Synthesis, Functionalization, and Biomedical Applications. Mater. Today 2025, 88, 597–642. [Google Scholar] [CrossRef]
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical Sensors Based on Molecularly Imprinted Polymer on Nanostructured Carbon Materials: A Review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical Sensing Based on Carbon Nanoparticles: A Review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal Oxide Based Non-Enzymatic Electrochemical Sensors for Glucose Detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.-H.; Choi, J.-W. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. High-Performing Conductive Hydrogels for Wearable Applications. Gels 2023, 9, 549. [Google Scholar] [CrossRef]
- Shrivastav, A.; Singh, S.; Rawat, R.; Mishra, A.; Kumar, P.; Kaushik, A.; Mathur, A. Vanadium MXene-Modified Disposable Screen-Printed Electrodes for Highly Sensitive Glucose Sensing. ECS Sens. Plus 2025, 4, 021601. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, N.; Bikkannavar, P.; Cordeiro, M.F.; Yetisen, A.K. Ophthalmic Sensing Technologies for Ocular Disease Diagnostics. Analyst 2021, 146, 6416–6444. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Sui, X.; Shao, X.; Li, K.; Zhang, H.; Xu, Z.; Zhang, D. An Artificial Intelligence-Assisted Microfluidic Colorimetric Wearable Sensor System for Monitoring of Key Tear Biomarkers. NPJ Flex. Electron. 2024, 8, 35. [Google Scholar] [CrossRef]
- Khanal, B.; Pokhrel, P.; Khanal, B.; Giri, B. Machine-Learning-Assisted Analysis of Colorimetric Assays on Paper Analytical Devices. ACS Omega 2021, 6, 33837–33845. [Google Scholar] [CrossRef]
- Cui, R.; Tang, H.; Huang, Q.; Ye, T.; Chen, J.; Huang, Y.; Hou, C.; Wang, S.; Ramadan, S.; Li, B.; et al. AI-Assisted Smartphone-Based Colorimetric Biosensor for Visualized, Rapid and Sensitive Detection of Pathogenic Bacteria. Biosens. Bioelectron. 2024, 259, 116369. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry. Anal. Chem. 2019, 92, 378–396. [Google Scholar] [CrossRef]
- Turner, A.P.; Magan, N. Electronic Noses and Disease Diagnostics. Nat. Rev. Microbiol. 2004, 2, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, Y.; Tisch, U.; Shuster, G.; Pisula, W.; Feng, X.; Müllen, K.; Haick, H. Carbon Nanotube/Hexa-Peri-Hexabenzocoronene Bilayers for Discrimination Between Nonpolar Volatile Organic Compounds of Cancer and Humid Atmospheres. Adv. Mater. 2010, 22, 4317–4320. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Zhao, H.; Su, R.; Teng, L.; Tian, Q.; Han, F.; Li, H.; Cao, Z.; Xie, R.; Li, G.; Liu, X.; et al. Recent Advances in Flexible and Wearable Sensors for Monitoring Chemical Molecules. Nanoscale 2022, 14, 1653–1669. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Novel Wearable Optical Sensors for Vital Health Monitoring Systems—A Review. Biosensors 2023, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, Y.-N.; Jiang, L.; Li, L.; Li, X.; Zhao, J. Flexible Optical Fiber Sensor for Non-Invasive Continuous Monitoring of Human Physiological Signals. Small Methods 2025, 9, 2401368. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, L.; Zhang, Z. Strain Engineering of 2D Materials: Issues and Opportunities at the Interface. Adv. Mater. 2019, 31, 1805417. [Google Scholar] [CrossRef]
- Adams, F.; Barbante, C. Chapter 4—Nanotechnology and Analytical Chemistry. In Chemical Imaging Analysis; Adams, F., Barbante, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 69, pp. 125–157. ISBN 0166-526X. [Google Scholar]
- Nam, S.H.; Jeon, P.J.; Min, S.W.; Lee, Y.T.; Park, E.Y.; Im, S. Highly Sensitive Non-Classical Strain Gauge Using Organic Heptazole Thin-Film Transistor Circuit on a Flexible Substrate. Adv. Funct. Mater. 2014, 24, 4413–4419. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Chen, C.-C.; Chang, C.-C.; Yang, C.-L. An Enhanced Sensing Application Based on a Flexible Projected Capacitive-Sensing Mattress. Sensors 2014, 14, 6922–6937. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A Wearable and Highly Sensitive Pressure Sensor with Ultrathin Gold Nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Lillehoj, P.B. Flexible Analytical Devices for Point-of-Care Testing. SLAS Technol. 2020, 25, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Kong, C.; Yang, C.; Yin, L.; Jeerapan, I.; Pu, F.; Zhang, X.; Yang, S.; Yang, Z. Wearable, Stable, Highly Sensitive Hydrogel–Graphene Strain Sensors. Beilstein J. Nanotechnol. 2019, 10, 475–480. [Google Scholar] [CrossRef]
- Chugh, V.; Basu, A.; Kaushik, A.; Basu, A.K. E-Skin—Based Advanced Wearable Technology for Health Management. Curr. Res. Biotechnol. 2023, 5, 100129. [Google Scholar] [CrossRef]
- Shirinov, A.V.; Schomburg, W.K. Pressure Sensor from a PVDF Film. Sens. Actuators A Phys. 2008, 142, 48–55. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, Y.; Fei, P.; Mai, W.; Gao, Y.; Yang, R.; Bao, G.; Wang, Z.L. Flexible Piezotronic Strain Sensor. Nano Lett. 2008, 8, 3035–3040. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Tee, B.C.-K.; Bettinger, C.J.; Tok, J.B.-H.; Bao, Z. Chemical and Engineering Approaches to Enable Organic Field-Effect Transistors for Electronic Skin Applications. Acc. Chem. Res. 2012, 45, 361–371. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible Polymer Transistors with High Pressure Sensitivity for Application in Electronic Skin and Health Monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Jung, H.-C.; Moon, J.-H.; Baek, D.-H.; Lee, J.-H.; Choi, Y.-Y.; Hong, J.-S.; Lee, S.-H. CNT/PDMS Composite Flexible Dry Electrodes for Long-Term ECG Monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, P.K.; Srivastava, A. A Review of Modern Technologies for Tackling COVID-19 Pandemic. Diabetes Metab. Syndr. 2020, 14, 569–573. [Google Scholar] [CrossRef]
- Aljumah, A. Assessment of Machine Learning Techniques in IoT-Based Architecture for the Monitoring and Prediction of COVID-19. Electronics 2021, 10, 1834. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable Artificial Intelligence Biosensor Networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Channa, A.; Popescu, N.; Skibinska, J.; Burget, R. The Rise of Wearable Devices during the COVID-19 Pandemic: A Systematic Review. Sensors 2021, 21, 5787. [Google Scholar] [CrossRef]
- Riaz, M.S.; Shaukat, M.; Saeed, T.; Ijaz, A.; Qureshi, H.N.; Posokhova, I.; Sadiq, I.; Rizwan, A.; Imran, A. IPREDICT: AI Enabled Proactive Pandemic Prediction Using Biosensing Wearable Devices. Inform. Med. Unlocked 2024, 46, 101478. [Google Scholar] [CrossRef]
- Mishra, T.; Wang, M.; Metwally, A.A.; Bogu, G.K.; Brooks, A.W.; Bahmani, A.; Alavi, A.; Celli, A.; Higgs, E.; Dagan-Rosenfeld, O.; et al. Pre-Symptomatic Detection of COVID-19 from Smartwatch Data. Nat. Biomed. Eng. 2020, 4, 1208–1220. [Google Scholar] [CrossRef]
- Chatterjee, A.; Prinz, A.; Riegler, M.A.; Das, J. A Systematic Review and Knowledge Mapping on ICT-Based Remote and Automatic COVID-19 Patient Monitoring and Care. BMC Health Serv. Res. 2023, 23, 1047. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Radin, J.M.; Gadaleta, M.; Baca-Motes, K.; Ariniello, L.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R. Wearable Sensor Data and Self-Reported Symptoms for COVID-19 Detection. Nat. Med. 2020, 27, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Shahshahani, A.; Bhadra, S.; Zilic, Z. A Piezo Transducer Based Flexible Hybrid Sensor for Health Monitoring. In Proceedings of the 2018 International Flexible Electronics Technology Conference (IFETC), Ottawa, ON, Canada, 7–9 August 2018; pp. 1–2. [Google Scholar]
- Bhardwaj, V.; Joshi, R.; Gaur, A.M. IoT-Based Smart Health Monitoring System for COVID-19. SN Comput. Sci. 2022, 3, 137. [Google Scholar] [CrossRef]
- Hasasneh, A.; Hijazi, H.; Talib, M.A.; Afadar, Y.; Nassif, A.B.; Nasir, Q. Wearable Devices and Explainable Unsupervised Learning for COVID-19 Detection and Monitoring. Diagnostics 2023, 13, 3071. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Böckelmann, I.; Waranski, M.; Kotewitsch, M.; Teschler, M.; Schäfer, H.; Schmitz, B. Autonomic Dysregulation in Long-Term Patients Suffering from Post-COVID-19 Syndrome Assessed by Heart Rate Variability. Sci. Rep. 2023, 13, 15814. [Google Scholar] [CrossRef]
- Islam, M.M.; Mahmud, S.; Muhammad, L.J.; Islam, M.R.; Nooruddin, S.; Ayon, S.I. Wearable Technology to Assist the Patients Infected with Novel Coronavirus (COVID-19). SN Comput. Sci. 2020, 1, 320. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakazawa, E.; Ichinohe, S.; Akabayashi, A.; Akabayashi, A. Wearable Technology for Monitoring Respiratory Rate and SpO2 of COVID-19 Patients: A Systematic Review. Diagnostics 2022, 12, 2563. [Google Scholar] [CrossRef]
- Costrada, A.N.; Arifah, A.G.; Putri, I.D.; Sara Sawita, I.K.A.; Harmadi, H.; Djamal, M. Design of Heart Rate, Oxygen Saturation, and Temperature Monitoring System for Covid-19 Patient Based on Internet of Things (IoT). J. Ilmu Fis. Univ. Andalas 2022, 14, 54–63. [Google Scholar] [CrossRef]
- Phillips, C.; Liaqat, D.; Gabel, M.; de Lara, E. WristO2: Reliable Peripheral Oxygen Saturation Readings from Wrist-Worn Pulse Oximeters. In Proceedings of the 2021 IEEE International Conference on Pervasive Computing and Communications Workshops and other Affiliated Events (PerCom Workshops), Kassel, Germany, 22–26 March 2021; pp. 623–629. [Google Scholar]
- Bubb, C.A.; Weber, M.; Kretsch, N.; Heim, R.; Zellhuber, I.; Schmid, S.; Kagerbauer, S.M.; Kreuzer, J.; Schaller, S.J.; Blobner, M.; et al. Wearable In-Ear Pulse Oximetry Validly Measures Oxygen Saturation between 70% and 100%: A Prospective Agreement Study. Digit. Health 2023, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Han, D.; Pierre, A.; Ting, J.; Wang, X.; Lochner, C.M.; Bovo, G.; Yaacobi-Gross, N.; Newsome, C.; Wilson, R.; et al. A Flexible Organic Reflectance Oximeter Array. Proc. Natl. Acad. Sci. USA 2018, 115, E11015–E11024. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Assaad, M. Noninvasive Non-Contact SpO2 Monitoring Using an Integrated Polarization-Sensing CMOS Imaging Sensor. Sensors 2022, 22, 7796. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Li, X.; Wang, Q.; Luo, Y.; Wang, X.; Huang, X.; Zhang, J.; Peng, J.; Wang, Q.; Fan, L.; et al. Reliability of Non-Contact Infrared Thermometers for Fever Screening Under COVID-19. Risk Manag. Healthc. Policy 2022, 15, 447–456. [Google Scholar] [CrossRef]
- Ahmed, S.; Yong, J.; Shrestha, A. The Integral Role of Intelligent IoT System, Cloud Computing, Artificial Intelligence, and 5G in the User-Level Self-Monitoring of COVID-19. Electronics 2023, 12, 1912. [Google Scholar] [CrossRef]
- Mukati, N.; Namdev, N.; Dilip, R.; Hemalatha, N.; Dhiman, V.; Sahu, B. Healthcare Assistance to COVID-19 Patient Using Internet of Things (IoT) Enabled Technologies. Mater. Today Proc. 2023, 80, 3777–3781. [Google Scholar] [CrossRef]
- Cai, J.; Du, M.; Li, Z. Flexible Temperature Sensors Constructed with Fiber Materials. Adv. Mater. Technol. 2022, 7, 2101182. [Google Scholar] [CrossRef]
- Kong, M.; Yang, M.; Li, R.; Long, Y.-Z.; Zhang, J.; Huang, X.; Cui, X.; Zhang, Y.; Said, Z.; Li, C. Graphene-Based Flexible Wearable Sensors: Mechanisms, Challenges, and Future Directions. Int. J. Adv. Manuf. Technol. 2024, 131, 3205–3237. [Google Scholar] [CrossRef]
- Arman Kuzubasoglu, B.; Kursun Bahadir, S. Flexible Temperature Sensors: A Review. Sens. Actuators A Phys. 2020, 315, 112282. [Google Scholar] [CrossRef]
- Zhao, Y.; Bergmann, J.H.M. Non-Contact Infrared Thermometers and Thermal Scanners for Human Body Temperature Monitoring: A Systematic Review. Sensors 2023, 23, 7439. [Google Scholar] [CrossRef] [PubMed]
- Al-Humairi, S.N.S.; Zainol, M.H.; Razalli, H.; Raya, L.; Abdullah, M.I.; Daud, R.J. Conceptual Design: A Novel Covid-19 Smart AI Helmet. Int. J. Emerg. Technol. 2020, 11, 389–396. [Google Scholar]
- Barnawi, A.; Chhikara, P.; Tekchandani, R.; Kumar, N.; Alzahrani, B. Artificial Intelligence-Enabled Internet of Things-Based System for COVID-19 Screening Using Aerial Thermal Imaging. Future Gener. Comput. Syst. 2021, 124, 119–132. [Google Scholar] [CrossRef]
- Khaloufi, H.; Abouelmehdi, K.; Beni-Hssane, A.; Rustam, F.; Jurcut, A.D.; Lee, E.; Ashraf, I. Deep Learning Based Early Detection Framework for Preliminary Diagnosis of COVID-19 via Onboard Smartphone Sensors. Sensors 2021, 21, 6853. [Google Scholar] [CrossRef] [PubMed]
- Kummer, R.L.; Marini, J.J. The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome-Lessons Learned? J. Clin. Med. 2024, 13, 1833. [Google Scholar] [CrossRef]
- Rubulotta, F.; Bahrami, S.; Marshall, D.C.; Komorowski, M. Machine Learning Tools for Acute Respiratory Distress Syndrome Detection and Prediction. Crit. Care Med. 2024, 52, 1768–1780. [Google Scholar] [CrossRef]
- Troyee, T.G.; Gani, M.M.; Hasan, M. Design and Implementation of Low-Cost Respiratory Rate Measurement Device. Arab. J. Sci. Eng. 2024, 49, 6959–6969. [Google Scholar] [CrossRef]
- Vitazkova, D.; Foltan, E.; Kosnacova, H.; Micjan, M.; Donoval, M.; Kuzma, A.; Kopani, M.; Vavrinsky, E. Advances in Respiratory Monitoring: A Comprehensive Review of Wearable and Remote Technologies. Biosensors 2024, 14, 90. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P.; Kumar, S. Advancements in Optical Fiber-Based Wearable Sensors for Smart Health Monitoring. Biosens. Bioelectron. 2024, 254, 116232. [Google Scholar] [CrossRef]
- Purnomo, A.T.; Komariah, K.S.; Lin, D.-B.; Hendria, W.F.; Sin, B.-K.; Ahmadi, N. Non-Contact Supervision of COVID-19 Breathing Behaviour with FMCW Radar and Stacked Ensemble Learning Model in Real-Time. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 664–678. [Google Scholar] [CrossRef]
- Kumar, A.K.; Ritam, M.; Han, L.; Guo, S.; Chandra, R. Deep Learning for Predicting Respiratory Rate from Biosignals. Comput. Biol. Med. 2022, 144, 105338. [Google Scholar] [CrossRef]
- Cannata, S.; Paviglianiti, A.; Pasero, E.; Cirrincione, G.; Cirrincione, M. Deep Learning Algorithms for Automatic COVID-19 Detection on Chest X-Ray Images. IEEE Access 2022, 10, 119905–119913. [Google Scholar] [CrossRef]
- Rohmetra, H.; Raghunath, N.; Narang, P.; Chamola, V.; Guizani, M.; Lakkaniga, N.R. AI-Enabled Remote Monitoring of Vital Signs for COVID-19: Methods, Prospects and Challenges. Computing 2023, 105, 783–809. [Google Scholar] [CrossRef]
- Palanisamy, P.; Padmanabhan, A.; Ramasamy, A.; Subramaniam, S. Remote Patient Activity Monitoring System by Integrating IoT Sensors and Artificial Intelligence Techniques. Sensors 2023, 23, 5869. [Google Scholar] [CrossRef]
- Somasundaram, S.K.; Sridevi, S.; Murugappan, M.; VinothKumar, B. Continuous Physiological Signal Monitoring Using Wearables for the Early Detection of Infectious Diseases: A Review. In Surveillance, Prevention, and Control of Infectious Diseases: An AI Perspective; Chowdhury, M.E.H., Kiranyaz, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 193–218. ISBN 978-3-031-59967-5. [Google Scholar]
- Chen, L.; Xia, C.; Zhao, Z.; Fu, H.; Chen, Y. AI-Driven Sensing Technology: Review. Sensors 2024, 24, 2958. [Google Scholar] [CrossRef] [PubMed]
- Kishor, A.; Chakraborty, C. Artificial Intelligence and Internet of Things Based Healthcare 4.0 Monitoring System. Wireless Pers. Commun. 2022, 127, 1615–1631. [Google Scholar] [CrossRef]
- Chen, X.; Xie, H.; Tao, X.; Wang, F.L.; Leng, M.; Lei, B. Artificial Intelligence and Multimodal Data Fusion for Smart Healthcare: Topic Modeling and Bibliometrics. Artif. Intell. Rev. 2024, 57, 91. [Google Scholar] [CrossRef]
- Raddad, Y.; Hasasneh, A.; Abdallah, O.; Rishmawi, C.; Qutob, N. Integrating Statistical Methods and Machine Learning Techniques to Analyze and Classify COVID-19 Symptom Severity. Big Data Cogn. Comput. 2024, 8, 192. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, H.; Mao, J.; Chen, Y.; Zhong, H.; Wang, Y. Review on the COVID-19 Pandemic Prevention and Control System Based on AI. Eng. Appl. Artif. Intell. 2022, 114, 105184. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, A.; Chen, X. Innovation and Possible Long-Term Impact Driven by COVID-19: Manufacturing, Personal Protective Equipment and Digital Technologies. Technol. Soc. 2021, 65, 101541. [Google Scholar] [CrossRef]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabete-2020. Diabetes Care 2020, 43, S135–S151. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous Glucose Monitoring Systems—Current Status and Future Perspectives of the Flagship Technologies in Biosensor Research. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef]
- Mansour, M.; Saeed Darweesh, M.; Soltan, A. Wearable Devices for Glucose Monitoring: A Review of State-of-the-Art Technologies and Emerging Trends. Alex. Eng. J. 2024, 89, 224–243. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Peng, S.; Jiang, X.; Xu, K.; Chen, C.; Wang, Z.; Dai, C.; Chen, W. A Review of Wearable and Unobtrusive Sensing Technologies for Chronic Disease Management. Comput. Biol. Med. 2021, 129, 104163. [Google Scholar] [CrossRef]
- Ellahham, S. Artificial Intelligence: The Future for Diabetes Care. Am. J. Med. 2020, 133, 895–900. [Google Scholar] [CrossRef]
- Contreras, I.; Vehi, J. Artificial Intelligence for Diabetes Management and Decision Support: Literature Review. J. Med. Internet Res. 2018, 20, e10775. [Google Scholar] [CrossRef]
- Nimri, R.; Phillip, M.; Kovatchev, B. Closed-Loop and Artificial Intelligence–Based Decision Support Systems. Diabetes Technol. Ther. 2023, 25, S-70–S-89. [Google Scholar] [CrossRef]
- Templer, S. Closed-Loop Insulin Delivery Systems: Past, Present, and Future Directions. Front. Endocrinol. 2022, 13, 919942. [Google Scholar] [CrossRef]
- Ying, L.P.; Yin, O.X.; Quan, O.W.; Jain, N.; Mayuren, J.; Pandey, M.; Gorain, B.; Candasamy, M. Continuous glucose monitoring data for artificial intelligence-based predictive glycemic event: A potential aspect for diabetic care. Int. J. Diabetes Dev. Ctries. 2025, 45, 272–287. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable Non-Invasive Epidermal Glucose Sensors: A Review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab. Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. [Google Scholar] [CrossRef]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and Limitations of Non-Invasive Blood Glucose Monitoring Using near-Infrared Spectroscopy. Biomed. Signal Process. Control 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Cistola, D.P.; Roberts, R.C.; Quinones, S. Non-Invasive Glucose Monitoring Using Optical Sensor and Machine Learning Techniques for Diabetes Applications. IEEE Access 2021, 9, 73029–73045. [Google Scholar] [CrossRef]
- Pal, D.; Kumar, A.; Avraham, N.; Eisenbach, Y.; Beiderman, Y.; Agdarov, S.; Beiderman, Y.; Zalevsky, Z. Noninvasive Blood Glucose Sensing by Secondary Speckle Pattern Artificial Intelligence Analyses. J. Biomed. Opt. 2023, 28, 87001. [Google Scholar] [CrossRef]
- Delbeck, S.; Vahlsing, T.; Leonhardt, S.; Steiner, G.; Heise, H.M. Non-Invasive Monitoring of Blood Glucose Using Optical Methods for Skin Spectroscopy-Opportunities and Recent Advances. Anal. Bioanal. Chem. 2019, 411, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling Interstitial Fluid from Human Skin Using a Microneedle Patch. Sci. Transl. Med. 2020, 12, eaaw0285. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Sun, C.; Zhou, Z.; Zhang, J.; Xu, Y.; Xiao, X.; Deng, H.; Zhong, Y.; Li, G.; et al. Fully Integrated Wearable Microneedle Biosensing Platform for Wide-Range and Real-Time Continuous Glucose Monitoring. Acta Biomater. 2024, 175, 199–213. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, S.; Ma, D.; Zhang, T.; Huang, X.; Huang, S.; Chen, H.; Wang, J.; Jiang, L.; Xie, X. Masticatory System—Inspired Microneedle Theranostic Platform for Intelligent and Precise Diabetic Management. Sci. Adv. 2022, 8, eabo6900. [Google Scholar] [CrossRef] [PubMed]
- Tawakey, S.H.; Mansour, M.; Soltan, A.; Salim, A.I. Early Detection of Hypo/Hyperglycemia Using a Microneedle Electrode Array-Based Biosensor for Glucose Ultrasensitive Monitoring in Interstitial Fluid. Lab. Chip 2024, 24, 3958–3972. [Google Scholar] [CrossRef]
- Martin, A.; McConville, A.; Anderson, A.; McLister, A.; Davis, J. Microneedle Manufacture: Assessing Hazards and Control Measures. Safety 2017, 3, 25. [Google Scholar] [CrossRef]
- He, W.; Kong, S.; Lin, R.; Xie, Y.; Zheng, S.; Yin, Z.; Huang, X.; Su, L.; Zhang, X. Machine Learning Assists in the Design and Application of Microneedles. Biomimetics 2024, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Tarar, C.; Aydın, E.; Yetisen, A.K.; Tasoglu, S. Machine Learning-Enabled Optimization of Interstitial Fluid Collection via a Sweeping Microneedle Design. ACS Omega 2023, 8, 20968–20978. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Kim, J.; Li, S.; Zhao, Y.; Bahari, J.; Eliahoo, P.; Li, G.; Kawakita, S.; Haghniaz, R.; et al. Skin-Interfaced Electronics: A Promising and Intelligent Paradigm for Personalized Healthcare. Biomaterials 2023, 296, 122075. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, C.; Tan, R.; Zhang, J.; Fang, Q.; Jin, R.; Mi, X.; Sun, D.; Xue, Y.; Wang, Y.; et al. Artificial Intelligence-Assisted Bioinformatics, Microneedle, and Diabetic Wound Healing: A “New Deal” of an Old Drug. ACS Appl. Mater. Interfaces 2022, 14, 37396–37409. [Google Scholar] [CrossRef]
- Cinar, A. Automated Insulin Delivery Algorithms. Diabetes Spectr. 2019, 32, 209–214. [Google Scholar] [CrossRef]
- Zhou, K.; Isaacs, D. Closed-Loop Artificial Pancreas Therapy for Type 1 Diabetes. Curr. Cardiol. Rep. 2022, 24, 1159–1167. [Google Scholar] [CrossRef]
- Guzman Gómez, G.E.; Burbano Agredo, L.E.; Martínez, V.; Bedoya Leiva, O.F. Application of Artificial Intelligence Techniques for the Estimation of Basal Insulin in Patients with Type I Diabetes. Int. J. Endocrinol. 2020, 2020, 7326073. [Google Scholar] [CrossRef]
- Zarkogianni, K.; Vazeou, A.; Mougiakakou, S.G.; Prountzou, A.; Nikita, K.S. An Insulin Infusion Advisory System Based on Autotuning Nonlinear Model-Predictive Control. IEEE Trans. Biomed. Eng. 2011, 58, 2467–2477. [Google Scholar] [CrossRef]
- Ahmed, B.M.; Ali, M.E.; Masud, M.M.; Naznin, M. Recent Trends and Techniques of Blood Glucose Level Prediction for Diabetes Control. Smart Health 2024, 32, 100457. [Google Scholar] [CrossRef]
- Nwokolo, M.; Hovorka, R. The Artificial Pancreas and Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Medanki, S.; Dommati, N.; Bodapati, H.H.; Katru, V.N.S.K.; Moses, G.; Komaraju, A.; Donepudi, N.S.; Yalamanchili, D.; Sateesh, J.; Turimerla, P. Artificial Intelligence Powered Glucose Monitoring and Controlling System: Pumping Module. World J. Exp. Med. 2024, 14, 87916. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, J.; Lee, S.J.; Kang, S.; Mo, J. Reinforcement Learning-Based Expanded Personalized Diabetes Treatment Recommendation Using South Korean Electronic Health Records. Expert. Syst. Appl. 2022, 206, 117932. [Google Scholar] [CrossRef]
- Boughton, C.K.; Hovorka, R. New Closed-Loop Insulin Systems. Diabetologia 2021, 64, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Campanella, S.; Paragliola, G.; Cherubini, V.; Pierleoni, P.; Palma, L. Towards Personalized AI-Based Diabetes Therapy: A Review. IEEE J. Biomed. Health Inform. 2024, 28, 6944–6957. [Google Scholar] [CrossRef]
- Nimri, R.; Battelino, T.; Laffel, L.M.; Slover, R.H.; Schatz, D.; Weinzimer, S.A.; Dovc, K.; Danne, T.; Phillip, M. Insulin Dose Optimization Using an Automated Artificial Intelligence-Based Decision Support System in Youths with Type 1 Diabetes. Nat. Med. 2020, 26, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.K.; et al. Wireless Smart Contact Lens for Diabetic Diagnosis and Therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.; Cheong, W.H.; Jang, J.; Park, Y.; Na, K.; Kim, Y.; Heo, J.H.; Lee, C.Y.; et al. Soft, Smart Contact Lenses with Integrations of Wireless Circuits, Glucose Sensors, and Displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.-C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A Machine Learning-Based on-Demand Sweat Glucose Reporting Platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef]
- Ye, Z.T.; Tseng, S.F.; Tsou, S.X.; Tsai, C.W. High-Sensitivity Flip Chip Blue Mini-LEDs Miniaturized Optical Instrument for Non-Invasive Glucose Detection. Discover Nano 2024, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Kim, S.K.; Kim, S.J.; Choi, I.; Mok, J.W.; Joo, C.K.; Shin, S.; Hahn, S.K. Long-Term Stable Wireless Smart Contact Lens for Robust Digital Diabetes Diagnosis. Biomaterials 2023, 302, 122315. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Khamis, M.; Fernandez, F.A.; Heidari, H.; Butt, H.; Ahmed, Z.; Wilkinson, T.; Ghannam, R. State-of-the-Art in Smart Contact Lenses for Human–Machine Interaction. IEEE Trans. Hum. Mach. Syst. 2023, 53, 187–200. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless Battery-Free Wearable Sweat Sensor Powered by Human Motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef] [PubMed]
- Klous, L.; de Ruiter, C.J.; Scherrer, S.; Gerrett, N.; Daanen, H.A.M. The (in)Dependency of Blood and Sweat Sodium, Chloride, Potassium, Ammonia, Lactate and Glucose Concentrations during Submaximal Exercise. Eur. J. Appl. Physiol. 2021, 121, 803–816. [Google Scholar] [CrossRef]
- Zhou, L.; Menon, S.S.; Li, X.; Zhang, M.; Malakooti, M.H. Machine Learning Enables Reliable Colorimetric Detection of PH and Glucose in Wearable Sweat Sensors. Adv. Mater. Technol. 2024, 10, 2401121. [Google Scholar] [CrossRef]
- Promphet, N.; Thanawattano, C.; Buekban, C.; Laochai, T.; Lormaneenopparat, P.; Sukmas, W.; Rattanawaleedirojn, P.; Puthongkham, P.; Potiyaraj, P.; Leewattanakit, W.; et al. Smartphone Based Wearable Sweat Glucose Sensing Device Correlated with Machine Learning for Real-Time Diabetes Screening. Anal. Chim. Acta 2024, 1312, 342761. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Li, J.; Yang, T.; Zhang, Z.; Wu, H.; Xu, H.; Meng, J.; Li, F. Explainable Deep-Learning-Assisted Sweat Assessment via a Programmable Colorimetric Chip. Anal. Chem. 2022, 94, 15864–15872. [Google Scholar] [CrossRef]
- Satuluri, V.K.R.R.; Ponnusamy, V. Diagnosis of Non-Invasive Glucose Monitoring by Integrating IoT and Machine Learning. IEIE Trans. Smart Process. Comput. 2022, 11, 435–443. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in Healthcare Wearable Devices. Npj Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Patle, S.; Rotake, D. Recent Advances, Technological Challenges and Requirements to Predict the Future Treads in Wearable Sweat Sensors: A Critical Review. Microchem. J. 2024, 200, 110457. [Google Scholar] [CrossRef]
- Makroum, M.A.; Adda, M.; Bouzouane, A.; Ibrahim, H. Machine Learning and Smart Devices for Diabetes Management: Systematic Review. Sensors 2022, 22, 1843. [Google Scholar] [CrossRef] [PubMed]
- Mirmomeni, M.; Fazio, T.; von Cavallar, S.; Harrer, S. Chapter 12—From Wearables to THINKables: Artificial Intelligence-Enabled Sensors for Health Monitoring. In Wearable Sensors, 2nd ed.; Sazonov, E., Ed.; Academic Press: Oxford, UK, 2021; pp. 339–356. ISBN 978-0-12-819246-7. [Google Scholar]
- Ramesh, J.; Aburukba, R.; Sagahyroon, A. A Remote Healthcare Monitoring Framework for Diabetes Prediction Using Machine Learning. Healthc. Technol. Lett. 2021, 8, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Aziz, S.; Qidwai, U.; Abd-Alrazaq, A.; Sheikh, J. Performance of Artificial Intelligence Models in Estimating Blood Glucose Level among Diabetic Patients Using Non-Invasive Wearable Device Data. Comput. Methods Programs Biomed. Update 2023, 3, 100094. [Google Scholar] [CrossRef]
- Zhu, T.; Uduku, C.; Li, K.; Herrero, P.; Oliver, N.; Georgiou, P. Enhancing Self-Management in Type 1 Diabetes with Wearables and Deep Learning. NPJ Digit. Med. 2022, 5, 78. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Moammeri, A.; Shamsabadipour, A.; Moghaddam, Y.F.; Rahdar, A.; Pandey, S. Application of Various Optical and Electrochemical Nanobiosensors for Detecting Cancer Antigen 125 (CA-125): A Review. Biosensors 2023, 13, 99. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Padmapriya, P.; Noorasafrin, A.; Pooja, B.; Hema, K.; Firus Khan, A.Y.; Nithyakalyani, K.; Samsuri, F. Skin Cancer Detection Using Non-Invasive Techniques. RSC Adv. 2018, 8, 28095–28130. [Google Scholar] [CrossRef]
- Parimbelli, E.; Wilk, S.; Cornet, R.; Sniatala, P.; Sniatala, K.; Glaser, S.L.C.; Fraterman, I.; Boekhout, A.H.; Ottaviano, M.; Peleg, M. A Review of AI and Data Science Support for Cancer Management. Artif. Intell. Med. 2021, 117, 102111. [Google Scholar] [CrossRef]
- Lipkova, J.; Chen, R.J.; Chen, B.; Lu, M.Y.; Barbieri, M.; Shao, D.; Vaidya, A.J.; Chen, C.; Zhuang, L.; Williamson, D.F.K.; et al. Artificial Intelligence for Multimodal Data Integration in Oncology. Cancer Cell 2022, 40, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Joe, C.; Mitchell, R.J.; Gu, M.B. Biosensors for Healthcare: Current and Future Perspectives. Trends Biotechnol. 2023, 41, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Rebelo, R.; Reis, R.L.; Correlo, V.M. Biosensors Advances: Contributions to Cancer Diagnostics and Treatment. Adv. Exp. Med. Biol. 2022, 1379, 259–273. [Google Scholar] [CrossRef]
- Zhao, A.; She, J.; Manoj, D.; Wang, T.; Sun, Y.; Zhang, Y.; Xiao, F. Functionalized Graphene Fiber Modified by Dual Nanoenzyme: Towards High-Performance Flexible Nanohybrid Microelectrode for Electrochemical Sensing in Live Cancer Cells. Sens. Actuators B Chem. 2020, 310, 127861. [Google Scholar] [CrossRef]
- Meng, G.; Long, F.; Zeng, Z.; Kong, L.; Zhao, B.; Yan, J.; Yang, L.; Yang, Y.; Liu, X.-Y.; Yan, Z.; et al. Silk Fibroin Based Wearable Electrochemical Sensors with Biomimetic Enzyme-like Activity Constructed for Durable and on-Site Health Monitoring. Biosens. Bioelectron. 2023, 228, 115198. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Adams, T.E.; Prieto-Simon, B.; Voelcker, N.H. Electrochemical Immunosensor for Breast Cancer Biomarker Detection Using High-Density Silicon Microneedle Array. Biosens. Bioelectron. 2021, 192, 113496. [Google Scholar] [CrossRef]
- Karimi, F.; Karimi-Maleh, H.; Rouhi, J.; Zare, N.; Karaman, C.; Baghayeri, M.; Fu, L.; Rostamnia, S.; Dragoi, E.N.; Ayati, A.; et al. Revolutionizing Cancer Monitoring with Carbon-Based Electrochemical Biosensors. Environ. Res. 2023, 239, 117368. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Liu, Y.; Huang, K.-J. Smartphone-Assisted Flexible Electrochemical Sensor Platform by a Homology DNA Nanomanager Tailored for Multiple Cancer Markers Field Inspection. Anal. Chem. 2023, 95, 13305–13312. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless Wearable Electrochemical Sensing Platform with Zero-Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef]
- Zhang, S.; Suresh, L.; Yang, J.; Zhang, X.; Tan, S.C. Augmenting Sensor Performance with Machine Learning Towards Smart Wearable Sensing Electronic Systems. Adv. Intell. Syst. 2022, 4, 2100194. [Google Scholar] [CrossRef]
- Enginler, S.Ö.; Küçükdeniz, T.; Dal, G.E.; Yıldırım, F.; Cilasun, G.E.; Alkan, F.Ü.; Gürgen, H.Ö.; Taşaltın, N.; Sabuncu, A.; Yılmaz, M.; et al. Enhancing Electrochemical Detection through Machine Learning-Driven Prediction for Canine Mammary Tumor Biomarker with Green Silver Nanoparticles. Anal. Bioanal. Chem. 2024, 416, 5071–5088. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Kim, K.; Kim, H.W.; Park, Y. Classification between Normal and Cancerous Human Urothelial Cells by Using Micro-Dimensional Electrochemical Impedance Spectroscopy Combined with Machine Learning. Sensors 2022, 22, 7969. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, P.; Tan, H.; Chen, X.; Wang, Q.; Chen, T. Exosomes as Smart Nanoplatforms for Diagnosis and Therapy of Cancer. Front. Oncol. 2021, 11, 743189. [Google Scholar] [CrossRef] [PubMed]
- Shahub, S.; Upasham, S.; Ganguly, A.; Prasad, S. Machine Learning Guided Electrochemical Sensor for Passive Sweat Cortisol Detection. Sens. Biosensing Res. 2022, 38, 100527. [Google Scholar] [CrossRef]
- Giordano, G.F.; Ferreira, L.F.; Bezerra, Í.R.S.; Barbosa, J.A.; Costa, J.N.Y.; Pimentel, G.J.C.; Lima, R.S. Machine Learning toward High-Performance Electrochemical Sensors. Anal. Bioanal. Chem. 2023, 415, 3683–3692. [Google Scholar] [CrossRef]
- Tukimin, S.N.; Karman, S.B.; Ahmad, M.Y.; Zaman, W.S.W.K. Polarized Light-Based Cancer Cell Detection Techniques: A Review. IEEE Sens. J. 2019, 19, 9010–9025. [Google Scholar] [CrossRef]
- Bogomolov, A.; Ageev, V.; Zabarylo, U.; Usenov, I.; Schulte, F.; Kirsanov, D.; Belikova, V.; Minet, O.; Feliksberger, E.; Meshkovsky, I.; et al. LED-Based near Infrared Sensor for Cancer Diagnostics. In Optical Diagnostics and Sensing XVI: Toward Point-of-Care Diagnostics; SPIE: Bellingham, WA, USA, 2016; Volume 9715, p. 971510. [Google Scholar] [CrossRef]
- Vitorino, R.; Barros, A.S.; Guedes, S.; Caixeta, D.C.; Sabino-Silva, R. Diagnostic and Monitoring Applications Using near Infrared (NIR) Spectroscopy in Cancer and Other Diseases. Photodiagn. Photodyn. Ther. 2023, 42, 103633. [Google Scholar] [CrossRef]
- Negi, S.; Mittal, P.; Kumar, B.; Juneja, P.K. Organic LED Based Light Sensor for Detection of Ovarian Cancer. Microelectron. Eng. 2019, 218, 111154. [Google Scholar] [CrossRef]
- Katchman, B.A.; Smith, J.T.; Obahiagbon, U.; Kesiraju, S.; Lee, Y.-K.; O’Brien, B.; Kaftanoglu, K.; Blain Christen, J.; Anderson, K.S. Application of Flat Panel OLED Display Technology for the Point-of-Care Detection of Circulating Cancer Biomarkers. Sci. Rep. 2016, 6, 29057. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, C.; Asghar, S.A.; Dutta, P.K.; Mahadevappa, M. Design and Evaluation of Graphene-Silicon Heterojunction LEDs for Breast Cancer Detection. IEEE Trans. Nanotechnol. 2024, 23, 95–101. [Google Scholar] [CrossRef]
- Ewing, K.J.; Major, K.J.; Sanghera, J.S. A Biomimetic Optical Approach to Skin Cancer Detection. In Proceedings of the BiOS, San Francisco, CA, USA, 1–6 February 2020. [Google Scholar]
- Bin Murshed Leon, M.J.; Disha, A.S. A Simple Structure of PCF Based Sensor for Sensing Sulfur Dioxide Gas with High Sensitivity and Better Birefringence. Sens. Int. 2021, 2, 100115. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huu, D.M.N.; Le, T.-H.; Phan, Q.-H.; Pham, T.-T.-H. Skin Cancer Detection Using Effective Optical Parameters and the Classification and Regression Tree Algorithm: A Novel Framework. Vietnam. J. Sci. Technol. Eng. 2023, 65, 63–69. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, X.; Zhao, S.; Cui, X.; Gong, T.; Song, Z.; Meng, H.; Zhang, X.; Yu, B. Aptamer-Based Fluorescent Sensors for the Detection of Cancer Biomarkers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119038. [Google Scholar] [CrossRef]
- Hussain, S.H.; Huertas, C.S.; Mitchell, A.; Deman, A.-L.; Laurenceau, E. Biosensors for Circulating Tumor Cells (CTCs)-Biomarker Detection in Lung and Prostate Cancer: Trends and Prospects. Biosens. Bioelectron. 2022, 197, 113770. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Z.; Zheng, J.; Zhao, Q.; Yuan, Z. Artificial Intelligence-Aided Optical Imaging for Cancer Theranostics. Semin. Cancer Biol. 2023, 94, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, H.; Peng, B.; Huang, X.; Hu, Y.; Zheng, C.; Zhang, Z. Illuminating the Future of Precision Cancer Surgery with Fluorescence Imaging and Artificial Intelligence Convergence. NPJ Precis. Oncol. 2024, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Luo, K. Advancing Smart Sensor Networks and Carbon-Based Biosensors Through Artificial Intelligence: A Deep Learning Approach to Optoelectronic Device Innovation. IEEE Access 2025, 13, 86083–86109. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near Real-Time Intraoperative Brain Tumor Diagnosis Using Stimulated Raman Histology and Deep Neural Networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Nadiminti, H.; Tan, I. Melanin Stacking Differences in Pigmented and Non-Pigmented Melanomas: Quantitative Differentiation between Pigmented and Non-Pigmented Melanomas Based on Light-Scattering Properties. Life 2023, 13, 1004. [Google Scholar] [CrossRef]
- Zha, B.; Wang, Z.; Ma, L.; Chen, J.; Wang, H.; Li, X.; Kumar, S.; Min, R. Intelligent Wearable Photonic Sensing System for Remote Healthcare Monitoring Using Stretchable Elastomer Optical Fiber. IEEE Internet Things J. 2024, 11, 17317–17329. [Google Scholar] [CrossRef]
- Taha, B.A.; Al-Tahar, I.A.; Addie, A.J.; Mahdi, A.B.; Haider, A.J.; Al Mashhadany, Y.; Chaudhary, V.; Arsad, N. Nanophotonic Catheters: A Lens into the Body for Biosensing and Biomedical Imaging. Appl. Mater. Today 2024, 38, 102229. [Google Scholar] [CrossRef]
- Faruqui, N.; Yousuf, M.A.; Whaiduzzaman, M.; Azad, A.K.M.; Barros, A.; Moni, M.A. LungNet: A Hybrid Deep-CNN Model for Lung Cancer Diagnosis Using CT and Wearable Sensor-Based Medical IoT Data. Comput. Biol. Med. 2021, 139, 104961. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Brucher, S.; Wilson, C.; Kesztyüs, T. Use of Infrared Thermography in Medical Diagnosis, Screening, and Disease Monitoring: A Scoping Review. Medicina 2023, 59, 2139. [Google Scholar] [CrossRef]
- Fernández-Ovies, F.J.; Santiago Alférez-Baquero, E.; de Andrés-Galiana, E.J.; Cernea, A.; Fernández-Muñiz, Z.; Fernández-Martínez, J.L. Detection of Breast Cancer Using Infrared Thermography and Deep Neural Networks BT. In Bioinformatics and Biomedical Engineering; Rojas, I., Valenzuela, O., Rojas, F., Ortuño, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 514–523. [Google Scholar]
- Magalhaes, C.; Vardasca, R.; Mendes, J. Recent Use of Medical Infrared Thermography in Skin Neoplasms. Skin. Res. Technol. 2018, 24, 587–591. [Google Scholar] [CrossRef]
- Kašalynas, I.; Venckevičius, R.; Minkevičius, L.; Sešek, A.; Wahaia, F.; Tamošiūnas, V.; Voisiat, B.; Seliuta, D.; Valušis, G.; Švigelj, A.; et al. Spectroscopic Terahertz Imaging at Room Temperature Employing Microbolometer Terahertz Sensors and Its Application to the Study of Carcinoma Tissues. Sensors 2016, 16, 432. [Google Scholar] [CrossRef]
- Mambou, S.J.; Maresova, P.; Krejcar, O.; Selamat, A.; Kuca, K. Breast Cancer Detection Using Infrared Thermal Imaging and a Deep Learning Model. Sensors 2018, 18, 2799. [Google Scholar] [CrossRef]
- Roslidar, R.; Rahman, A.; Muharar, R.; Syahputra, M.R.; Arnia, F.; Syukri, M.; Pradhan, B.; Munadi, K. A Review on Recent Progress in Thermal Imaging and Deep Learning Approaches for Breast Cancer Detection. IEEE Access 2020, 8, 116176–116194. [Google Scholar] [CrossRef]
- Aggarwal, A.K.; Alpana; Pandey, M. Machine Learning Approach for Breast Cancer Detection Using Thermal Imaging. In Proceedings of the 2022 Second International Conference on Next Generation Intelligent Systems (ICNGIS), Kottayam, India, 29–31 July 2022; pp. 1–5. [Google Scholar]
- Perez-Raya, I.; Kandlikar, S.G. Thermal Modeling of Patient-Specific Breast Cancer with Physics-Based Artificial Intelligence. ASME J. Heat Mass Transf. 2022, 145, 31201. [Google Scholar] [CrossRef]
- Khan, S.U.R.; Raza, A.; Meeran, M.T.; Bilhaj, U. Enhancing Breast Cancer Detection through Thermal Imaging and Customized 2D CNN Classifiers. VFAST Trans. Softw. Eng. 2023, 11, 80–92. [Google Scholar] [CrossRef]
- Kakileti, S.T.; Madhu, H.J.; Manjunath, G.; Wee, L.; Dekker, A.; Sampangi, S. Personalized Risk Prediction for Breast Cancer Pre-Screening Using Artificial Intelligence and Thermal Radiomics. Artif. Intell. Med. 2020, 105, 101854. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Kim, J.H.; Yu, C.C.; Dagdeviren, C. An Emerging Era: Conformable Ultrasound Electronics. Adv. Mater. 2024, 36, 2307664. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, R.S.; Lin, M.; Xu, S. Emerging Wearable Ultrasound Technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 713–729. [Google Scholar] [CrossRef]
- Du, W.; Zhang, L.; Suh, E.; Lin, D.; Marcus, C.; Ozkan, L.; Ahuja, A.; Fernandez, S.; Shuvo, I.I.; Sadat, D.; et al. Conformable Ultrasound Breast Patch for Deep Tissue Scanning and Imaging. Sci. Adv. 2023, 9, eadh5325. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Luo, Y.; Zhuang, W.; Xu, T. A Fully Integrated Conformal Wearable Ultrasound Patch for Continuous Sonodynamic Therapy. Adv. Mater. 2024, 36, 2409528. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Chen, W.; Shang, D.; Zhang, Q.; Li, Y.; Zheng, H.; Gu, D.; Wu, D.; Ma, T. Skin-Conformable Flexible and Stretchable Ultrasound Transducer for Wearable Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 811–820. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, Z.; Ru, J.; Meng, Y.; Wang, K. Application and Prospects of AI-Based Radiomics in Ultrasound Diagnosis. Vis. Comput. Ind. Biomed. Art 2023, 6, 20. [Google Scholar] [CrossRef]
- Shen, Y.-T.; Chen, L.; Yue, W.-W.; Xu, H.-X. Artificial Intelligence in Ultrasound. Eur. J. Radiol. 2021, 139, 109717. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Ren, J.-Y.; Xu, X.-L.; Xiong, L.-Y.; Peng, Y.-X.; Pan, X.-F.; Dietrich, C.F.; Cui, X.-W. Ultrasound-Based Artificial Intelligence in Gastroenterology and Hepatology. World J. Gastroenterol. 2022, 28, 5530–5546. [Google Scholar] [CrossRef]
- Tagnamas, J.; Ramadan, H.; Yahyaouy, A.; Tairi, H. Multi-Task Approach Based on Combined CNN-Transformer for Efficient Segmentation and Classification of Breast Tumors in Ultrasound Images. Vis. Comput. Ind. Biomed. Art 2024, 7, 2. [Google Scholar] [CrossRef]
- Brown, C.; Chauhan, J.; Grammenos, A.; Han, J.; Hasthanasombat, A.; Spathis, D.; Xia, T.; Cicuta, P.; Mascolo, C. Exploring Automatic Diagnosis of COVID-19 from Crowdsourced Respiratory Sound Data. In ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; Association for Computing Machinery: New York, NY, USA, 2020; Volume 11, pp. 3474–3484. [Google Scholar] [CrossRef]
- Vettoretti, M.; Cappon, G.; Facchinetti, A.; Sparacino, G. Advanced Diabetes Management Using Artificial Intelligence and Continuous Glucose Monitoring Sensors. Sensors 2020, 20, 3870. [Google Scholar] [CrossRef]
- Sánchez-Cauce, R.; Pérez-Martín, J.; Luque, M. Multi-Input Convolutional Neural Network for Breast Cancer Detection Using Thermal Images and Clinical Data. Comput. Methods Programs Biomed. 2021, 204, 106045. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Dyachenko, E.I. Salivary Biomarkers in Breast Cancer: From Salivaomics to Salivaoncoomics. Front. Biosci. Landmark 2024, 29, 253. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; Martínez-Reglero, C.; Salgado-Barreira, Á.; Takkouche, B.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. Salivary Biomarkers for Cancer Diagnosis: A Meta-Analysis. Ann. Med. 2020, 52, 131–144. [Google Scholar] [CrossRef]
- Arora, A.; Kaur, D.; Patiyal, S.; Kaur, D.; Tomer, R.; Raghava, G.P.S. SalivaDB—A Comprehensive Database for Salivary Biomarkers in Humans. Database 2023, 2023, baad002. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pati, S.; Duggal, M.; Sethi, A.; Patil, A.; Malekar, G.; Kowe, N.; Kumar, J.; Kashyap, J.; Rout, D.; et al. A Cytology Dataset for Early Detection of Oral Squamous Cell Carcinoma. arXiv 2025, arXiv:2506.09661. [Google Scholar] [CrossRef]
- Ordun, C.; Cha, A.N.; Raff, E.; Gaskin, B.; Hanson, A.; Rule, M.; Purushotham, S.; Gulley, J.L. Intelligent Sight and Sound: A Chronic Cancer Pain Dataset. Adv. Neural Inf. Process Syst. arXiv 2022, arXiv:2204.04214. [Google Scholar]
- Tschandl, P.; Rosendahl, C.; Kittler, H. The HAM10000 Dataset, a Large Collection of Multi-Source Dermatoscopic Images of Common Pigmented Skin Lesions. Scientific Data 2018, 5, 180161. [Google Scholar] [CrossRef]
- Wen, D.; Khan, S.M.; Xu, A.J.; Ibrahim, H.; Smith, L.; Caballero, J.; Zepeda, L.; de Blas Perez, C.; Denniston, A.K.; Liu, X.; et al. Characteristics of Publicly Available Skin Cancer Image Datasets: A Systematic Review. Lancet Digit. Health 2022, 4, e64–e74. [Google Scholar] [CrossRef]
- Marling, C.; Bunescu, R. The OhioT1DM Dataset for Blood Glucose Level Prediction: Update 2020. CEUR Workshop Proc. 2020, 2675, 71. [Google Scholar]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. Review The Cancer Genome Atlas (TCGA): An Immeasurable Source of Knowledge. Contemp. Oncol./Współczesna Onkol. 2015, 2015, 68–77. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Radanliev, P. Privacy, Ethics, Transparency, and Accountability in AI Systems for Wearable Devices. Front. Digit. Health 2025, 7, 1431246. [Google Scholar] [CrossRef] [PubMed]
- Dankan Gowda, V.; Chaithra, S.M.; Gujar, S.S.; Shaikh, S.F.; Ingole, B.S.; Reddy, N.S. Scalable AI Solutions for IoT-Based Healthcare Systems Using Cloud Platforms. In Proceedings of the 8th International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud), I-SMAC 2024–Proceedings, Kirtipur, Nepal, 3–5 October 2024; pp. 156–162. [Google Scholar] [CrossRef]
- Prateek, M.; Rathore, S.P.S. Clinical Validation of AI Disease Detection Models—An Overview of the Clinical Validation Process for AI Disease Detection Models, and How They Can Be Validated for Accuracy and Effectiveness. AI in Disease Detection: Advancements and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 215–237. [Google Scholar] [CrossRef]
- Nazer, L.H.; Zatarah, R.; Waldrip, S.; Ke, J.X.C.; Moukheiber, M.; Khanna, A.K.; Hicklen, R.S.; Moukheiber, L.; Moukheiber, D.; Ma, H.; et al. Bias in Artificial Intelligence Algorithms and Recommendations for Mitigation. PLOS Digit. Health 2023, 2, e0000278. [Google Scholar] [CrossRef] [PubMed]
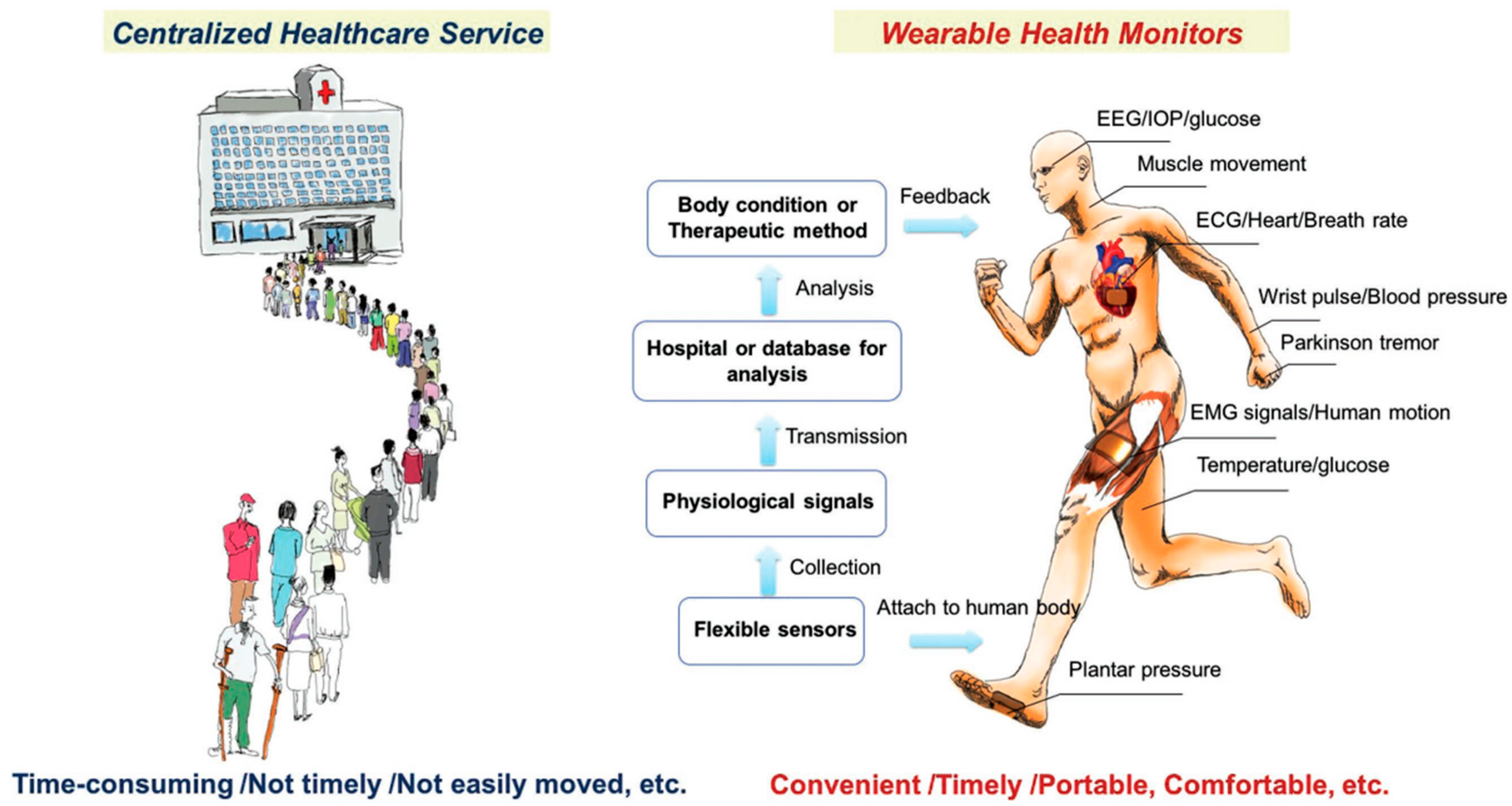
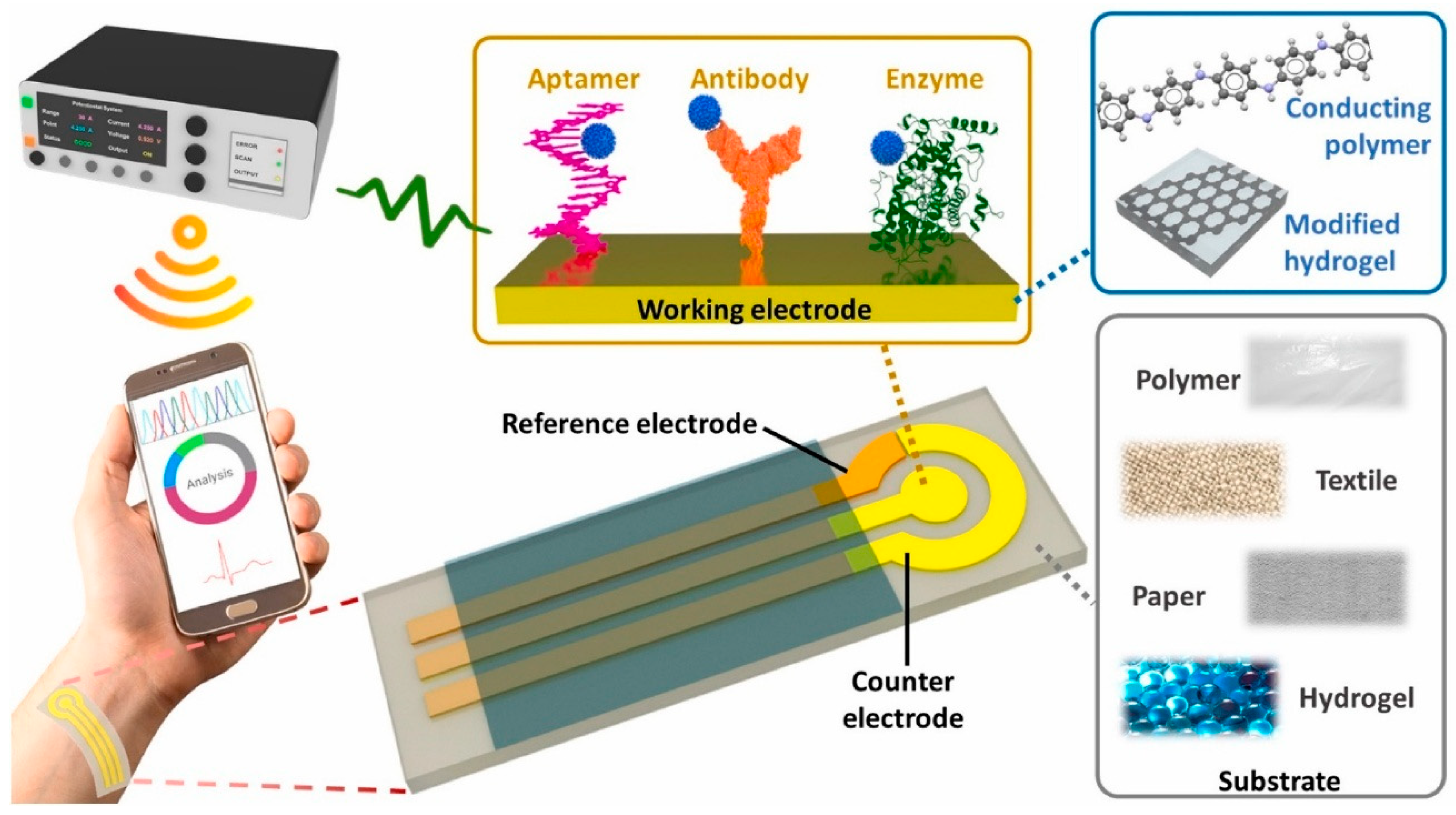
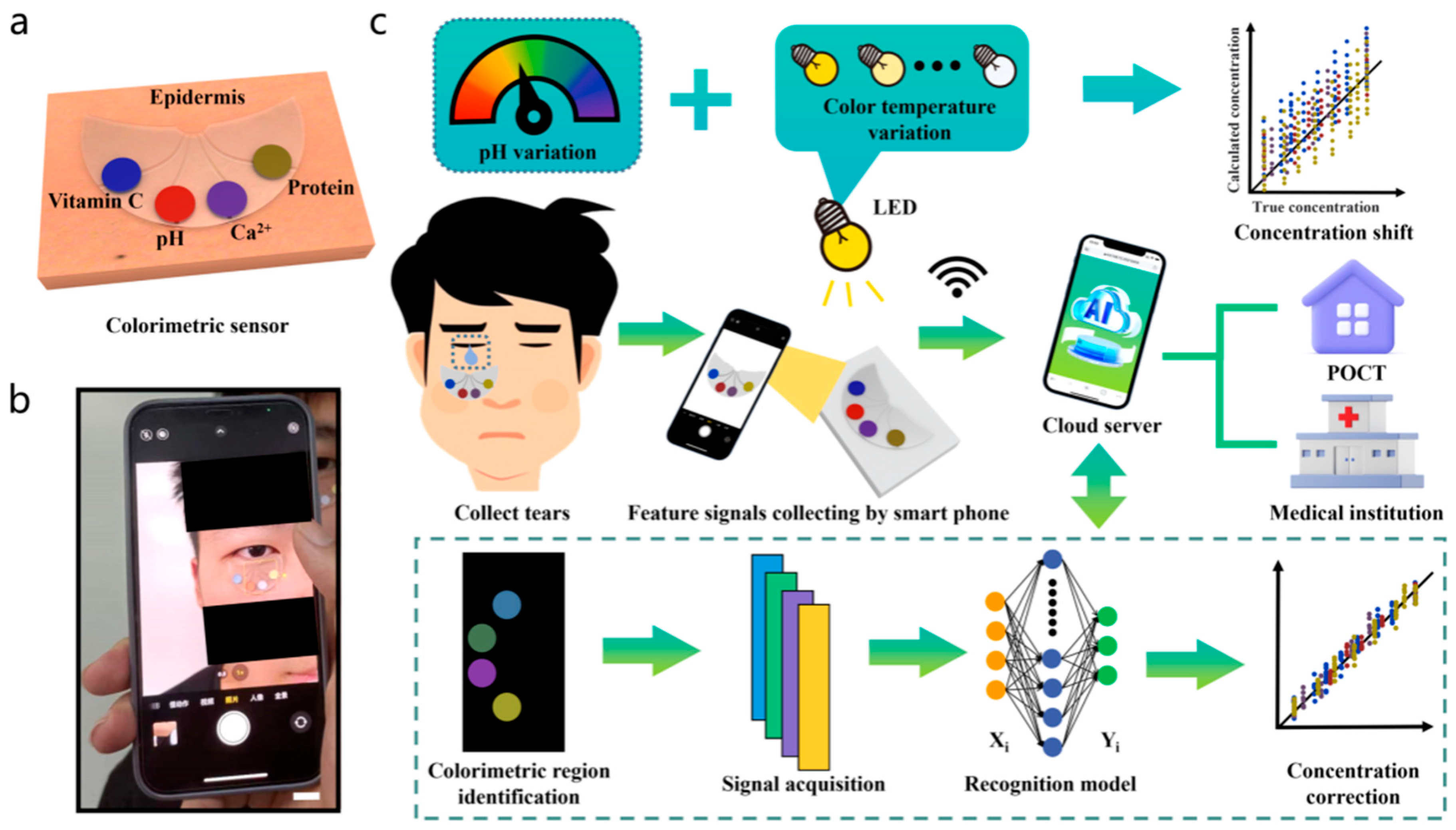
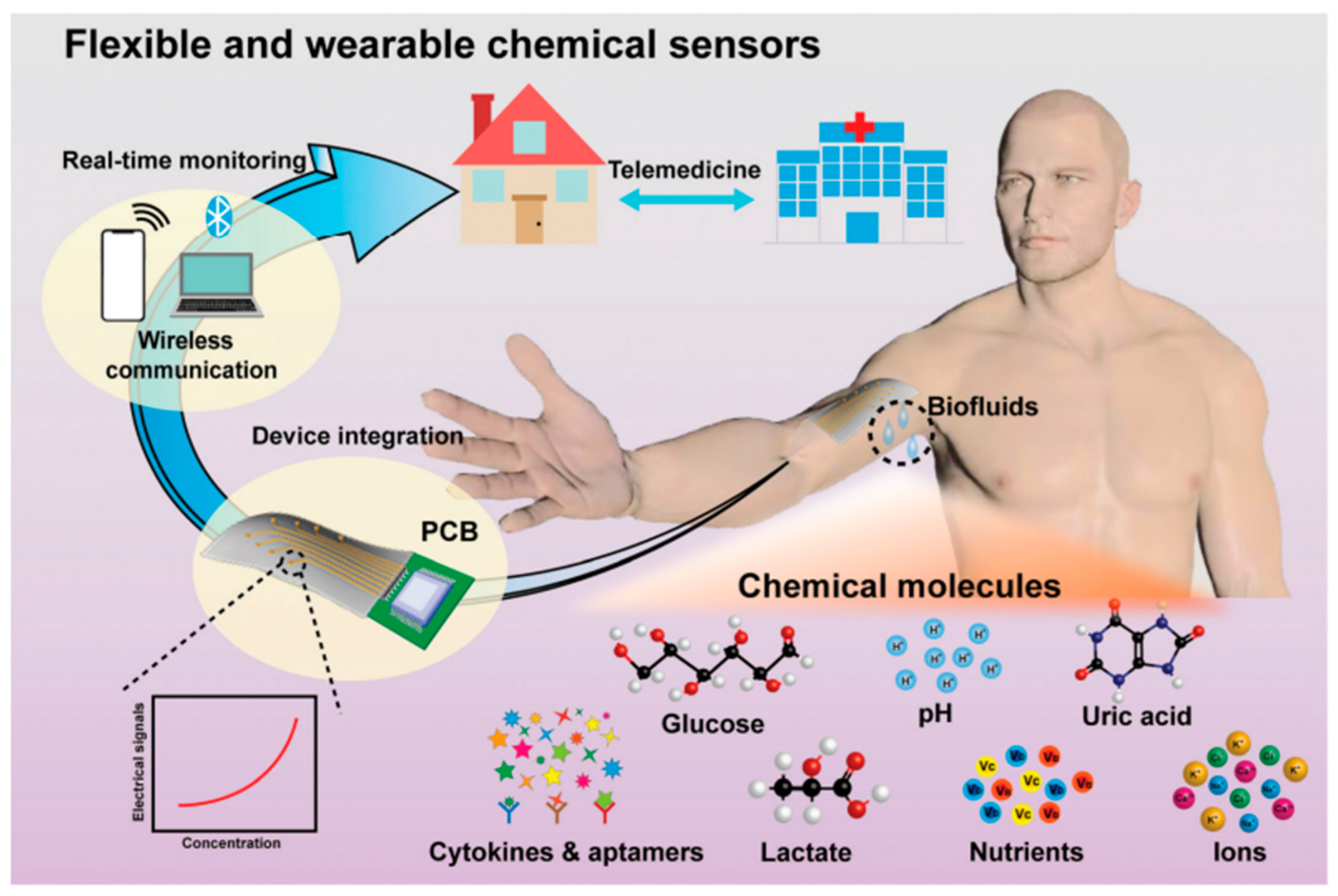
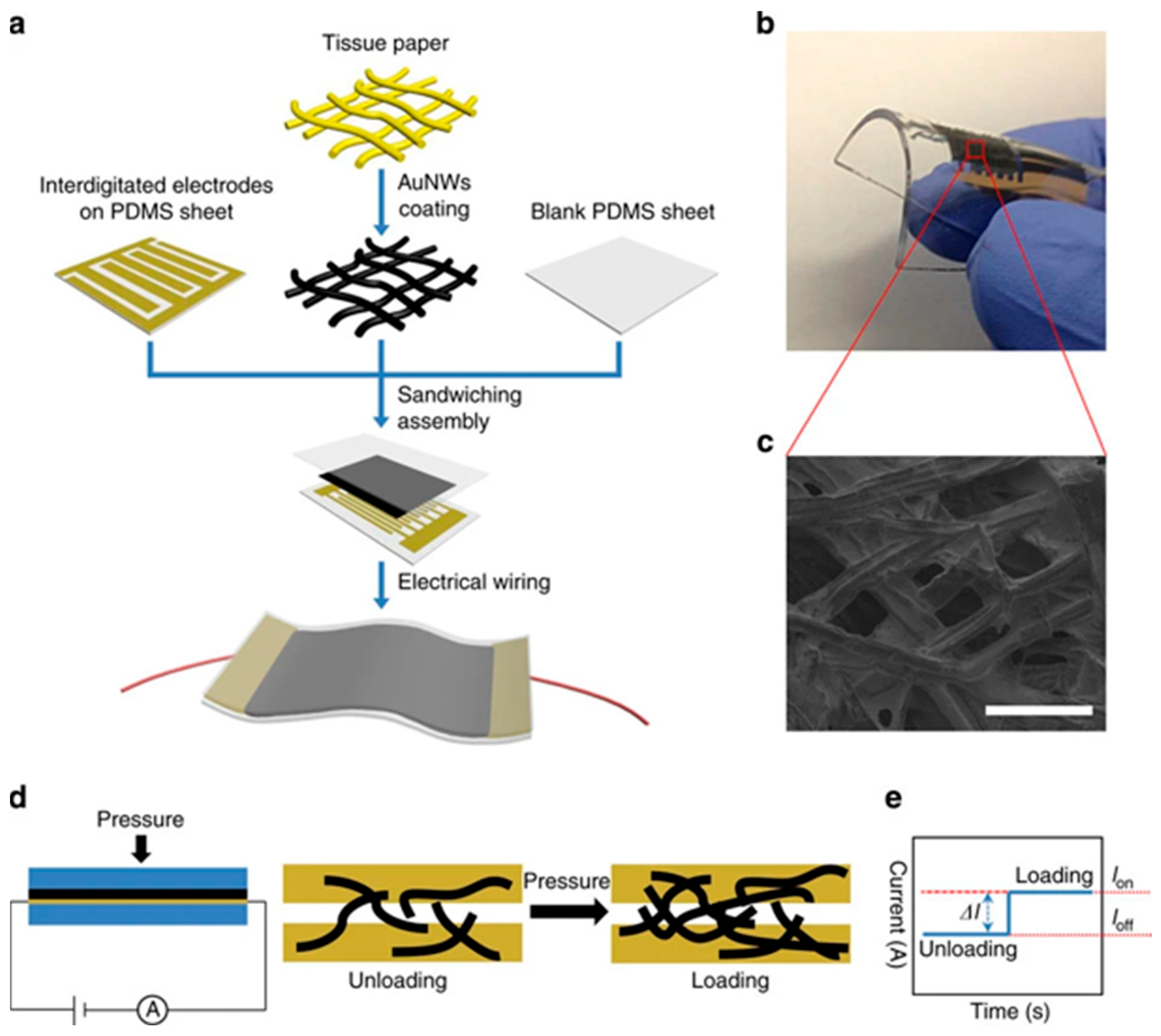
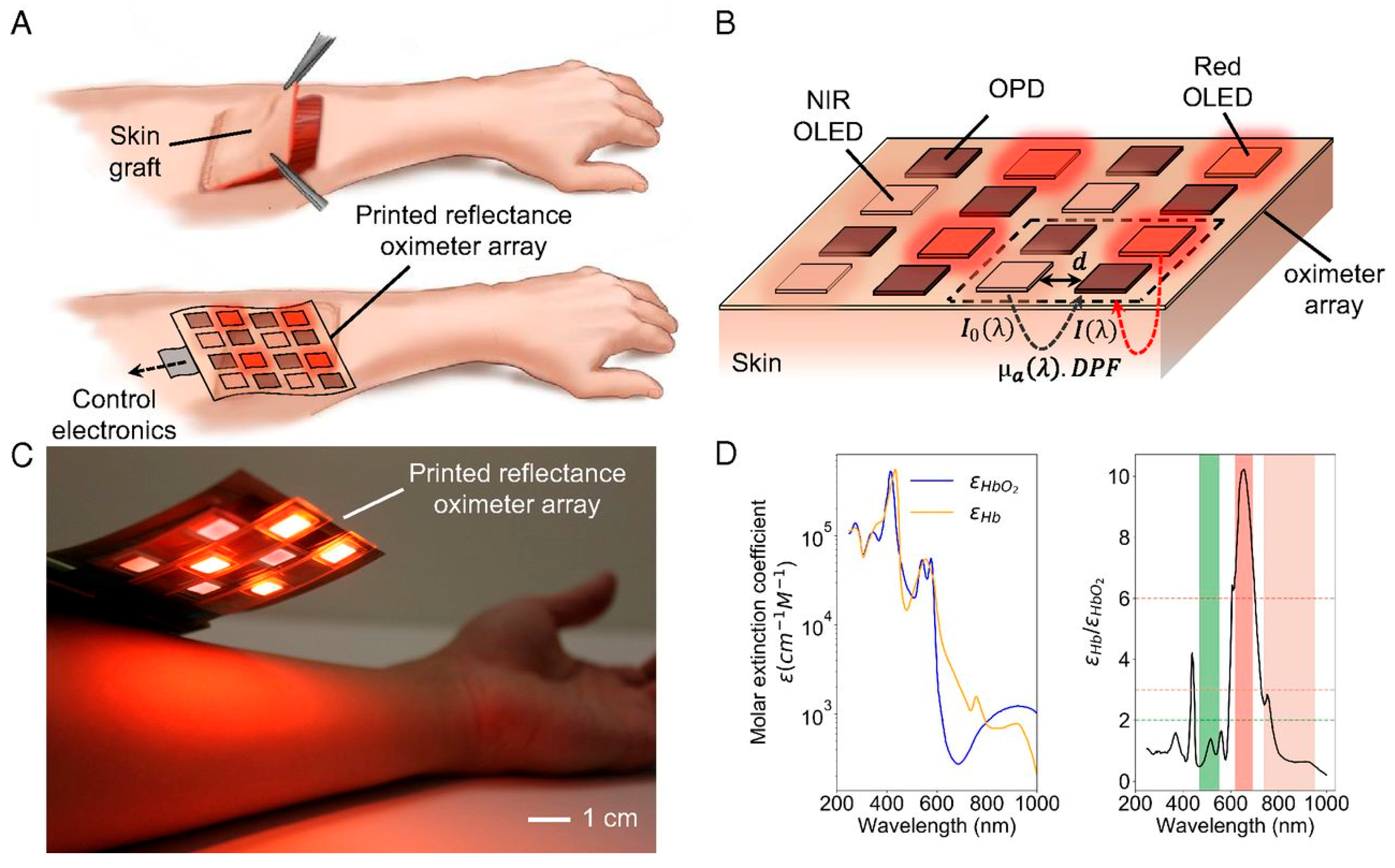
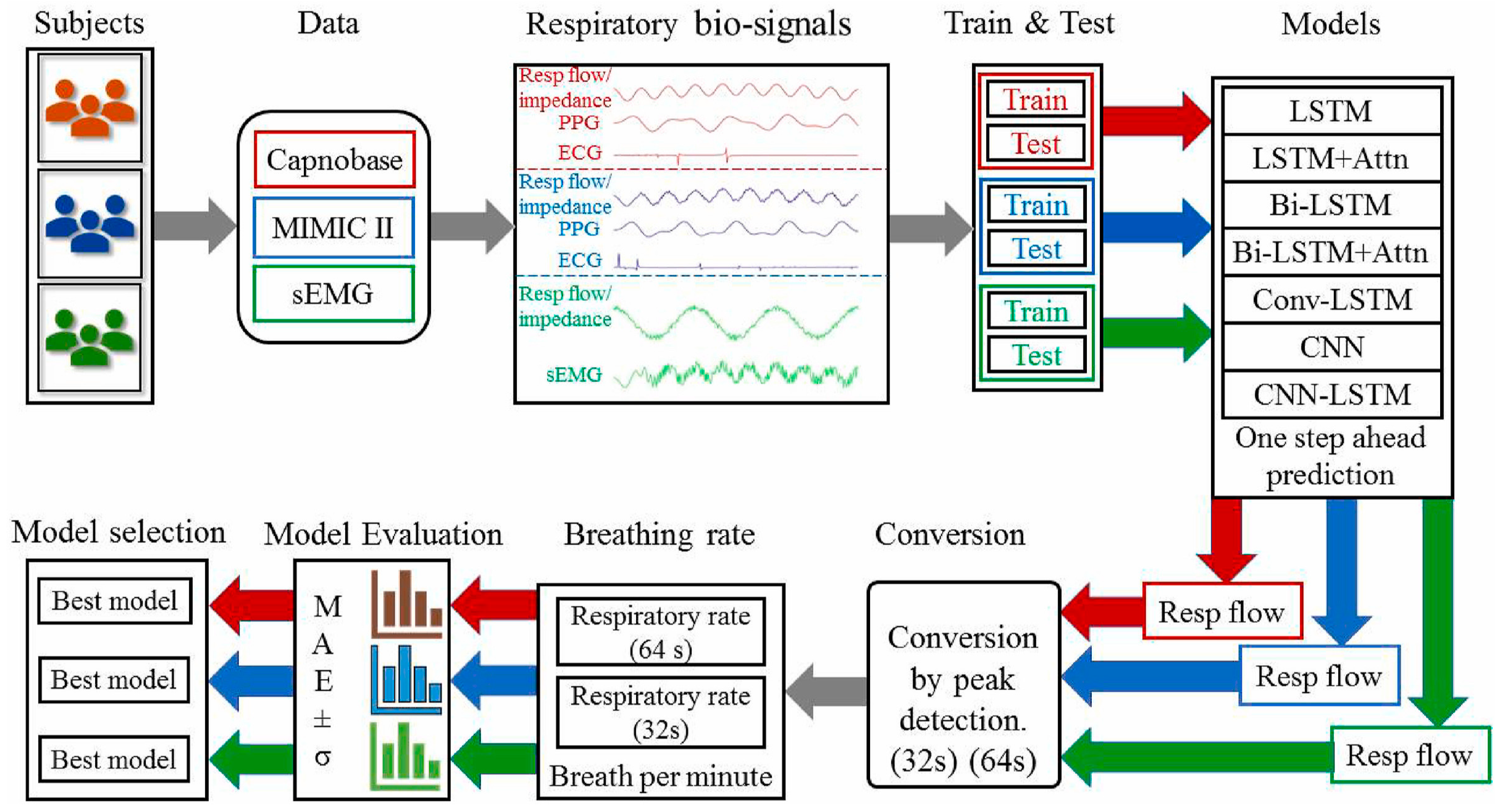
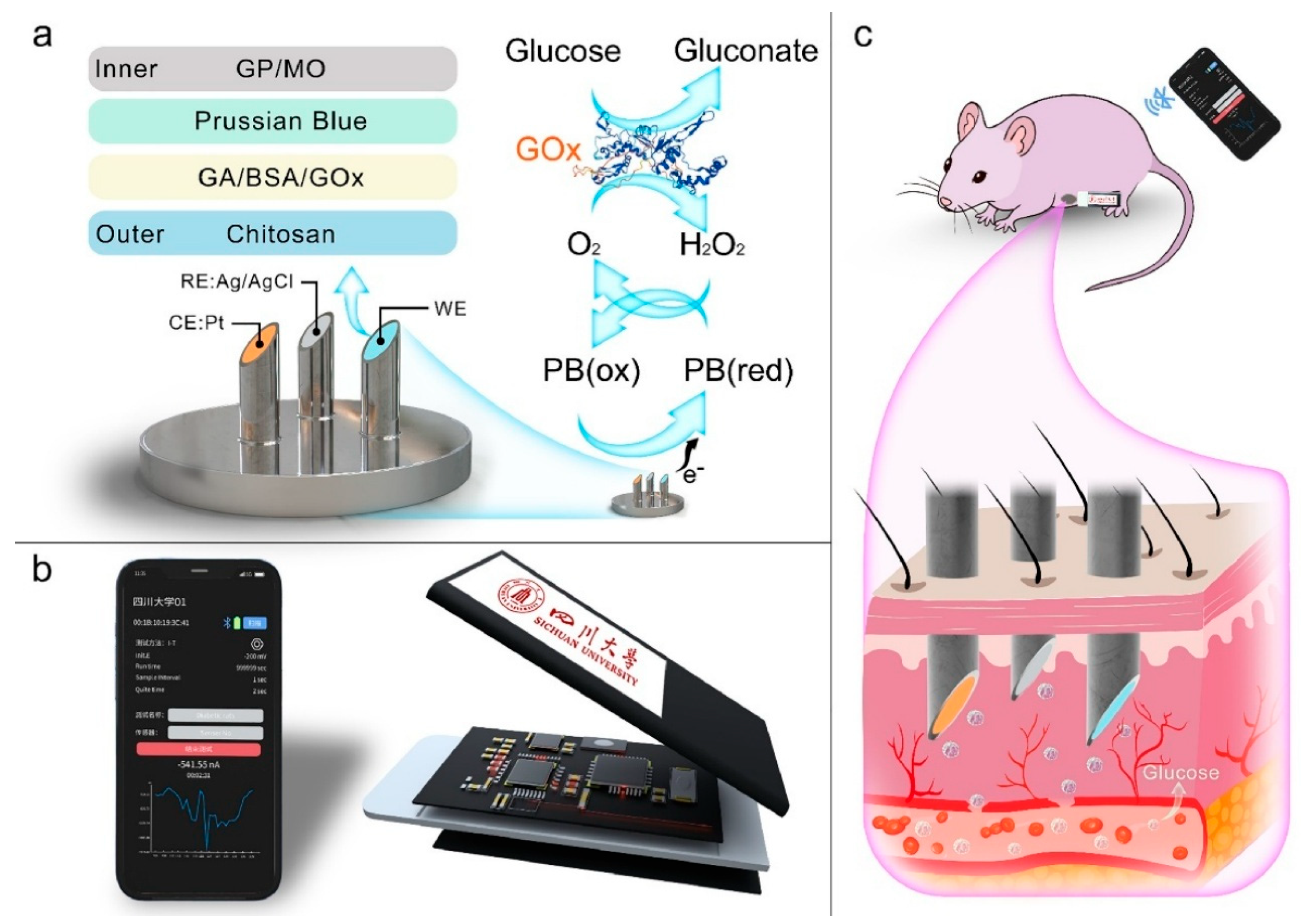
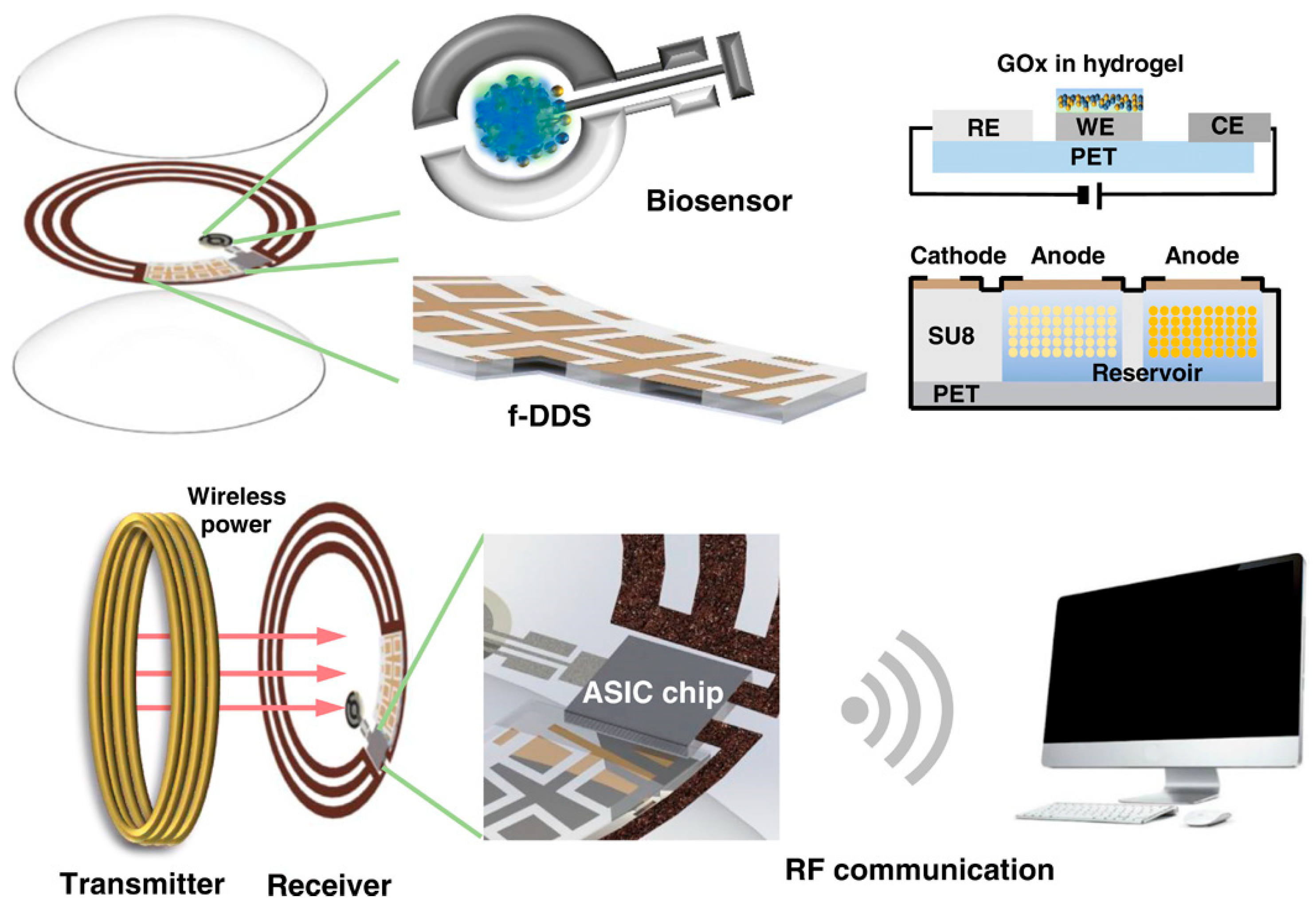
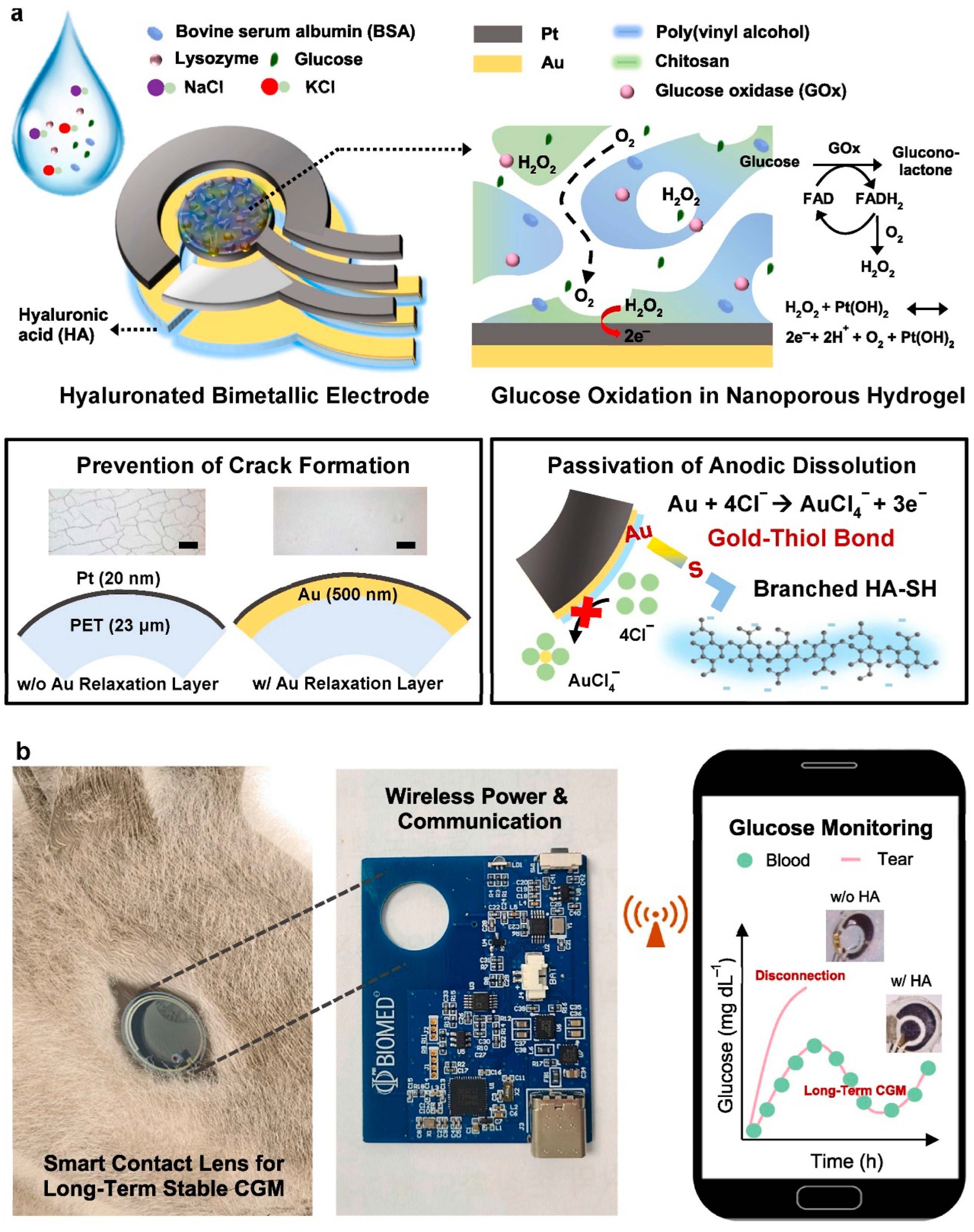
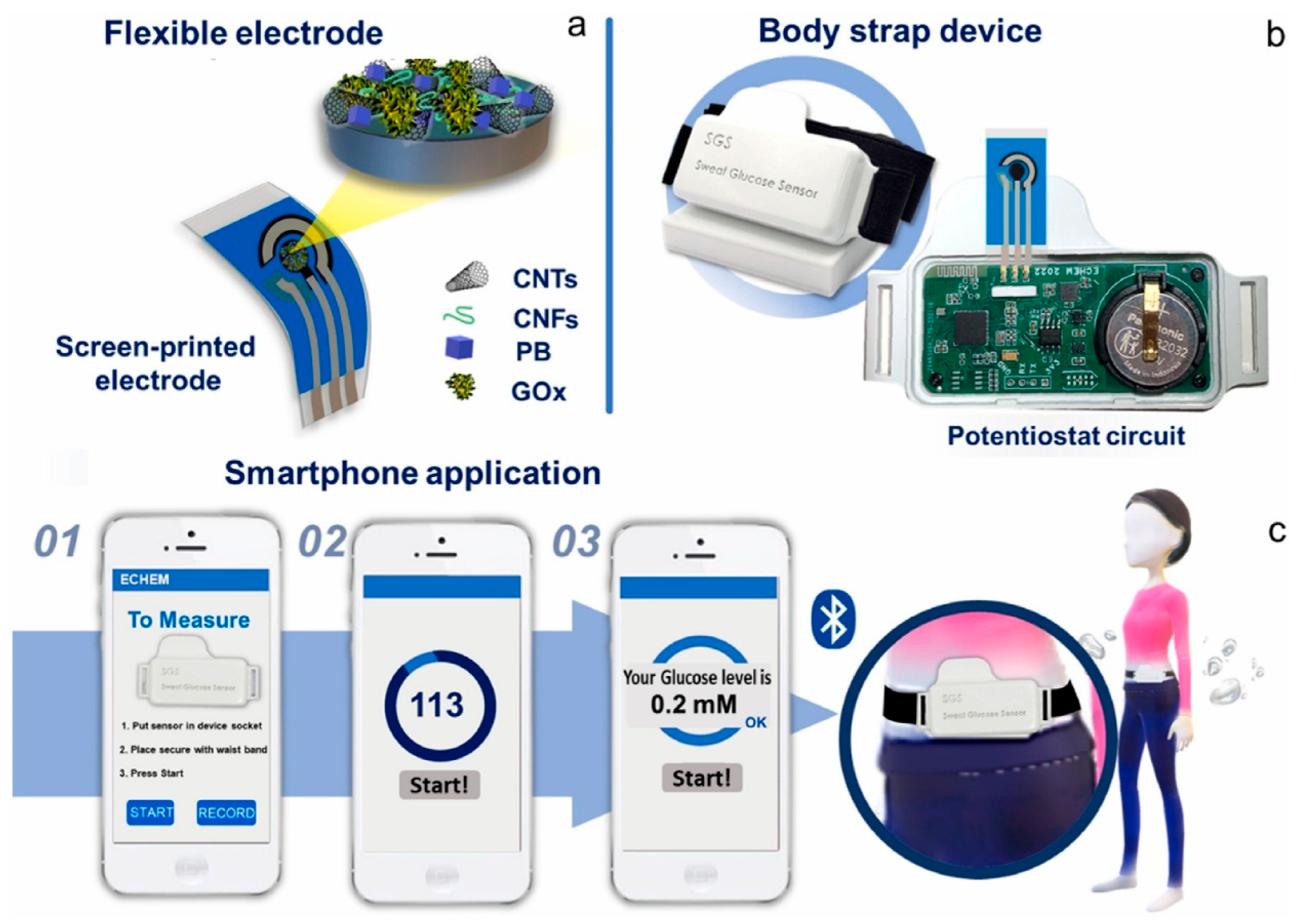
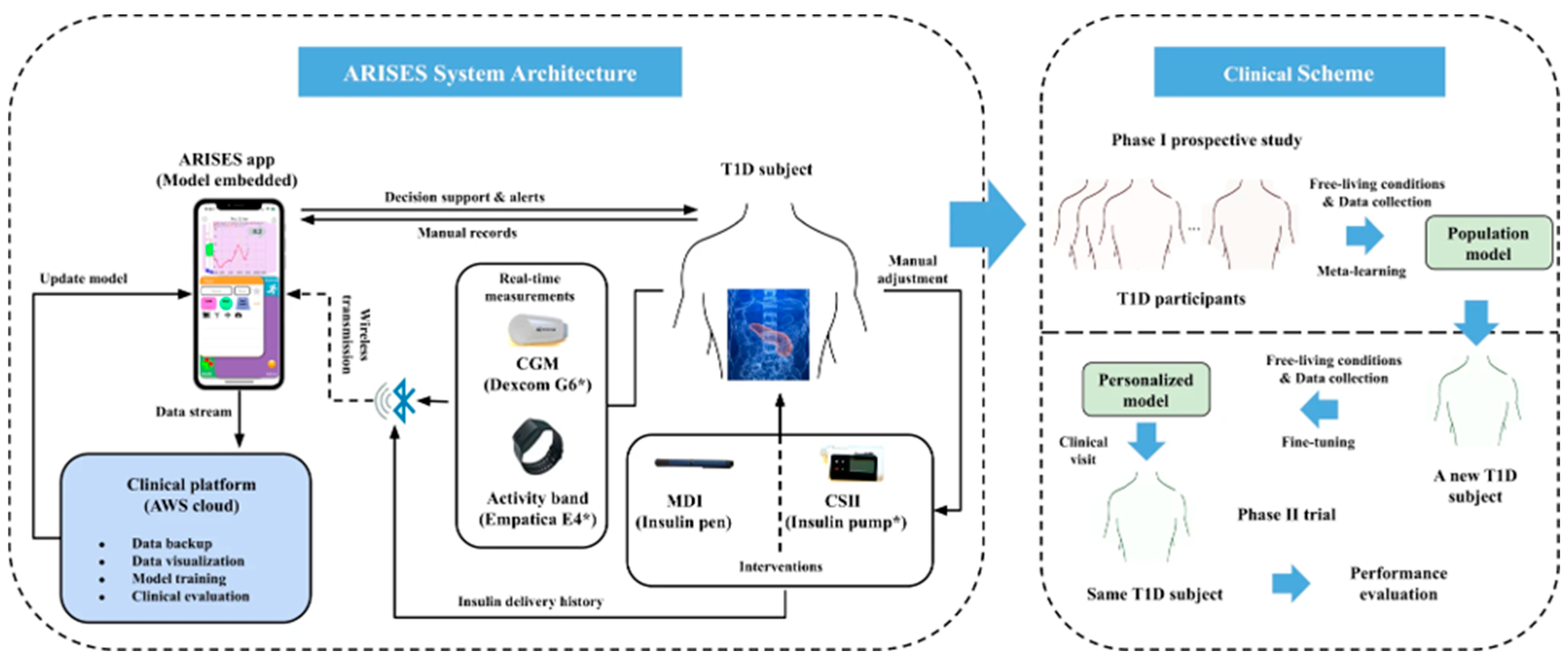
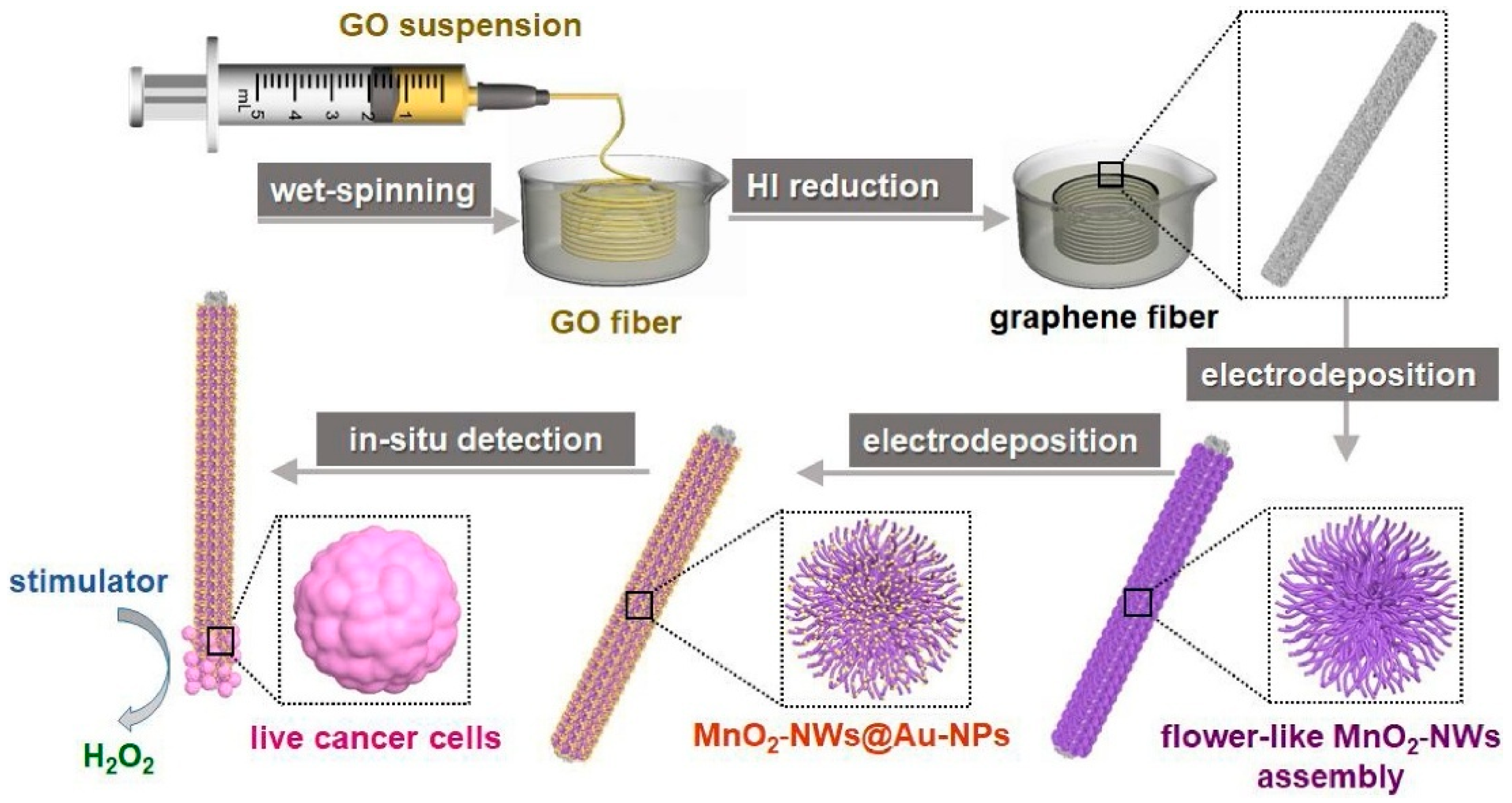
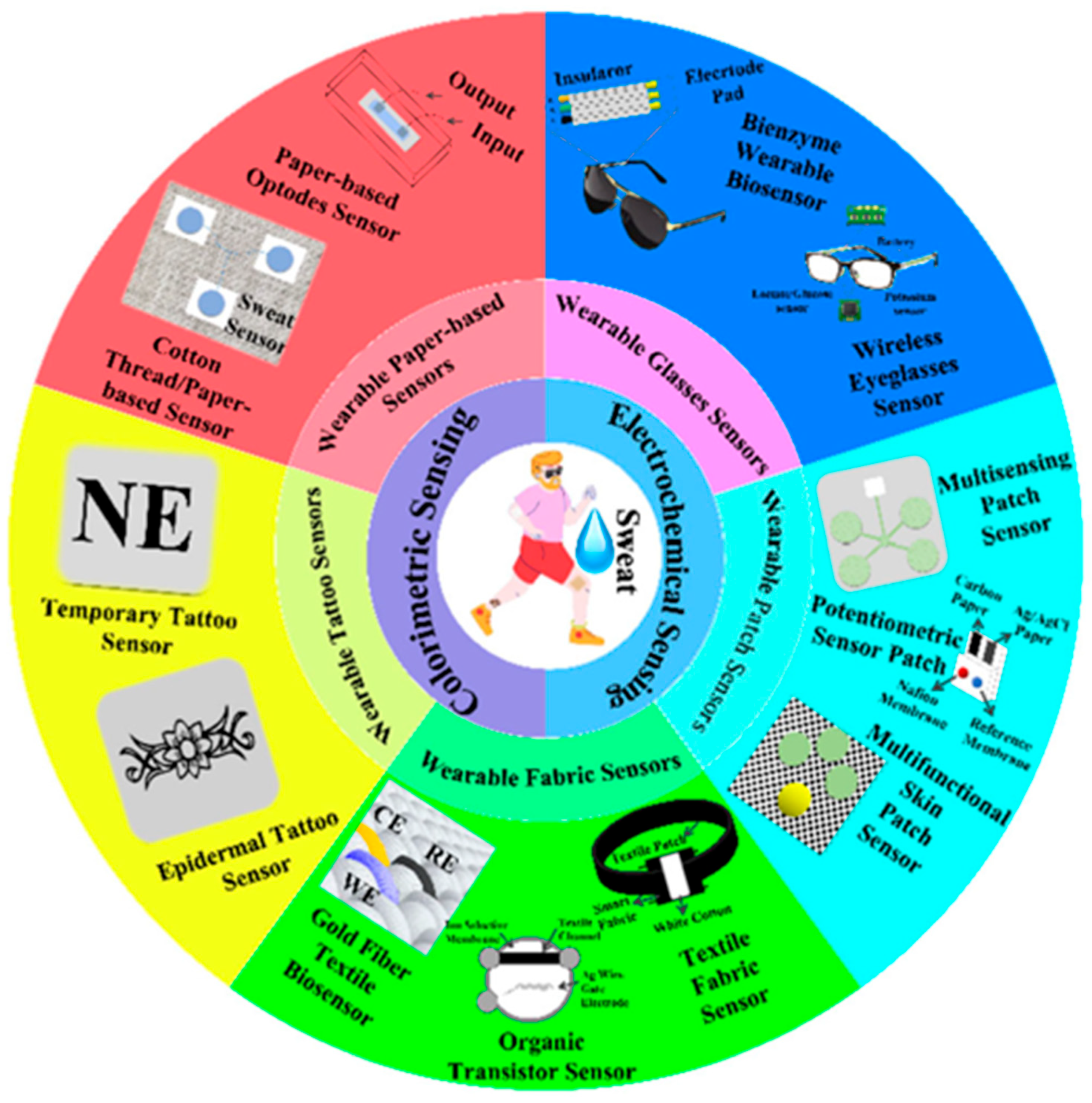
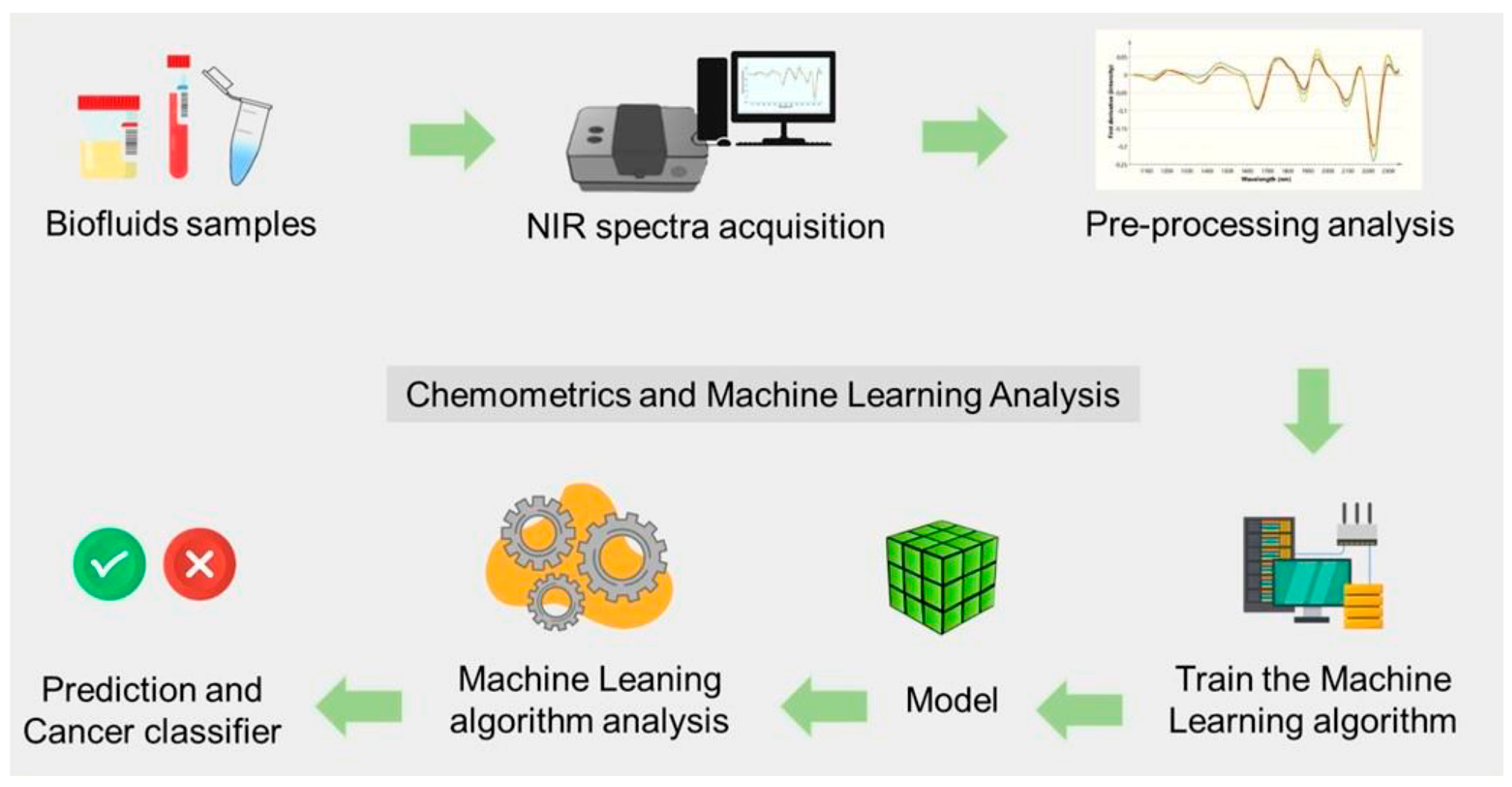
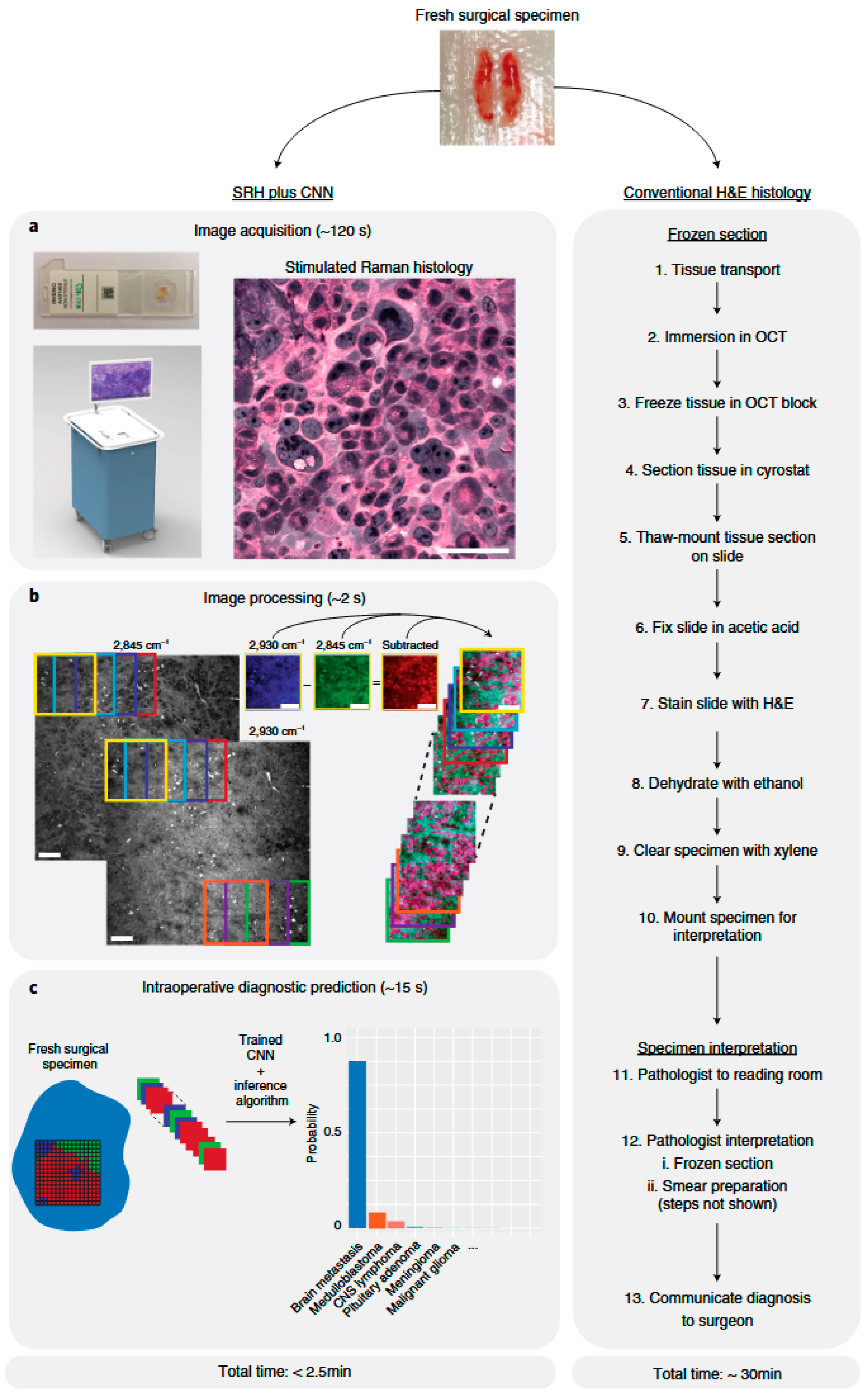
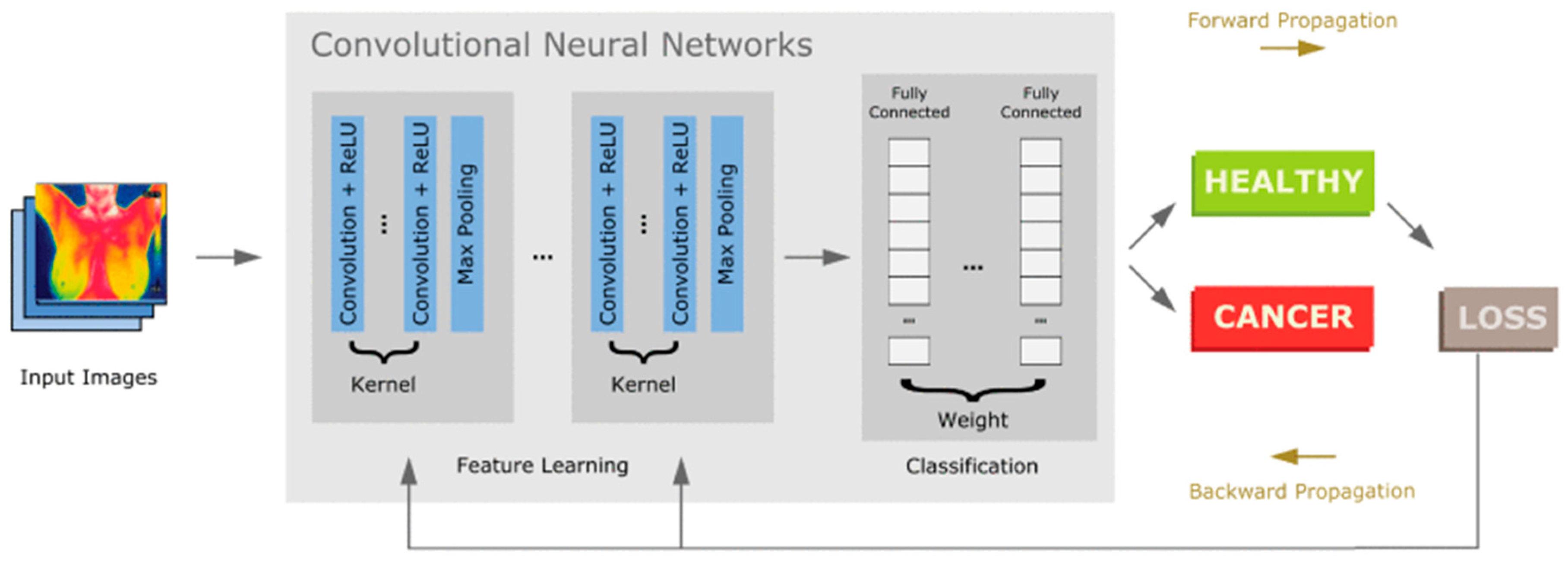
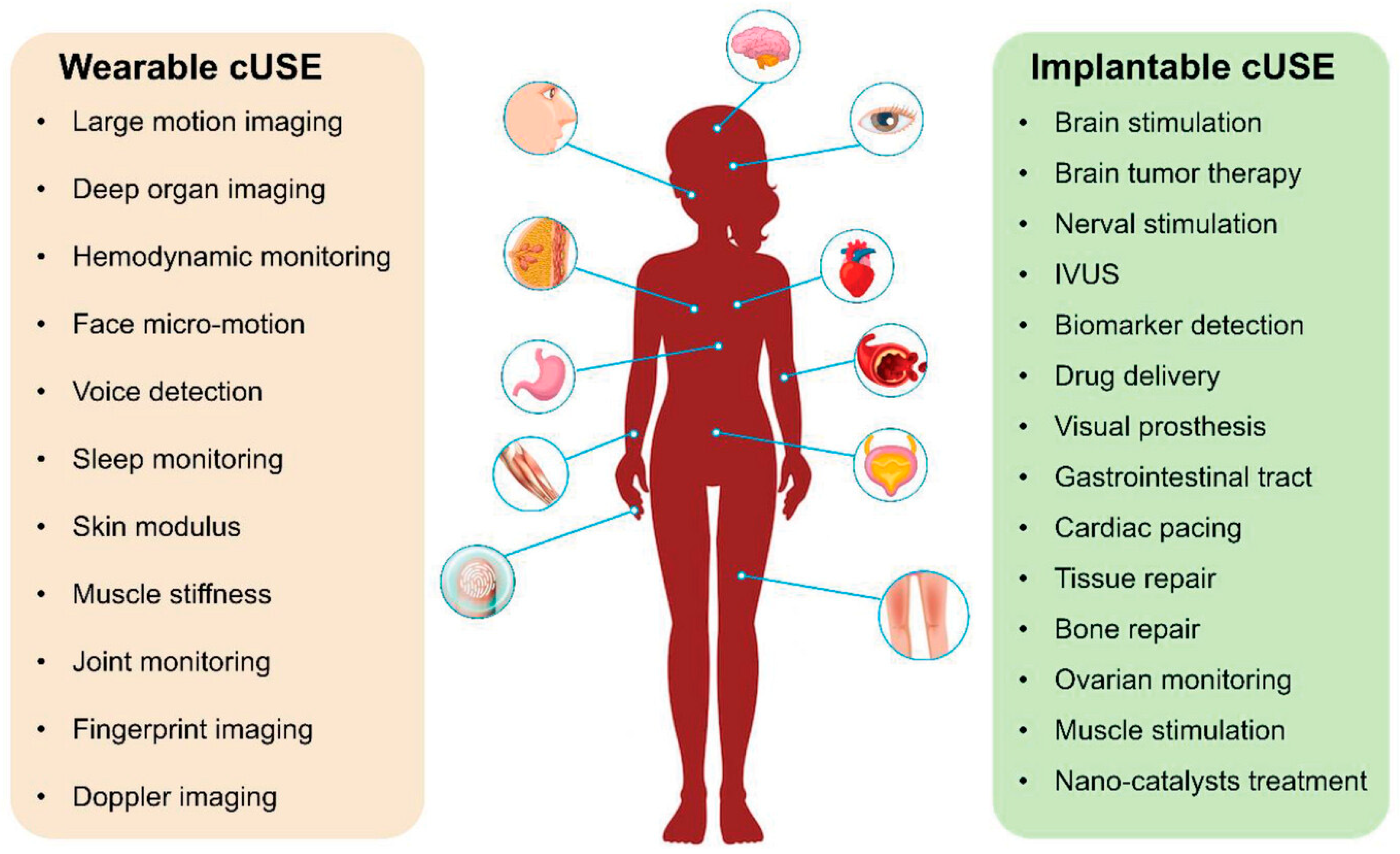
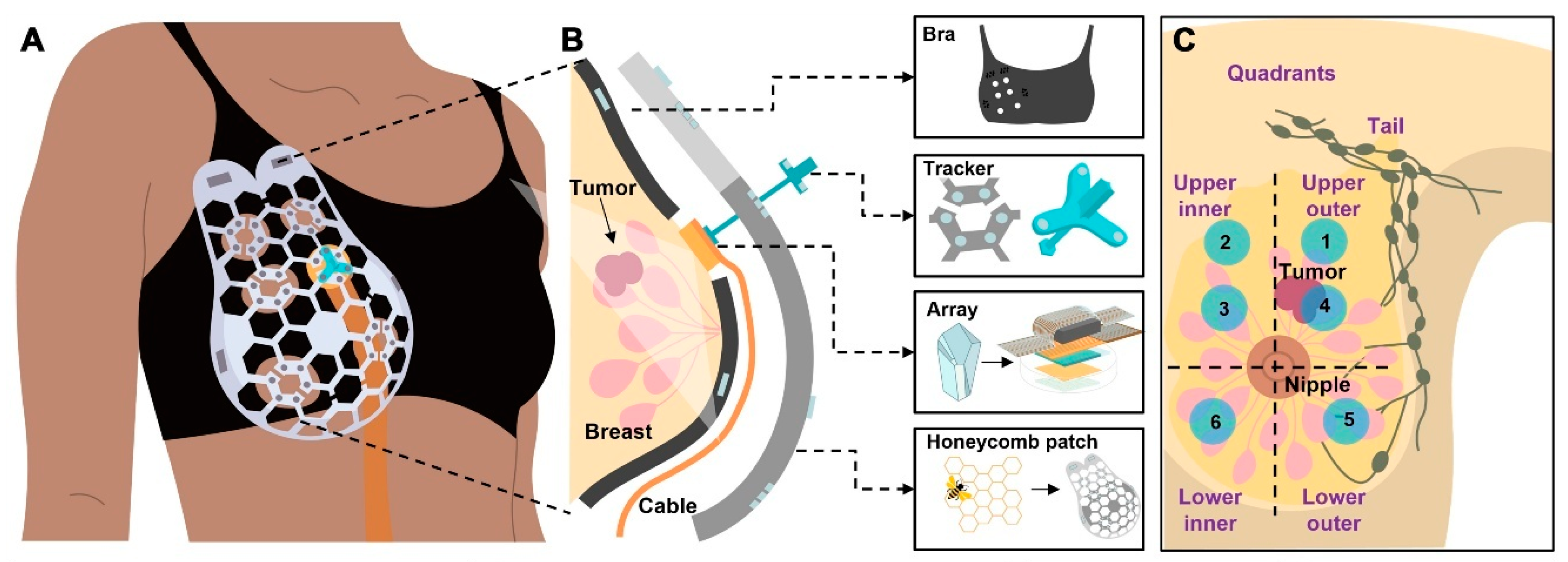
| Disease | Target Parameter for Wearable Sensor | Significant AI Algorithm |
|---|---|---|
| COVID-19 | Imaging, Respiration, and Cough | CNN, RNN, SVM |
| Diabetes | Electrochemical Patches and CGM | CNN, LSTM, RF, Gradient Boost |
| Cancer | Ultrasound, Thermal, Sweat Patches | CNN, CNN–CNN-Transformer, SVM, KNN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Goel, S.; Chaudhary, A.; Dutt, S.; Mishra, V.K.; Kumar, R. Artificial Intelligence-Based Wearable Sensing Technologies for the Management of Cancer, Diabetes, and COVID-19. Biosensors 2025, 15, 756. https://doi.org/10.3390/bios15110756
Kumar A, Goel S, Chaudhary A, Dutt S, Mishra VK, Kumar R. Artificial Intelligence-Based Wearable Sensing Technologies for the Management of Cancer, Diabetes, and COVID-19. Biosensors. 2025; 15(11):756. https://doi.org/10.3390/bios15110756
Chicago/Turabian StyleKumar, Amit, Shubham Goel, Abhishek Chaudhary, Sunil Dutt, Vivek K. Mishra, and Raj Kumar. 2025. "Artificial Intelligence-Based Wearable Sensing Technologies for the Management of Cancer, Diabetes, and COVID-19" Biosensors 15, no. 11: 756. https://doi.org/10.3390/bios15110756
APA StyleKumar, A., Goel, S., Chaudhary, A., Dutt, S., Mishra, V. K., & Kumar, R. (2025). Artificial Intelligence-Based Wearable Sensing Technologies for the Management of Cancer, Diabetes, and COVID-19. Biosensors, 15(11), 756. https://doi.org/10.3390/bios15110756







