Aptamer-Based Strategies for Colorectal Cancer Detection: Emerging Technologies and Future Directions
Abstract
1. Introduction
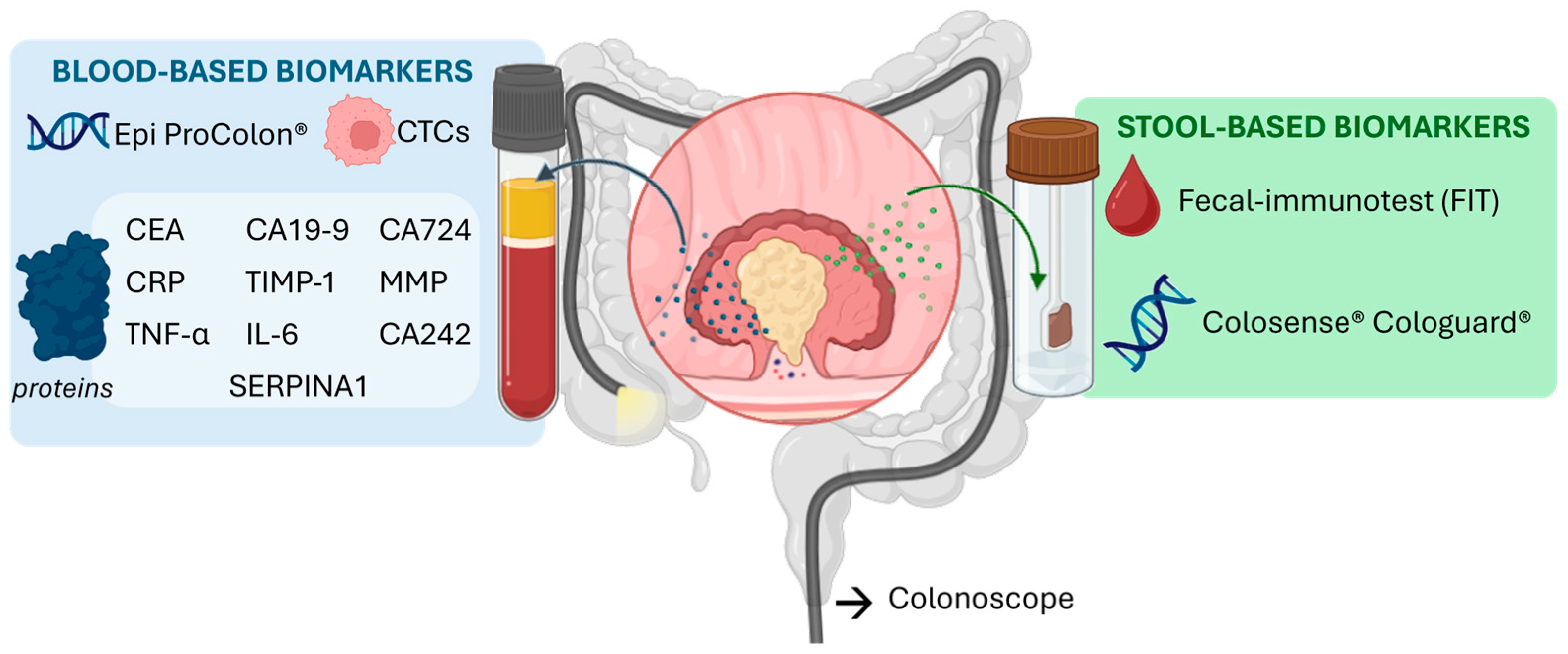
2. Colorectal Cancer Biomarkers
2.1. Genetic and Epigenetic Biomarkers
2.2. Circulating Nucleic Acids
2.3. Circulating Tumor Cells
2.4. Protein Biomarkers
| Name | Clinically Relevant Parameters | Ref. | |||
|---|---|---|---|---|---|
| Specificity | Sensitivity | AUC | Cut-Off Value | ||
| CEA | 89.2% | 64.5% | 0.789 | 3.36 ng mL−1 | [57] |
| CA19-9 | 90.1% | 47.8% | 0.690 | 23.9 U mL−1 | [57] |
| CA72-4 | 86% | 50% | 0.73 | - | [43] |
| CA242 | 59.7% | 66% | 0.651 | 20 U mL−1 | [58] |
| TIMP-1 | 95% | 42–65% | 0.83 | - | [45] |
| CRP | 90% | 17% | 0.64 | 14.6 ng mL−1 | [49] |
| IL6 | 61% | 84% | 0.794 | 4.2 pg mL−1 | [50] |
| TNF-α | 85% | 80% | 0.90 | - | [51] |
| STK4 | 92.3% | 100% | - | - | [59] |
| SerpinA1 | 95% | 95% | 0.97 | 817 µg mL−1 | [48] |
| MMP-9 | 67% | 80% | 0.8 | 204 ng mL−1 | [60] |
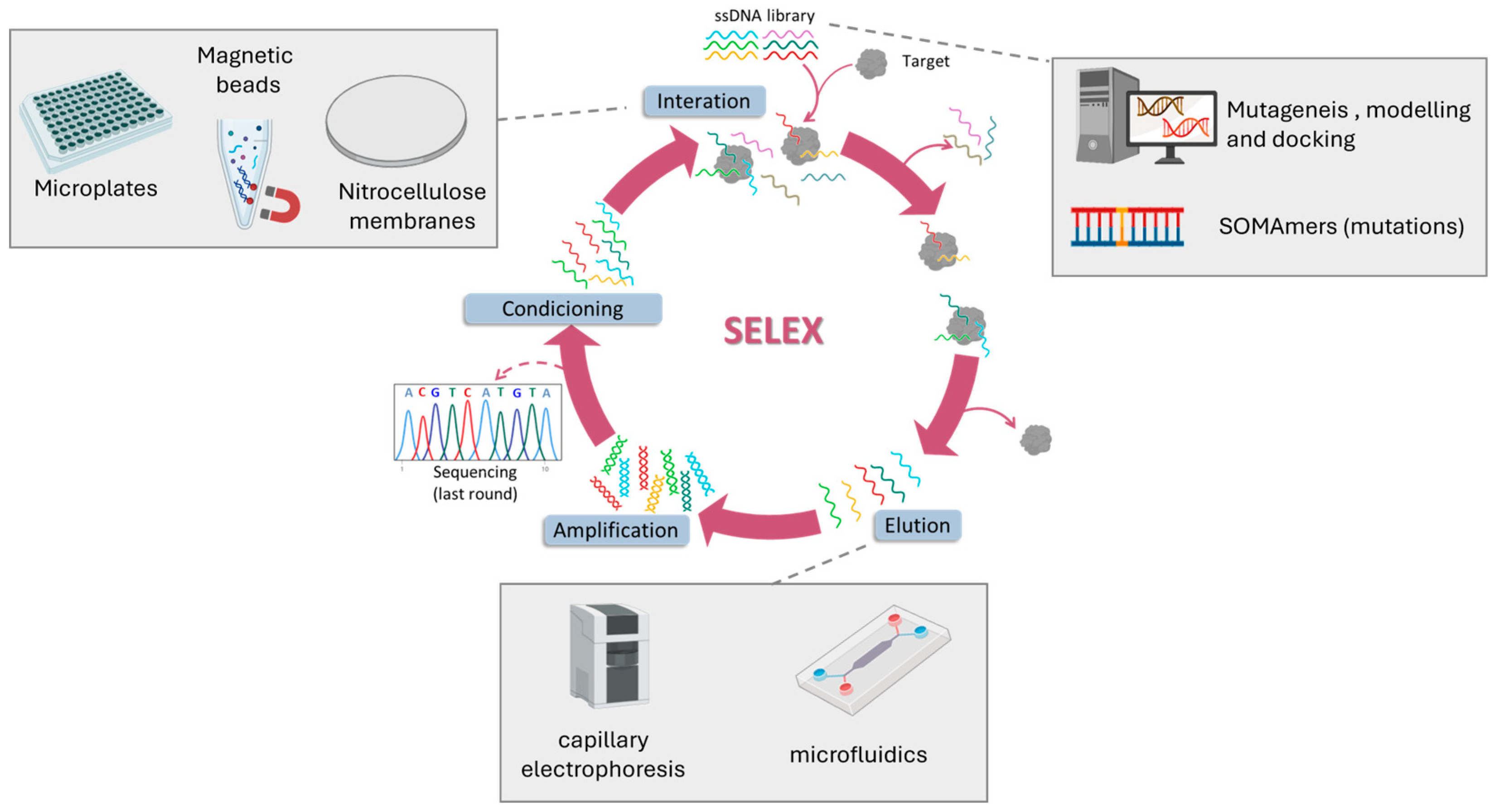
3. SELEX for CRC Biomarkers
4. Aptasensors for CRC Diagnosis
4.1. Traditional Approaches
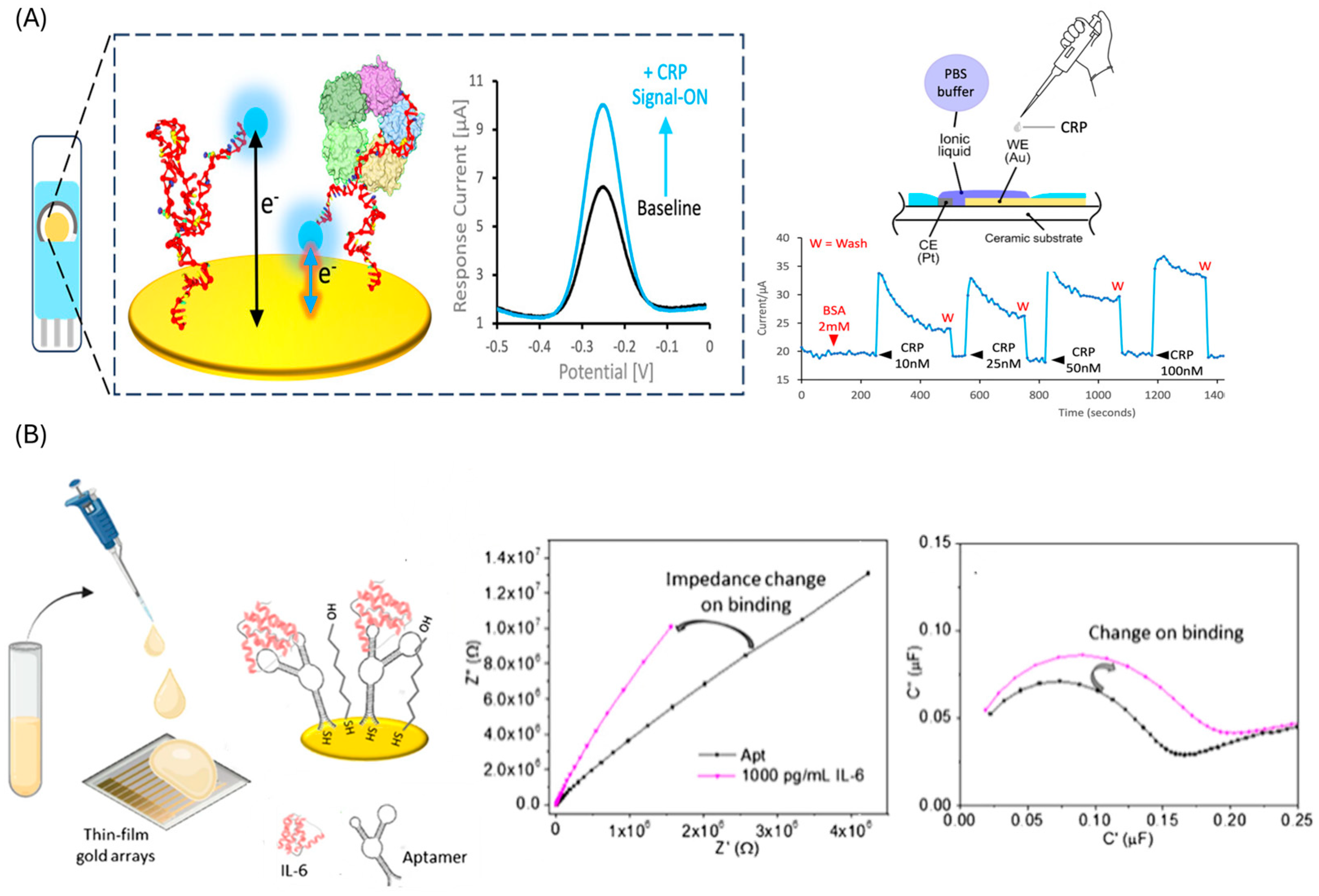
| Protein | Sensing Platform/ Assay Format | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CEA | ITO/Fe3O4@SiO2@CdS/Apt1/SiO2–Au-Apt2 Sandwich assay | Photo- electrochemical | 1–6000 pg mL−1/ 0.3 pg mL−1 | AFP, PSA, BSA, HCG | 2 h | - | 4 weeks | [75] |
| PtμEs/Au/aptamer Direct assay | Electrochemistry (SWV) | 10 pg mL−1–100 ng mL−1/ 7.7 pg mL−1 | AFP, BSA, pancreatin, IgG | 1 h | 5 Blood samples | - | [84] | |
| IDE/Apt/MCH Direct assay | Electrochemistry (EIS) | 2 pg mL−1–2 ng mL−1/ 2.4 pg mL−1 | HSA, IL-6, SARS-CoV-2-S | 20 min | 1% spiked serum | - | [85] | |
| Apt + 1,1′-biphenyl]-4,4′-diyldiboronic acid Direct assay | Fluorescence | 0.003–10 ng mL−1/ 1 pg mL−1 | AFP, IgG, VEGF, BSA, CA125, IgE, PSA, PTK7 | 55 min | 3 cancer serum | - | [78] | |
| CRP | Au/Apt-MB/MCH Direct assay | Electrochemistry (SWV) | 1–200 nM/ 20 nM | pfLDH, PCT | 1 min | - | 1 week | [81] |
| Au/apt-MB/MCH Direct assay | Electrochemistry (SWV) | 1 pM to 100 pM/ 1 pM | BSA, IgE | 30 min | 10% spiked serum | - | [82] | |
| Au/aptamer/MCH/BSA Direct assay | Electrochemistry (DPV) | 0–1000 ng mL−1/ 1.84 ng mL−1 | cTnT, cTnI | - | 60 blood samples | - | [83] | |
| Fiber/Au/aptamer/MCH Direct assay | SPR | 0–100 nM/ 1.7 nM | Hemoglobin, fibrinogen, cTn-I | - | - | - | [76] | |
| PTB7-Th/GCE-Au/Apt/HT Direct assay | Photo- electrochemical | 1 pM–1000 nM/ 0.33 pM | BSA, IgG, Thrombin | 30 min | - | - | [77] | |
| IL-6 | Au/apt-MB/MCH Direct assay | Electrochemistry (SWV) | 50–200 nM/ 50 nM | TNF-α, HSA, H-calprotectin | - | - | 7 days | [79] |
| TFGA-Au/Apt/MCH Direct assay | Electrochemistry (non-faradaic EIS) | 10–10,000 pg mL−1/ 10 pg mL−1 | MMP3 | 30 min | Spiked serum | 2 weeks | [86] | |
| IDE-strep/btn-apt Direct assay | Electrochemistry (current changes) | 1 fM–100 pM/ 10 fM | - | 5 min | 1% spiked serum | - | [87] | |
| MMP-9 | Apt1/apt2-btn/strep Sandwich assay | Piezoelectric (QZM) | 8.3–2075 ng mL−1/ 100 pg mL −1 | HSA, IgG | 1 h | - | - | [74] |
| TIMP-1 | MBs-strep/btn-apt/Ab-AE Sandwich assay | Chemi- luminescence | 1–500 ng mL−1/ 1 ng mL−1 | AFP, CEA, CA199, BSA, 8HIS peptide | - | 40 serum samples | - | [65] |
| TNF-α | Au/apt-MB/MCH | Electrochemistry (SWV) | 10–100 ng mL−1/ 10 ng ml−1 | - | 15 min | - | 10 h (blood) | [80] |
4.2. Nanomaterial-Based Sensors
4.2.1. Gold Nanoparticles

| Protein | Sensing Platform/ Assay Format | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CA125 | DNA-AgNCs-apt+ AuNPs Direct assay | Fluorescence | 0.01–2.0 U mL−1/ 0.015 U mL−1 | DP, BSA, OVA, GOD, Tb | 25 min | serum (n = 4) | 2 h | [99] |
| CEA | Au/4-mercaptophenyl/AuNPs/apt Direct assay | QCM | 0.1–25 ng mL−1/ 102 pg mL−1 | AFP, CA-125, VEGF | 50 min | Spiked serum | - | [95] |
| GrSPE/AuNPs/apt/MCH Direct assay | Electrochemistry (EIS) | 0.2–15.0 ng mL−1/ 0.085 ng mL−1 | DHEA, AFP, Leptin, AA, BSA | 1 h | - | - | [93] | |
| Au@AgNPs/4-MBA/apt Direct assay | SERS | 0.01–200 ng mL−1/ 3.24 pg mL−1 | AFP, DP, IgG, BSA | 20 min | serum (n = 3) | - | [96] | |
| ITO/E-Si/ZrO2/AuNPS/apt Direct assay | Electrochemistry (DPV) | 0.01 pg mL−1–100 ng mL−1 42.504 fg mL−1 | cTnI, CA199, NSE, l-cys | 30 min | serum (n = 43) | - | [100] | |
| AuE/apt/MCH/CEA/apt2-Au@PtNPs Sandwich assay | Electrochemistry (amperometry) | 0.1–100 ng mL−1/ 0.02–0.31 ng mL−1 | AFP, AA, BSA, CA125, | 2 h 30 min | spiked 20% serum | 2 weeks | [101] | |
| DNA-AgNCs-apta+ AuNPs Direct assay | Fluorescence | 0.01–0.9 ng mL−1/ 0.03–7.5 pg mL−1 | DP, BSA, OVA, GOD, Tb | 25 min | Serum (n = 4) | 2 h | [99] | |
| CRP | ePAD/EGaIn-PPD/AuNPs/Apt/ MCH/AuNPs Direct assay | Electrochemistry (DPV) | 1–100 ng mL−1/ 0.039 ng mL−1 | Cys, Glu, urea, Hcy | 1 h | Saliva (n = 38) | - | [92] |
| SPCE/AuNPs/Apt/casein/JNP-AuNPs/HRP Direct assay | Electrochemistry (amperometry) | 10 pg mL−1–1 ng mL−1/ 3.1 pg mL−1 | cTnT, cTnI, TB, HigG, HSA, Myo | 1 h | serum (n = 3) | 32 days | [97] | |
| IL-6 | Electrode/AuNPs/apt/MB Direct assay | Electrochemistry (CV) | 5–5000 pg mL−1/ 1.6 pg mL−1 | IL-1, IL-18, BSA, HSA | 2 h | 15 saliva, 15 sweat | 3 days | [89] |
| TNF-α | Electrode/AuNPs/apt/ferrocene Direct assay | Electrochemistry (CV) | 5–5000 pg mL−1/ 1.6 pg mL−1 | IL-1, IL-18, BSA, HSA | 2 h | 15 saliva, 15 sweat | 3 days | [89] |
| GrSPECoHCF-AuNps/Apt/1-HT/Ab-HRP Direct assay | Electrochemistry (DPV) | 1–100 pg mL−1/ 0.52 pg mL−1 | APC, HSA, IgG | 80 min | Serum (n = 3) | - | [90] | |
| GCE/AuNPS/Apt/MCH/TNF-α apt2-Ru(phen)2+/GO Sandwich assay | ECL | 0.05–50 ng mL−1/ 36 pg mL−1 | MMP-2, IL-2/6, BSA, IFN-γ | 1h 45 min | - | - | [94] | |
| Fe3O4/AuNPs/Apt1/cApt-MB AuNPs Direct-displacement assay | Electrochemistry (SWV) | 10 pg mL−1–100 ng mL−1/ 10 pg mL−1 | IL-1, IL-2, IL-6, IL-12, IFN-γ | 30 min | Spiked serum | - | [91] | |
| Quantum dot/apt/AuNPs Direct assay | FRET | 0–22.3 nM/ 97.2 pM | HSA, CRP, transferrin, Tb | 1 h | spiked serum (n = 5) | - | [98] |
4.2.2. Carbon-Based Nanomaterials
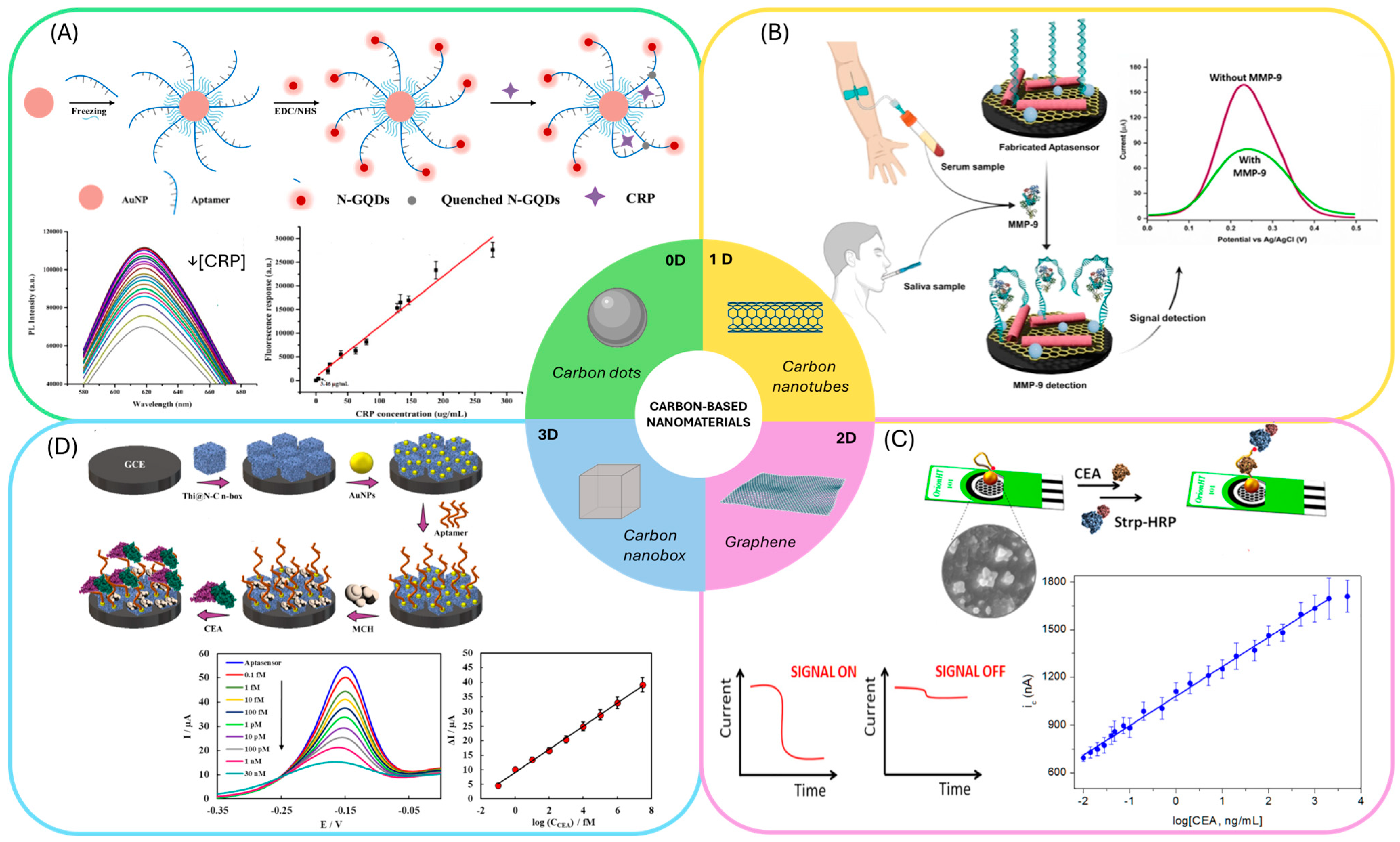
| Protein | Sensing Platform/ Assay Format | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CA19-9 | GCE/GO-cMWCNTs/AuNPs cDNA-AQ/BSA/Apt-Fc Displacement assay | Electrochemistry (ACV) | 1.0–1.0 × 106 mUmL−1/ 0.65 mU mL−1 | CA242, AFP, PSA | 1 h | 1% serum | 15 days | [120] |
| CEA | MACNTs/apt/DNA-Ag@ZIF Displacement assay | Chemiluminescence | 0.05–500 ng mL−1/ 4.53 pg mL−1 | AFP, glu, adrenaline, Na+, BSA, Carbamide, | 20 min | - | 21 days | [107] |
| GCE/AgNPs/SWCNT/MCF/Apt/BSA Direct assay | Electrochemistry (DPV) | 1 fg mL−1–20 ng mL−1/ 0.30 fg mL−1 | Glu, insulin, urea, gly, AA, HSA, Hb | 1 h | Serum (n = 5) | 16 days | [109] | |
| GO/AgNPS/aptamer Direct assay | Electrochemistry (DPV) | 0.001–10 pg mL−1/ 0.5 fg mL-1 | HER2, VEGF, IgG, MUC1 CFP10 | - | Serum (n = 2) | - | [117] | |
| IDE/GO/apt1/BSA/CEA/apt2 Sandwich assay | Electrochemistry (LSW) | 0.5–500 ng mL−1/ 0.96 ng mL−1 | - | - | 1% serum | - | [112] | |
| SPCE/rGO/AuNPs/casein/apt-btn/strep-HRP Direct assay | Electrochemistry (amperometry) | 20 pg mL−1–2 μg mL−1/ 16 pg mL−1 | cTnI, CRP, HSA, TBA, IgG | 35 min | - | 60 days | [116] | |
| PEDOT:PSS/GO/APTES/apt Direct assay | Electrochemistry (EIS) | 0.77–14 ng mL−1/ 0.45 ngmL−1 | BSA, PSA, insulin | - | Spiked serum | - | [113] | |
| Cu3(PO4)2HNF/GO/strep/btn-apt/Cu2+ Direct assay | Electrochemistry (SWV) | 10 fg mL−1–500 ng mL−1/2.4 fg mL−1 | PSA, TB, Hb | 1 h | Serum | 15 days | [119] | |
| GCE/Thi@N-C n-box/AuNPs /apt/MCH Direct assay | Electrochemistry (SWV) | 0.1 fM–30 nM/ 0.03 fM | HCG, AFP, PSA | 25 min | Spiked 0.1% serum | 15 days | [123] | |
| GNSPE/PdNPs/apt/BSA Direct assay | Electrochemistry (DPV) | 0.002–200 ng mL−1/ 1.0 pg mL−1 | insulin, cys, glu, arg, gly, BSA, cholesterol | 30 min | 1% serum | 5 weeks | [122] | |
| Au/apt/BSA/Apt2-CNTs- PFcGE Sandwich assay | Electrochemistry (SWV) | 1 fg mL−1–10 ng mL−1/ 0.28 fg mL−1 | DA, AA, UA, l-cys, | 1 h | 5% spiked serum | 2 weeks | [121] | |
| CRP | IDE/SWCNTs/Apt-AuNPs/ PEG-COOH Direct assay | Electrochemistry (SWV) | 1 pM–10 nM/ 10 pM | Troponin I | 30 min | - | - | [108] |
| GCE/PDES/GO/AuNPs/Apt Direct assay | Electrochemistry (EIS) | 0.001–50 ng mL−1/ 0.0003 ng mL−1 | AFP, ly, UA | 80 min | serum (n = 3) | 10 days | [115] | |
| CSPE/CNFs-CHIT/RNA-apt/BSA/MB Direct assay | Electrochemistry (SWV) | 1–150 pM/ 0.37 pM | HSA, IgG | 1 h | Spiked 2.5% serum | 15 days | [122] | |
| AuNPs/apt/N-GQDs Direct assay | FRET | 0.2–300 ng mL−1/ 0.2 ng mL−1 | BSA, cTNI, MYO | 40 min | Serum | - | [104] | |
| IL-6 | GCE/MWCNTs/CoHCF/ /AuNPs/Apt/MCH Direct assay | Electrochemistry (DPV) | 0.5–1000 pg mL−1/ 0.17 pg mL−1 | BSA, IgG, CEA, MUC1 | 1 h | serum (n = 5) | 15 days | [106] |
| AuNPS-apt/NCD Direct assay | FRET | 1.5–5.9 pg mL−1/ 0.84 pg mL−1 | BSA, TNF-α | 1 h | 5% serum | - | [103] | |
| MMP-9 | GCE/MWCNTS-Gc3n4/OZrO2NPs/apt/BSA Direct assay | Electrochemistry (DPV) | 50–1250 pg mL−1 10.51 pg mL-1 | leptin, TB, VEGF, MUC-1, IL-6, Apo-A1, NGAL | 45 min | 1% serum, 10% saliva | 14 days | [105] |
| IDE/Graphene/apt/6-FHH Direct assay | Electrochemistry (DPV) | 10 fM–1000 nM/ 0.1 pM | - | - | Wound fluids | 1 month | [110] | |
| TNF-α | GO/strep/btn-PEG-Apt-Fc Direct assay | Electrochemistry (SWV) | 5 –200 pg mL−1/ 5 pg mL−1 | BSA, IL-6, IL-1β | 1 h | Serum, sweat | 30 days | [111] |
| AuSPE/GO/Ag@PtNPs/Apt Direct assay | Electrochemistry (DPV) | 0–60 pg mL−1/ 2.07 pg mL−1 | BSA, Hb, cys, l-arg | 2 h | serum (n = 3) | 15 days | [124] | |
| SPCE/Au-GO/CS/apt/MCH/Apt-Ag@Pt-GRs Sandwich assay | Electrochemistry (DPV) | 5–70 pg mL−1 1.64 pg mL−1 | BSA, Hb, L-cys, L-arg | 2 h+ overnight | serum (n = 3) | 14 days | [114] |
4.2.3. Metal–Organic and Covalent Organic Frameworks
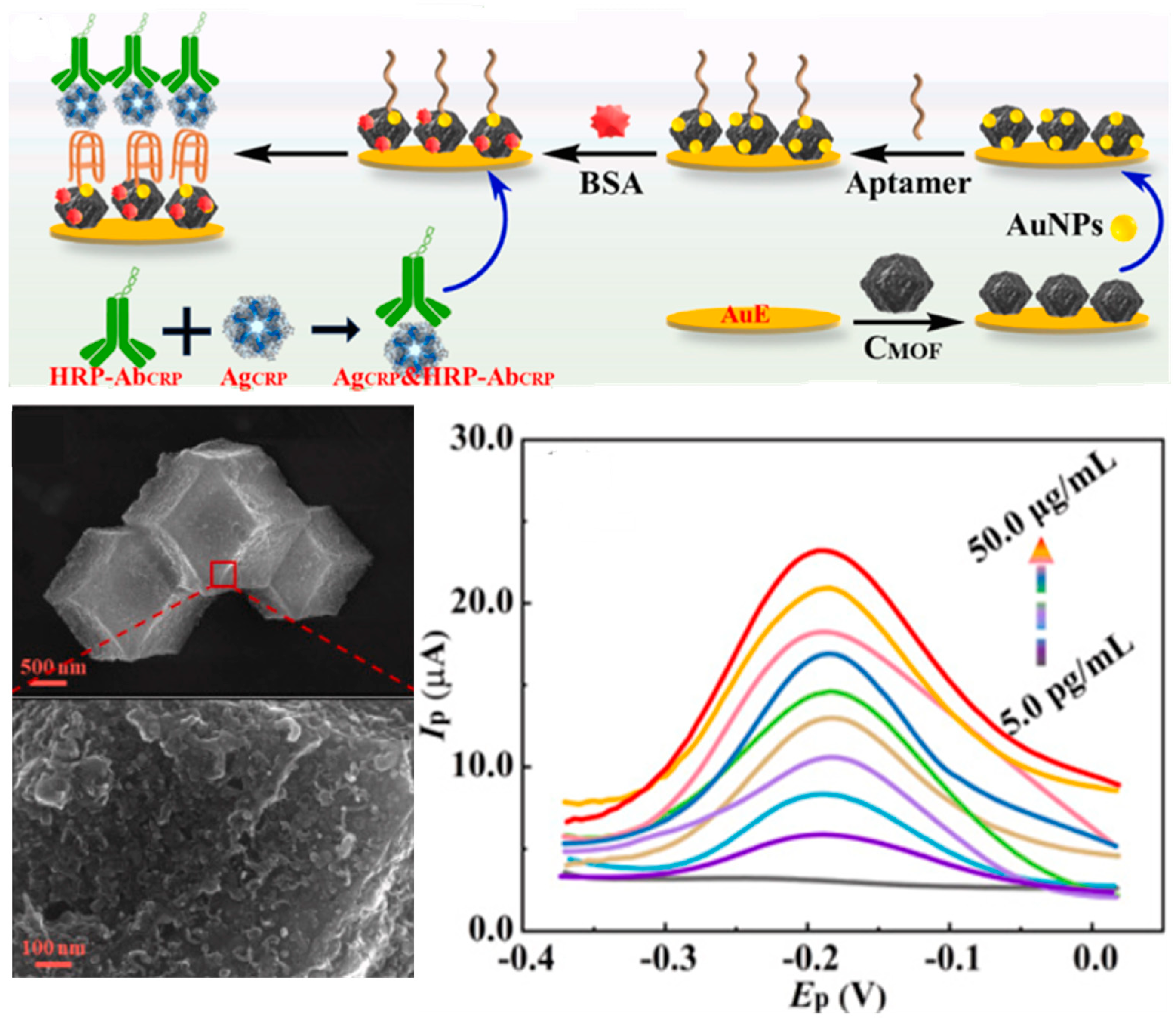
| Protein | Sensing Platform/ Assay Format | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CEA | 6FAM-apt/COF Displacement assay | Fluorescence | 0.01–15 ng mL−1/ 5 pg mL−1 | CA125, BSA, HSA, PSA, AGP, VEGF | 55 min | serum | - | [135] |
| GCE/HEANPs-Fe-MOF/apt/BSA Direct assay | Electrochemistry (DPV) | 1 fg mL−1–10 μg mL−1 0.115 fg mL−1 | AFP, PSA, CP | 35 min | 10% plasma | 12 days | [131] | |
| GE/rGO/AuNPS-MOF/apt Direct assay | Electrochemistry (EIS) | 0.0025–0.25 ng L−1/ 0.8 pg L−1 | MUC-1, HSA, IgG, HGB, BSA | 1 h | Spiked serum | - | [132] | |
| AuE/ZrCo-MOF/apt/BSA Direct assay | Electrochemistry (DPV) | 0.001–100 pg mL−1/ 0.35 fgmL−1 | BSA, PSA, VEGF, IgG, MUC1, HER2, HSA, Tb, HGB | 80 min | Spiked serum | 15 days | [129] | |
| GCE/CdS QDs@MOF/TEOA@ AuNPs/apt/b-ME Direct assay | ECL | 0.0001–10 ng mL−1 0.085 pg mL−1 | BSA, thrombin, lysozyme, trypsin | 90 min | 0.1% serum | - | [134] | |
| GCE/Fe-MOFAu@PDA/apt/BSA Direct assay | Electrochemistry (DPV) | 1 fg mL−1–1 μg mL−1/ 0.33 fg mL−1 | AFP, PSA | 35 min | serum (n = 3; SA) | 12 days | [133] | |
| CRP | PCB/AuE/CMOF/AuNPs/aptamer/BSA/Ag-CRP+Ab-HRP Sandwich Assay | Electrochemistry (DPV) | 5 pgmL−1–50 µgmL−1 0.3 pg mL−1 | IGF-I, GHBP, IL-6, IL-10, CA125, BSA | 2 h | serum (n = 3) | 21 days | [127] |
| Cu-MOF/apt Displacement assay | Colorimetric/ Fluorometry | 0.1–50 ng mL−1 40 pg mL−1 | Glu, GSH, AA, Fe, Cr, Ca, albumin, | 15 min /8 h | 10% serum | - | [128] | |
| ITO/NiS/pCOFs/AgNP/apt/MCH Direct assay | Photo-electrochemical | 0.5–100 ng mL−1/ 0.1 ng mL−1 | PSA, BSA, HCG, MC-LR | 50 min | 10% serum | 20 days | [136] | |
| MMP-9 | GCE/rGO-AuNRs/cDNA/BSA/ apt-ZIF-8@Au NPs@S QDs Nanorod displacement | DPV Colorimetric Fluorescence | 1 ng mL−1–10 μg mL−1 10 fg mL−1–10 μg mL−1 0.01–5 μM | annexin CAVIN2-, FEN1-, RAB26 | 40 min | - | 30 days | [126] |
4.2.4. Other Nanomaterials

| Protein | Sensing Platform/ Assay Format | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CEA | ITO/SNF-ngqdS/apt/BSA Direct Assay | ECL | 0.001–10 ng mL−1/ 0.4 pg mL−1 | Glucosa, NGAL, S100, AFP | - | 2% FBS (SA) | - | [139] |
| ITO/VMSF/PtNPs/Apt/BSA Direct assay | ECL | 0 fg mL−1–100 ngmL−1/ 0.4 fg mL−1 | AFP, CA19-9, S100, Hb, HSA, IgG, AA, UA, Glu, DA | 90 min | 2% spiked serum | - | [140] | |
| AuSPE/cDA-Fc/apt/PdPt@PCN-224 Displacement assay | Electrochemistry (DPV) | 1 pg mL−1–100 ng mL−1/ 0.98 pg mL−1 | Thrombin, PSA, HSA, AFP, IgG | 30 min | Serum (n = 2) | - | [144] | |
| CRP | ITO/bp-SNF/Ru(bpy)32+/apt Direct assay | ECL | 0.01–1000 ng mL−1/ 8.5 pg mL−1 | CEA, SAA, CA-15-3, CA19-9, Glu, TRP | 1 h | Spiked FBS | 11 days | [137] |
| ITO/SNF/AuNPs/Apt/BSA Direct assay | ECL | 10 pgmL−1–1 μgmL−1 4 pg mL−1 | AFP, CA15-3, glu, l-serines, Na, K, Ca | 30 min | 2% spiked serum | 15 days | [138] | |
| SPCE/Ti3C2Tx/PBA/Chit/AuNP/Apt/MCH Direct assay | Electrochemistry (DPV) | 0.1–15 μg mL−1/ 0.075 μg mL−1 | Hb, cholesterol, cys, LPS, BSA, Glu | 30 min | serum (n = 3) | 30 days | [141] | |
| SPCE/MSA-NiSe2/QD/apt/BSA Direct assay | Electrochemistry (CC) | 10–110 pg mL−1/ 2.80 pg mL−1 | l-cys, BSA, cTnI, | - | 10% serum (n = 3) | 15 days | [147] | |
| SPCE/Ti3C2Tx-AgNO3/AuNPS/apt/MCH Direct assay | Electrochemistry (DPV) | 0.1–200 ng mL−1/ 41 pg mL−1 | IL-6, BSA, Hb, Glu, Cys | 40 min | Spiked serum (n = 2) | 10 days | [143] | |
| GCE/ZIF67-C@AuNPs/apt/BSA/CRP/ Ab-HRP Sandwich assay | Electrochemistry (DPV) | 10 pg mL−1–10 μg mL−1 0.44 pg mL−1 | IL-6/10, GHBP, IFN-γ, CA-125, BSA | 1 h | Plasma (n = 4) | 15 days | [130] | |
| GCE/PDA/AuNPs/Ab/BSA/CRP/aptamer@ Mn3(PO4)2 Sandiwch assay | Electrochemistry (DPV) | 1 pg mL−1–1 ng mL−1 0.37 pg mL−1 | CEA, cTnI, NMP22, PSA | 2 h 40 min | Serum (n = 8) | - | [146] | |
| IL-6 | SPCE/Ti3C2Tx/PBA/Chit/AuNP/apt/MCH Direct assay | Electrochemistry (DPV) | 0.01–100 ng mL−1/ 7 pg mL−1 | Hb, cholesterol, cys, LPS, BSA, Glu | 40 min | serum (n = 3) | 30 days | [141] |
| SPE/Ti3C2Tx/MoS2/Au/NPs/apt/MCH Direct assay | Electrochemistry (CA) | 5 pg mL−1–100 ng mL−1/ 2.9 pg mL−1 | CRP, bovine l-cys, AA, BSA, UA | 30 min | 1% serum | - | [142] | |
| TNF-α | ITO/CdS/Apt/MCH/TNF-α/NiCo3O4-Au-Apt2 Sandwich assay | Photoelectrochemistry | 1 fg mL−1–1 ng mL−1/ 0.63 fg mL−1 | h-FABP, CEA, l-cys | 2 h 30 min | Spiked 10% serum | 20 days | [145] |
4.3. DNA-Based Amplifications
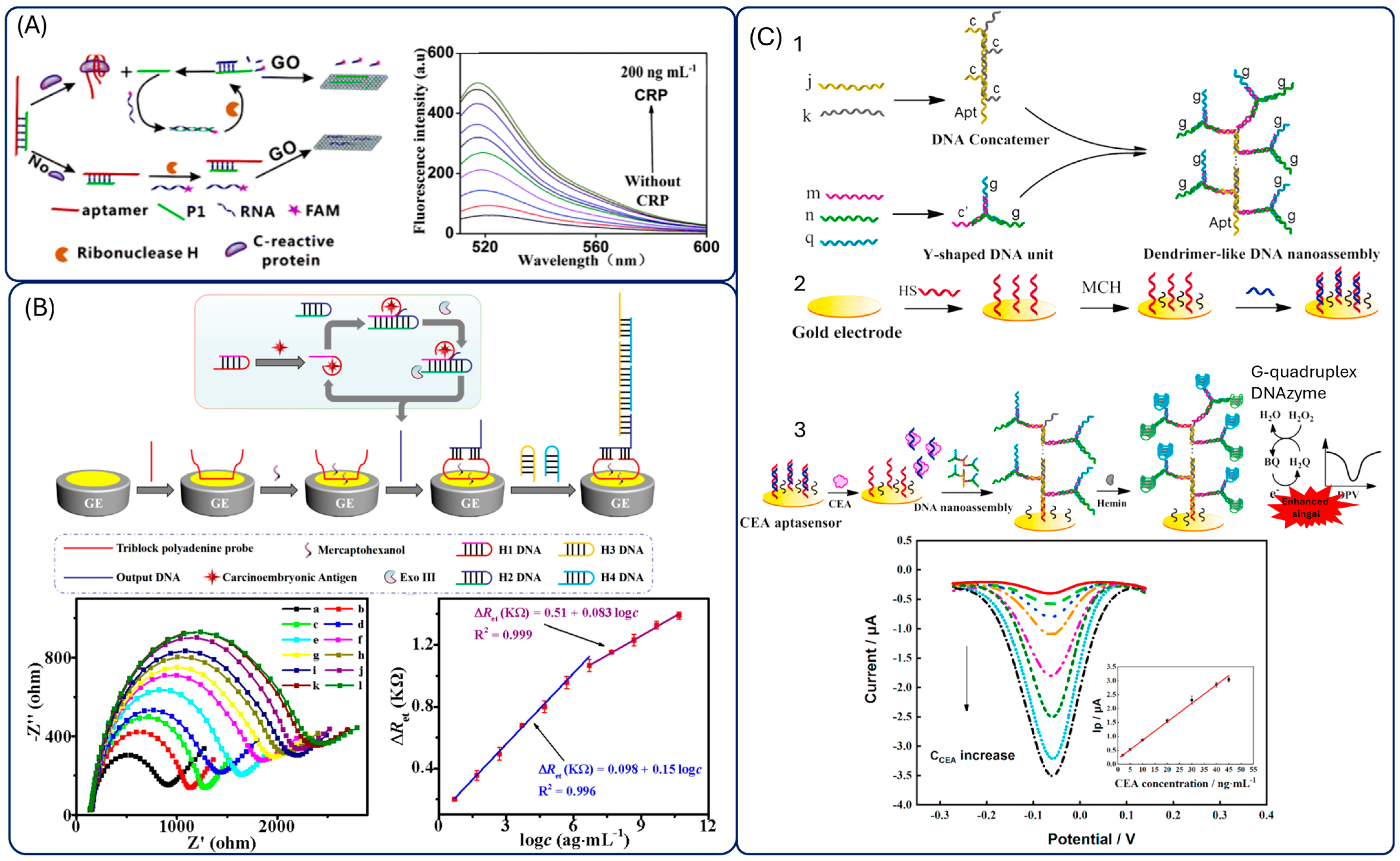
| Protein | Sensing Platform | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CEA | AuE/E1/MCH/T1-L2-L1/CEA ExoIII | Electrochemistry (DPV) | 10 fg mL−1–50 ng mL−1/ 4.88 fg mL−1 | TB, Hb, BSA | 2 h 40 min | Serum (n = 3) | 5 days | [153] |
| AuE/CTF-ssDNA/MCH/rHp/ CuxMn3-x(HITP)2+ Exo-III | ECL | 1 pg mL−1–50 ng mL−1/ 2.91 fg mL−1 | AFP, CD63, NCL, CA-50 | 1 h 40 min | Serum (SA) | 15 days | [150] | |
| AuE/AuNPs/Fc-HP2/MCH/exoIII/T4-DNA ligase+ Phi29-DNA polymerase+ dNTPs/MB | Ratiometric | 1 pg mL−1–100 ng mL−1/ 0.59 pg mL−1 | AFP, PSA, HSA | 4 h 30 min | 10% serum (n= 5) | - | [151] | |
| GCE/Au/cDNA/MCH/apt/ CEA+ExoI+ Ag+ | ECL | 100 ag mL−1–10 ng mL1/ 38.86 ag mL−1 | HSA, PSA, AFP, IgG | 1 h 30 min | 1% serum (n = 3) | - | [154] | |
| AuE/AuNPs/hpDNA/MCH/Fc-apt/exoI+CEA | Ratiometric | 10 pg mL−1–100 ng mL−1/ 1.9 pg mL−1 | AFP, PSA, HSA | 2 h | 10% serum (SA, n = 3) | 1 week | [155] | |
| AuE/AuNP/cpDNA-MCH/fcDNA ExoI+ CEA/tDNA/H1+H2+MB | Ratiometric | 1 pg mL−1–100 ng mL−1/ 0.479 pg mL−1 | AFP, PSA, HSA | 1 h 30 min | 10% serum (n =4) | 21 days | [156] | |
| AuE/H2/apt/Exo-III+CEA/S1/S2/MB | Electrochemistry (DPV) | 10 pg mL−1–100 ng mL−1/ 0.84 pg.mL−1 | AFP, CA125, CA199,CA153 | 1 h 15 min | 10% serum (n = 3) | 7 days | [152] | |
| Photoanode: ITO/BVO/ZIS Photocathode: AuNP/CuBi2O4/ ITO/Apt1/CEA/Apt2/G4/hemin | Photo-electrochemistry | 0.1 pg mL−1–10 ng mL−1/ 0.021 pg mL−1 | AFP, BSA, IgG, PSA | 2 h 20 min | Serum (n = 4) | 600 s | [159] | |
| MCH/cpDNA/Au/Apt/MCH /cpDNA/Au | Electrochemistry (DPV) | 2–45 ng mL−1/ 0.24 ng mL−1 | BSA, HSA, IgG, CRP, CA125, AFP | 3 h 24 min | Serum (n = 9) | 31 days | [160] | |
| Aptamer/Harpins/DNA/hemin/G4+ HCR | Fluorescence | 0.25–1.5 nM/ 0.2 nM | IgG, AFP, PSA | 8 h 20 min | 0.1% serum | - | [164] | |
| ExoI+ExoIII on MBs/AuE | Electrochemistry (DPV) | 10 fg mL−1–100 ng mL−1/1.26 fg mL−1 | BSA, AFP, UA CA125, Hb, AA | 3 h | Spiked serum | 30 days | [161] | |
| GCE/rGO-IL/PtNPs/Apt-G4/ hemin + exoI | Ratiometric- ECL | 10 fg mL−1–10 ng mL−1/0.85 fg mL−1 | HSA, AFP, PSA, IgG, | 30 min | 1% serum | 10 days | [162] | |
| Fe3O4@AuNPs-S1-S2-S3 + exo III+ G4. | Electrochemistry (DPV) | 0.1–200 ng mL−1/ 0.4 pg mL−1 | HSA, Hb, l-cys, BSA | 2 h 35 min | serum | - | [163] | |
| MNPs/strep/btn-cDNA/apt | Fluorescence | 1–500 ng mL−1/ 0.7 ng mL−1 | SCD146, IgG, BSA | 3 h | plasma | - | [149] | |
| AuSPE/TPP+ exoIII | Electrochemistry (EIS) | Up to 1010 ag mL−1/ 0.39 ag mL−1 | HSA, Hb, AFP, lysz ,BSAVEGF | 4h 30 min | 2% serum (SA) | 20 days | [157] | |
| CRP | GO+ aptamer+RNA-FAM+ ribonuclease H | Fluorescence | 50 pg mL−1–100 ng mL−1/ 0.01 ng mL−1 | d-dimer, MB, MUC1 | 50 min | 2% serum, urine, saliva | - | [148] |
5. Adaptations to POC (Point-of-Care) Formats

| Protein | Sensing Platform/POC Device | Detection Method | Linear Range/ LOD | Selectivity | Time | Samples | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| CEA | Ti3C2-He@CCNT/Apt/BSA/CEA microfluidic chip | Electrochemistry (DPV) | 10 pg mL−1–1 µg mL−1/ 2.88 pg mL−1 | HSA, IgG, glu | 1 h | Serum (n = 3) | - | [167] |
| CRP | uPAD/AuNPs/apt/salt paper-based + microfluidic | Colorimetric | 0 -1000 mg L−1 1.9 mg L−1/ | BSA, IL-6, TNF-α | 40 min | plasma (spiked) | 1 year | [170] |
| HFGN-SPCE/MB-apt Microneedles | Electrochemistry (SWV) | 1 ngmL−1–100 μgmL−1/ 0.85 ng mL−1 | UA, AA, Hb BSA, cTnI | seconds | In vivo (rats) | 16 days | [172] | |
| IL-6 | Fe3O4AuNPs/apt/AuNC-RTG-cDNA Microfluidic chip | SERS | 1 pg mL−1–1 µg mL−1 /0.178 pg mL−1 | OPN, AFP | 15 min | Serum (n = 30) | - | [169] |
| uPAD/AuNPs/apt/salt paper-based + microfluidic | Colorimetric | 1–25 ng L−1/ 0.07 ng L−1 | BSA, CRP, TNF-α | 40 min | plasma (spiked) | 1 year | [170] | |
| MMP9 | Fe3O4AuNPs/apt/AuNC-RTG-cDNA Microfluidic chip | SERS | 1 pg mL−1–1 µg mL−1/ 0.178 pg mL−1 | OPN, AFP | 15 min | Serum (n = 30) | [169] | |
| TNF-α | Glass/Au/apt-MB/MCH + PDMS microfluidic device | Electrochemistry (SWV) | 9–88ng mL−1/ 5.46 ng mL−1 | IL-12, IL-6, IL-10, BSA, IFN-γ, | 1 h | - | - | [168] |
6. Market Transfer
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| Ab | Antibody |
| ACV | Alternating Current Voltammetry |
| AE | Acridinium ester |
| AFP | Alpha-feto protein |
| AgNPS | Silver nanoparticles |
| AGP | Arabinogalactan protein |
| APC | Activated protein-C |
| Apo | Apolipoprotein |
| Apt | Aptamer |
| APTES | 3-Aminopropyltriethoxysilane |
| Arg | Arginine |
| Au | Gold |
| AUC | Area under the curve |
| AuE | Gold electrode |
| AuNCs | Gold nanocages |
| AuNPs | Gold nanoparticles |
| AuNRs | Gold nanorods |
| AuSPE | Gold screen printed electrode |
| b-ME | β-mercaptoethanol |
| Bp-SNF | Bipolar silica nanochannel film |
| BSA | Bovine serum albumin |
| btn | Biotin |
| BVO | BiVO4 |
| CA | Carbohydrate antigen |
| cAPT | Aptamer complementary strand |
| CAVIN | Caveolae-associated protein |
| CCNT | Carboxylic carbon nanotube |
| cDNA | Complementary DNA |
| CDRH | Center for Devices and Radiological Health |
| CD63 | Granulophysin |
| CEA | Carcinoembryonic antigen |
| CHIT | Chitosan |
| CNTs | Carbon nanotubes |
| COFs | Covalent organic frameworks |
| CP | Calprotectin |
| cpDNA | Complementry DNA |
| CQDs | Carbon quantum dots |
| CR | Creatinine |
| CRC | Colorectal cancer |
| CRP | C-reactive protein |
| CSPE | Carbon screen printed electrode |
| CTCs | Ciculating tumor cells |
| ctDNA | Circulating tumor DNA |
| CTF | Covalent triazine framework |
| cTnI | Troponin I |
| cTnT | Cardiac troponin |
| CV | Cyclic voltammetry |
| cys | Cysteine |
| DA | Dopamine |
| DHEA | Dehydroepiandrosterone |
| DNA | Deoxyribonucleic acid |
| dNTPs | Nucleotides |
| DP | Dopamine |
| DPV | Differential pulse voltammetry |
| ECL | Electrochemiluminescence |
| EGaIn | Eutectic gallium indium |
| EIS | Electrochemical impedance spectroscopy |
| ePAD | Electrochemical paper-based analytical devices |
| Exo | Exonuclease |
| FBS | Fetal bovine serum |
| Fc | Ferrocene |
| Fc-HP | Ferrocene-hairpin DNA |
| FDA | Food and drug administration |
| FEN | Flap endonuclease |
| FH | Ferrocenylhexanethiol |
| FIT | Fecal immunotest |
| FRET | Förster resonance energy transfer |
| GCE | Glassy carbon electrode |
| GE | Graphene electrode |
| GHBP | Growth hormone receptor |
| Glu | Glucose |
| Gly | Glycine |
| GNPs | gold nanoparticles |
| GO | Graphene oxide |
| GOD | Glucose oxidase |
| GrSPE | Graphene screen printed electrodes |
| GSH | Glutathione |
| G4 | G-quadruplex |
| Hb | Hemoglobulin |
| HCG | Human chorionic gonadotropin |
| HCR | Hybridization chain reaction |
| Hcy | Homocysteine |
| He | Hemin |
| HER-2 | Human Epidermal growth factor Receptor 2 |
| hFBPA | Heart-type fatty acid-binding protein |
| HFGN | Hierarchical flower-like gold nanostructure |
| HITP | 2,3,6,7,10,11-hexaiminotriphenylene |
| HRP | Horse radish peroxidase |
| HSA | Human serum albumin |
| HT | Hexanothiol |
| IDE | Interdigitated electrodes |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| ISF | Interstitial fluid |
| ITO | Indium tin oxide |
| IVDR | In vitro diagnostic regulation |
| IVDs | In vitro diagnostic devices |
| JNP | janus particles |
| KD | Dissociation constant |
| lncRNAs | Long non-coding RNA |
| LPS | Lipopolysaccharide |
| LSW | Linear sweep voltammetry |
| Lyz | Lysozymes |
| m-SELEX | Membrane-SELEX |
| MACE | Microbead-assisted capillary electrophoresis |
| MACNTs | Magnetic Carbon nanotubes |
| MARAS | Magnetic-Assisted Rapid Aptamer Selection |
| MB | Methylene blue |
| MBs | Magnetic beads |
| MCF | Mesoporous carbon foam |
| MCH | Mercaptohexanol |
| MC-LR | Microcystin |
| miRNAs | microRNAs |
| MMPs | Matrix metalloproteinases |
| MNs | Microneedles |
| MNTPS | Magneitc nanoparticles |
| MOFs | Metal–organic frameworks |
| MRD | Molecular residual disease |
| MSA | Mercaptosuccinic acid |
| MSI | Microsatellite instability |
| MUC | Mucin |
| MWCNTs | Multi-walled carbon nanotubes |
| Myo | Myoglobin |
| NCD | nitrogen dopped carbon dots |
| NCL | Nucleolin |
| NGAL | Lipocalin-2 |
| N-GQDSNPs | Nitrogen-graphene quantum dots nanoparticles |
| NMP | Nuclear matrix protein |
| NPs | Nanoparticles |
| NSE | Neuron-specific enolase |
| OPN | Osteopotin |
| OVA | Ovalbumin |
| PBA | Phenylboronic acid |
| PCB | Printed circuit board |
| PCR | Polymerase chain reaction |
| PCT | Procalcitonin |
| PDA | Polymerized dopamine |
| PDMS | Polydimethylsiloxane |
| PEDOT:PSS | 3,4-(ethylenedioxythiophene):poly(styrenesulfonate) |
| PEG | Polyethylene glycol |
| PFcGE | Poly(ferrocenyl glycidyl ether)-grafted |
| pfLDH | Plasmodium lactate dehydrogenase |
| PMA | Premarket Approval |
| PNAs | Peptide nucleic acids |
| PSA | Prostate specific antigen |
| PTB7-Th | poly{4,8-bis[5-(2-ethylhexyl) thiophen-2-yl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl-alt-3-fluoro-2-[(2-ethylhexyl)carbonyl] thieno[3,4-b]-thiophene-4,6-diyl} |
| PTK7 | Tyrosine-protein kinase-like 7 |
| PtNPs | Platinum nanoparticles |
| QCM | Quartz crystal microbalance |
| QDs | Quantum dots |
| RAB26 | Ras-related protein |
| RCA | Rolling circle amplification |
| rGO | Reduce graphene oxide |
| rGO/IL | Reduced graphene oxide ionic liquid |
| rHP | Residual hairpins strands |
| RTG | Rammna-tag |
| SA | Standard addition |
| SAA | Serum Amyloid A |
| SCD146 | Soluble melanoma cell adhesion molecule |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| SERS | Surface-Enhanced Raman Scattering |
| SOMAMERS | Slow Off-rate Modified Aptamers |
| SPCE | Screen printed carbon electrodes |
| SPR | Surface plasmon resonance |
| ssDNA | Single stranded DNA |
| strep | Streptavidin |
| SWCNTs | Single well carbon nanotubes |
| SWV | Square wave voltammetry |
| S100 | Calcium related proteins |
| POC | Point-of-care |
| PtμEs: | Platinum microelectrode |
| TB | Thrombin |
| TcTn-I | Troponin I |
| tDNA | Trigger DNA |
| TFGA | Surface flexible thin film gold arrays chips |
| TIMP-1 | Metalloproteinases-1 |
| TNF-α | Tumor necrosis factor-alpha |
| TPP | Triblock polyadenine probe |
| TRP | Tryptophan |
| UA | Uric acid |
| VEGF | Vascular endothelial growth factor |
| VMSF | Vertically ordered mesoporous silica film |
| ZIS | ZnIn2S4 |
| 4-MBA | 4-mercaptobenzoic acid |
| 6-FAM | Fluorescein |
| µPADs | Microfluidic paper-based analytical devices |
References
- Disc Klimeck, L.; Heisser, T.; Hoffmeister, M.; Brenner, H. Colorectal cancer: A health and economic problem. Best Pract. Res. Clin. Gastroenterol. 2023, 66, 101839. [Google Scholar] [CrossRef]
- Mannucci, A.; Zuppardo, R.A.; Rosati, R.; Di Leo, M.; Perea, J.; Cavestro, G.M. Colorectal cancer screening from 45 years of age: Thesis, antithesis and synthesis. World J. Gastroenterol. 2019, 25, 2565. [Google Scholar] [CrossRef]
- McCabe, M.A.; Mauro, A.J.; Schoen, R.E. Novel colorectal cancer screening methods—Opportunities and challenges. Nat. Rev. Clin. Oncol. 2025, 22, 581–591. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Darmadi, D.; Darabi, R.; Al-Aouadi, R.F.A.; Ivraghi, M.S.; Akkol, E.K. Biomarkers for colorectal cancer detection: An insight into colorectal cancer and FDA-approved biomarkers. Bioimpacts 2025, 15, 31211. [Google Scholar] [CrossRef]
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef]
- López Salas, M.; De Haro Gázquez, D.; Fernández Sánchez, B.; Amador Muñoz, M.L. Knowledge, Compliance, and Inequities in Colon Cancer Screening in Spain: An Exploratory Study. Healthcare 2023, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Berg-Beckhoff, G.; Leppin, A.; Nielsen, J.B. Reasons for participation and non-participation in colorectal cancer screening. Public Health 2022, 205, 83–89. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Jiang, J.T.; Zhou, X. Recent advances in design strategies of aptamer-based liquid biopsy. J. Polym. Sci. 2024, 62, 2848–2870. [Google Scholar] [CrossRef]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Ahmadyousefi, Y.; Malih, S.; Mirzaee, Y.; Saidijam, M. Nucleic acid aptamers in diagnosis of colorectal cancer. Biochimie 2019, 156, 1–11. [Google Scholar] [CrossRef]
- Goh, K.W.; Stephen, A.; Wu, Y.S.; Sim, M.S.; Batumalaie, K.; Gopinath, S.C.B.; Guad, R.M.; Kumar, A.; Sekar, M.; Subramaniyan, V.; et al. Molecular Targets of Aptamers in Gastrointestinal Cancers: Cancer Detection, Therapeutic Applications, and Associated Mechanisms. J. Cancer 2023, 14, 2491–2516. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, S.; Cai, Y.; Tang, F. Nucleic acid aptamer application in diagnosis and therapy of colorectal cancer based on cell-SELEX technology. npj Precis. Oncol. 2017, 1, 37. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, H.; Liu, W.; Miao, J.; Mao, Y.; Li, Q. Prognostic and predictive molecular biomarkers in colorectal cancer. Front. Oncol. 2025, 15, 1532924. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Wang, K.; Xu, C.; Hao, M.; Ding, L. Perspectives of the Application of Liquid Biopsy in Colorectal Cancer. BioMed Res. Int. 2020, 2020, 6843180. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef] [PubMed]
- Nikolouzakis, T.K.; Vassilopoulou, L.; Fragkiadaki, P.; Sapsakos, T.M.; Papadakis, G.Z.; Spandidos, D.A.; Tsatsakis, A.M.; Tsiaoussis, J. Improving diagnosis, prognosis and prediction by using biomarkers in CRC patients (Review). Oncol. Rep. 2018, 39, 2455–2472. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, B.; Li, P.; Zhao, Y. Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. Biomed. Pharmacother. 2023, 163, 114786. [Google Scholar] [CrossRef]
- Łukaszewicz-zając, M.; Mroczko, B. Circulating Biomarkers of Colorectal Cancer (CRC)—Their Utility in Diagnosis and Prognosis. J. Clin. Med. 2021, 10, 2391. [Google Scholar] [CrossRef]
- Marshall, K.W.; Mohr, S.; El Khettabi, F.; Nossova, N.; Chao, S.; Bao, W.; Ma, J.; Li, X.J.; Liew, C.C. A blood-based biomarker panel for stratifying current risk for colorectal cancer. Int. J. Cancer 2010, 126, 1177–1186. [Google Scholar] [CrossRef]
- Koch, A.; Joosten, S.C.; Feng, Z.; De Ruijter, T.C.; Draht, M.X.; Melotte, V.; Smits, K.M.; Veeck, J.; Herman, J.G.; Van Neste, L.; et al. Analysis of DNA methylation in cancer: Location revisited. Nat. Rev. Clin. Oncol. 2018, 15, 459–466. [Google Scholar] [CrossRef]
- DeVos, T.; Tetzner, R.; Model, F.; Weiss, G.; Schuster, M.; Distler, J.; Steiger, K.V.; Grützmann, R.; Pilarsky, C.; Habermann, J.K.; et al. Circulating Methylated SEPT9 DNA in Plasma Is a Biomarker for Colorectal Cancer. Clin. Chem. 2009, 55, 1337–1346. [Google Scholar] [CrossRef]
- Shirley, M. Epi proColon® for Colorectal Cancer Screening: A Profile of Its Use in the USA. Mol. Diagn. Ther. 2020, 24, 497–503. [Google Scholar] [CrossRef]
- Han, Y.J.; Shao, C.Y.; Yao, Y.; Zhang, Z.; Fang, M.Z.; Gong, T.; Zhang, Y.J.; Li, M. Immunotherapy of microsatellite stable colorectal cancer: Resistance mechanisms and treatment strategies. Postgrad. Med. J. 2024, 100, 373–381. [Google Scholar] [CrossRef]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.; Wu, H.T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients with Stages i to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B.; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal–Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757. [Google Scholar] [CrossRef]
- Ma, D.; Gao, X.; Wang, L.; Yin, H.; Feng, L.; Zhu, Y. Circulating tumor DNA for MRD detection in colorectal cancer: Recent advances and clinical implications. Biomark. Res. 2025, 13, 89. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Shen, Y.; Nagasaka, T.; Lozano, J.J.; Boland, C.R.; Goel, A. Fecal microRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1766–1774. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, S.; Zhang, B.; Shi, W.; Li, A.; Tian, Q. The critical role of the miR-21–MEG2 axis in colorectal cancer. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Horimatsu, T.; Okugawa, Y.; Nishida, N.; Honjo, H.; Ida, H.; Kou, T.; Kusaka, T.; Sasaki, Y.; Yagi, M.; et al. Serum miR-21, miR-29a, and miR-125b Are Promising Biomarkers for the Early Detection of Colorectal Neoplasia. Clin. Cancer Res. 2015, 21, 4234–4242. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.; Fawzy, A.; Akel, S.Y.; Gamal, H.; Elshimy, R.A.A. Evaluation of microRNA 92a Expression and Its Target Protein Bim in Colorectal Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 723–730. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, T.; Duan, J.; Liu, X.; Liu, L. MicroRNA-223-induced inhibition of the FBXW7 gene affects the proliferation and apoptosis of colorectal cancer cells via the Notch and Akt/mTOR pathways. Mol. Med. Rep. 2020, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.A.; El Amin, H.A.; Ahmed, E.S.M.; Kenawy, A.G.; El-Ebidi, A.M.; Elnakeeb, I.; Kholef, E.F.M.; Elsewify, W.A.E. Role of MicroRNA-223 and MicroRNA-182 as Novel Biomarkers in Early Detection of Colorectal Cancer. Int. J. Gen. Med. 2022, 15, 3281–3291. [Google Scholar] [CrossRef]
- Eslamizadeh, S.; Zare, A.-A.; Talebi, A.; Tabaeian, S.P.; Eshkiki, Z.S.; Heydari-Zarnagh, H.; Akbari, A. Differential Expression of miR-20a and miR-145 in Colorectal Tumors as Potential Location-specific miRNAs. MicroRNA 2021, 10, 66–73. [Google Scholar] [CrossRef]
- Humphreys, K.J.; McKinnon, R.A.; Michael, M.Z. miR-18a Inhibits CDC42 and Plays a Tumour Suppressor Role in Colorectal Cancer Cells. PLoS ONE 2014, 9, e112288. [Google Scholar] [CrossRef]
- Alaiyan, B.; Ilyayev, N.; Stojadinovic, A.; Izadjoo, M.; Roistacher, M.; Pavlov, V.; Tzivin, V.; Halle, D.; Pan, H.; Trink, B.; et al. Differential expression of colon cancer associated transcript1 (CCAT1) along the colonic adenoma-carcinoma sequence. BMC Cancer 2013, 13, 196. [Google Scholar] [CrossRef]
- Ling, H.; Spizzo, R.; Atlasi, Y.; Nicoloso, M.; Shimizu, M.; Redis, R.S.; Nishida, N.; Gafà, R.; Song, J.; Guo, Z.; et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013, 23, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Badowski, C.; He, B.; Garmire, L.X. Blood-derived lncRNAs as biomarkers for cancer diagnosis: The Good, the Bad and the Beauty. Npj Precis. Oncol. 2022, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Betsou, F.; Cadamuro, J.; Cornes, M.; Fleischhacker, M.; Fruekilde, P.; Neumaier, M.; Nybo, M.; Padoan, A.; Plebani, M.; et al. Preanalytical challenges-time for solutions. Clin. Chem. Lab. Med. 2019, 57, 974–981. [Google Scholar] [CrossRef]
- Hauptman, N.; Glavač, D. Colorectal Cancer Blood-Based Biomarkers. Gastroenterol. Res. Pract. 2017, 2017, 2195361. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Carpelan-Holmström, M.; Louhimo, J.; Stenman, U.H.; Alfthan, H.; Järvinen, H.; Haglund, C. CEA, CA 242, CA 19-9, CA 72-4 and hCGbeta in the diagnosis of recurrent colorectal cancer. Tumour Biol. 2004, 25, 228–234. [Google Scholar] [CrossRef]
- Yanqing, H.; Cheng, D.; Ling, X. Serum CA72-4 as a Biomarker in the Diagnosis of Colorectal Cancer: A Meta-analysis. Open Med. 2018, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, H.; Shao, Y.; Han, X.; Zhang, Y. Significance of cea and CA242 in the diagnosis of colorectal carcinoma. Chin. J. Cancer Res. 1996, 8, 272–275. [Google Scholar] [CrossRef]
- Holten-Andersen, M.; Christensen, I.; Nielsen, H.; Stephens, R.; Jensen, V.; Nielsen, O.; Sørensen, S.; Overgaard, J.; Lilja, H.; Harris, A.; et al. Total levels of tissue inhibitor of metalloproteinases 1 in plasma yield high diagnostic sensitivity and specificity in patients with colon cancer. Clin. Cancer Res. 2002, 8, 156–164. [Google Scholar] [PubMed]
- Hadler-Olsen, E.; Winberg, J.O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Girondo, M.; Rodríguez-Berrocal, F.J.; Cubiella, J.; Castro, I.; Hernández, V.; Martínez-Zorzano, V.S. Serum matrix metalloproteinase-9 in colorectal cancer family-risk population screening. Sci. Rep. 2015, 5, 13030. [Google Scholar] [CrossRef]
- Peltier, J.; Roperch, J.P.; Audebert, S.; Borg, J.P.; Camoin, L. Quantitative proteomic analysis exploring progression of colorectal cancer: Modulation of the serpin family. J. Proteom. 2016, 148, 139–148. [Google Scholar] [CrossRef]
- Bünger, S.; Haug, U.; Kelly, M.; Posorski, N.; Klempt-Giessing, K.; Cartwright, A.; Fitzgerald, S.P.; Toner, V.; McAleer, D.; Gemoll, T.; et al. A novel multiplex-protein array for serum diagnostics of colon cancer: A case-control study. BMC Cancer 2012, 12, 393. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Zhang, H.; Szmitkowski, M.; Mäkinen, M.J.; Li, P.; Xia, D.; Yang, J.; Wu, Y.; Wu, H. Diagnostic and Prognostic Value of Serum Interleukin-6 in Colorectal Cancer. Medicine 2016, 95, e2502. [Google Scholar] [CrossRef]
- Dressen, K.; Hermann, N.; Manekeller, S.; Walgenbach-Bruenagel, G.; Schildberg, F.A.; Hettwer, K.; Uhlig, S.; Kalff, J.C.; Hartmann, G.; Holdenrieder, S. Diagnostic Performance of a Novel Multiplex Immunoassay in Colorectal Cancer. Anticancer Res. 2017, 37, 2477–2486. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yu, F.; Chen, J.; Zhao, S.; Zhang, D.; Yu, Y.; Liu, X.; Tang, H.; Peng, Z. Combined detection of preoperative serum CEA, CA19-9 and CA242 improve prognostic prediction of surgically treated colorectal cancer patients. Int. J. Clin. Exp. Pathol. 2015, 8, 14853. [Google Scholar] [PubMed]
- Alves Martins, B.A.; de Bulhões, G.F.; Cavalcanti, I.N.; Martins, M.M.; de Oliveira, P.G.; Martins, A.M.A. Biomarkers in Colorectal Cancer: The Role of Translational Proteomics Research. Front. Oncol. 2019, 9, 1284. [Google Scholar] [CrossRef]
- Yu, J.; Zhai, X.; Li, X.; Zhong, C.; Guo, C.; Yang, F.; Yuan, Y.; Zheng, S. Identification of MST1 as a potential early detection biomarker for colorectal cancer through a proteomic approach. Sci. Rep. 2017, 7, 14265. [Google Scholar] [CrossRef] [PubMed]
- Djaballah, S.A.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in C olorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 219–232. [Google Scholar] [CrossRef]
- Benli, Y.; Arıkan, H.; Akbulut-Çalışkan, Ö. HER2-targeted therapy in colorectal cancer: A comprehensive review. Clin. Transl. Oncol. 2025, 27, 3607–3624. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Lin, M.; Zhang, H.B. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9404. [Google Scholar]
- Luo, H.; Shen, K.; Li, B.; Li, R.; Wang, Z.; Xie, Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncol. Lett. 2020, 20, 742. [Google Scholar] [CrossRef]
- Babel, I.; Barderas, R.; Diaz-Uriarte, R.; Moreno, V.; Suarez, A.; Fernandez-Aceñero, M.J.; Salazar, R.; Capellá, G.; Casal, J.I. Identification of MST1/STK4 and SULF1 Proteins as Autoantibody Targets for the Diagnosis of Colorectal Cancer by Using Phage Microarrays. Mol. Cell. Proteom. 2011, 10, M110.001784. [Google Scholar] [CrossRef]
- Janer, A.; Warsinggih, W.; Uwuratuw, J.A.; Ladju, R.B.; Sampetoding, S.; Syarifuddin, E. Accuracy of Faecal Matrix-Metalloproteinase-9 (MMP-9) for Colorectal Cancer Detection in Makassar, Indonesia. Asian Pac. J. Cancer Prev. 2025, 26, 603. [Google Scholar] [CrossRef] [PubMed]
- Yunussova, N.; Sypabekova, M.; Zhumabekova, Z.; Matkarimov, B.; Kanayeva, D. A Novel ssDNA Aptamer Targeting Carcinoembryonic Antigen: Selection and Characterization. Biology 2022, 11, 1540. [Google Scholar] [CrossRef]
- Pan, Q.; Law, C.O.K.; Yung, M.M.H.; Han, K.C.; Pon, Y.L.; Lau, T.C.K. Novel RNA aptamers targeting gastrointestinal cancer biomarkers CEA, CA50 and CA72-4 with superior affinity and specificity. PLoS ONE 2018, 13, e0198980. [Google Scholar] [CrossRef]
- Gu, L.; Yan, W.; Liu, S.; Ren, W.; Lyu, M.; Wang, S. Trypsin enhances aptamer screening: A novel method for targeting proteins. Anal. Biochem. 2018, 561–562, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Manea, I.; Casian, M.; Hosu-Stancioiu, O.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Cristea, C. A review on magnetic beads-based SELEX technologies: Applications from small to large target molecules. Anal. Chim. Acta 2024, 1297, 342325. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Yao, L.; Li, H.; Zhang, L.; Wang, Y.; Li, J.; Chen, T.; Chai, K.; Gao, J.; et al. High-affinity ssDNA aptamer and chemiluminescent aptasensor for TIMP-1 detection in human serum. Anal. Sci. 2025, 41, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Kataoka, Y.; Fujita, H.; Kuwahara, M.; Horii, K.; Shiratori, I.; Waga, I. Modified DNA Aptamers for C-Reactive Protein and Lactate Dehydrogenase-5 with Sub-Nanomolar Affinities. Int. J. Mol. Sci. 2020, 21, 2683. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K.; Ganji, A.; Sankian, M. Designing a new dimerized anti human TNF-α aptamer with blocking activity. Biotechnol. Prog. 2020, 36, e2969. [Google Scholar] [CrossRef]
- Tsao, S.M.; Lai, J.C.; Horng, H.E.; Liu, T.C.; Hong, C.Y. Generation of aptamers from A primer-free randomized ssDNA library using magnetic-assisted rapid aptamer selection. Sci. Rep. 2017, 7, 45478. [Google Scholar] [CrossRef]
- Nagano, M.; Oguro, T.; Sawada, R.; Yoshitomi, T.; Yoshimoto, K. Accelerated Discovery of Potent Bioactive anti-TNFα Aptamers by Microbead-Assisted Capillary Electrophoresis (MACE)-SELEX. ChemBioChem 2021, 22, 3341–3347. [Google Scholar] [CrossRef]
- Huang, C.J.; Lin, H.I.; Shiesh, S.C.; Lee, G. Bin Integrated microfluidic system for rapid screening of CRP aptamers utilizing systematic evolution of ligands by exponential enrichment (SELEX). Biosens. Bioelectron. 2010, 25, 1761–1766. [Google Scholar] [CrossRef]
- Gupta, S.; Hirota, M.; Waugh, S.M.; Murakami, I.; Suzuki, T.; Muraguchi, M.; Shibamori, M.; Ishikawa, Y.; Jarvis, T.C.; Carter, J.D.; et al. Chemically Modified DNA Aptamers Bind Interleukin-6 with High Affinity and Inhibit Signaling by Blocking Its Interaction with Interleukin-6 Receptor. J. Biol. Chem. 2014, 289, 8706–8719. [Google Scholar] [CrossRef]
- Wang, Q.L.; Cui, H.F.; Du, J.F.; Lv, Q.Y.; Song, X. In silico post-SELEX screening and experimental characterizations for acquisition of high affinity DNA aptamers against carcinoembryonic antigen. RSC Adv. 2019, 9, 6328–6334. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, G.; Chen, J.; Wang, L.; Hu, Q.; Wu, J.; Zhang, W.; Song, M.; Qiao, J.; Xu, C. Electrochemical biosensors for measurement of colorectal cancer biomarkers. Anal. Bioanal. Chem. 2021, 413, 2407–2428. [Google Scholar] [CrossRef]
- Scarano, S.; Dausse, E.; Crispo, F.; Toulmé, J.J.; Minunni, M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal. Chim. Acta 2015, 897, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Shen, Y.; Guo, L.; Yin, H.; Fang, X.; Yang, Z.; Xu, Q.; Li, H. Superparamagnetic Nanostructures for Split-Type and Competitive-Mode Photoelectrochemical Aptasensing. Anal. Chem. 2020, 92, 8607–8613. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Wang, R.; Li, D.A.; Li, X.; Wang, Y.; Hua, Y.; Wang, R.; Li, D. A Fiber-Based SPR Aptasensor for the In Vitro Detection of Inflammation Biomarkers. Micromachines 2022, 13, 1036. [Google Scholar] [CrossRef]
- Li, M.J.; Wang, H.J.; Yuan, R.; Chai, Y.Q. A sensitive label-free photoelectrochemical aptasensor based on a novel PTB7-Th/H2O2 system with unexpected photoelectric performance for C-reactive protein analysis. Biosens. Bioelectron. 2021, 181, 113162. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, M.; Yang, Y.; Tang, Q.; Zeng, Y.; Wang, H.; Zhang, L.; Pan, C.; Hu, C.; Fu, Z.; et al. Detecting carcinoembryonic antigen based on the aggregation-induced emission enhancement effect. Chem. Commun. 2024, 60, 13570–13573. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Duan, W.; Deng, F.; Tang, W.; Payne, S.C.; Guo, T.; Goldys, E.M.; Lovell, N.H.; Shivdasani, M.N. Towards an Implantable Aptamer Biosensor for Monitoring in Inflammatory Bowel Disease. Biosensors 2025, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, W.L.; Lo, L.H.Y.; Kinghorn, A.B.; Shiu, S.C.C.; Tanner, J.A. Structure-Switching Electrochemical Aptasensor for Rapid, Reagentless, and Single-Step Nanomolar Detection of C-Reactive Protein. ACS Appl. Bio Mater. 2024, 7, 3721–3730. [Google Scholar] [CrossRef] [PubMed]
- Jarczewska, M.; Rębiś, J.; Górski, Ł.; Malinowska, E. Development of DNA aptamer-based sensor for electrochemical detection of C-reactive protein. Talanta 2018, 189, 45–54. [Google Scholar] [CrossRef]
- Beduk, D.; Beduk, T.; Ait Lahcen, A.; Mani, V.; Guler Celik, E.; Iskenderoglu, G.; Demirci, F.; Turhan, S.; Ozdogan, O.; Ozgur, S.; et al. Multiplexed aptasensor for detection of acute myocardial infraction (AMI) biomarkers. Sens. Diagn. 2024, 3, 1020–1027. [Google Scholar] [CrossRef]
- Zhai, J.; Ji, P.; Xin, Y.; Liu, Y.; Qu, Q.; Han, W.; Zhao, G. Development of Carcinoembryonic Antigen Rapid Detection System Based on Platinum Microelectrode. Front. Chem. 2022, 10, 899276. [Google Scholar] [CrossRef]
- Yunussova, N.; Tilegen, M.; Pham, T.T.; Kanayeva, D. Rapid detection of carcinoembryonic antigen by means of an electrochemical aptasensor. iScience 2024, 27, 109637. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; De-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Corrigan, D.K. Comparing nanobody and aptamer-based capacitive sensing for detection of interleukin-6 (IL-6) at physiologically relevant levels. Anal. Bioanal. Chem. 2023, 415, 7035–7045. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Li, Q.; Song, L.; Gopinath, S.C.B.; Wu, D. An interdigitated aptasensor to detect interleukin-6 for diagnosing rheumatoid arthritis in serum. Biotechnol. Appl. Biochem. 2021, 68, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Kumalasari, M.R.; Alfanaar, R.; Andreani, A.S. Gold nanoparticles (AuNPs): A versatile material for biosensor application. Talanta Open 2024, 9, 100327. [Google Scholar] [CrossRef]
- Irimes, M.B.; Pusta, A.; Coker, D.; Martian, P.C.; Suciu, M.; Pandrea, S.L.; Tertis, M.; Cristea, C.; Oprean, R. Dual-target electrochemical aptasensor for the simultaneous detection of interleukin-6 and tumor necrosis factor-α in biological fluids. Sens. Bio-Sens. Res. 2025, 49, 100866. [Google Scholar] [CrossRef]
- Hosseini Ghalehno, M.; Mirzaei, M.; Torkzadeh-Mahani, M. Electrochemical aptasensor for tumor necrosis factor α using aptamer–antibody sandwich structure and cobalt hexacyanoferrate for signal amplification. J. Iran. Chem. Soc. 2019, 16, 1783–1791. [Google Scholar] [CrossRef]
- Miao, P.; Yang, D.; Chen, X.; Guo, Z.; Tang, Y. Voltammetric determination of tumor necrosis factor-α based on the use of an aptamer and magnetic nanoparticles loaded with gold nanoparticles. Microchim. Acta 2017, 184, 3901–3907. [Google Scholar] [CrossRef]
- Diao, W.; Zhou, C.; Zhang, Z.; Cao, Y.; Li, Y.; Tang, J.; Liu, G. EGaIn-Modified ePADs for Simultaneous Detection of Homocysteine and C-Reactive Protein in Saliva toward Early Diagnosis of Cardiovascular Disease. ACS Sens. 2024, 9, 4265–4276. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Xuan, T.; Wang, J.; Wang, X. Label-free Electrochemical Impedance Spectroscopy Aptasensor for Ultrasensitive Detection of Lung Cancer Biomarker Carcinoembryonic Antigen. Front. Chem. 2021, 9, 721008. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, X.; Li, M.; Qi, H.; Gao, Q.; Zhang, C. Ultrasensitive Electrochemiluminescence Aptasensor for Assessment of Protein Heterogeneity in Small Cell Population. ACS Appl. Bio Mater. 2019, 2, 3052–3058. [Google Scholar] [CrossRef]
- Erkal-Aytemur, A.; Mülazımoğlu, İ.E.; Üstündağ, Z.; Caglayan, M.O. A novel aptasensor platform for the detection of carcinoembryonic antigen using quartz crystal microbalance. Talanta 2024, 277, 126376. [Google Scholar] [CrossRef]
- Lu, T.; Wang, L.; Xia, Y.; Jin, Y.; Zhang, L.; Du, S. A multimer-based SERS aptasensor for highly sensitive and homogeneous assay of carcinoembryonic antigens. Analyst 2021, 146, 3016–3024. [Google Scholar] [CrossRef]
- Villalonga, A.; Sánchez, A.; Vilela, D.; Mayol, B.; Martínez-Ruíz, P.; Villalonga, R. Electrochemical aptasensor based on anisotropically modified (Janus-type) gold nanoparticles for determination of C-reactive protein. Microchim. Acta 2022, 189, 309. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Datta, D.; Chaudhry, S.; Dutta, M.; Stroscio, M.A. Rapid Detection of Tumor Necrosis Factor-Alpha Using Quantum Dot-Based Optical Aptasensor. IEEE Trans. Nanobiosci. 2018, 17, 417–423. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Chen, P.; Wu, J.; Jin, Y.; Zhang, L.; Du, S. Salt-induced gold nanoparticles aggregation lights up fluorescence of DNA-silver nanoclusters to monitor dual cancer markers carcinoembryonic antigen and carbohydrate antigen 125. Anal. Chim. Acta 2020, 1125, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Meng, F.; Zhang, R.; Huang, L.; Qian, K. Integrating zirconia-gold hybrid into aptasensor capable of ultrasensitive and robust carcinoembryonic antigen determination. Electrochim. Acta 2024, 507, 145063. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Chang, A.; Cai, S.; Zhou, N. Amperometric sandwich-type aptasensor based on peroxidase mimetic Au@Pt for the carcinoembryonic antigen. Electroanalysis 2023, 35, e202200443. [Google Scholar] [CrossRef]
- Jin, J.; Guo, J.; Guo, J.; Li, D. Carbon-Based Biosensor in Point of Care Setting. Adv. Sens. Res. 2024, 3, 2400037. [Google Scholar] [CrossRef]
- Mahani, M.; Faghihi-Fard, M.; Divsar, F.; Torkzadeh-Mahani, M.; Khakbaz, F. Ultrasensitive FRET-based aptasensor for interleukin-6 as a biomarker for COVID-19 progression using nitrogen-doped carbon quantum dots and gold nanoparticles. Microchim. Acta 2022, 189, 472. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Zhang, C.; Meng, H.; Liu, B.; Wei, X. A rapid fluorescent aptasensor for point-of-care detection of C-reactive protein. Talanta 2022, 249, 123661. [Google Scholar] [CrossRef]
- Ansari, M.A.; Mohd-Naim, N.F.; Ahmed, M.U. Electrochemical Nanoaptasensor Based on Graphitic Carbon Nitride/Zirconium Dioxide/Multiwalled Carbon Nanotubes for Matrix Metalloproteinase-9 in Human Serum and Saliva. ACS Appl. Bio Mater. 2024, 7, 1579–1587. [Google Scholar] [CrossRef]
- Li, Y.; Hua, X.; Wang, J.; Jin, B. cMWCNT/CoHCF/AuNPs nanocomposites aptasensor for electrochemical detection of interleukin-6. Talanta Open 2023, 7, 100188. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Liu, S.; Sun, Y.; Dai, Y.; Luo, C.; Wang, X. A novel flower-shaped Ag@ZIF-67 chemiluminescence sensor for sensitive detection of CEA. Talanta 2023, 253, 123938. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Xu, J.; Zhao, X.; Song, S.; Zhang, H. Myocardial infarction biomarker C-reactive protein detection on nanocomposite aptasensor. Biotechnol. Appl. Biochem. 2022, 69, 166–171. [Google Scholar] [CrossRef]
- Hoseynidokht, F.; Mazloum-Ardakani, M.; Sahraei, N.; Hakimian, F.; Behnam Rad, M. Highly sensitive electrochemical detection of carcinoembryonic antigen by silver nanoparticles/carboxylated single-walled carbon nanotubes embedded in mesoporous carbon foam. J. Solid State Electrochem. 2024, 28, 377–387. [Google Scholar] [CrossRef]
- Mishyn, V.; Aslan, M.; Hugo, A.; Rodrigues, T.; Happy, H.; Sanyal, R.; Knoll, W.; Baudoux, F.; Bouchiat, V.; Bilyy, R.O.; et al. Catch and release strategy of matrix metalloprotease aptamers via thiol–disulfide exchange reaction on a graphene based electrochemical sensor. Sens. Diagn. 2022, 1, 739–749. [Google Scholar] [CrossRef]
- Shen, Z.; Ni, S.; Yang, W.; Sun, W.; Yang, G.; Liu, G. Redox probes tagged electrochemical aptasensing device for simultaneous detection of multiple cytokines in real time. Sens. Actuators B Chem. 2021, 336, 129747. [Google Scholar] [CrossRef]
- Ma, R.; Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y. Carbon Material Hybrid Construction on an Aptasensor for Monitoring Surgical Tumors. J. Anal. Methods Chem. 2022, 2022, 9740784. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.K.; Chao, C.H.; Yeh, Y.S. A Graphene-PEDOT:PSS Modified Paper-Based Aptasensor for Electrochemical Impedance Spectroscopy Detection of Tumor Marker. Sensors 2020, 20, 1372. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L. A Sensitive Electrochemical Aptasensor for TNF-α Based on Bimetallic Ag@Pt Core-Shell Nanoparticle Functionalized Graphene Nanostructures as Labels for Signal Amplification. J. Electrochem. Soc. 2016, 163, B119. [Google Scholar] [CrossRef]
- Mahyari, M.; Hooshmand, S.E.; Sepahvand, H.; Gholami, S.; Rezayan, A.H.; Zarei, M.A. Gold nanoparticles anchored onto covalent poly deep eutectic solvent functionalized graphene: An electrochemical aptasensor for the detection of C-reactive protein. Mater. Chem. Phys. 2021, 269, 124730. [Google Scholar] [CrossRef]
- Villalonga, A.; Vegas, B.; Paniagua, G.; Eguílaz, M.; Mayol, B.; Parrado, C.; Rivas, G.; Díez, P.; Villalonga, R. Amperometric aptasensor for carcinoembryonic antigen based on a reduced graphene oxide/gold nanoparticles modified electrode. J. Electroanal. Chem. 2020, 877, 114511. [Google Scholar] [CrossRef]
- Yan, M.; Fu, L.L.; Feng, H.C.; Namadchian, M. Application of Ag nanoparticles decorated on graphene nanosheets for electrochemical sensing of CEA as an important cancer biomarker. Environ. Res. 2023, 239, 117363. [Google Scholar] [CrossRef]
- Agrahari, S.; Kumar Singh, A.; Tiwari, I.; Srikrishna, S. Label free and ultrasensitive electrochemical detection of carcinoembryonic antigen using a disposable aptasensor based on Pd and graphene. Microchem. J. 2024, 205, 111359. [Google Scholar] [CrossRef]
- Xu, L.; Zou, L.; Guo, J.; Cao, Y.; Feng, C.; Ye, B. Simple “Signal-Off” Electrochemical Aptasensor Based on Aptamer-Cu3(PO4)2 Hybrid Nanoflowers/Graphene Oxide for Carcinoembryonic Antigen Detection. ChemElectroChem 2020, 7, 1660–1665. [Google Scholar] [CrossRef]
- Lin, F.; Li, X.; Zhang, J.; Zhang, H.; Zhang, Z.; Hou, L.; Shen, B.; Jin, L. Ratiometric electrochemical aptasensor based on functionalized graphene nanocomposites for detection of CA19-9. Anal. Sci. 2025, 41, 825–838. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Wang, W.; Liu, Y.; Yang, H.; Kong, J.; Si, F. Polymer-functionalized carbon nanotubes prepared via ring-opening polymerization for electrochemical detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2021, 328, 129031. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Acedo, P. Highly Sensitive RNA-Based Electrochemical Aptasensor for the Determination of C-Reactive Protein Using Carbon Nanofiber-Chitosan Modified Screen-Printed Electrode. Nanomaterials 2022, 12, 415. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H. Thionine functionalized hollow N-doped carbon nanoboxes: As a high-performance substrate for fabrication of label-free electrochemical aptasensor toward ultrasensitive detection of carcinoembryonic antigen. J. Electroanal. Chem. 2021, 903, 115858. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Taleat, Z. Synthesis and electrocatalytic effect of Ag@Pt core–shell nanoparticles supported on reduced graphene oxide for sensitive and simple label-free electrochemical aptasensor. Biosens. Bioelectron. 2015, 74, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Moharramnejad, M.; Karim, A.; Gharanli, S.; Malekshah, R.E.; Amini, A.H.; Sharifi, M.S.; Basmenj, Z.S.; Salariyeh, Z.; Mohammadkhani, M.; Shahi, M.; et al. A comprehensive review of MOFs based on electrochemical biosensors as smart platforms in cancer biomarkers detection. Microchem. J. 2025, 208, 112498. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, L.; Tian, J.; Han, Y.; Zhai, D.; Cui, L.; Zhang, P.; Zhang, X.; Yang, S.; Zhang, L. Aversatile MOF as an electrochemical/fluorescence/colorimetric signal probe for the tri-modal detection of MMP-9 secretion in the extracellular matrix to identify the efficacy of chemotherapeutic drugs. Anal. Chim. Acta 2024, 1315, 342798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, S.; Yan, Y.; Pang, W.; Zhong, F.; Huang, Q.; Caddeo, F.; Zhang, M.; Jin, M.; Shui, L. Multiplex signal amplification for ultrasensitive CRP assay via integrated electrochemical biosensor array using MOF-derived carbon material and aptamers. Talanta 2024, 272, 125735. [Google Scholar] [CrossRef]
- Ali, G.K.; Omer, K.M. Ultrasensitive aptamer-functionalized Cu-MOF fluorescent nanozyme as an optical biosensor for detection of C-reactive protein. Anal. Biochem. 2022, 658, 114928. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, K.; Li, S.; He, L.; Wang, M.; Zhou, N.; Du, M. Impedimetric aptasensor based on zirconium–cobalt metal–organic framework for detection of carcinoembryonic antigen. Microchim. Acta 2022, 189, 338. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Z.; Yan, Y.; Chen, J.; Yang, R.; Huang, Q.; Jin, M.; Shui, L. Triple signal-enhancing electrochemical aptasensor based on rhomboid dodecahedra carbonized-ZIF67 for ultrasensitive CRP detection. Biosens. Bioelectron. 2022, 207, 114129. [Google Scholar] [CrossRef]
- Chandio, I.; Cheung, S.; Ai, Y.; Liang, Q. High-entropy alloy nanoparticles integrated with metal-organic framework for enhanced aptasensing of carcinoembryonic antigen. Microchem. J. 2025, 209, 112760. [Google Scholar] [CrossRef]
- He, P.; Zhang, Q.; Liu, Q. Impedimetric aptasensor based on MOF based composite for measuring of carcinoembryonic antigen as a tumor biomarker. Chemosphere 2023, 338, 139339. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Ai, Y.; Liu, Y.; Sun, H.; Liang, Q. Self-Polymerized Dopamine-Decorated Au NPs and Coordinated with Fe-MOF as a Dual Binding Sites and Dual Signal-Amplifying Electrochemical Aptasensor for the Detection of CEA. ACS Appl. Mater. Interfaces 2020, 12, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.H.; Qiao, X.; Fan, J.; Hao, Y.Q.; Zhang, Y.T.; Zhou, Y.L.; Xu, M.T. A label-free ECL aptasensor for sensitive detection of carcinoembryonic antigen based on CdS QDs@MOF and TEOA@Au as bi-coreactants of Ru(bpy)32+. Microchem. J. 2022, 173, 106910. [Google Scholar] [CrossRef]
- Chen, S.; Yan, H.; Tang, Q.; Gu, Y.; Zhang, J.; Yang, Y.; Wang, H.; Qian, Z.; Li, L.; Guo, L.; et al. A novel FAM-based fluorescent aptasensor with covalent organic framework as quencher for sensitive determination of carcinoembryonic antigen. Microchem. J. 2025, 215, 114197. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, K.N.; Li, D.L.; Deng, Y.; Ma, Y.C.; Xu, Q.Q.; Hu, R.; Yang, Y.H. 2D-porphrinic covalent organic framework-based aptasensor with enhanced photoelectrochemical response for the detection of C-reactive protein. Biosens. Bioelectron. 2019, 129, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Q.; Xi, F. The Fabrication of a Probe-Integrated Electrochemiluminescence Aptasensor Based on Double-Layered Nanochannel Array with Opposite Charges for the Sensitive Determination of C-Reactive Protein. Molecules 2023, 28, 7867. [Google Scholar] [CrossRef]
- Ma, N.; Xu, S.; Wu, W.; Liu, J. Electrochemiluminescence Aptasensor with Dual Signal Amplification by Silica Nanochannel-Based Confinement Effect on Nanocatalyst and Efficient Emitter Enrichment for Highly Sensitive Detection of C-Reactive Protein. Molecules 2023, 28, 7664. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, X.; Liu, J. Boosted electrochemiluminescence of luminol/oxygen by nanochannel-confined nanocatalyst for sensitive aptasensing of tumor biomarker. Microchem. J. 2025, 216, 114671. [Google Scholar] [CrossRef]
- An, J.; Zhang, C.; Yan, F.; Ma, P. Nanochannel-confined platinum nanostructure for enhancement of luminol-dissolved oxygen electrochemiluminescence coupled with gated aptasensor for sensitive detection of carcinoembryonic antigen. Microchem. J. 2024, 206, 111413. [Google Scholar] [CrossRef]
- Shi, Z.; Li, K.; Wang, Y.; Li, Z.; Zhu, Z.; Wang, L. Development of a dual-channel electrochemical aptasensor for simultaneous detection of CRP and IL-6 in sepsis diagnosis. Electrochim. Acta 2025, 539, 147110. [Google Scholar] [CrossRef]
- Shi, Z.; Li, K.; Wang, Y.; Hu, Y.; Li, Z.; Zhu, Z. An innovative label-free electrochemical aptamer sensor: Utilizing Ti3C2Tx/MoS2/Au NPs for accurate interleukin-6 detection. Talanta 2024, 276, 126281. [Google Scholar] [CrossRef]
- Li, K.; Shi, Z.; Wang, Y.; Yan, F.; Li, Z.; Wang, Z.; Zhu, Z. A label-free electrochemical aptasensor based on Ti3C2Tx-Ag/Au nanoparticles as a signal amplification strategy for CRP detection. Microchem. J. 2023, 195, 109479. [Google Scholar] [CrossRef]
- Shi, S.S.; Li, X.J.; Ma, R.N.; Shang, L.; Zhang, W.; Zhao, H.Q.; Jia, L.P.; Wang, H.S. A novel dual-signal output strategy for POCT of CEA based on a smartphone electrochemical aptasensing platform. Microchim. Acta 2024, 191, 407. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Fan, B.; Chang, S.; Guo, D.; Wang, F.; Pan, Q. An ultrasensitive photoelectrochemical assay for tumor necrosis factor-alpha based on hollow CdS cubes as a signal generator and NiCo2O4-Au as a signal extinguisher. Analyst 2023, 148, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, X.; Li, Y.; Zeng, Y.; Gong, J.; Wang, Z.; An, Y.; Li, H. Biomineralized Mn3(PO4)2/aptamer nanosheets for enhanced electrochemical determination of C-reactive protein. Sens. Actuators B Chem. 2021, 333, 129510. [Google Scholar] [CrossRef]
- Oranzie, M.; January, J.L.; Sanga, N.A.; Leve, Z.D.; Mini, S.; Cupido, C.; Douman, S.F.; Iwuoha, E.I. Aptamer-Driven Biosensor Technology for the Quantitative Analysis of C-Reactive Protein. ChemElectroChem 2025, 12, e202400667. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; Luo, D.; Ren, F.; Ran, F.; Chen, W.; Zhang, B.; Wang, C.; Chen, H.; Wei, J. Ultrasensitive fluorescent aptasensor for CRP detection based on the RNase H assisted DNA recycling signal amplification strategy. RSC Adv. 2019, 9, 11960–11967. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Du, M.; Li, Q.; Wu, S.; Su, S.; Jian, N.; Wu, Y.; Wang, Y. A fluorescent platform integrated with a “one-pot” nicking endonuclease signal amplification and magnetic separation for simultaneous detection of tumor markers. Talanta 2025, 282, 127011. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Wang, Y.; Guo, C.; Guo, R.R.; Guo, Y.; Zhang, Z. Development of an electrochemiluminescence aptasensor combining covalent-triazine framework emitter with exonuclease III-driven DNA walker for sensitive CEA detection. Microchem. J. 2025, 215, 114388. [Google Scholar] [CrossRef]
- Wang, P.; Xie, Y.; Ma, H.; Liu, J.; Liu, C.; Feng, W.; Xi, S. A novel ratiometric electrochemical aptasensor for highly sensitive detection of carcinoembryonic antigen. Anal. Biochem. 2022, 659, 114957. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Lin, X.; Jiang, X.; Guo, F.; Liu, J.; Liu, X.; Huang, H.; Huang, Y. An electrochemical aptasensor for highly sensitive detection of CEA based on exonuclease III and hybrid chain reaction dual signal amplification. Bioelectrochemistry 2022, 143, 107986. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, J.; Ye, B.; Peng, G.; Zhang, C.; Zou, L. An ultrasensitive carcinoembryonic antigen electrochemical aptasensor based on 3D DNA nanoprobe and Exo III. Biosens. Bioelectron. 2022, 196, 113741. [Google Scholar] [CrossRef]
- Shang, L.; Shi, B.J.; Zhang, W.; Jia, L.P.; Ma, R.N.; Xue, Q.W.; Wang, H.S.; Yan, W. Electrochemical stripping chemiluminescence coupled with recycling amplification strategy for sensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2022, 368, 132191. [Google Scholar] [CrossRef]
- Ma, H.; Wang, P.; Xie, Y.; Liu, J.; Feng, W.; Li, S. Ratiometric electrochemical aptasensor for the sensitive detection of carcinoembryonic antigen based on a hairpin DNA probe and exonuclease I-assisted target recycling. Anal. Biochem. 2022, 649, 114694. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, H.; Zhu, Y.; Feng, W.; Su, M.; Li, S.; Mao, H. A new ratiometric electrochemical aptasensor for the sensitive detection of carcinoembryonic antigen based on exonuclease I-assisted target recycling and hybridization chain reaction amplification strategy. J. Electroanal. Chem. 2022, 910, 116167. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Liu, S.; Li, H.; Yang, M.; Fang, Y.; Xiao, Q. Triblock polyadenine-based electrochemical aptasensor for ultra-sensitive detection of carcinoembryonic antigen via exonuclease III-assisted target recycling and hybridization chain reaction. Bioelectrochemistry 2024, 159, 108749. [Google Scholar] [CrossRef]
- Díaz-Fernández, A.; Ferapontova, E.E. Covalent Hemin/G4 complex-linked sandwich bioassay on magnetic beads for femtomolar HER-2/neu detection in human serum via direct electrocatalytic reduction of oxygen. Anal. Chim. Acta 2022, 1219, 340049. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, W.; Che, S.; Zhang, X.; Yuan, X. Self-powered dual-photoelectrode photoelectrochemical aptasensor amplified by hemin/G-quadruplex-based DNAzyme. Microchim. Acta 2025, 192, 74. [Google Scholar] [CrossRef]
- Zhai, X.J.; Wang, Q.L.; Cui, H.F.; Song, X.; Lv, Q.Y.; Guo, Y. A DNAzyme-catalyzed label-free aptasensor based on multifunctional dendrimer-like DNA assembly for sensitive detection of carcinoembryonic antigen. Biosens. Bioelectron. 2021, 194, 113618. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Guo, Z.; Wang, X.; Zhou, N. Immobilization-free electrochemical homogeneous aptasensor for highly sensitive detection of carcinoembryonic antigen by dual amplification strategy. Anal. Chim. Acta 2023, 1274, 341586. [Google Scholar] [CrossRef]
- Gao, Z.; Shang, L.; Zhang, W.; Jia, L.P.; Ma, R.N.; Li, X.J.; Wang, H.S. Simplified ratiometric electrochemiluminescence aptasensor for carcinoembryonic antigen detection based on unmodified aptamer using single luminophore and coreactant. Microchim. Acta 2025, 192, 566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Weng, C.; Wang, J.; Yang, W.; Lu, Q.; Yan, X.; Sakran, M.A.; Hong, J.; Zhu, W.; Zhou, X. A label-free electrochemical magnetic aptasensor based on exonuclease III–assisted signal amplification for determination of carcinoembryonic antigen. Microchim. Acta 2020, 187, 492. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Zhao, L.; Wang, Y.; Chen, X.; Zhai, H.; Tian, M.; Zhao, R.; Wang, T.; Xu, H.; et al. A novel aptasensor based on HCR and G-quadruplex DNAzyme for fluorescence detection of Carcinoembryonic Antigen. Talanta 2021, 221, 121451. [Google Scholar] [CrossRef]
- Díaz-Fernández, A.; Lorenzo-Gómez, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Electrochemical aptasensors for cancer diagnosis in biological fluids—A review. Anal. Chim. Acta 2020, 1124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, J.; Liang, Y.; Tian, F.; Peng, L.; Huo, D.; Hou, C. Functionalized Carbon Nanotube-Decorated MXene Nanosheet-Enabled Microfluidic Electrochemical Aptasensor for Carcinoembryonic Antigen Determination. ACS Sustain. Chem. Eng. 2021, 9, 15386–15393. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Matharu, Z.; Rahimian, A.; Revzin, A. Detecting multiple cell-secreted cytokines from the same aptamer-functionalized electrode. Biosens. Bioelectron. 2015, 64, 43–50. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, M.; Jiang, F.; Lu, C.; Zhu, Q.; Yang, Y.; Fu, L.; Li, L.; Liu, J.; Wang, Z.; et al. Microfluidic-SERS sensing system based on dual signal amplification and aptamer for gastric cancer detection. Microchim. Acta 2024, 191, 668. [Google Scholar] [CrossRef]
- Malekmohamadi, M.; Mirzaei, S.; Rezayan, A.H.; Abbasi, V.; Abouei Mehrizi, A. µPAD-based colorimetric nanogold aptasensor for CRP and IL-6 detection as sepsis biomarkers. Microchem. J. 2024, 197, 109744. [Google Scholar] [CrossRef]
- Conejo-Cuevas, G.; Pellitero, M.A.; Ruiz-Rubio, L.; Javier, F.; Campo, D. Microneedles for Continuous, Minimally Invasive Monitoring: A Technology Overview. Adv. Sens. Res. 2025, 4, 2500057. [Google Scholar] [CrossRef]
- Yuan, R.; Cai, J.; Li, J.; Xu, Y.; Ma, J.; Wang, L.; Su, S. Integrated Microneedle Aptasensing Platform toward Point-of-Care Monitoring of Bacterial Infections and Treatment. ACS Sens. 2025, 10, 5684–5693. [Google Scholar] [CrossRef] [PubMed]
- Overview of IVD Regulation|FDA. Available online: https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation (accessed on 25 September 2025).
- 510(k) Third Party Review Program|FDA. Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/510k-third-party-review-program (accessed on 25 September 2025).
- Regulation—2017/746—EN—Medical Device Regulation—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2017/746/oj/eng (accessed on 25 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo-Castañón, M.J.; Díaz-Fernández, A. Aptamer-Based Strategies for Colorectal Cancer Detection: Emerging Technologies and Future Directions. Biosensors 2025, 15, 726. https://doi.org/10.3390/bios15110726
Lobo-Castañón MJ, Díaz-Fernández A. Aptamer-Based Strategies for Colorectal Cancer Detection: Emerging Technologies and Future Directions. Biosensors. 2025; 15(11):726. https://doi.org/10.3390/bios15110726
Chicago/Turabian StyleLobo-Castañón, María Jesús, and Ana Díaz-Fernández. 2025. "Aptamer-Based Strategies for Colorectal Cancer Detection: Emerging Technologies and Future Directions" Biosensors 15, no. 11: 726. https://doi.org/10.3390/bios15110726
APA StyleLobo-Castañón, M. J., & Díaz-Fernández, A. (2025). Aptamer-Based Strategies for Colorectal Cancer Detection: Emerging Technologies and Future Directions. Biosensors, 15(11), 726. https://doi.org/10.3390/bios15110726






