NanoArrayPAD−X: Nanoprobe Array and 3D-µPAD for the Simultaneous Detection of Respiratory Pathogens and Biomarkers at the Point of Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
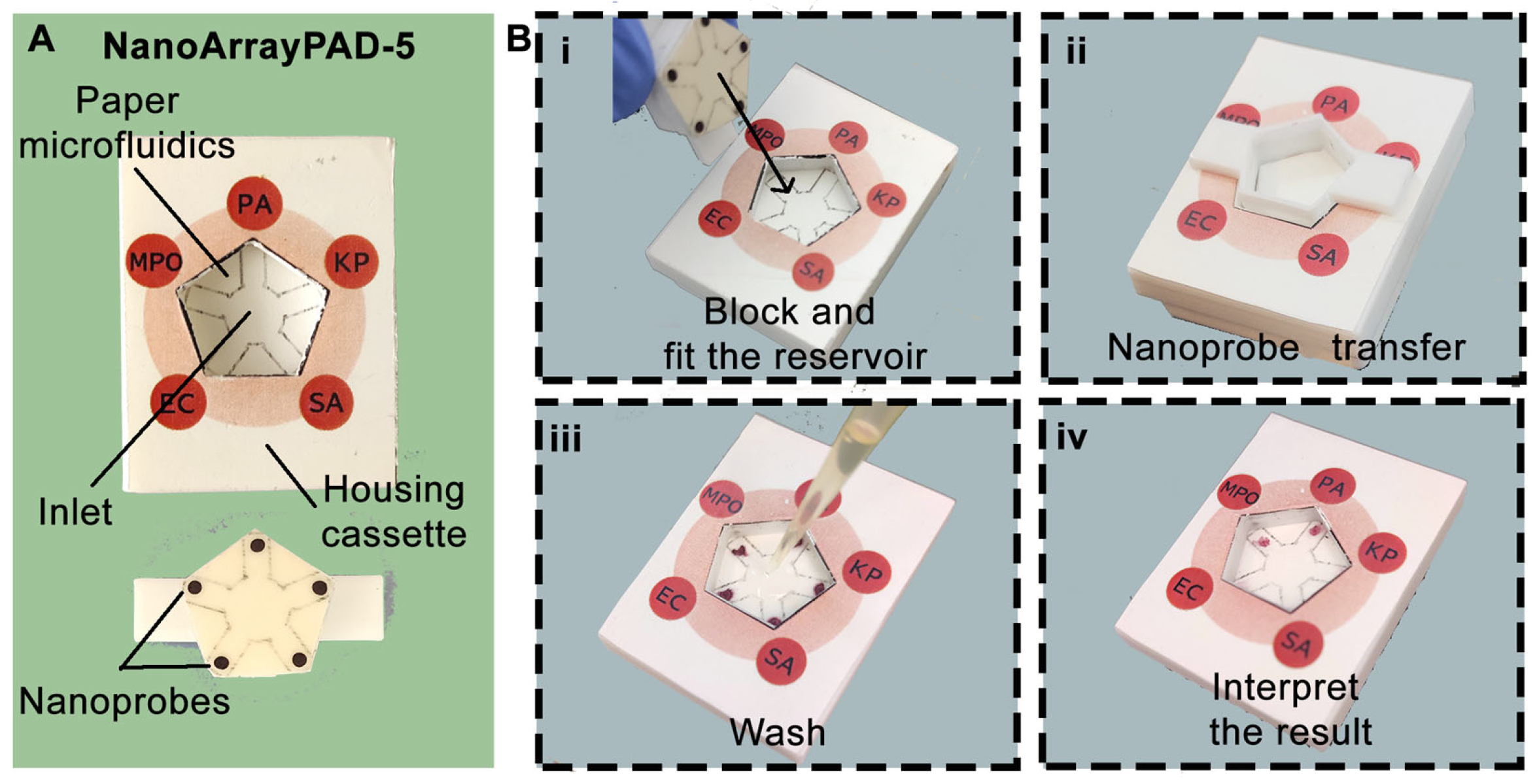
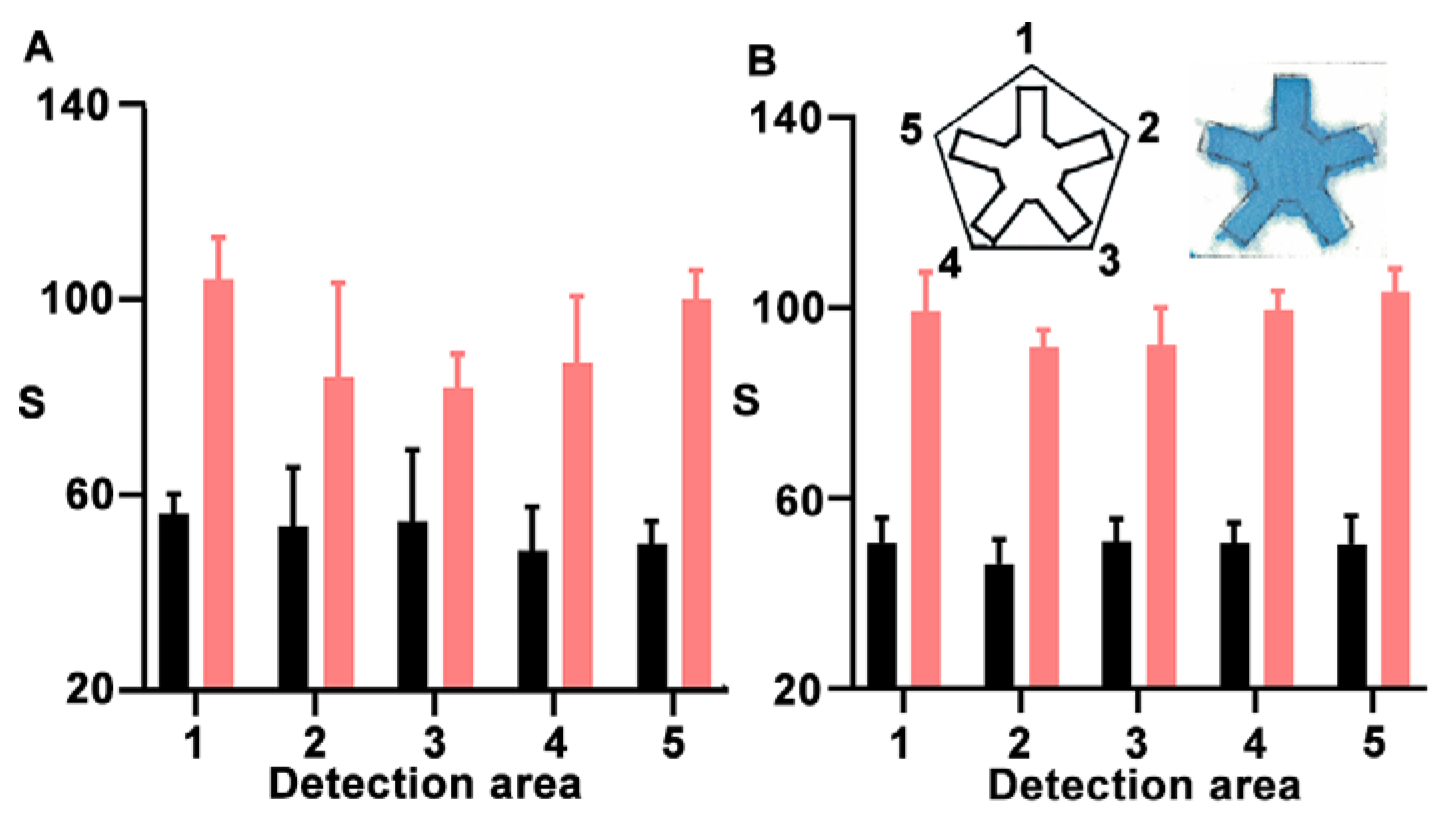
2.2.1. Manufacturing of NanoArrayPAD−X
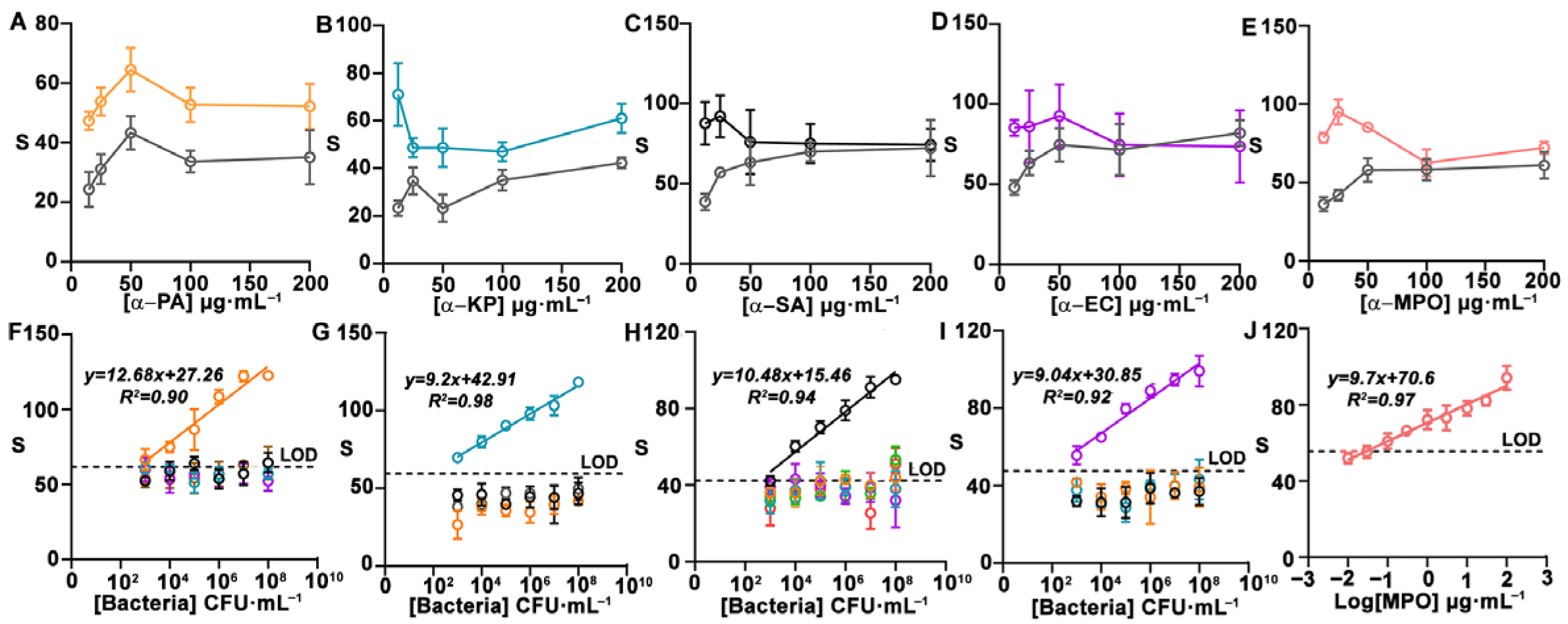
2.2.2. Detection of Bacteria and MPO
2.2.3. Quantification of Colorimetric Signals
2.2.4. Processing and Analysis of BAS Samples
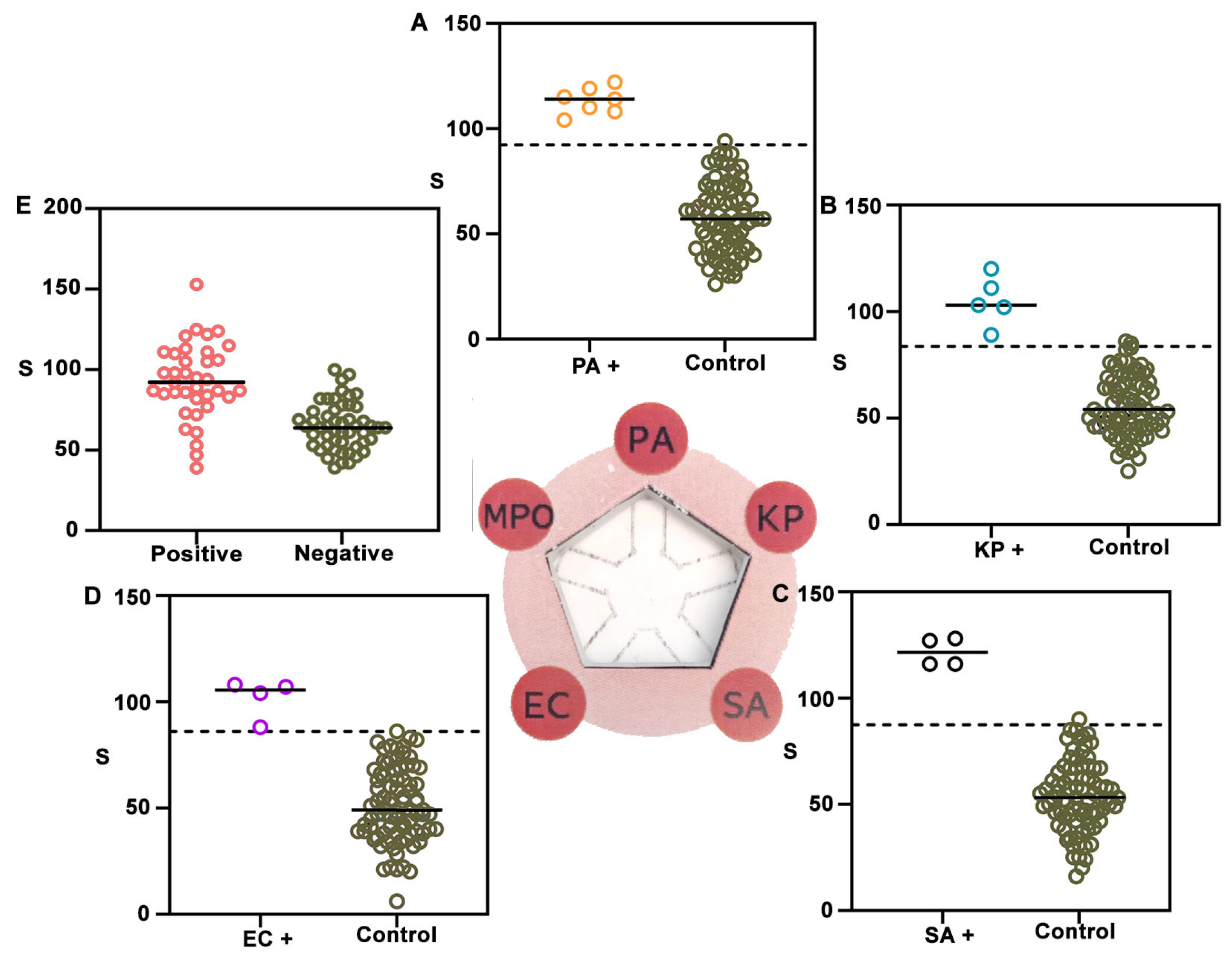
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VAP | Ventilator-associated pneumonia |
| ICU | Intensive Care Unit |
| 3D-µPAD | Three-dimensional microfluidic paper-based analytical device |
| µPAD | Microfluidic paper-based analytical device |
| PA | Pseudomonas aeruginosa |
| KP | Klebsiella pneumoniae |
| SA | Staphylococcus aureus |
| EC | Escherichia coli |
| MPO | Myeloperoxidase |
| BSA | Bovine serum albumin |
| PBS | Phosphate-buffered saline |
| PBST | Phosphate-buffered saline with 0.1% TWEEN 20 |
| AuNPs | Gold nanoprobes |
| PI | Pixel intensity |
| BAS | Bronchial aspirate sample |
| SD | Standard deviation |
References
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Howroyd, F.; Chacko, C.; MacDuff, A.; Gautam, N.; Pouchet, B.; Tunnicliffe, B.; Weblin, J.; Gao-Smith, F.; Ahmed, Z.; Duggal, N.A.; et al. Ventilator-Associated Pneumonia: Pathobiological Heterogeneity and Diagnostic Challenges. Nat. Commun. 2024, 15, 6447. [Google Scholar] [CrossRef]
- Metersky, M.L.; Kalil, A.C. Management of Ventilator-Associated Pneumonia. Infect. Dis. Clin. N. Am. 2024, 38, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Ljungcrantz, E.G.; Askman, S.; Sjövall, F.; Paulsson, M. Biomarkers in Lower Respiratory Tract Samples in the Diagnosis of Ventilator-Associated Pneumonia: A Systematic Review. Eur. Resp. Rev. 2025, 34, 240229. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Forveille, S.; Lascarrou, J.-B.; Seguin, A.; Canet, E.; Lemarié, J.; Agbakou, M.; Desmedt, L.; Blonz, G.; Zambon, O.; et al. Immediate vs. Culture-Initiated Antibiotic Therapy in Suspected Non-Severe Ventilator-Associated Pneumonia: A before–after Study (DELAVAP). Ann. Intensive Care 2024, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Cojuc-Konigsberg, G.; Moscona-Nissan, A.; Guijosa, A.; Mireles Dávalos, C.D.; Martínez, M.E.J.; Mújica Sánchez, M.A.; Hernández Huizar, V.F.; Durán Barrón, M.A.; Gómez, K.V.; Andrade-Galindo, R.; et al. Diagnostic Accuracy of the BioFire® FilmArray® Pneumonia Panel in COVID-19 Patients with Ventilator-Associated Pneumonia. BMC Infect. Dis. 2023, 23, 524. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Hinic, V.; Goldenberger, D.; Gensch, A.; Schweitzer, M.; Bättig, V.; Siegemund, M.; Bassetti, S.; Bingisser, R.; Tamm, M.; et al. Evaluation of the Clinical Relevance of the Biofire© FilmArray Pneumonia Panel among Hospitalized Patients. Infection 2024, 52, 173–181. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Shao, S.; Ci, Q.; Chen, L.; Lu, X.; Liu, Q.; Chen, J. CRISPR/Cas14 and G-Quadruplex DNAzyme-Driven Biosensor for Paper-Based Colorimetric Detection of African Swine Fever Virus. ACS Sens. 2024, 9, 2413–2420. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Liu, G. Signal Amplification Strategies for Paper-Based Analytical Devices. Biosens. Bioelectron. 2019, 136, 60–75. [Google Scholar] [CrossRef]
- Boehle, K.E.; Carrell, C.S.; Caraway, J.; Henry, C.S. Paper-Based Enzyme Competition Assay for Detecting Falsified β-Lactam Antibiotics. ACS Sens. 2018, 3, 1299–1307. [Google Scholar] [CrossRef]
- Jeon, J.; Choi, H.; Han, G.-R.; Ghosh, R.; Palanisamy, B.; Di Carlo, D.; Ozcan, A.; Park, S. Paper-Based Vertical Flow Assays for in Vitro Diagnostics and Environmental Monitoring. ACS Sens. 2025, 10, 3317–3339. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zheng, Z.; Feng, Y.; Feng, S.; Zhang, Y.; Miao, Y.; Liu, C. Thymine-Capped Mesoporous Silica Nanoparticles as Ion-Responsive Release System: A Paper-Based Colorimetric Sensing Platform for Rapid and Selective Mercuric Identification. Biosens. Bioelectron. 2025, 272, 117101. [Google Scholar] [CrossRef]
- Suvanasuthi, R.; Chimnaronk, S.; Promptmas, C. 3D Printed Hydrophobic Barriers in a Paper-Based Biosensor for Point-of-Care Detection of Dengue Virus Serotypes. Talanta 2022, 237, 122962. [Google Scholar] [CrossRef]
- Jackson, S.; Lee, S.; Badu-Tawiah, A.K. Automated Immunoassay Performed on a 3D Microfluidic Paper-Based Device for Malaria Detection by Ambient Mass Spectrometry. Anal. Chem. 2022, 94, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, E.; Calvert, V.; Hodge, A.; VanMeter, A.; Petricoin, E.F.; Pierobon, M. Reverse Phase Protein Microarrays. In Molecular Profiling: Methods and Protocols; Espina, V., Ed.; Springer: New York, NY, USA, 2017; pp. 149–169. ISBN 978-1-4939-6990-6. [Google Scholar]
- Cao, X.; Han, Y.; Wang, X.; Zhang, L. High-Throughput Capture and Sensitive In Situ Visualization of Protein N-Phosphorylation on Membranes Based on DpaZn-Nanopolymers Functionalized Reverse Phase N-Phosphoprotein Array. Anal. Chem. 2025, 97, 14905–14911. [Google Scholar] [CrossRef]
- Vaquer, A.; Adrover-Jaume, C.; Clemente, A.; Iglesias, A.; López, M.; Martínez, R.; Roig, I.M.; Cosío, B.G.; de la Rica, R. Immunosensors Made of Polymer-Infused Porous Paper for the Non-Invasive Detection of Airways Cytokines Trapped by Porous Face Masks. Sens. Actuators B Chem. 2023, 379, 133233. [Google Scholar] [CrossRef]
- Alba-Patiño, A.; Adrover-Jaume, C.; de la Rica, R. Nanoparticle Reservoirs for Paper-Only Immunosensors. ACS Sens. 2020, 5, 147–153. [Google Scholar] [CrossRef]
- Clemente, A.; Alba-Patiño, A.; Rojo-Molinero, E.; Russell, S.M.; Borges, M.; Oliver, A.; de la Rica, R. Rapid Detection of Pseudomonas Aeruginosa Biofilms via Enzymatic Liquefaction of Respiratory Samples. ACS Sens. 2020, 5, 3956–3963. [Google Scholar] [CrossRef]
- Clemente, A.; Alba-Patiño, A.; Santopolo, G.; Barón, E.; Rojo-Molinero, E.; Oliver, A.; Pérez-Bárcena, J.; Cos, P.M.d.; Aranda, M.; del Castillo, A.; et al. Optimized Detection of Lung IL-6 via Enzymatic Liquefaction of Low Respiratory Tract Samples: Application for Managing Ventilated Patients. Analyst 2021, 146, 6537–6546. [Google Scholar] [CrossRef] [PubMed]
- Ioanas, M.; Ferrer, R.; Angrill, J.; Ferrer, M.; Torres, A. Microbial Investigation in Ventilator-Associated Pneumonia. Eur. Resp. J. 2001, 17, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.A.; Gonzalez, J.L.; Vazquez-Alvarado, M.; Martinez, N.W.; Martinez, A.W. Beyond Wax Printing: Fabrication of Paper-Based Microfluidic Devices Using a Thermal Transfer Printer. Anal. Chem. 2022, 94, 8833–8837. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Torres, A. Multidrug Resistant Gram-Negative Bacteria in Community-Acquired Pneumonia. Crit. Care 2019, 23, 79. [Google Scholar] [CrossRef]
- Torres, A.; Cillóniz, C.; Blasi, F.; Chalmers, J.D.; Gaillat, J.; Dartois, N.; Schmitt, H.-J.; Welte, T. Burden of Pneumococcal Community-Acquired Pneumonia in Adults across Europe: A Literature Review. Respir. Med. 2018, 137, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef]
- Maurer, F.P.; Christner, M.; Hentschke, M.; Rohde, H. Advances in Rapid Identification and Susceptibility Testing of Bacteria in the Clinical Microbiology Laboratory: Implications for Patient Care and Antimicrobial Stewardship Programs. Infect. Dis. Rep. 2017, 9, 6839. [Google Scholar] [CrossRef]
- Maxson, T.; Mitchell, D.A. Targeted Treatment for Bacterial Infections: Prospects for Pathogen-Specific Antibiotics Coupled with Rapid Diagnostics. Tetrahedron 2016, 72, 3609–3624. [Google Scholar] [CrossRef]
- Vaquer, A.; Adrover-Jaume, C.; Clemente, A.; Viana, J.; Rodríguez, R.; Rojo-Molinero, E.; Oliver, A.; de la Rica, R. OriPlex: Origami-Enabled Multiplexed Detection of Respiratory Pathogens. Biosens. Bioelectron. 2024, 257, 116341. [Google Scholar] [CrossRef]
- Adrover-Jaume, C.; Clemente, A.; Viana-Ramírez, J.; Rojo-Molinero, E.; Oliver, A.; de la Rica, R. A Multirange Paper-Based Analytical Device for Identifying Low, Moderate, and High P. Aeruginosa Bacterial Loads in Sputum Samples. Biosens. Bioelectron. 2025, 272, 117097. [Google Scholar] [CrossRef] [PubMed]
- Adrover-Jaume, C.; Rojo-Molinero, E.; Clemente, A.; Russell, S.M.; Arranz, J.; Oliver, A.; Rica, R. de la Mobile Origami Immunosensors for the Rapid Detection of Urinary Tract Infections. Analyst 2021, 145, 7916–7921. [Google Scholar] [CrossRef]
- Hickey, M.J. MPO and Neutrophils: A Magnetic Attraction. Blood 2011, 117, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.-J.; Schröder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase Attracts Neutrophils by Physical Forces. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.C.; Santos, A.M.; Nguyen, E.K.; Gelston, A.A.; Majumdar, S.; Weiss, G.A. Phage vs. Phage: Direct Selections of Sandwich Binding Pairs. Viruses 2023, 15, 807. [Google Scholar] [CrossRef]
- Kaewarsa, P.; Schenkel, M.S.; Rahn, K.L.; Laiwattanapaisal, W.; Henry, C.S. Improving Design Features and Air Bubble Manipulation Techniques for a Single-Step Sandwich Electrochemical ELISA Incorporating Commercial Electrodes into Capillary-Flow Driven Immunoassay Devices. Analyst 2024, 149, 2034–2044. [Google Scholar] [CrossRef]
- Nandhakumar, P.; Muñoz San Martín, C.; Arévalo, B.; Ding, S.; Lunker, M.; Vargas, E.; Djassemi, O.; Campuzano, S.; Wang, J. Redox Cycling Amplified Electrochemical Lateral-Flow Immunoassay: Toward Decentralized Sensitive Insulin Detection. ACS Sens. 2023, 8, 3892–3901. [Google Scholar] [CrossRef]
- Horati, H.; Margaroli, C.; Chandler, J.D.; Kilgore, M.B.; Manai, B.; Andrinopoulou, E.-R.; Peng, L.; Guglani, L.; Tiddens, H.A.M.W.; Caudri, D.; et al. Key Inflammatory Markers in Bronchoalveolar Lavage Predict Bronchiectasis Progression in Young Children with CF. J. Cyst. Fibros. 2024, 23, 450–456. [Google Scholar] [CrossRef]
- Zhu, T.; Li, S.; Wang, J.; Liu, C.; Gao, L.; Zeng, Y.; Mao, R.; Cui, B.; Ji, H.; Chen, Z. Induced Sputum Metabolomic Profiles and Oxidative Stress Are Associated with Chronic Obstructive Pulmonary Disease (COPD) Severity: Potential Use for Predictive, Preventive, and Personalized Medicine. EPMA J. 2020, 11, 645–659. [Google Scholar] [CrossRef]
- Jiang, E.; Fu, Y.; Wang, Y.; Ying, L.; Li, W. The Role and Clinical Significance of Myeloperoxidase (MPO) and TNF-α in Prognostic Evaluation of T-COPD. BMC Pulm. Med. 2025, 25, 192. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.A.-E.; Kinawy, S.A.-E.; Mahmoud, E.M. The Role of Serum Myeloperoxidase in Prediction of Severe Bronchial Asthma. Int. J. Med. Arts 2023, 5, 3373–3378. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaquer, A.; Bouzada, F.M.; Tejada, S.; Clemente, A.; Socias, A.; Aranda, M.; del Castillo, A.; Mena, J.; Montaner, M.; Rodríguez, R.; et al. NanoArrayPAD−X: Nanoprobe Array and 3D-µPAD for the Simultaneous Detection of Respiratory Pathogens and Biomarkers at the Point of Care. Biosensors 2025, 15, 715. https://doi.org/10.3390/bios15110715
Vaquer A, Bouzada FM, Tejada S, Clemente A, Socias A, Aranda M, del Castillo A, Mena J, Montaner M, Rodríguez R, et al. NanoArrayPAD−X: Nanoprobe Array and 3D-µPAD for the Simultaneous Detection of Respiratory Pathogens and Biomarkers at the Point of Care. Biosensors. 2025; 15(11):715. https://doi.org/10.3390/bios15110715
Chicago/Turabian StyleVaquer, Andreu, Francisco M. Bouzada, Sofia Tejada, Antonio Clemente, Antonia Socias, Maria Aranda, Alberto del Castillo, Joana Mena, Maria Montaner, Rocío Rodríguez, and et al. 2025. "NanoArrayPAD−X: Nanoprobe Array and 3D-µPAD for the Simultaneous Detection of Respiratory Pathogens and Biomarkers at the Point of Care" Biosensors 15, no. 11: 715. https://doi.org/10.3390/bios15110715
APA StyleVaquer, A., Bouzada, F. M., Tejada, S., Clemente, A., Socias, A., Aranda, M., del Castillo, A., Mena, J., Montaner, M., Rodríguez, R., Rojo-Molinero, E., Oliver, A., Borges, M., & de la Rica, R. (2025). NanoArrayPAD−X: Nanoprobe Array and 3D-µPAD for the Simultaneous Detection of Respiratory Pathogens and Biomarkers at the Point of Care. Biosensors, 15(11), 715. https://doi.org/10.3390/bios15110715




