Development of CPE/ssDNA-Based Electrochemical Sensor for the Detection of Leucine to Assess Soil Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents for the Development of DNA Biosensor
2.2. Solutions
2.3. Apparatus
2.4. UV–Vis Study of ssDNA Along with Leucine
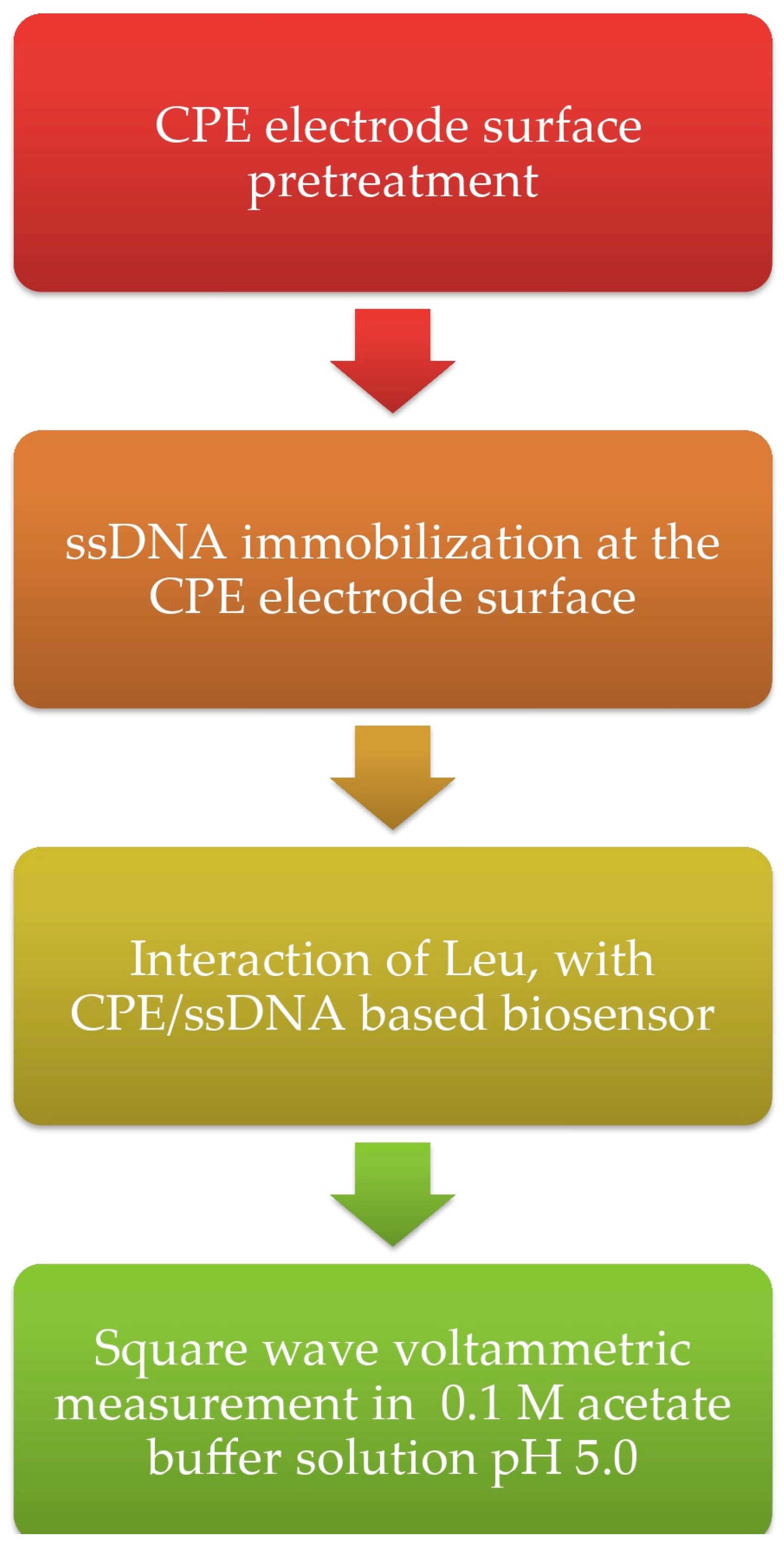
3. Procedures
Interaction of ssDNA with Leu at the CPE/ssDNA-Based Biosensor with Leucine
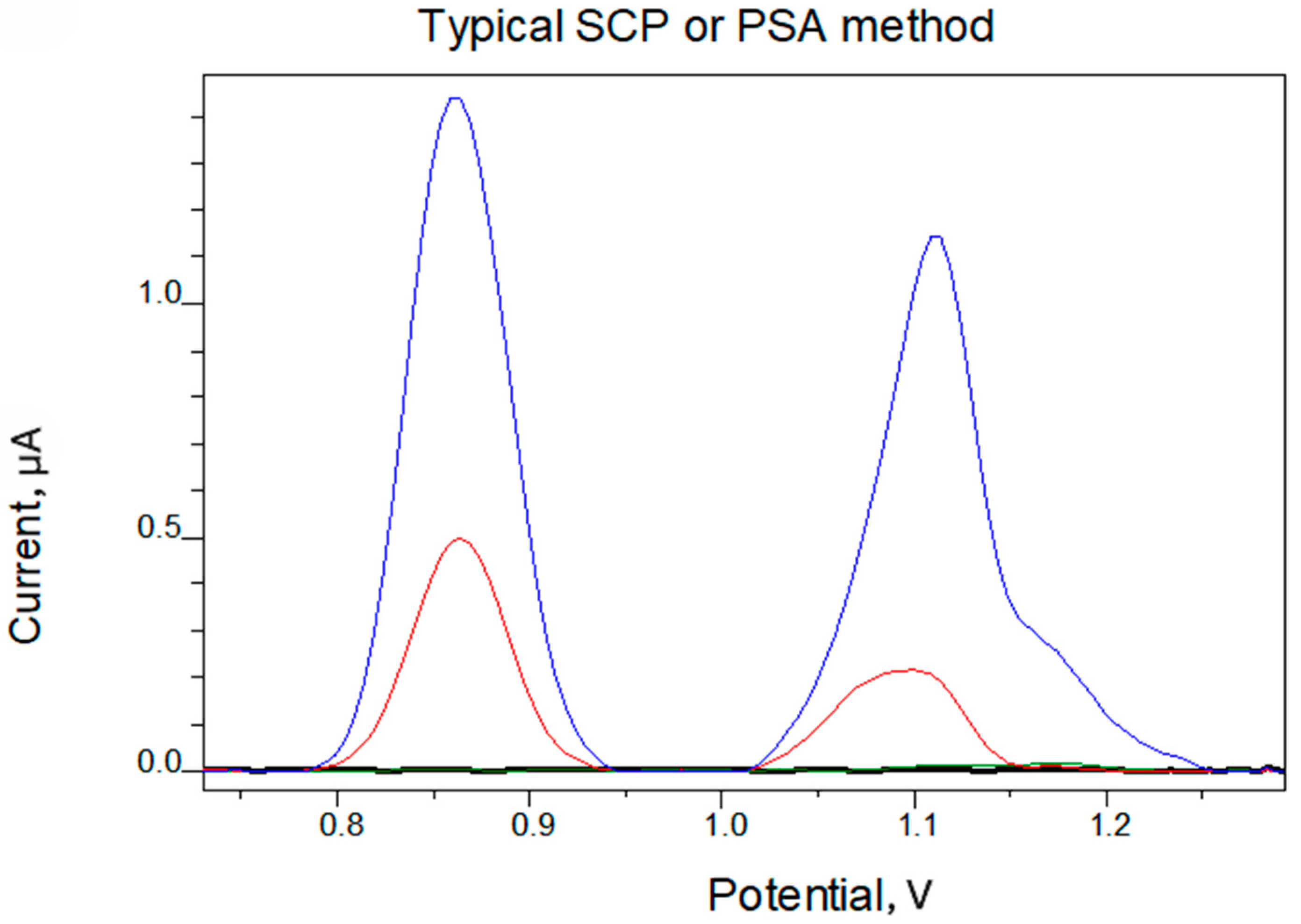
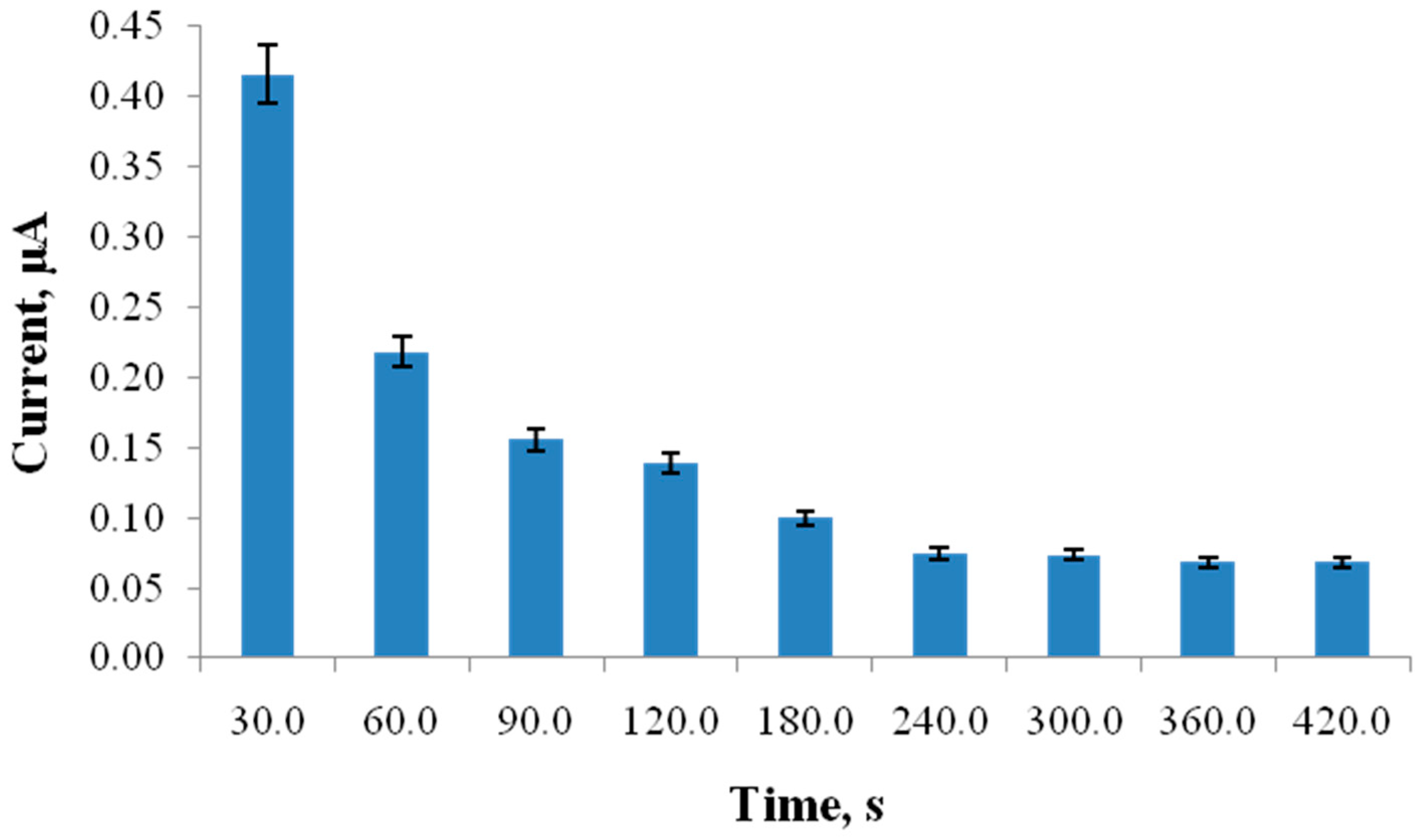
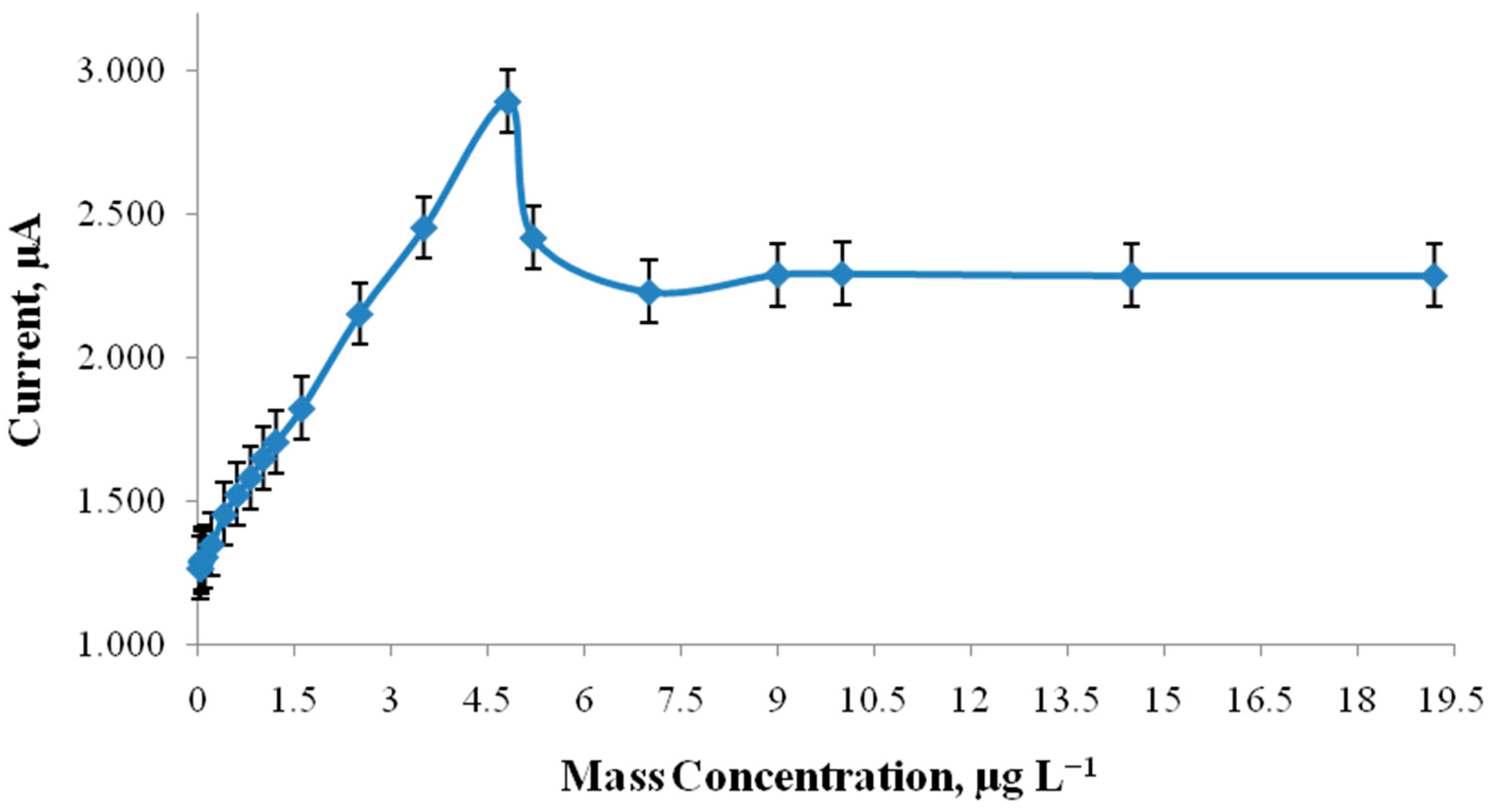
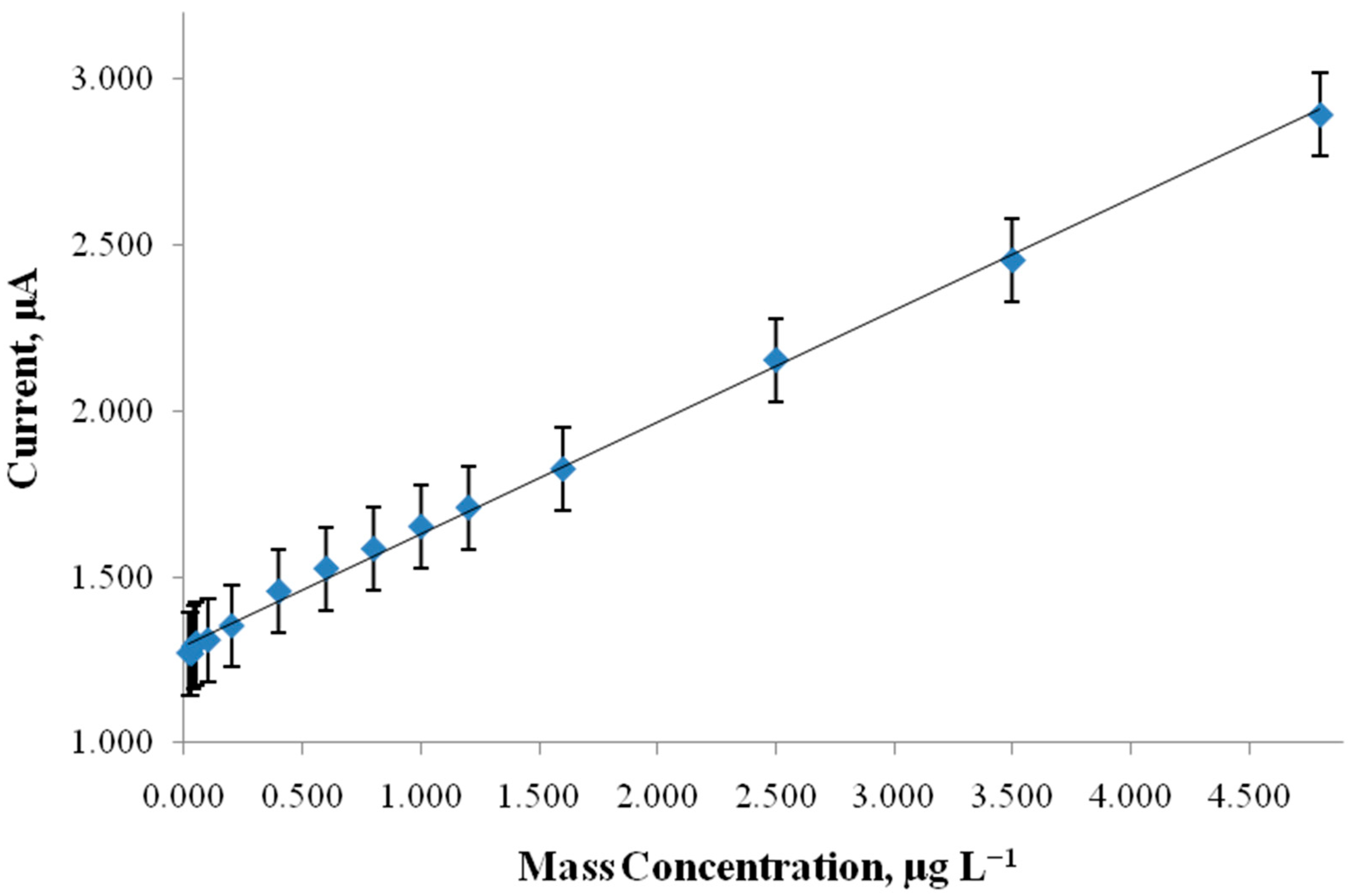
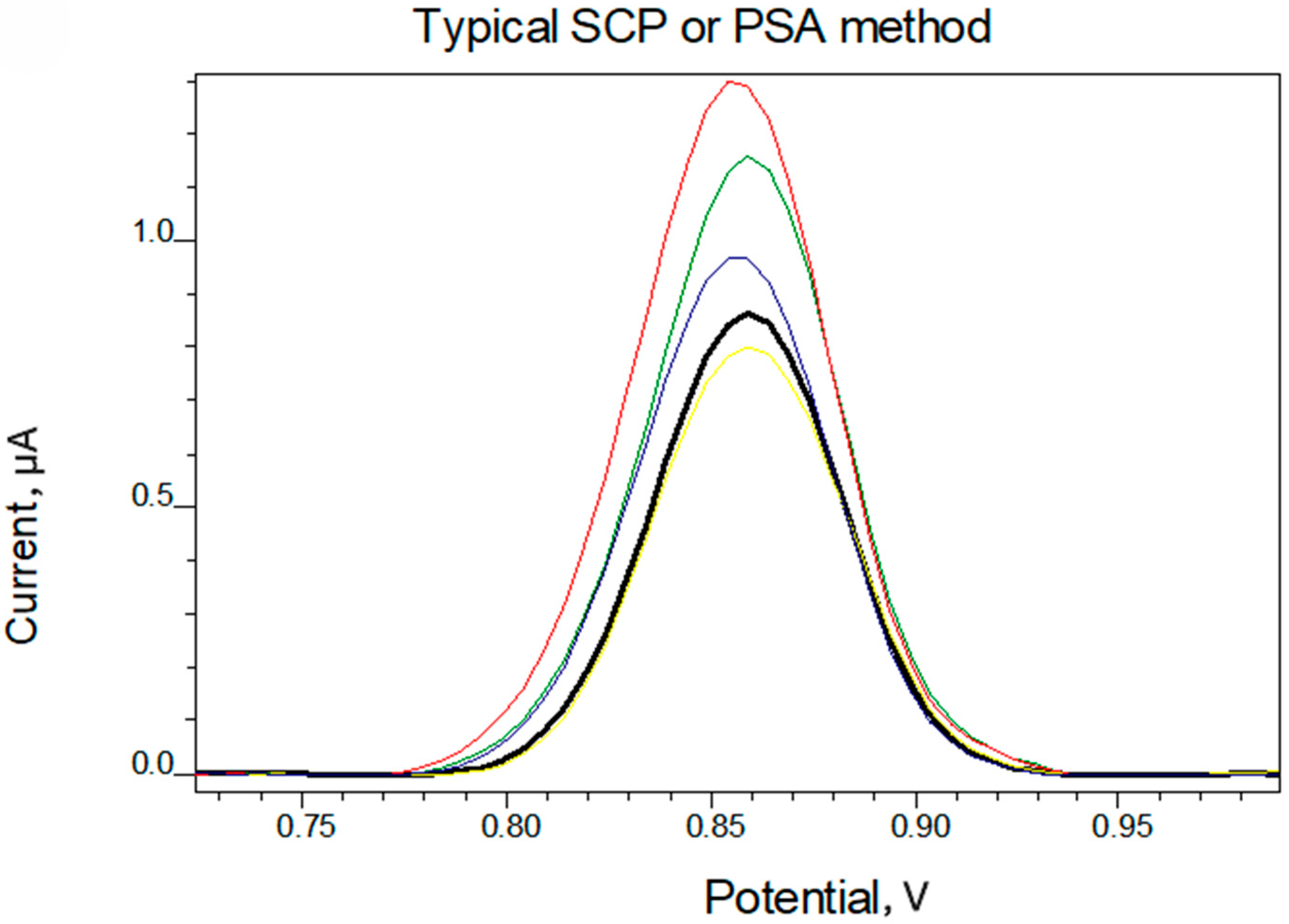
4. Results and Discussion
Interferences Study
5. Application in Soil Sample
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haghighi, M.; Barzegar Sadeghabad, A.; Abolghasemi, R. Effect of exogenous amino acids application on the biochemical, antioxidant, and nutritional value of some leafy cabbage cultivars. Sci. Rep. 2022, 12, 17720. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Shan, Y.; Ren, X.N.; Li, X.M. Research progress on the effects of abiotic stress on plant carbohydrates and related enzymes in their metabolism. J. Anhui Agric. Sci. 2021, 49, 6–9. [Google Scholar] [CrossRef]
- Feng, D.; Gao, Q.; Liu, J.; Tang, J.; Hua, Z.; Sun, X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agron. 2023, 142, 126656. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Gong, Q.; Xiao, Y.; Peng, F. Leucine Contributes to Copper Stress Tolerance in Peach (Prunus persica) Seedlings by Enhancing Photosynthesis and the Antioxidant Defense System. Antioxidants 2022, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.; Fan, Y.; Zuo, D.; Xu, J.; Liu, Y.; Mei, X.; Huang, H.; Yang, M.; Zhu, S. Exogenous leucine alleviates heat stress and improves saponin synthesis in Panax notoginseng by improving antioxidant capacity and maintaining metabolic homeostasis. Front. Plant Sci. 2023, 14, 1175878. [Google Scholar] [CrossRef]
- Bayranvand, M.; Akbarinia, M.; Salehi Jouzani, G.; Gharechahi, J.; Baldrian, P. Distribution of Soil Extracellular Enzymatic, Microbial, and Biological Functions in the C and N-Cycle Pathways Along a Forest Altitudinal Gradient. Front. Microbiol. 2021, 12, 660603. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. The Role of Leucine in the Regulation of Protein Metabolism11. J. Nutr. 2005, 135, 1553S–1556S. [Google Scholar] [CrossRef]
- Golia, E.E.; Aslanidis, P.S.; Papadimou, S.G.; Kantzou, O.D.; Chartodiplomenou, M.A.; Lakiotis, K.; Androudi, M.; Tsiropoulos, N.G. Assessment of remediation of soils, moderately contaminated by potentially toxic metals, using different forms of carbon (charcoal, biochar, activated carbon). Impacts on contamination, metals availability and soil indices. Sustain. Chem. Pharm. 2022, 28, 100724. [Google Scholar] [CrossRef]
- Kgathi, D.; Sekhwela, M.; Almendros, G. Changes in Soil Organic Matter Associated with Land Use of Arenosols from Southern Botswana. Agronomy 2024, 14, 1869. [Google Scholar] [CrossRef]
- Matisic, M.; Dugan, I.; Bogunovic, I. Challenges in Sustainable Agriculture—The Role of Organic Amendments. Agriculture 2024, 14, 643. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.S.; Niazi, N.K.; Lee, Y.-H.; Kim, K.H.; Park, J.-H.; Moon, D.H.; Ok, Y.S. Assessment of Soil Health in Urban Agriculture: Soil Enzymes and Microbial Properties. Sustainability 2017, 9, 310. [Google Scholar] [CrossRef]
- Feliziani, G.; Bordoni, L.; Gabbianelli, R. Regenerative Organic Agriculture and Human Health: The Interconnection Between Soil, Food Quality, and Nutrition. Antioxidants 2025, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Clunes, J.; Pinochet, D. Leucine retention by the clay-sized mineral fraction. An indicator of C storage. Agro Sur 2020, 48, 37–46. [Google Scholar] [CrossRef]
- D’Alò, F.; Odriozola, I.; Baldrian, P.; Zucconi, L.; Ripa, C.; Cannone, N.; Malfasi, F.; Brancaleoni, L.; Onofri, S. Microbial activity in alpine soils under climate change. Sci. Total Environ. 2021, 783, 147012. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.; Causeret, F. Changes in soil carbon inputs and outputs along a tropical altitudinal gradient of volcanic soils under intensive agriculture. Geoderma 2018, 320, 95–104. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of soil analysis part 3-chemical methods. In Soil Science Society of America Book Series: 5; ACSESS: Madison, WI, USA, 1996. [Google Scholar]
- Hou, S.; He, H.; Zhang, W.; Xie, H.; Zhang, X. Determination of soil amino acids by high performance liquid chromatography-electro spray ionization-mass spectrometry derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. Talanta 2009, 80, 440–447. [Google Scholar] [CrossRef]
- Jones, D.L.; Owen, A.G.; Farrar, J.F. Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biol. Biochem. 2002, 34, 1893–1902. [Google Scholar] [CrossRef]
- Warren, C.R. Rapid and sensitive quantification of amino acids in soil extracts by capillary electrophoresis with laser-induced fluorescence. Soil Biol. Biochem. 2008, 40, 916–923. [Google Scholar] [CrossRef]
- Gao, J.; Helmus, R.; Cerli, C.; Jansen, B.; Wang, X.; Kalbitz, K. Robust analysis of underivatized free amino acids in soil by hydrophilic interaction liquid chromatography coupled with electrospray tandem mass spectrometry. J. Chromatogr. A 2016, 1449, 78–88. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Shields, M.R.; Bianchi, T.S.; Schuur, E.A. A rapid and precise method for the analysis of underivatized amino acids in natural samples using volatile-ion-pairing reverse-phase liquid chromatography–electrospray ionization tandem mass spectrometry. Org. Geochem. 2018, 115, 46–56. [Google Scholar] [CrossRef]
- Ionela Raluca Comnea-Stancu, I.R.; Stefan-van Staden, R.I.; van Staden, J.K.F. Enantioanalysis of Leucine and Arginine: A Key Factor in Lung Cancer Metabolomics. J. Electrochem. Soc. 2024, 171, 067513. [Google Scholar] [CrossRef]
- García-Carmona, L.; González, M.C.; Escarpa, A. Electrochemical On-site Amino Acids Detection of Maple Syrup Urine Disease Using Vertically Aligned Nickel Nanowires. Electroanalysis 2018, 30, 1505–1510. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Asiri, A.M. Sensitive L-leucine sensor based on a glassy carbon electrode modified with SrO nanorods. Microchim. Acta 2016, 183, 3265–3273. [Google Scholar] [CrossRef]
- Stefan-van Staden, R.I.; Shirley Muvhulawa, L. Determination of L-and D-Enantiomers of Leucine Using Amperometric Biosensors Based on Diamond Paste. Instrum. Sci. Technol. 2006, 34, 475–481. [Google Scholar] [CrossRef]
- Labroo, P.; Cui, Y. Amperometric bienzyme screen-printed biosensor for the determination of leucine. Anal. Bioanal. Chem. 2014, 406, 367–372. [Google Scholar] [CrossRef]
- Rezaei, B.; Zare, Z.M. Modified glassy carbon electrode with multiwall carbon nanotubes as a voltammetric sensor for determination of leucine in biological and pharmaceutical samples. Anal. Lett. 2008, 41, 2267–2286. [Google Scholar] [CrossRef]
- Karastogianni, S.; Girousi, S. Electrochemical (Bio)Sensing of Maple Syrup Urine Disease Biomarkers Pointing to Early Diagnosis: A Review. Appl. Sci. 2020, 10, 7023. [Google Scholar] [CrossRef]
- Luscombe, N.M.; Laskowski, R.A.; Thornton, J.M. Amino acid–base interactions: A three-dimensional analysis of protein–DNA interactions at an atomic level. Nucleic Acids Res. 2001, 29, 2860–2874. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-T.; Malik, F. Single-Stranded DNA Binding Proteins and Their Identification Using Machine Learning-Based Approaches. Biomolecules 2022, 12, 1187. [Google Scholar] [CrossRef]
- Andrews, C.T.; Campbell, B.A.; Elcock, A.H. Direct comparison of amino acid and salt interactions with double-stranded and single-stranded DNA from explicit-solvent molecular dynamics simulations. J. Chem. Theory Comput. 2017, 13, 1794–1811. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Malik, F.K.; Guo, J.T. A comparative study of protein–ssDNA interactions. NAR Genom. Bioinform. 2021, 3, lqab006. [Google Scholar] [CrossRef]
- Wolfe, A.; Shimer, G., Jr.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalaye into duplex regions of denaturated DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef]
- Gherghi, I.C.; Girousi, S.T.; Pantazaki, A.A.; Voulgaropoulos, A.N.; Tzimou-Tsitouridou, R. Electrochemical DNA biosensors applicable to the study of interactions between DNA and DNA intercalators. Int. J. Environ. Anal. Chem. 2003, 83, 693–700. [Google Scholar] [CrossRef]
- Gherghi, I.C.; Girousi, S.T.; Voulgaropoulos, A.N.; Tzimou-Tsitouridou, R. Interaction of the mutagen ethidium bromide (EB) with DNA, using a carbon paste electrode (CPE) and a Hanging mercury drop electrode (HMDE). Anal. Chim. Acta 2004, 505, 135–144. [Google Scholar] [CrossRef]
- Gherghi, I.C.; Girousi, S.T.; Voulgaropoulos, A.N.; Tzimou-Tsitouridou, R. Adsorptive transfer stripping voltammetry applied to the study of the interaction between DNA and actinomycin D. Int. J. Environ. Anal. Chem. 2004, 84, 865–874. [Google Scholar] [CrossRef]
- Karastogianni, S.; Dendrinou-Samara, C.; Ioannou, E.; Raptopoulou, C.P.; Hadjipavlou-Litina, D.; Girousi, S. Synthesis, characterization, DNA binding properties and antioxidant activity of a manganese (II) complex with NO6 chromophore. J. Inorg. Biochem. 2013, 118, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Gherghi, I.C.; Girousi, S.T.; Voulgaropoulos, A.N.; Tzimou-Tsitouridou, R. DNA modified carbon paste electrode applied to the voltammetric study of the interaction between DNA and acridine orange. Chem. Anal. 2004, 49, 467. [Google Scholar]
- Zheng, X.; Guo, Y.; Zheng, J.; Zhou, X.; Li, Q.; Lin, R. Simultaneous determination of ascorbic acid, dopamine and uric acid using poly (l-leucine)/DNA composite film modified electrode. Sens. Actuators B Chem. 2015, 213, 188–194. [Google Scholar] [CrossRef]
- Li, W.; Ji, Y.Y.; Wang, J.W.; Zhu, Y.M. Cytotoxic Activities and DNA Binding Properties of 1-Methyl-7H-indeno[1,2-b]Quinolinium-7-(4-dimethylamino) Benzylidene Triflate. DNA Cell Biol. 2012, 31, 1046–1053. [Google Scholar] [CrossRef]
- Werdin-Pfisterer, Ν.R.; Kielland, K.; Boone, R.D. Soil amino acid composition across a boreal forest successional sequence. Soil Biol. Biochem. 2009, 41, 1210–1220. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Olk, D.C.; Angers, D.A. Investigating the nature of soil carbohydrates and amino compounds with liquid chromatography. Soil Sci. Soc. Am. J. 2025, 89, e70018. [Google Scholar] [CrossRef]
| Ref. | Application | LOD mol/L | Linearity mol/L | Electrode |
|---|---|---|---|---|
| [23] | blood | 3 × 10−12 | 10−11–10−8 | α-CD/ZnO/nanoC |
| [24] | 8 × 10−16 | 25–700 × 10−6 | v-NiNWs | |
| [25] | spiked urine, milk and serum | 37.5 ± 0.2 × 10−12 | 0.1–100 × 10−9 | SrO NR |
| [26] | - | Diamond paste | ||
| [27] | - | 2 × 10−6 | 10–600 × 10−6 | Amperometric bienzyme ScPE |
| [28] | blood, urine samples | 3.0 × 10−6 | 9.0 × 10−6–5 × 10−3 | MWNTs |
| This work | spiked soil | 4.9 × 10−10 | 1.4 × 10−9–3.5 × 10−8 | CPE/ssDNA |
| Interfering Amino Acid | Recovery, R% at 3 μg L−1 |
|---|---|
| Leucine | 100 |
| Isoleucine | 108.75 |
| Valine | 100.95 |
| Phenylalanine | 102.4 |
| Methionine | 97.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girousi, S.; Banti, Z.; Karastogianni, S.; Papi, R.; Ozkan Ariksoysal, D.; Golia, E.E. Development of CPE/ssDNA-Based Electrochemical Sensor for the Detection of Leucine to Assess Soil Health. Biosensors 2025, 15, 708. https://doi.org/10.3390/bios15110708
Girousi S, Banti Z, Karastogianni S, Papi R, Ozkan Ariksoysal D, Golia EE. Development of CPE/ssDNA-Based Electrochemical Sensor for the Detection of Leucine to Assess Soil Health. Biosensors. 2025; 15(11):708. https://doi.org/10.3390/bios15110708
Chicago/Turabian StyleGirousi, Stella, Zoi Banti, Sophia Karastogianni, Rigini Papi, Dilsat Ozkan Ariksoysal, and Evangelia E. Golia. 2025. "Development of CPE/ssDNA-Based Electrochemical Sensor for the Detection of Leucine to Assess Soil Health" Biosensors 15, no. 11: 708. https://doi.org/10.3390/bios15110708
APA StyleGirousi, S., Banti, Z., Karastogianni, S., Papi, R., Ozkan Ariksoysal, D., & Golia, E. E. (2025). Development of CPE/ssDNA-Based Electrochemical Sensor for the Detection of Leucine to Assess Soil Health. Biosensors, 15(11), 708. https://doi.org/10.3390/bios15110708








