Conductometric Chemosensor for Saccharides Based on Thin Films of Poly(3-Thienylboronic) Acid: Measurements of Transversal Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
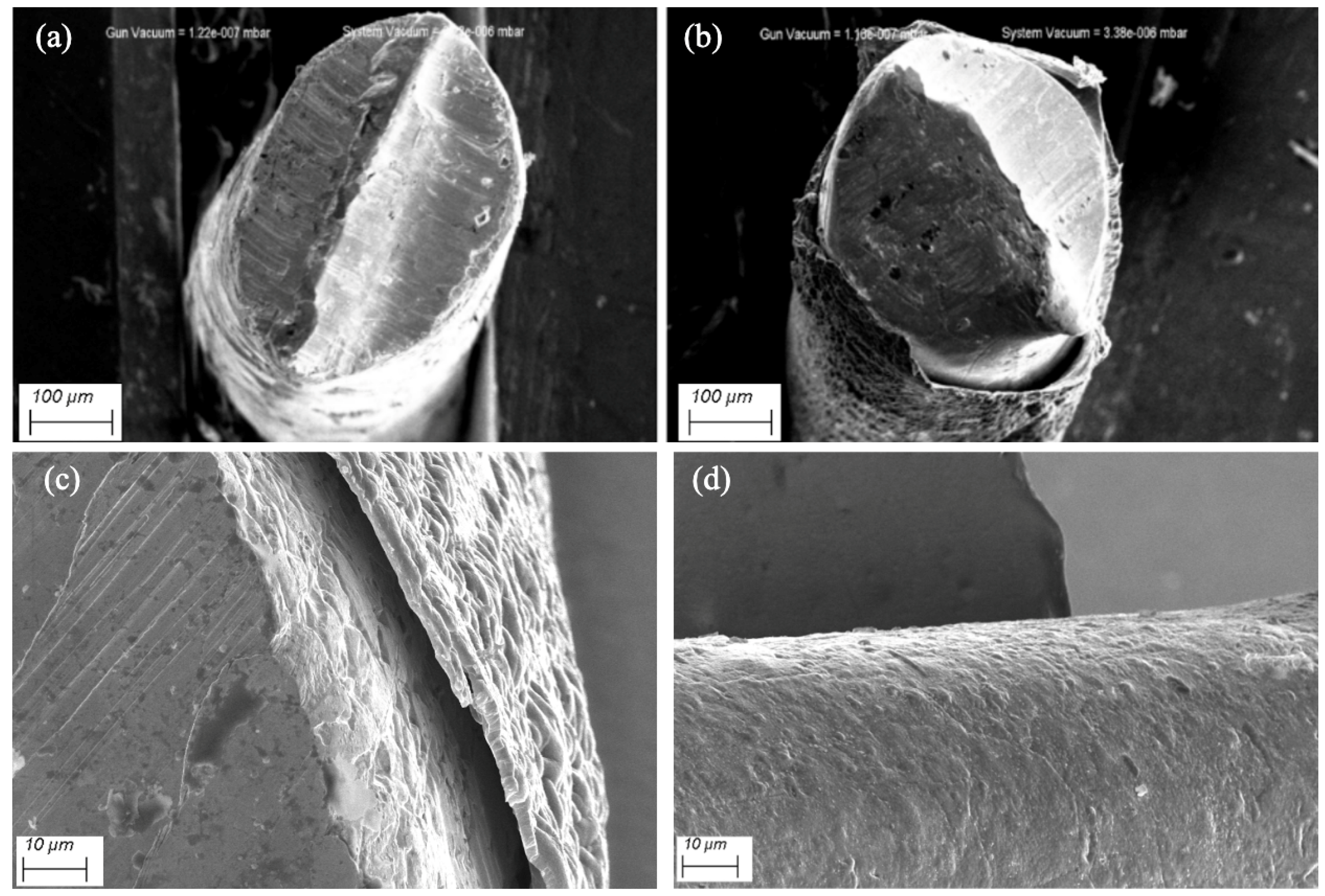
2.2. Instrumentation and Procedures
3. Results and Discussion
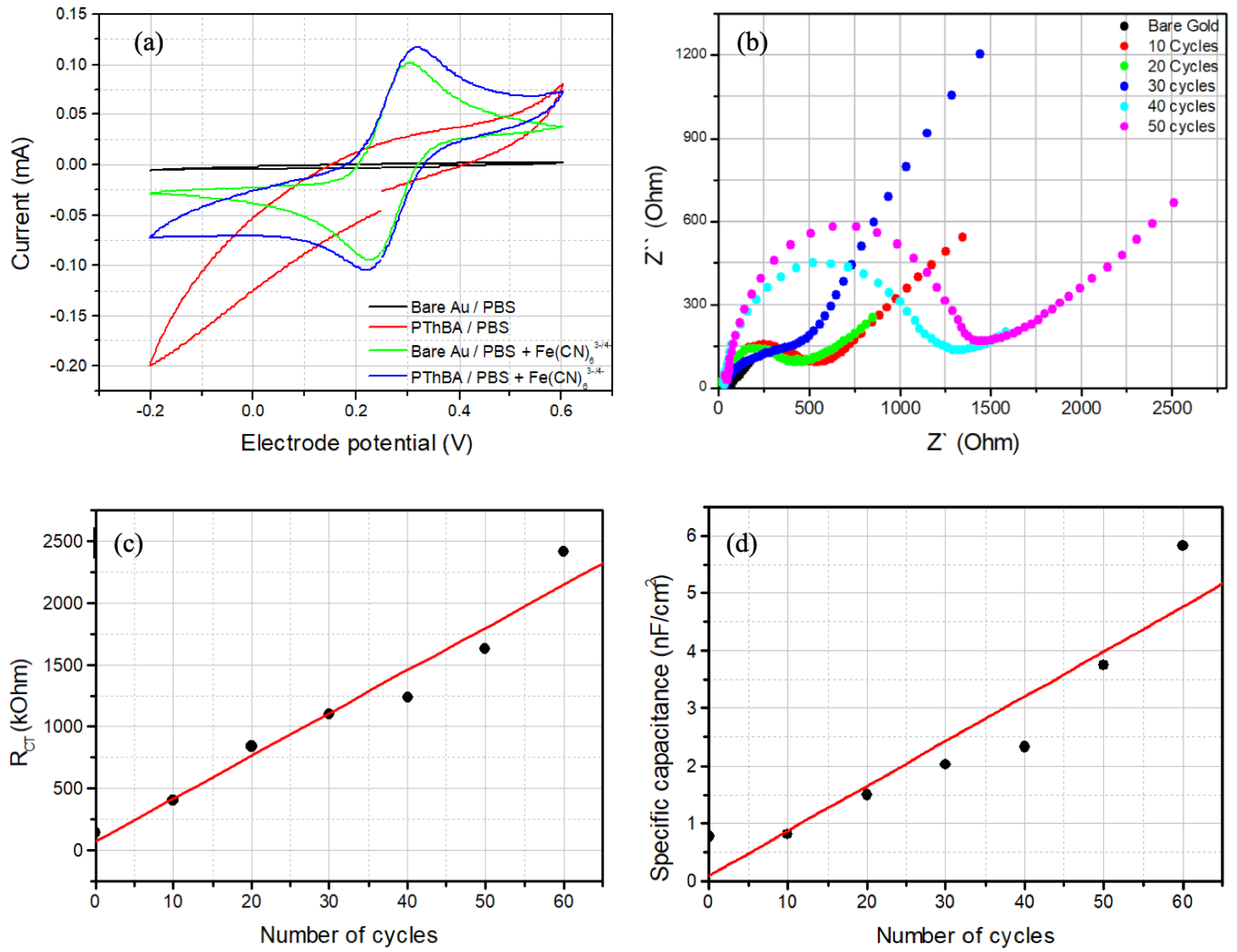
3.1. Electrochemical Deposition of Thin Films of PThBA
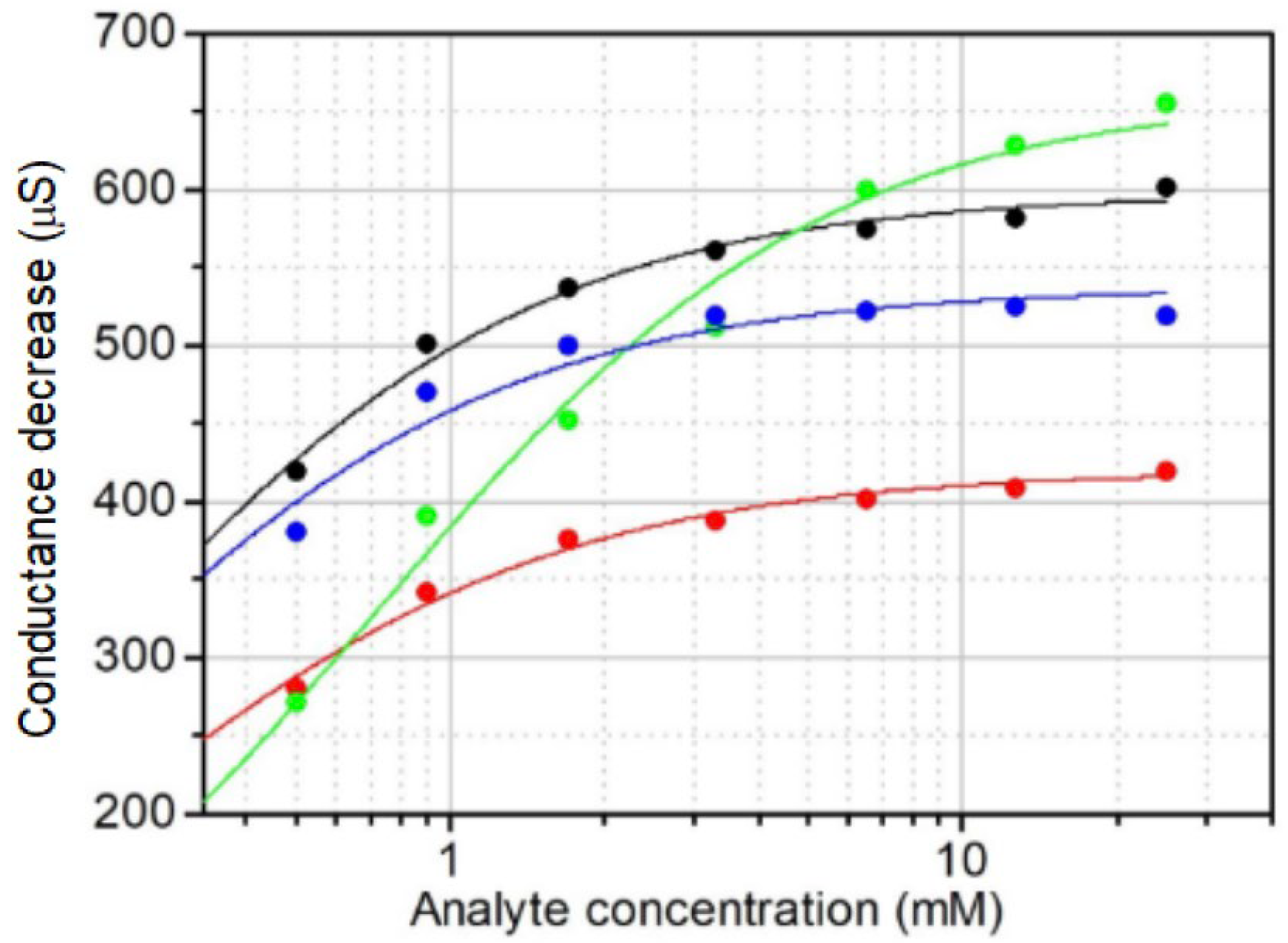
3.2. Effect of Fructose on the Resistance of PThBA Films in Aerobic and Anoxic Conditions
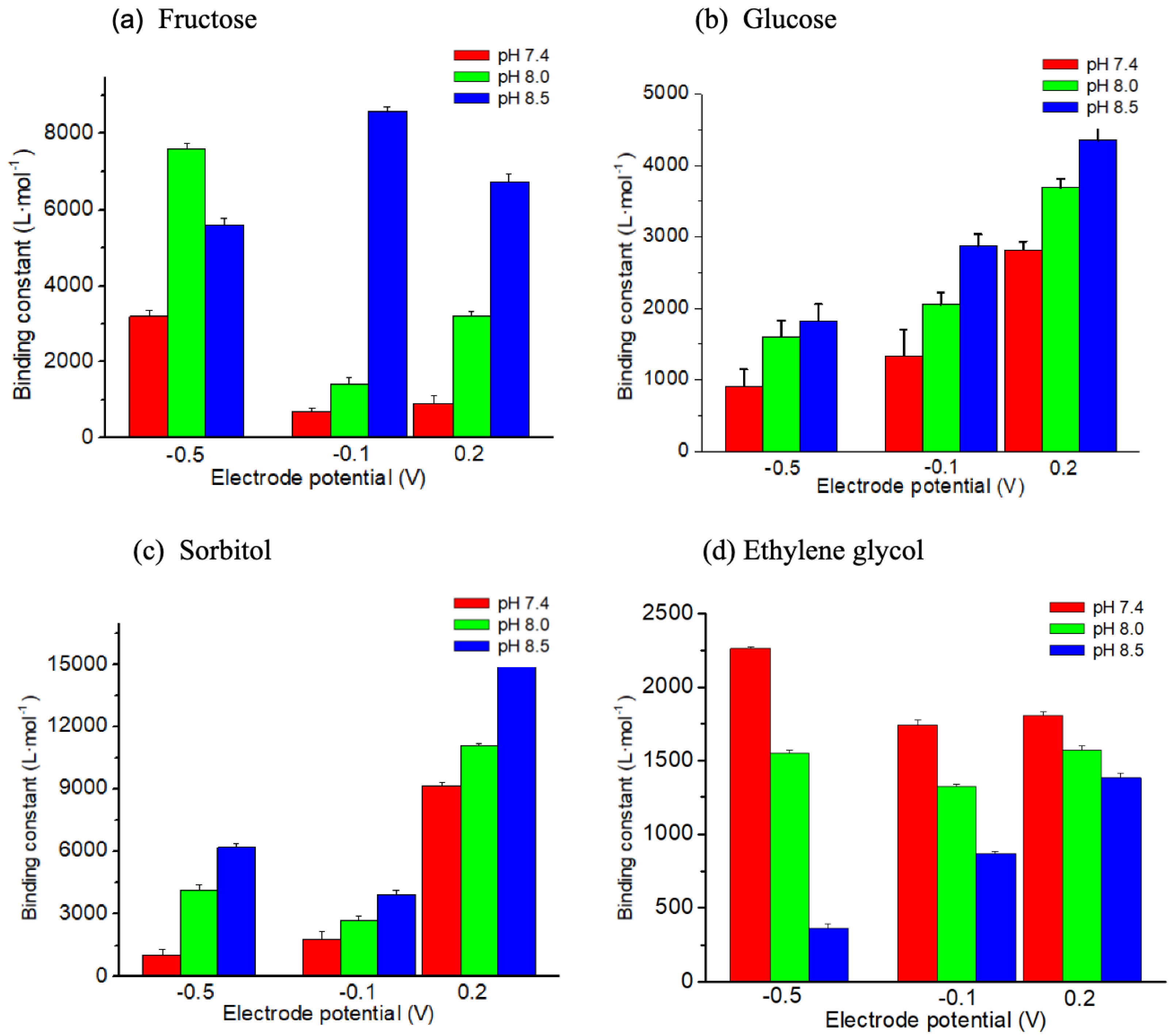
3.3. Influence of pH of the Electrolyte and Redox State of the Sensing Polymer on the Affinity Towards Various Saccharides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Lehninger. Principles of Biochemistry, 7th ed.; W.H. Freeman and Company: New York, NY, USA, 2017. [Google Scholar]
- Shum, C.; Asha, A.B.; Narain, R. Carbohydrate Biosensors and Applications. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Krenzelok, E.P.; Olson, K.; Watson, W. American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 537–560. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Banton, M.; Klapacz, J.; Shen, H. A toxicological review of the ethylene glycol series: Commonalities and differences in toxicity and modes of action. Toxicol. Lett. 2017, 278, 66–83. [Google Scholar] [CrossRef] [PubMed]
- von Woedtke, T.; Jülich, W.-D.; Hartmann, V.; Stieber, M.; Abel, P.U. Sterilization of enzyme glucose sensors: Problems and concepts. Biosens. Bioelectron. 2002, 17, 373–382. [Google Scholar] [CrossRef]
- Schmid, T.; Baumann, B.; Himmelsbach, M.; Klampfl, C.W.; Buchberger, W. Analysis of saccharides in beverages by HPLC with direct UV detection. Anal. Bioanal. Chem. 2016, 408, 1871–1878. [Google Scholar] [CrossRef]
- Jernelv, I.L.; Milenko, K.; Fuglerud, S.S.; Hjelme, D.R.; Ellingsen, R.; Aksnes, A. A review of optical methods for continuous glucose monitoring. Appl. Spectrosc. Rev. 2019, 54, 543–572. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A critical review of electrochemical glucose sensing: Evolution of biosensor platforms based on advanced nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Kurniawan, F.; Tsakova, V.; Mirsky, V.M. Gold nanoparticles in nonenzymatic electrochemical detection of sugars. Electroanalysis 2006, 18, 1937–1942. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Hassan, M.N.; Vyas, C.; Grieve, B.; Bartolo, P. Recent advances in enzymatic and non-enzymatic electrochemical glucose sening. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Anzai, J.-I. Recent progress in electrochemical biosensors based on phenylboronic acid and derivatives. Mater. Sci. Eng. C 2016, 67, 737–746. [Google Scholar] [CrossRef]
- Shi, T.; Kou, D.; Xue, Y.; Zhang, S.; Ma, W. Saccharide sensors based on phenylboronic acid derivatives. Prog. Chem. 2024, 36, 106–119. [Google Scholar] [CrossRef]
- Nan, K.; Jiang, Y.-N.; Li, M.; Wang, B. Recent progress in diboronic-acid-based glucose sensors. Biosensors 2023, 13, 618. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. Chemosensitive properties of electrochemically synthesized poly-3-thienylboronic acid: Conductometric detection of glucose and other diol-containing compounds under electrical affinity control. Polymers 2024, 16, 1938. [Google Scholar] [CrossRef] [PubMed]
- Hall, D. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Liu, G.-J.; Ning, Z.-W.; Xing, G.-W. Boronic acid-based chemical sensors for saccharides. Carbohydr. Res. 2017, 452, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Kearns, F.L.; Robart, C.; Kemp, M.T.; Vankayala, S.L.; Chapin, B.M.; Anslyn, E.V.; Woodcock, H.L.; Larkin, J.D. Modeling boronic acid-based fluorescent saccharide sensors: Computational investigation of D-fructose binding to dimethylaminomethylphenylboronic acid. J. Chem. Inf. Model. 2019, 59, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, P.; Chaudhary, V.; Kumar, P.; Huertas, C.S.; Kumar, P.; Kumar, S. Nonenzymatic dual glucose sensing on boronic acid modified zeolitic imidazolate framework-67 nanoparticles for diabetes management. Microchim. Acta 2024, 191, 306. [Google Scholar] [CrossRef]
- Williams, G.T.; Kedge, J.L.; Fossey, J.S. Molecular boronic acid-based saccharide sensors. ACS Sens. 2021, 6, 1508–1528. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. 3-Thienylboronic acid as a receptor for diol-containing compounds: A study by isothermal titration calorimetry. Chemosensors 2022, 10, 251. [Google Scholar] [CrossRef]
- Fang, G.; Wang, H.; Bian, Z.; Sun, J.; Liu, A.; Fang, H.; Liu, B.; Yaoabcd, Q.; Wu, Z. Recent development of boronic acid-based fluorescent sensors. RSC Adv. 2018, 8, 29400–29427. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2015, 116, 1375–1397. [Google Scholar] [CrossRef]
- Vancoillie, G.; Hoogenboom, R. Synthesis and polymerization of boronic acid containing monomers. Polym. Chem. 2016, 7, 5484–5495. [Google Scholar] [CrossRef]
- Lange, U.; Mirsky, V.M. Integrated electrochemical transistor as a fast recoverable gas sensor. Analyt. Chim. Acta 2011, 687, 7–11. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. Electrically controlled variation of receptor affinity. Anal. Bioanal. Chem. 2016, 408, 7283–7287. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, Y.; Mirsky, V.M. Virtual sensor array consisting of a single sensor element with variable affinity: An application for analysis of fish freshness. Sens. Actuators B Chem. 2017, 241, 652–657. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. Poly-3-thienylboronic acid: A chemosensitive derivative of polythiophene. J. Solid State Electrochem. 2020, 24, 3105–3111. [Google Scholar] [CrossRef]
- Kang, H.; Xu, L.; Cai, Y.; Liu, Y.; Jiang, F.; Xu, J.; Zhou, W. Using boronic acid functionalization to simultaneously enhance electrical conductivity and thermoelectric performance of free-standing polythiophene film. Eur. Polym. J. 2021, 144, 110208. [Google Scholar] [CrossRef]
- Kolosova, O.S.; Efremenko, Y.; Laurinavichyute, V.K.; Nizamov, S.; Petrushenko, S.I.; Mirsky, V.M. Poly-3-thienylboronic acid nanoparticles: Synthesis, characterization, and interaction with saccharides studied at the level of individual nanoparticles. ACS Appl. Nano Mater. 2024, 7, 11120–11135. [Google Scholar] [CrossRef]
- Pringsheim, E.; Terpetschnig, E.; Piletsky, S.A.; Wolfbeis, O.S. A Polyaniline with near-infrared optical response to saccharides. Adv. Mater. 1999, 11, 865–868. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Chen, X.; Fossey, J.S.; James, T.D.; Jiang, Y.-B. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rew. 2013, 42, 8032–8048. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Kulikov, V.; Mirsky, V.M. Investigation of contact and bulk resistance of conducting polymers by simultaneous two- and four-point technique. Sens. Actuators B Chem. 2003, 94, 352–357. [Google Scholar] [CrossRef]
- Lange, U.; Mirsky, V. Separated analysis of bulk and contact resistance of conducting polymers: Comparison of simultaneous two- and four-point measurements with impedance measurements. J. Electroanal. Chem. 2008, 622, 246–251. [Google Scholar] [CrossRef]
- Takahashi, S.; Anzai, J.-i. Phenylboronic acid monolayer-modified electrodes sensitive to sugars. Langmuir 2005, 21, 5230–5236. [Google Scholar] [CrossRef]
- Ćwik, P.; Wawrzyniak, U.E.; Jańczyk, M.; Wróblewski, W. Electrochemical studies of self-Assembled monolayers composed of Various Phenylboronic Acid Derivatives. Talanta 2014, 120, 188–193. [Google Scholar] [CrossRef]
- Lange, U.; Mirsky, V.M. Polythiophene films on gold electrodes: A comparison of bulk and contact resistances in aqueous and organic media. J. Solid State Electrochem. 2011, 15, 2377–2382. [Google Scholar] [CrossRef]
- James, T.D. Boronic acid-based receptors and sensors for saccharides. In Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine; Hall, D.G., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp. 441–479. [Google Scholar]
- James, T.D.; Sandanayake, K.R.A.S.; Shinkai, S. Saccharide sensing with molecular receptors based on boronic acid. Angew. Chem. Int. Ed. 1996, 35, 1910–1922. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Deng, C.C.; Sumerlin, B.S. Structure–reactivity relationships in boronic acid–diol complexation. ACS Omega 2018, 3, 17863–17870. [Google Scholar] [CrossRef]
- Bindra, P.; Gerischer, H.; Peter, L.M. The dependence of the rate of the Fe(CN)63−/Fe(CN)64− couple on ionic strength in concentrated solutions. J. Electroanal. Chem. 1974, 57, 435–438. [Google Scholar] [CrossRef]
- Beriet, C.; Pletcher, D. A microelectrode study of the mechanism and kinetics of the ferro/ferricyanide couple in aqueous media: The influence of the electrolyte and its concentration. J. Electroanal. Chem. 1993, 361, 93–101. [Google Scholar] [CrossRef]
- Zhi, Z.-l.; Drazan, V.; Wolfbeis, O.S.; Mirsky, V.M. Electrocatalytic activity of DNA on electrodes as an indication of hybridization. Bioelectrochemistry 2006, 68, 1–6. [Google Scholar] [CrossRef]



| Analyte | Interfering Compound | SA/B | pH | Electrode Potential, V |
|---|---|---|---|---|
| Fructose | Glucose | 4.7 | 8.0 | −0.5 |
| Sorbitol | 3.2 | 7.4 | −0.5 | |
| Ethylene glycol | 15 | 8.5 | −0.5 | |
| Glucose | Fructose | 3.1 | 7.4 | 0.2 |
| Sorbitol | 0.89 | 7.4 | −0.5 | |
| Ethylene glycol | 5.0 | 8.5 | −0.5 | |
| Sorbitol | Fructose | 10 | 7.4 | 0.2 |
| Glucose | 3.4 | 8.5 | 0.2 | |
| Ethylene glycol | 17 | 8.5 | −0.5 | |
| Ethylene glycol | Fructose | 2.4 | 7.4 | −0.1 |
| Glucose | 2.5 | 7.4 | −0.5 | |
| Sorbitol | 2.2 | 7.4 | −0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, B.; Efremenko, Y.; Mirsky, V.M. Conductometric Chemosensor for Saccharides Based on Thin Films of Poly(3-Thienylboronic) Acid: Measurements of Transversal Resistance. Biosensors 2025, 15, 679. https://doi.org/10.3390/bios15100679
Kaya B, Efremenko Y, Mirsky VM. Conductometric Chemosensor for Saccharides Based on Thin Films of Poly(3-Thienylboronic) Acid: Measurements of Transversal Resistance. Biosensors. 2025; 15(10):679. https://doi.org/10.3390/bios15100679
Chicago/Turabian StyleKaya, Berfinsu, Yulia Efremenko, and Vladimir M. Mirsky. 2025. "Conductometric Chemosensor for Saccharides Based on Thin Films of Poly(3-Thienylboronic) Acid: Measurements of Transversal Resistance" Biosensors 15, no. 10: 679. https://doi.org/10.3390/bios15100679
APA StyleKaya, B., Efremenko, Y., & Mirsky, V. M. (2025). Conductometric Chemosensor for Saccharides Based on Thin Films of Poly(3-Thienylboronic) Acid: Measurements of Transversal Resistance. Biosensors, 15(10), 679. https://doi.org/10.3390/bios15100679







