Optimized Aptamer-Conjugated Gold Nanoparticles for Specific Detection of GII.4 Human Norovirus in Feces
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
2.2. Preparation of Norovirus VLP
2.3. Enzyme-Linked Aptamer Sorbent Assay (ELASA)
2.4. Optimization of Aptamer Concentration of the Colorimetric Assay
2.5. General Procedure for Detecting GII.4 HuNoV VLPs
2.6. Specificity and Broad Spectrum of the Colorimetric Assay
2.7. Detection of GII.4 HuNoV in Real Sample
2.8. Recovery of GII.4 HuNoV VLPs in Fecal Sample
2.9. Aptamer Structure Modification and Simulated Docking with GII.4 HuNoV VP1
3. Results and Discussion
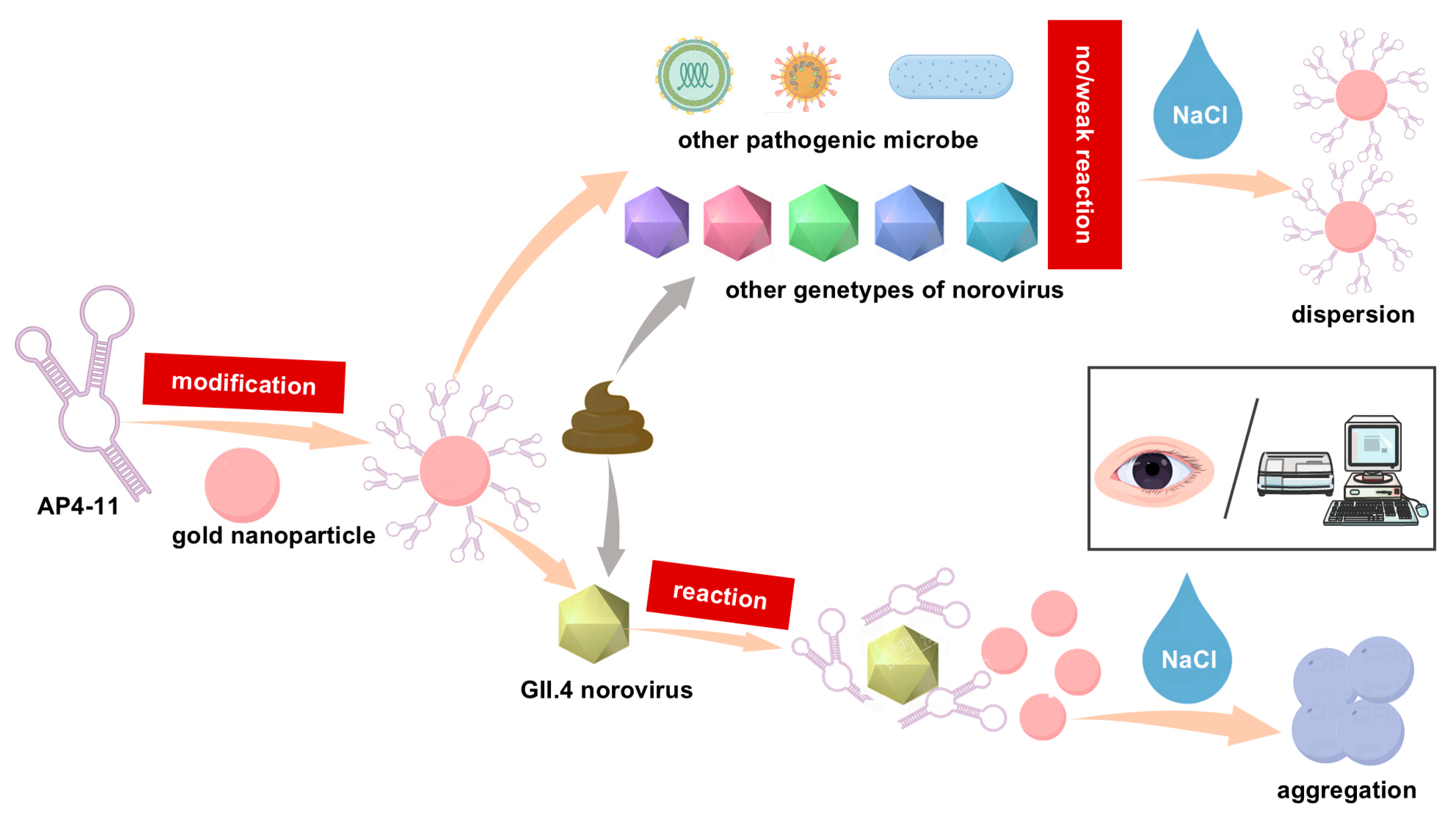
3.1. Principle of Colorimetric Detection
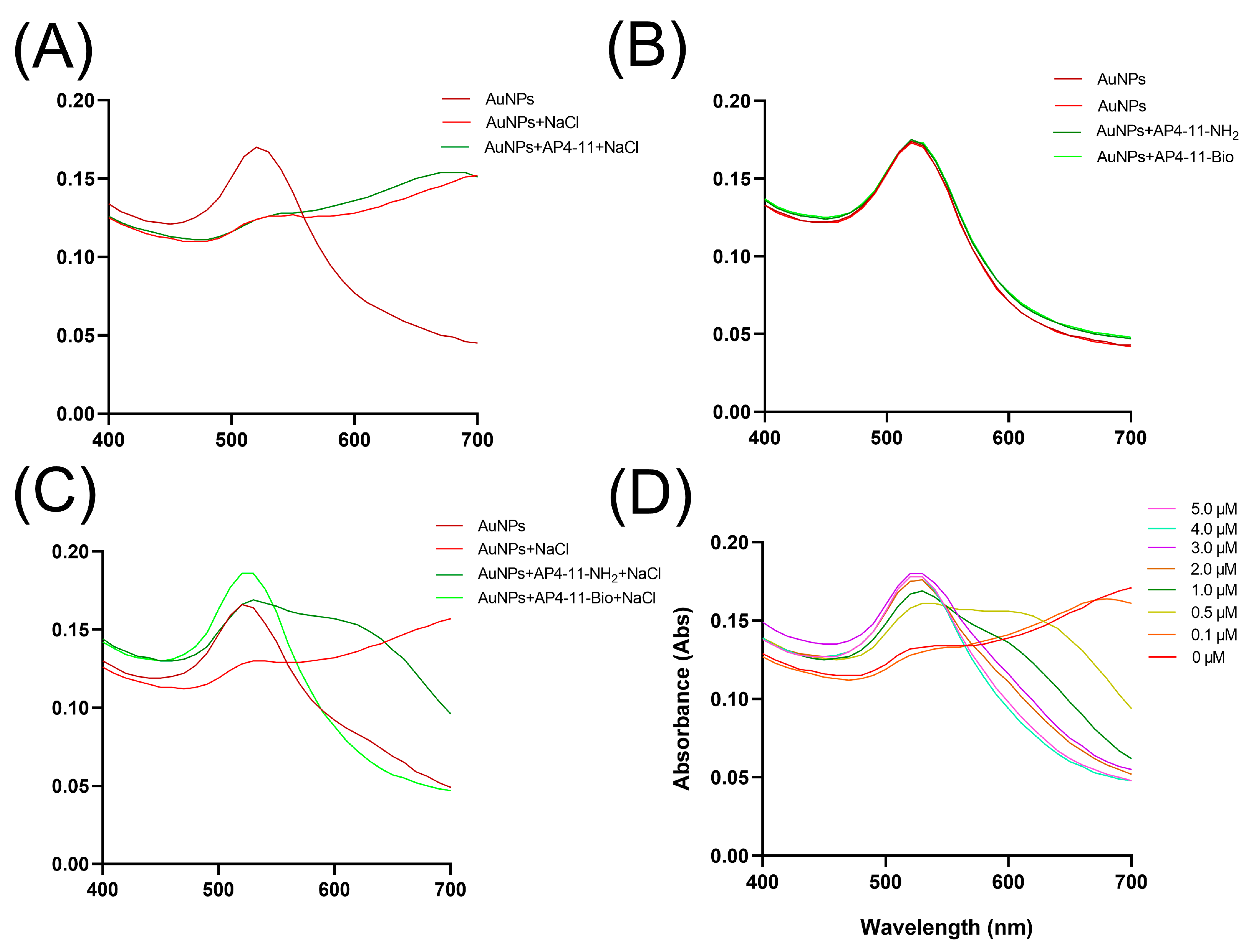
3.2. Aptamer Modification and Characterization
3.3. Characterization of AuNPs
3.4. Optimization of NaCl and Aptamer Concentrations
3.5. Detection of GII.4 HuNoV VLPs with Aptasensor
3.6. Kinetic Analysis of the Colorimetric Assay
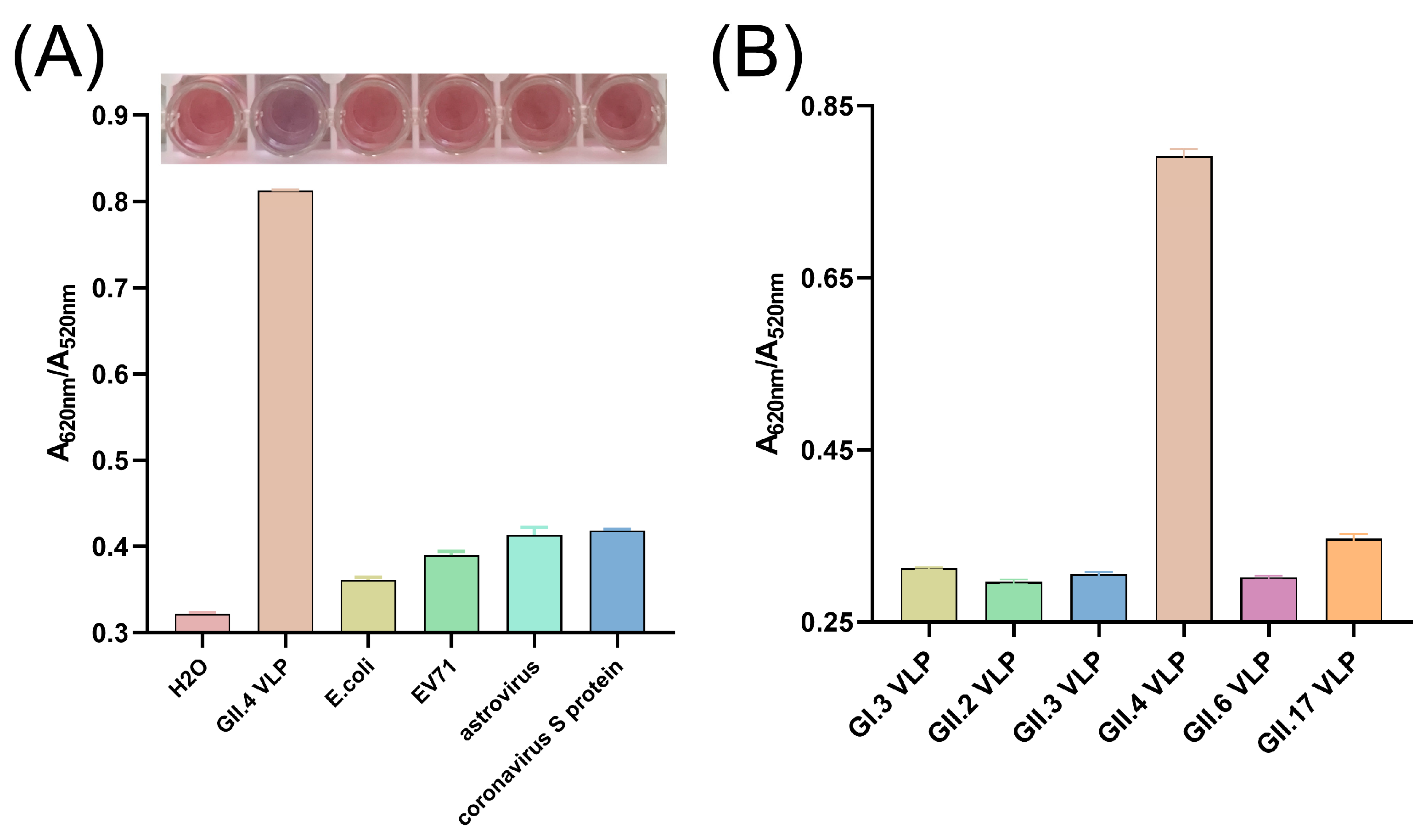
3.7. Specificity and Broad-Spectrum of the Colorimetric Assay
3.8. Detection of HuNoV in Clinical Fecal Samples
3.9. Recovery and Stability of the Colorimetric Assay
3.10. Simulated Docking and Validation of AP4-11 with HuNoV VP1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HuNoV | Human Norovirus |
| AuNPs | Gold Nanoparticles |
| VLP | Virus-Like Particle |
| PT-qPCR | Real-time Reverse Transcription Quantitative Polymerase Chain Reaction |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| WB | Western blotting |
| TEM | Transmission Electron Microscopy |
| ELASA | Enzyme-Linked Aptamer Sorbent Assay |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| RSD | Relative Standard Deviation |
| CD | Circular Dichroism |
References
- Kapikian, A.Z.; Wyatt, R.G.; Dolin, R.; Thornhill, T.S.; Kalica, A.R.; Chanock, R.M. Visualization by Immune Electron-Microscopy of a 27-Nm Particle Associated with Acute Infectious Nonbacterial Gastroenteritis. J. Virol. 1972, 10, 1075–1081. [Google Scholar] [CrossRef]
- Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Estes, M.K.; Crawford, S.E.; Neill, F.H.; Ramani, S.; Hill, H.; Ferreira, J.; Graham, D.Y. Determination of the 50% human infectious dose for Norwalk virus. J. Infect. Dis. 2014, 209, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.; Cannon, J.L. Environmental persistence and transfer of enteric viruses. Curr. Opin. Virol. 2014, 4, 37–43. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Moe, C.L.; Liu, P.; Miller, S.E.; Lindesmith, L.; Baric, R.S.; Le Pendu, J.; Calderon, R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008, 80, 1468–1476. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinjé, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII. 4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef]
- Costantini, V.; Grenz, L.; Fritzinger, A.; Lewis, D.; Biggs, C.; Hale, A.; Vinjé, J. Diagnostic Accuracy and Analytical Sensitivity of IDEIA Norovirus Assay for Routine Screening of Human Norovirus. J. Clin. Microbiol. 2010, 48, 2770–2778. [Google Scholar] [CrossRef]
- Tan, X.H.; Dey, S.K.; Telmer, C.; Zhang, X.L.; Armitage, B.A.; Bruchez, M.P. Aptamers Act as Activators for the Thrombin Mediated-Hydrolysis of Peptide Substrates. ChemBioChem 2014, 15, 205–208. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment—Rna Ligands to Bacteriophage-T4 DNA-Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Bunka, D.H.J.; Platonova, O.; Stockley, P.G. Development of aptamer therapeutics. Curr. Opin. Pharmacol. 2010, 10, 557–562. [Google Scholar] [CrossRef]
- Luan, Y.X.; Chen, J.Y.; Xie, G.; Li, C.; Ping, H.; Ma, Z.H.; Lu, A.X. Visual and microplate detection of aflatoxin B2 based on NaCl-induced aggregation of aptamer-modified gold nanoparticles. Microchim. Acta 2015, 182, 995–1001. [Google Scholar] [CrossRef]
- Liu, Z.C.; Zhang, Y.F.; Xie, Y.; Sun, Y.; Bi, K.W.; Cui, Z.; Zhao, L.J.; Fan, W.F. An Aptamer-based Colorimetric Sensor for Streptomycin and Its Application in Food Inspection. Chem. Res. Chin. Univ. 2017, 33, 714–720. [Google Scholar] [CrossRef]
- Goux, E.; Dausse, E.; Guieu, V.; Azéma, L.; Durand, G.; Henry, M.; Choisnard, L.; Toulmé, J.J.; Ravelet, C.; Peyrin, E. A colorimetric nanosensor based on a selective target-responsive aptamer kissing complex. Nanoscale 2017, 9, 4048–4052. [Google Scholar] [CrossRef]
- Hu, X.R.; Chang, K.K.; Wang, S.; Sun, X.Q.; Hu, J.D.; Jiang, M. Aptamer-functionalized AuNPs for the high- sensitivity colorimetric detection of melamine in milk samples. PLoS ONE 2018, 13, e0201626. [Google Scholar] [CrossRef]
- Gao, H.; Tian, Y.L.; Zhang, M.; Liu, J.H.; Yuan, Y.W.; Tan, J.X.; Ma, A.J. Selection, Identification, and Application of Aptamers against Lectin to Establish an Aptamer-AuNPs Colorimetric Method for Detection of ABL. J. Food Qual. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Rao, X.Y.; Zhang, J.J.; Cui, J.; Hu, Y.; Liu, T.; Chai, J.F.; Cheng, G.F.; He, P.G.; Fang, Y.Z. Au nanoparticle-DNAzyme dual catalyst system for sensitively colorimetric detection of thrombin. Chem. Res. Chin. Univ. 2013, 29, 868–873. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Fried, M.G.; Crothers, D.M. Kinetics and Mechanism in the Reaction of Gene Regulatory Proteins with DNA. J. Mol. Biol. 1984, 172, 263–282. [Google Scholar] [CrossRef]
- Kozlov, A.G.; Lohman, T.M. Calorimetric studies of E. coli SSB protein-single-stranded DNA interactions. Effects of monovalent salts on binding enthalpy. J. Mol. Biol. 1998, 278, 999–1014. [Google Scholar] [CrossRef]
- Samanta, A.; Medintz, I.L. Nanoparticles and DNA—A powerful and growing functional combination in bionanotechnology. Nanoscale 2016, 8, 9037–9095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.Y.; Li, H.T.; Wen, Y.Q.; Fan, X.Y.; Lin, F.B.; Tan, L.A.; Yao, S.Z. Ultrasensitive electrochemical aptasensor for thrombin based on the amplification of aptamer-AuNPs-HRP conjugates. Biosens. Bioelectron. 2011, 26, 2297–2303. [Google Scholar] [CrossRef]
- Papavlassopoulos, H.; Mishra, Y.K.; Kaps, S.; Paulowicz, I.; Abdelaziz, R.; Elbahri, M.; Maser, E.; Adelung, R.; Röhl, C. Toxicity of Functional Nano-Micro Zinc Oxide Tetrapods: Impact of Cell Culture Conditions, Cellular Age and Material Properties. PLoS ONE 2014, 9, e84983. [Google Scholar] [CrossRef]
- Lee, J.S.; Ulmann, P.A.; Han, M.S.; Mirkin, C.A. A DNA-gold nanoparticle-based colorimetric competition assay for the detection of cysteine. Nano Lett. 2008, 8, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.; Jo, E.J.; Li, T.H.; Joung, H.A.; Hong, D.G.; Shim, W.B.; Jung, C.; Kim, M.G. Homogeneous assay of target molecules based on chemiluminescence resonance energy transfer (CRET) using DNAzyme-linked aptamers. Biosens. Bioelectron. 2014, 58, 308–313. [Google Scholar] [CrossRef]
- Pamies, R.; Cifre, J.G.H.; Espín, V.F.; Collado-González, M.; Baños, F.G.D.; de la Torre, J.G. Aggregation behaviour of gold nanoparticles in saline aqueous media. J. Nanopart. Res. 2014, 16, 2376. [Google Scholar] [CrossRef]
- Zhou, X.T.; Wang, L.M.; Shen, G.Q.; Zhang, D.W.; Xie, J.L.; Mamut, A.; Huang, W.W.; Zhou, S.S. Colorimetric determination of ofloxacin using unmodified aptamers and the aggregation of gold nanoparticles. Microchim. Acta 2018, 185, 355. [Google Scholar] [CrossRef]
- Ye, H.; Duan, N.; Gu, H.J.; Wang, H.T.; Wang, Z.P. Fluorometric determination of lipopolysaccharides via changes of the graphene oxide-enhanced fluorescence polarization caused by truncated aptamers. Microchim. Acta 2019, 186, 173. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, M.J.; Li, J.J.; Xue, Y.T.; Cai, X.; Liu, J.; Wang, X.L.; Xu, S.H.; Xie, Y.H.; Zhang, J.Q. Nucleic Acid Aptamers for Human Norovirus GII.4 and GII.17 Virus-like Particles (VLPs) Exhibit Specific Binding and Inhibit VLPs from Entering Cells. Int. J. Nanomed. 2025, 20, 1789–1805. [Google Scholar] [CrossRef] [PubMed]
- Moe, C.L.; Sair, A.; Lindesmith, L.; Estes, M.K.; Jaykus, L.A. Diagnosis of Norwalk virus infection by indirect enzyme immunoassay detection of salivary antibodies to recombinant Norwalk virus antigen. Clin. Diagn. Lab. Immunol. 2004, 11, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.L.; Chen, L.N.; Wang, S.M.; Tan, M.; Qiu, C.; Qiu, T.Y.; Wang, X.Y. Prevalence and Evolution of Noroviruses between 1966 and 2019, Implications for Vaccine Design. Pathogens. 2021, 10, 1012. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Liu, X.M.; Cao, G.J.; Ding, H.M.; Zhang, D.J.; Yang, G.; Liu, N.L.; Fan, M.; Shen, B.F.; Shao, N.S. Screening of functional antidotes of RNA aptamers against bovine thrombin. FEBS Lett. 2004, 562, 125–128. [Google Scholar] [CrossRef]
- Hermann, T.; Patel, D.J. Biochemistry—Adaptive recognition by nucleic acid aptamers. Science. 2000, 287, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.H.; Liu, R.B.; Zhang, F.Y.; Chitrakar, B.; Wang, X.H. Screening of Single-Stranded DNA Aptamer Specific for Florfenicol and Application in Detection of Food Safety. Biosensors 2022, 12, 701. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold-sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Liu, Z.F.; Li, H.X.; Campos, L.M.; Neaton, J.B.; Venkataraman, L. Non-chemisorbed gold-sulfur binding prevails in self-assembled monolayers. Nat. Chem. 2019, 11, 351–358. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; Sang, Y.; Liu, M.; Shi, M.; Wang, X. Selection and Characterization of DNA Aptamers for Constructing Aptamer-AuNPs Colorimetric Method for Detection of AFM1. Foods 2022, 11, 1802. [Google Scholar] [CrossRef]
- Martorell, S.; Santiso-Bellón, C.; Gozalbo-Rovira, R.; Quintero-Campos, P.; Luque, D.; Maquieira, A.; Rodríguez-Díaz, J.; Morais, S. Synthetic biology-driven optoelectronic biosensor for rapid and highly sensitive norovirus detection in fecal samples. Biosens. Bioelectron. 2025, 288, 117787. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Takemeura, K.; Li, T.C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Size-controlled preparation of peroxidase-like graphene-gold nanoparticle hybrids for the visible detection of norovirus-like particles. Biosens. Bioelectron. 2017, 87, 558–565. [Google Scholar] [CrossRef]
- Hernández, O.H.; Gutiérrez-Escolano, A.L.; Cancio-Lonches, C.; Iturriaga, M.H.; Pacheco-Aguilar, J.R.; Morales-Rayas, R.; Arvizu-Medrano, S.M. Multiplex PCR method for the detection of human norovirus, Salmonella spp., Shigella spp., and shiga toxin producing Escherichia coli in blackberry, coriander, lettuce and strawberry. Food Microbiol. 2022, 102, 103926. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhao, Y.Q.; Gu, W.C.; Qian, Y.; Huang, Q.; Hu, X.J.; Xing, H.B. A novel dual-mode aptasensor based colorimetry and electrochemical detection of norovirus in fecal sample. Anal. Biochem. 2024, 687, 115444. [Google Scholar] [CrossRef]
- Nasrin, F.; Khoris, I.M.; Chowdhury, A.D.; Boonyakida, J.; Park, E.Y. Impedimetric biosensor of Norovirus with low variance using simple bioconjugation on conductive polymer-Au nanocomposite. Sensor Actuators B Chem. 2022, 369, 132390. [Google Scholar] [CrossRef]
- Gao, J.S.; Xue, L.; Li, Y.J.; Cai, W.C.; Miao, S.D.; Meng, L.B.; Ren, S.L.; Zhang, J.M.; Wang, J.; Wu, S.; et al. Rapid and sensitive lateral flow biosensor for the detection of GII human norovirus based on immunofluorescent nanomagnetic microspheres. J. Med. Virol. 2024, 96, e29487. [Google Scholar] [CrossRef]
- Nasrin, F.; Chowdhury, A.D.; Takemura, K.; Lee, J.; Adegoke, O.; Deo, V.K.; Abe, F.; Suzuki, T.; Park, E.Y. Single-step detection of norovirus tuning localized surface plasmon resonance-induced optical signal between gold nanoparticles and quantum dots. Biosens. Bioelectron. 2018, 122, 16–24. [Google Scholar] [CrossRef]
- Qian, W.D.; Huang, J.; Wang, X.F.; Wang, T.; Li, Y.D. CRISPR-Cas12a combined with reverse transcription recombinase polymerase amplification for sensitive and specific detection of human norovirus genotype GII.4. Virology 2021, 564, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Lee, J.Y.; Gao, L.F.; Yin, J.K.; Duan, Y.K.; Jimenez, L.A.; Adkins, B.; Ren, W.D.; Li, L.H.; Fang, J.; et al. A DNA aptamer for binding and inhibition of DNA methyltransferase 1. Nucleic Acids Res. 2019, 47, 11527–11537. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Zhao, J.J.; Yu, H.; Wang, C.; Huang, Y.Y.; Zhao, Q.; Zhou, X.; Li, C.G.; Liu, M.L. Structural Insights into the Mechanism of High-Affinity Binding of Ochratoxin A by a DNA Aptamer. J. Am. Chem. Soc. 2022, 144, 7731–7740. [Google Scholar] [CrossRef]
- Wei, L.K.; Wang, H.L.; Wu, J.M.; Mao, J.; Wang, S.J.; Qiu, J.Q. Ultra-sensitive label-free biosensor for doxorubicin detection by doxorubicin optimized aptamer. J. Food Compos. Anal. 2025, 139, 107141. [Google Scholar] [CrossRef]
- Xie, Y.; Yin, J.W.; Deng, F.; Xu, L.F.; Salminen, K.; Liu, L.M. Sandwich-type electrochemical aptamer-based sensor for rapid nanomolar detection of anesthetic drug procaine in biofluids. Microchem. J. 2025, 211, 113147. [Google Scholar] [CrossRef]




| Sample | Spiked (μg/mL) | Found (μg/mL) | Mean Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| 1 | 1.0 | 0.96 | 95.72 | 6.70 |
| 2 | 2.0 | 2.13 | 106.43 | 9.03 |
| 3 | 2.5 | 2.43 | 97.31 | 0.46 |
| 4 | 3.0 | 2.75 | 91.74 | 2.38 |
| Detection | Bioreceptor | Assay Time (min) | Detection Limit | Reference |
|---|---|---|---|---|
| Colorimetry | Antibody | 200 | 4.02 × 106 copies/mL | [40] |
| Colorimetry | scFv | 45 | 3 × 105 copies/mL | [39] |
| Colorimetry | Aptamer | 75 | 27.2 copies/mL | This work |
| Electrochemical | Antibody | – | 121 copies/mL | [43] |
| Fluorescence | Antibody | <5 | 1.56 × 104 copies/mL | [44] |
| SPR | Antibody | <5 | 96 copies/mL | [45] |
| RT-RPA-CRISPR/Cas12a | – | 40 | 6.95 × 102 copies/mL | [46] |
| RT-qPCR | – | 360 | 10–100 pfu | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Zhang, X.; Li, G.; Sun, M.; Zheng, W.; Li, J.; Liu, J.; Wang, X.; Xie, Y.; Xu, S.; et al. Optimized Aptamer-Conjugated Gold Nanoparticles for Specific Detection of GII.4 Human Norovirus in Feces. Biosensors 2025, 15, 713. https://doi.org/10.3390/bios15110713
Cheng C, Zhang X, Li G, Sun M, Zheng W, Li J, Liu J, Wang X, Xie Y, Xu S, et al. Optimized Aptamer-Conjugated Gold Nanoparticles for Specific Detection of GII.4 Human Norovirus in Feces. Biosensors. 2025; 15(11):713. https://doi.org/10.3390/bios15110713
Chicago/Turabian StyleCheng, Chao, Xiaomeng Zhang, Gaoyang Li, Minjia Sun, Wenjing Zheng, Jingjing Li, Jing Liu, Xuanyi Wang, Youhua Xie, Shouhong Xu, and et al. 2025. "Optimized Aptamer-Conjugated Gold Nanoparticles for Specific Detection of GII.4 Human Norovirus in Feces" Biosensors 15, no. 11: 713. https://doi.org/10.3390/bios15110713
APA StyleCheng, C., Zhang, X., Li, G., Sun, M., Zheng, W., Li, J., Liu, J., Wang, X., Xie, Y., Xu, S., & Zhang, J. (2025). Optimized Aptamer-Conjugated Gold Nanoparticles for Specific Detection of GII.4 Human Norovirus in Feces. Biosensors, 15(11), 713. https://doi.org/10.3390/bios15110713







