Actomyosin-Based Nanodevices for Sensing and Actuation: Bridging Biology and Bioengineering
Abstract
1. Introduction
- (1)
- Delivering drugs to precise locations in the body.
- (2)
- Monitoring physiological signals through molecular-scale sensors
- (3)
- Detecting diseases through tiny sensors.
- (4)
- Stimulating or repairing parts of the nervous system.
- (5)
- Transporting materials inside cells.
- (6)
- Performing microsurgery.
- (7)
- Aiding in tissue healing and regeneration.
2. Nanotechnology at the Molecular Scale
2.1. Rotary Enzymes
2.2. DNA Walkers
2.3. Synthetic Molecular Motors
2.4. Stimuli-Responsive Polymers
2.5. Nanoparticles
3. Molecular Motors and Their Tracks
3.1. Molecular Motor Types
3.2. Potential Uses in Nanotechnology
- (1)
- Transport of tiny objects, such as drug-filled, enzyme-containing, or antibody-coated vesicles, to specific locations on a surface;
- (2)
- Sensor-triggered, localized actuation—precise responses initiated when a molecular sensor is activated;
- (3)
- Sorting molecules in miniaturized lab-on-a-chip systems;
- (4)
- Powering devices where these proteins carry components or move fluids.
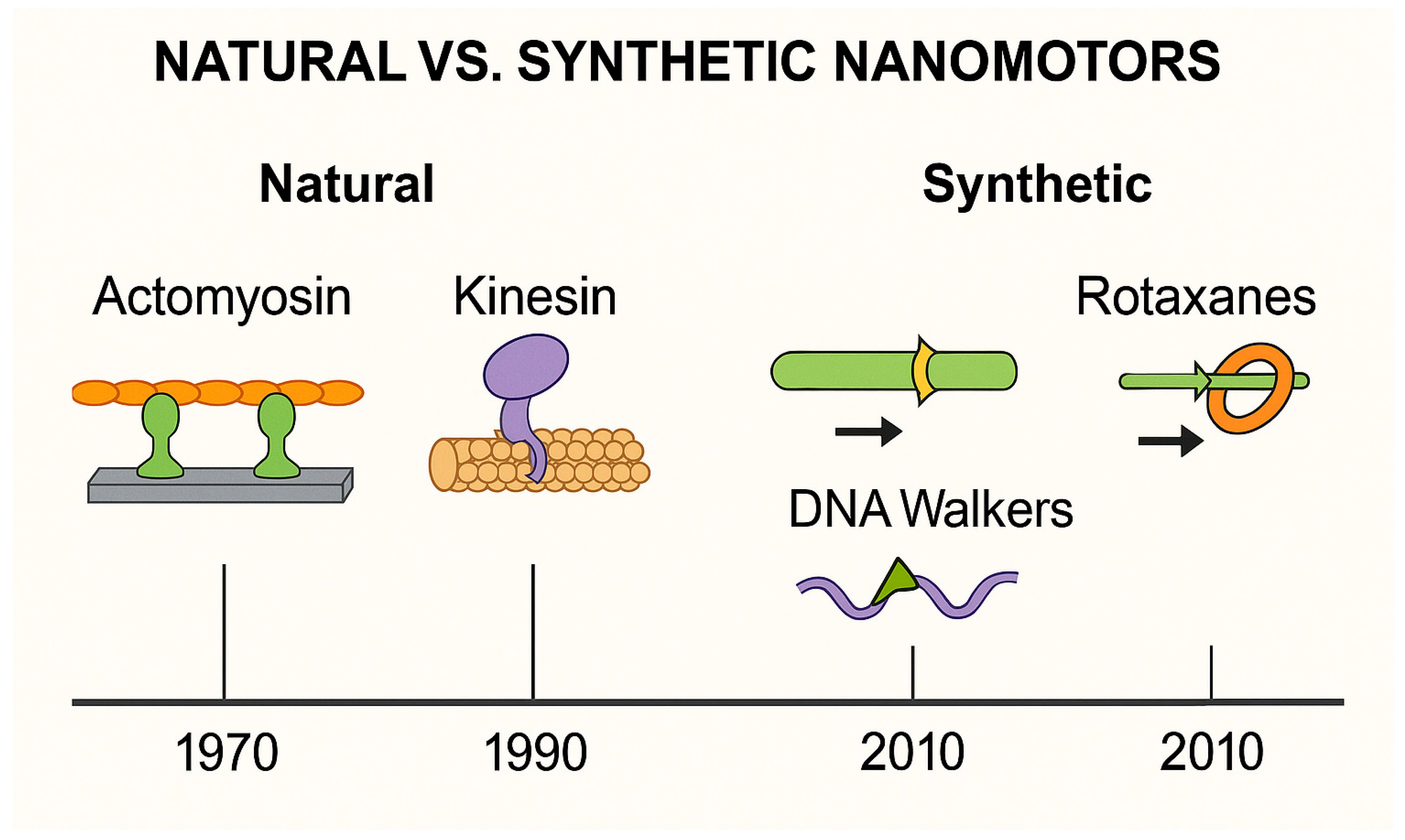
3.3. Natural vs. Synthetic Nanomotors
4. The Actomyosin System as a Bio-Nanomachine
4.1. Actomyosin’s Role in the Body
4.1.1. Muscles: Built for Contraction
- (1)
- Molecules of tropomyosin, an α-helical coiled-coil dimeric protein, bind “head-to-tail” to form two strands on a thin filament that block myosin motors’ access to binding sites on actin at resting levels of cytoplasmic Ca2+ (~10−7 M Ca2+).
- (2)
- Troponin C subunit of the troponin complex binds Ca2+ when a striated muscle is activated due to a transient increase in the cytoplasmic concentration of Ca2+ (Ca2+ transient).
- (3)
- Troponin I subunit holds the troponin complex together by binding both the troponin C and troponin T subunits; the C-terminus of troponin I is located between actin and tropomyosin at resting Ca2+ levels, keeping tropomyosin in position to prevent formation of actomyosin crossbridges; when cytoplasmic Ca2+ rises during a Ca2+ transient, Ca2+ binds troponin C, which allows troponin C to bind the C-terminus of troponin I, in turn allowing tropomyosin to move on the surface of actin such that actomyosin crossbridge cycling can occur.
- (4)
- Troponin T subunit binds the troponin complex to tropomyosin in a stoichiometry of the structural regulatory unit of 1:1:7 (troponin:tropomyosin:actin) and, due to its elongated structure, participates in communication along and between the two strands of tropomyosin on a thin filament.
4.1.2. Beyond Muscles: A Cellular Workhorse
- (1)
- During cell division, actomyosin forms a contractile ring that tightens to divide one cell into two.
- (2)
- For cell movement, it drives the extension and retraction of structures like lamellipodia and filopodia.
- (3)
- In intracellular transport, especially in actin-rich environments such as neurons or epithelial layers, it helps to move organelles and vesicles.
- (4)
- In mechanical sensing, it allows cells to respond to tension or pressure in their environment [83].
4.1.3. Why Actomyosin Is Ideal for Nanotechnology
- (1)
- It is powered by MgATP, a natural energy source, but can also utilize other nucleotides. For example, substituting deoxyATP (dATP) for ATP has been shown to enhance both the force and velocity of actomyosin interactions. This biochemical flexibility underscores the system’s adaptability and suggests opportunities to fine-tune motor output for specialized nanotechnological tasks [86,87,88].
- (2)
- It responds to calcium, temperature and pH, offering built-in control.
- (3)
- Although Ca2+ is the primary physiological regulator, other divalent cations such as Sr2+ and Ba2+ can also substitute to activate thin filaments, albeit with altered kinetics and sensitivity [89,90]. This property has been widely used experimentally and expands the biochemical flexibility of the system.
- (4)
- It operates with remarkable efficiency, with theoretical estimates suggesting that up to 50% of chemical energy can be converted into mechanical work under optimal conditions [91].
- (5)
- It exerts force at the piconewton level and enables motion at the nanometer scale, but organizes function across micrometer-scale cellular structures.
- (6)
- It is reversible and durable, capable of repeating its cycle over and over.
4.2. Recreating Actomyosin Function in the Laboratory
- (1)
- Gliding assays, where myosin motors are immobilized on a surface (typically glass or plastic), and actin filaments (typically fluorescently labeled) are allowed to glide across in the presence of ATP [96]. These setups are ideal for studying motor performance—such as speed and processivity—and for screening the effects of drugs or genetic mutations, especially in cardiac or skeletal muscle proteins.
- (2)
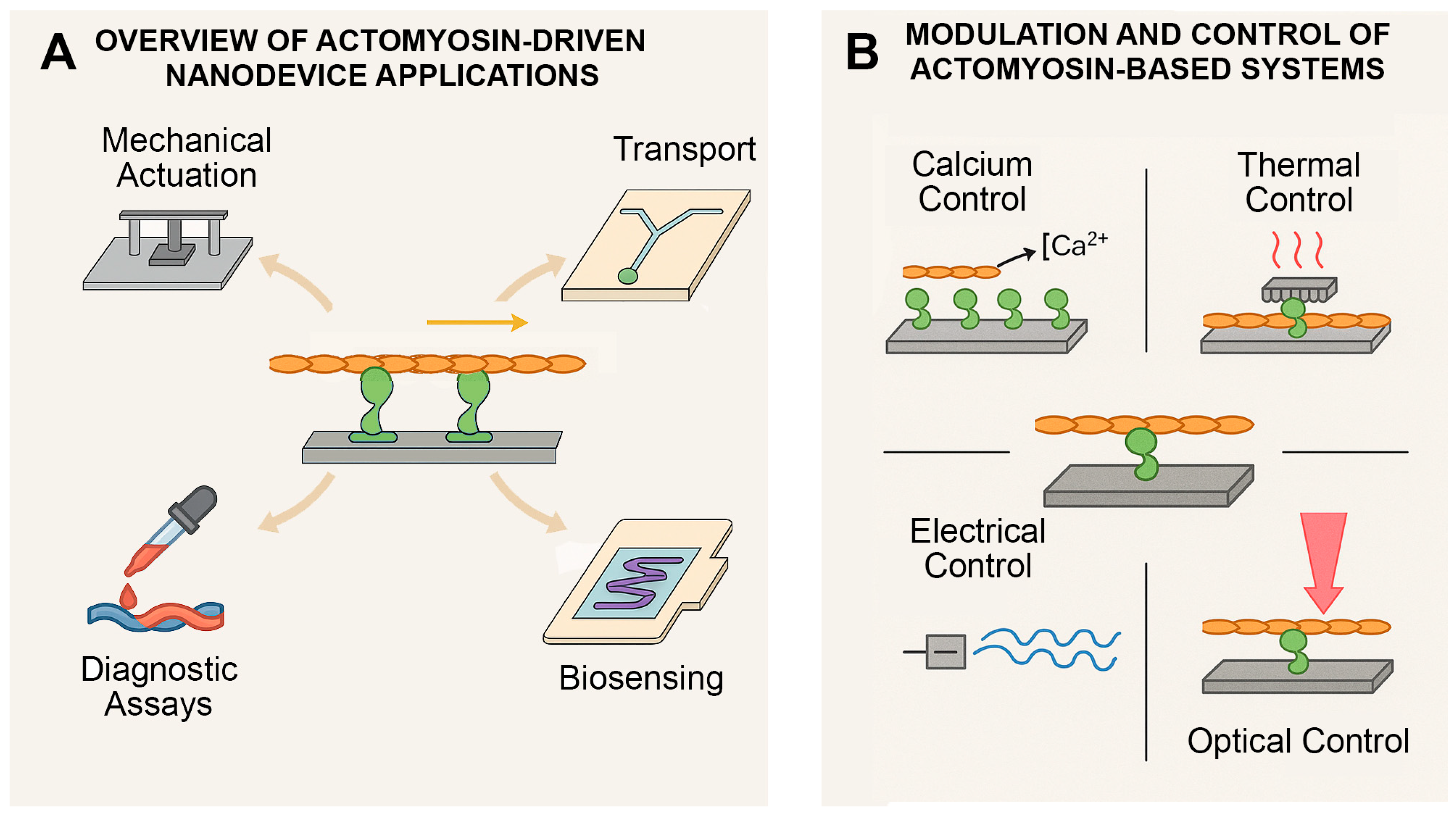
4.3. Controlling Actomyosin in Artificial Systems
4.3.1. Biochemical Control (ATP and Calcium Ions)
4.3.2. Thermal Control (Heat)
4.3.3. Electrical or Electrochemical Stimulation
4.3.4. Light Activation (Optogenetics or Photothermal Methods)
4.3.5. Surface Patterning and Environment Control
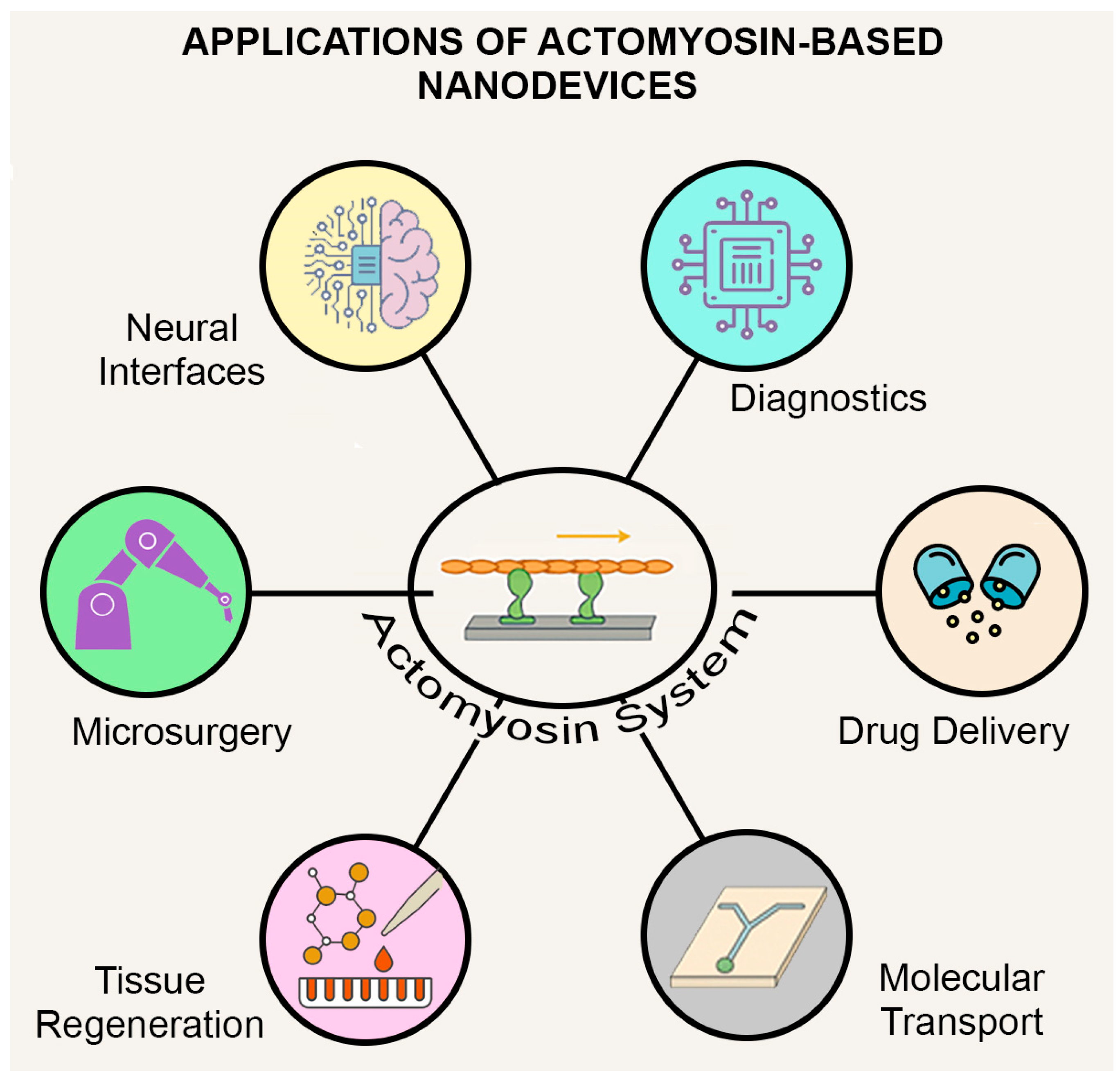
4.4. Applications in Biomedicine and Engineering
- (1)
- During early development, cells use actomyosin to pinch and fold into complex tissue shapes [161].
- (2)
- In cell division, it tightens like a drawstring to divide one cell into two [162].
- (3)
- Large cells, like egg cells, use it to stir their contents [163].
- (4)
- In the ear, actomyosin helps to maintain delicate structures that let us hear [164].
4.4.1. Bioactuators and Contractile Elements
4.4.2. Electrically and Thermally Triggered Systems
4.4.3. Biosensing Applications
4.4.4. Vision and Neural Interfaces
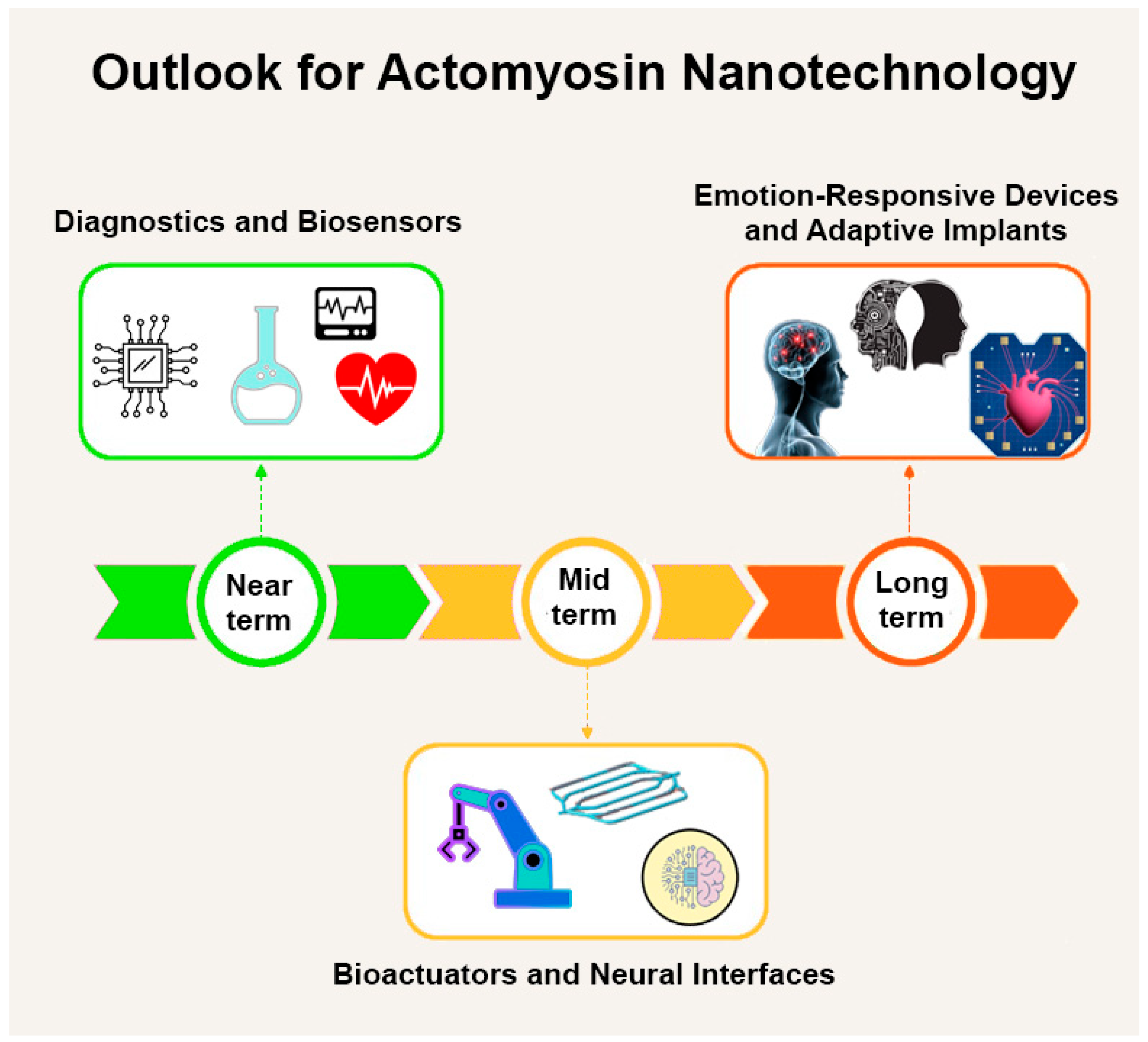
4.4.5. Looking Ahead: Emotion-Responsive Devices
4.5. Engineering Challenges and Design Considerations
4.5.1. Stability over Time
4.5.2. Proper Attachment and Orientation
4.5.3. Spatial Control
4.5.4. Scaling up
4.5.5. The Path Forward
- (1)
- Better chemistry to keep proteins stable;
- (2)
- Advanced surfaces for guiding movement;
- (3)
- Smarter control systems using light, heat, or electricity;
- (4)
- Integration with readout mechanisms for biosensing applications;
- (5)
- Computer modeling to plan system-wide behavior.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drexler, K.E. Nanosystems: Molecular Machinery, Manufacturing, and Computation; John Wiley & Sons: New York, NY, USA, 1992; p. 576. [Google Scholar]
- Drexler, K.E. Engines of Creation: Challenges and Choices of the Last Technological Revolution; Anchor/Doubleday: New York, NY, USA, 1986; p. 320. [Google Scholar]
- Weiss, P.S. Nanotechnology. Molecules join the assembly line. Nature 2001, 413, 585–586. [Google Scholar] [CrossRef]
- Weiss, P.S. Nanotechnology: A molecular four-wheel drive. Nature 2011, 479, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Hess, H. Toward devices powered by biomolecular motors. Science 2006, 312, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Hess, H. Engineering applications of biomolecular motors. Annu. Rev. Biomed. Eng. 2011, 13, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.; Saper, G. Engineering with Biomolecular Motors. Acc. Chem. Res. 2018, 51, 3015–3022. [Google Scholar] [CrossRef]
- Xu, M.; Tang, D. Recent advances in DNA walker machines and their applications coupled with signal amplification strategies: A critical review. Anal. Chim. Acta 2021, 1171, 338523. [Google Scholar] [CrossRef]
- Dinu, C.Z.; Chrisey, D.B.; Diez, S.; Howard, J. Cellular motors for molecular manufacturing. Anat. Rec. 2007, 290, 1203–1212. [Google Scholar] [CrossRef]
- Hess, H.; Bachand, G.D.; Vogel, V. Powering nanodevices with biomolecular motors. Chemistry 2004, 10, 2110–2116. [Google Scholar] [CrossRef]
- Saper, G.; Hess, H. Synthetic Systems Powered by Biological Molecular Motors. Chem. Rev. 2020, 120, 288–309. [Google Scholar] [CrossRef]
- Månsson, A. The potential of myosin and actin in nanobiotechnology. J. Cell. Sci. 2023, 136, jcs261025. [Google Scholar] [CrossRef]
- Berg, H.C. Keeping up with the F1-ATPase. Nature 1998, 394, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Kato-Yamada, Y.; Noji, H.; Yasuda, R.; Kinosita, K., Jr.; Yoshida, M. Direct observation of the rotation of ε subunit in F1-ATPase. J. Biol. Chem. 1998, 273, 19375–19377. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Noji, H.; Kinosita, K., Jr.; Yoshida, M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell 1998, 93, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Noji, H.; Yoshida, M.; Kinosita, K., Jr.; Itoh, H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature 2001, 410, 898–904. [Google Scholar] [CrossRef]
- Berry, R.M.; Berg, H.C. Torque generated by the flagellar motor of Escherichia coli while driven backward. Biophys. J. 1999, 76, 580–587. [Google Scholar] [CrossRef]
- Meister, M.; Caplan, S.R.; Berg, H.C. Dynamics of a tightly coupled mechanism for flagellar rotation. Bacterial motility, chemiosmotic coupling, protonmotive force. Biophys. J. 1989, 55, 905–914. [Google Scholar] [CrossRef]
- Ryu, W.S.; Berry, R.M.; Berg, H.C. Torque-generating units of the flagellar motor of Escherichia coli have a high duty ratio. Nature 2000, 403, 444–447. [Google Scholar] [CrossRef]
- Chen, X.; Berg, H.C. Torque-speed relationship of the flagellar rotary motor of Escherichia coli. Biophys. J. 2000, 78, 1036–1041. [Google Scholar] [CrossRef]
- Fung, D.C.; Berg, H.C. Powering the flagellar motor of Escherichia coli with an external voltage source. Nature 1995, 375, 809–812. [Google Scholar] [CrossRef]
- Berry, R.M.; Berg, H.C. Torque generated by the bacterial flagellar motor close to stall. Biophys. J. 1996, 71, 3501–3510. [Google Scholar] [CrossRef]
- Berry, R.M.; Turner, L.; Berg, H.C. Mechanical limits of bacterial flagellar motors probed by electrorotation. Biophys. J. 1995, 69, 280–286. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Wang, B.; Yan, M.; Liu, Y.; Zhao, R.; Wang, X.; Shao, T.; Li, Y.; Imran, M.; Ji, M.; et al. Progress in enzyme-powered micro/nanomotors in diagnostics and therapeutics. Bioact. Mater. 2025, 46, 555–568. [Google Scholar] [CrossRef]
- Hortelão, A.C.; Patiño, T.; Perez-Jiménez, A.; Blanco, À.; Sánchez, S. Enzyme-Powered Nanobots Enhance Anticancer Drug Delivery. Adv. Funct. Mater. 2018, 28, 1705086. [Google Scholar] [CrossRef]
- Ma, X.; Hortelão, A.C.; Patiño, T.; Sánchez, S. Enzyme Catalysis To Power Micro/Nanomachines. ACS Nano 2016, 10, 9111–9122. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, X.; Wang, L.; Ma, X. Fundamentals and applications of enzyme powered micro/nano-motors. Bioact. Mater. 2021, 6, 1727–1749. [Google Scholar] [CrossRef]
- Mason, S.D.; Wang, G.A.; Yang, P.; Li, Y.; Li, F. Probing and Controlling Dynamic Interactions at Biomolecule-Nanoparticle Interfaces Using Stochastic DNA Walkers. ACS Nano 2019, 13, 8106–8113. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, Y.; Mason, S.D.; Chen, J.; Li, F. Enzyme-Powered Three-Dimensional DNA Nanomachine for DNA Walking, Payload Release, and Biosensing. ACS Nano 2016, 10, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Liu, S.; Yan, Y.; Zhang, P.; Che, K. Artificial molecular motors in biological applications. Front. Mol. Biosci. 2024, 11, 1510619. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, M.; Silvi, S.; Credi, A. Photo- and Redox-Driven Artificial Molecular Motors. Chem. Rev. 2020, 120, 200–268. [Google Scholar] [CrossRef]
- Corra, S.; Curcio, M.; Credi, A. Photoactivated Artificial Molecular Motors. JACS Au 2023, 3, 1301–1313. [Google Scholar] [CrossRef]
- Credi, A. Artificial molecular motors powered by light. Aust. J. Chem. 2006, 59, 157–169. [Google Scholar] [CrossRef]
- Kassem, S.; van Leeuwen, T.; Lubbe, A.S.; Wilson, M.R.; Feringa, B.L.; Leigh, D.A. Artificial molecular motors. Chem. Soc. Rev. 2017, 46, 2592–2621. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.A.; Frazar, E.M.; Hilt, J.Z. Recent developments in stimuli responsive nanomaterials and their bionanotechnology applications. Curr. Opin. Chem. Eng. 2020, 30, 103–111. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2024, 24, e202300217. [Google Scholar] [CrossRef]
- Agasti, S.S.; Rana, S.; Park, M.-H.; Kim, C.K.; You, C.-C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010, 62, 316–328. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Hutter, E.; Maysinger, D. Gold nanoparticles and quantum dots for bioimaging. Microsc. Res. Tech. 2011, 74, 592–604. [Google Scholar] [CrossRef]
- Rarokar, N.; Yadav, S.; Saoji, S.; Bramhe, P.; Agade, R.; Gurav, S.; Khedekar, P.; Subramaniyan, V.; Wong, L.S.; Kumarasamy, V. Magnetic nanosystem a tool for targeted delivery and diagnostic application: Current challenges and recent advancement. Int. J. Pharm. X 2024, 7, 100231. [Google Scholar] [CrossRef]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. 2014, 6, 9–26. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, S.C.; Holliday, L.S. Biochemistry of mechanoenzymes: Biological motors for nanotechnology. Biomed. Microdevices 2003, 5, 269–280. [Google Scholar] [CrossRef]
- van den Heuvel, M.G.L.; Dekker, C. Motor proteins at work for nanotechnology. Science 2007, 317, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.V.; Nag, S.; Spudich, A.; Ruppel, K.M.; Spudich, J.A. The Myosin Family of Mechanoenzymes: From Mechanisms to Therapeutic Approaches. Annu. Rev. Biochem. 2020, 89, 667–693. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.A.; Spudich, J.A. The myosin superfamily at a glance. J. Cell. Sci. 2012, 125, 1627–1632. [Google Scholar] [CrossRef]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682–696. [Google Scholar] [CrossRef]
- Hajne, J.; Hanson, K.L.; van Zalinge, H.; Nicolau, D.V. Motility of actin filaments on micro-contact printed myosin patterns. IEEE Trans. Nanobioscience 2015, 14, 313–322. [Google Scholar] [CrossRef]
- Månsson, A. Translational actomyosin research: Fundamental insights and applications hand in hand. J. Muscle Res. Cell Motil. 2012, 33, 219–233. [Google Scholar] [CrossRef]
- de Lanerolle, P. Nuclear actin and myosins at a glance. J. Cell Sci. 2012, 125 Pt 21, 4945–4949. [Google Scholar] [CrossRef]
- de Lanerolle, P.; Serebryannyy, L. Nuclear actin and myosins: Life without filaments. Nat. Cell Biol. 2011, 13, 1282–1288. [Google Scholar] [CrossRef]
- Pestic-Dragovich, L.; Stojiljkovic, L.; Philimonenko, A.A.; Nowak, G.; Ke, Y.; Settlage, R.E.; Shabanowitz, J.; Hunt, D.F.; Hozak, P.; de Lanerolle, P. A myosin I isoform in the nucleus. Science 2000, 290, 337–341. [Google Scholar] [CrossRef]
- Philimonenko, V.V.; Zhao, J.; Iben, S.; Dingova, H.; Kysela, K.; Kahle, M.; Zentgraf, H.; Hofmann, W.A.; de Lanerolle, P.; Hozak, P.; et al. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 2004, 6, 1165–1172. [Google Scholar] [CrossRef]
- Khataee, H.R.; Khataee, A.R. Kinesin and Dynein Smart Nanomotors: Towards Bio-Nanorobotic Systems. Nano 2010, 5, 13–23. [Google Scholar] [CrossRef]
- Miki, H.; Okada, Y.; Hirokawa, N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Amino, M.; Yoshio, M.; Oiwa, K.; Kojima, H.; Furuta, K. Creating biomolecular motors based on dynein and actin-binding proteins. Nat. Nanotechnol. 2017, 12, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Höök, P.; Vallee, R.B. The dynein family at a glance. J. Cell Sci. 2006, 119, 4369–4371. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Kon, T.; Knight, P.J.; Sutoh, K.; Burgess, S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 713–726. [Google Scholar] [CrossRef]
- Howard, J. Mechanics of Motor Proteins and the Cytoskeleton; Sinaur Associates: Sunderland, MA, USA, 2001; p. 367. [Google Scholar]
- Howard, J. Molecular motors: Structural adaptations to cellular functions. Nature 1997, 389, 561–567. [Google Scholar] [CrossRef]
- Vale, R.D. The molecular motor toolbox for intracellular transport. Cell 2003, 112, 467–480. [Google Scholar] [CrossRef]
- Bath, J.; Turberfield, A.J. DNA nanomachines. Nat. Nanotechnol. 2007, 2, 275–284. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef]
- Hess, H.; Bachand, G.D. Biomolecular motors. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966, 184, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F.; Niedergerke, R. Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature 1954, 173, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.; Hanson, J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 1954, 173, 973–976. [Google Scholar] [CrossRef]
- Cooke, R. The sliding filament model: 1972–2004. J. Gen. Physiol. 2004, 123, 643–656. [Google Scholar] [CrossRef]
- Huxley, A.F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef]
- Huxley, H.E. The mechanism of muscular contraction. Science 1969, 164, 1356–1366. [Google Scholar] [CrossRef]
- Spudich, J.A. The myosin swinging cross-bridge model. Nat. Rev. Mol. Cell Biol. 2001, 2, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Namba, K.; Fujii, T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yanagisawa, H.; Wakabayashi, T. Cryo-EM structures of cardiac thin filaments reveal the 3D architecture of troponin. J. Struct. Biol. 2020, 209, 107450. [Google Scholar] [CrossRef] [PubMed]
- Risi, C.M.; Pepper, I.; Belknap, B.; Landim-Vieira, M.; White, H.D.; Dryden, K.; Pinto, J.R.; Chase, P.B.; Galkin, V.E. The Structure of the Native Cardiac Thin Filament at Systolic Ca2+ Levels. Proc. Natl. Acad. Sci. USA 2021, 118, e2024288118. [Google Scholar] [CrossRef]
- Risi, C.M.; Belknap, B.; Atherton, J.; Coscarella, I.L.; White, H.D.; Chase, P.B.; Pinto, J.R.; Galkin, V.E. Troponin structural dynamics in the native cardiac thin filament revealed by cryo electron microscopy. J. Mol. Biol. 2024, 436, 168498. [Google Scholar] [CrossRef]
- Risi, C.M.; Landim-Vieira, M.; Belknap, B.; Chase, P.B.; Pinto, J.R.; Galkin, V.E. The role of the troponin T interactions with actin in regulation of cardiac thin filament revealed by the troponin T pathogenic variant Ile79Asn. J. Mol. Cell. Cardiol. 2025, 204, 55–67. [Google Scholar] [CrossRef]
- Ebashi, S.; Endo, M. Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 1968, 18, 123–183. [Google Scholar] [CrossRef]
- Lehman, W.; Craig, R. Tropomyosin and the steric mechanism of muscle regulation. Adv. Exp. Med. Biol. 2008, 644, 95–109. [Google Scholar] [CrossRef]
- Wilson, C.; Naber, N.; Cooke, R. The role of the super-relaxed state of myosin in human metabolism. Metabol. Open 2021, 9, 100068. [Google Scholar] [CrossRef]
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, jcs186254. [Google Scholar] [CrossRef]
- Munjal, A.; Lecuit, T. Actomyosin networks and tissue morphogenesis. Development 2014, 141, 1789–1793. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef]
- Regnier, M.; Rivera, A.J.; Chen, Y.; Chase, P.B. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ. Res. 2000, 86, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Lee, D.M.; Homsher, E. ATP analogs and muscle contraction: Mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophys. J. 1998, 74, 3044–3058. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Homsher, E. The effect of ATP analogs on posthydrolytic and force development steps in skinned skeletal muscle fibers. Biophys. J. 1998, 74, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Moisescu, D.G.; Thieleczek, R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J. Physiol. 1978, 275, 241–262. [Google Scholar] [CrossRef]
- Stephenson, D.G.; Williams, D.A. Activation of skinned arthropod muscle fibres by Ca2+ and Sr2+. J. Muscle Res. Cell Motil. 1980, 1, 73–87. [Google Scholar] [CrossRef]
- Vilfan, A.; Šarlah, A. Theoretical efficiency limits and speed-efficiency trade-off in myosin motors. PLoS Comput. Biol. 2023, 19, e1011310. [Google Scholar] [CrossRef]
- Gordon, A.M.; LaMadrid, M.; Chen, Y.; Luo, Z.; Chase, P.B. Calcium regulation of skeletal muscle thin filament motility in vitro. Biophys. J. 1997, 72, 1295–1307. [Google Scholar] [CrossRef]
- Kron, S.J.; Toyoshima, Y.Y.; Uyeda, T.Q.P.; Spudich, J.A. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991, 196, 399–416. [Google Scholar] [CrossRef]
- Murrell, M.; Thoresen, T.; Gardel, M. Reconstitution of contractile actomyosin arrays. Methods Enzymol. 2014, 540, 265–282. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Spudich, J.A. Movement of myosin-coated fluorescent beads on actin cables in vitro. Nature 1983, 303, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Faulstich, H.; Zobeley, S.; Rinnerthaler, G.; Small, J.V. Fluorescent phallotoxins as probes for filamentous actin. J. Muscle Res. Cell Motil. 1988, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Spudich, J.A.; Kron, S.J.; Sheetz, M.P. Movement of myosin-coated beads on oriented filaments reconstituted from purified actin. Nature 1985, 315, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Margossian, S.S.; Lowey, S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982, 85, 55–71. [Google Scholar]
- Pardee, J.D.; Spudich, J.A. Purification of muscle actin. Methods Enzymol. 1982, 85, 164–181. [Google Scholar]
- Muthu, P.; Liang, J.; Schmidt, W.; Moore, J.R.; Szczesna-Cordary, D. In vitro rescue study of a malignant familial hypertrophic cardiomyopathy phenotype by pseudo-phosphorylation of myosin regulatory light chain. Arch. Biochem. Biophys. 2014, 552–553, 29–39. [Google Scholar] [CrossRef]
- Pinto, J.R.; Parvatiyar, M.S.; Jones, M.A.; Liang, J.; Potter, J.D. A troponin T mutation that causes infantile restrictive cardiomyopathy increases Ca2+ sensitivity of force development and impairs the inhibitory properties of troponin. J. Biol. Chem. 2008, 283, 2156–2166. [Google Scholar] [CrossRef]
- Schoffstall, B.; Brunet, N.M.; Wang, F.; Williams, S.; Barnes, A.T.; Miller, V.F.; Compton, L.A.; McFadden, L.A.; Taylor, D.W.; Dhanarajan, R.; et al. Ca2+-sensitivity of regulated cardiac thin filament sliding does not depend on myosin isoform. J. Physiol. 2006, 577 Pt 3, 935–944. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Straceski, A.J.; Leinwand, L.A.; Tikunov, B.A.; Faust, L. Heterologous expression of a cardiomyopathic myosin that is defective in its actin interaction. J. Biol. Chem. 1994, 269, 1603–1605. [Google Scholar] [CrossRef]
- Kron, S.J.; Spudich, J.A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc. Natl. Acad. Sci. USA 1986, 83, 6272–6276. [Google Scholar] [CrossRef]
- Desai, R.; Geeves, M.A.; Kad, N.M. Using fluorescent myosin to directly visualize cooperative activation of thin filaments. J. Biol. Chem. 2015, 290, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Kad, N.M.; Kim, S.; Warshaw, D.M.; Vanburen, P.; Baker, J.E. Single-myosin crossbridge interactions with actin filaments regulated by troponin-tropomyosin. Proc. Natl. Acad. Sci. USA 2005, 102, 16990–16995. [Google Scholar] [CrossRef] [PubMed]
- Kad, N.M.; Patlak, J.B.; Fagnant, P.M.; Trybus, K.M.; Warshaw, D.M. Mutation of a Conserved Glycine in the SH1-SH2 Helix Affects the Load-Dependent Kinetics of Myosin. Biophys. J. 2007, 92, 1623–1631. [Google Scholar] [CrossRef]
- Snyder, G.E.; Sakamoto, T.; Hammer, J.A., III; Sellers, J.R.; Selvin, P.R. Nanometer localization of single green fluorescent proteins: Evidence that myosin V walks hand-over-hand via telemark configuration. Biophys. J. 2004, 87, 1776–1783. [Google Scholar] [CrossRef]
- Sakamoto, T.; Wang, F.; Schmitz, S.; Xu, Y.; Xu, Q.; Molloy, J.E.; Veigel, C.; Sellers, J.R. Neck length and processivity of myosin V. J. Biol. Chem. 2003, 278, 29201–29207. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yildez, A.; Selvin, P.R.; Sellers, J.R. Step-size is determined by neck length in myosin V. Biochemistry 2005, 44, 16203–16210. [Google Scholar] [CrossRef]
- Yang, Y.; Kovacs, M.; Sakamoto, T.; Zhang, F.; Kiehart, D.P.; Sellers, J.R. Dimerized Drosophila myosin VIIa: A processive motor. Proc. Natl. Acad. Sci. USA 2006, 103, 5746–5751. [Google Scholar] [CrossRef]
- Ruff, C.; Furch, M.; Brenner, B.; Manstein, D.J.; Meyhöfer, E. Single-molecule tracking of myosins with genetically engineered amplifier domains. Nat. Struct. Biol. 2001, 8, 226–229. [Google Scholar] [CrossRef]
- Ikezaki, K.; Komori, T.; Sugawa, M.; Arai, Y.; Nishikawa, S.; Iwane, A.H.; Yanagida, T. Simultaneous observation of the lever arm and head explains myosin VI dual function. Small 2012, 8, 3035–3040. [Google Scholar] [CrossRef]
- Lynch, G.S.; Stephenson, D.G.; Williams, D.A. Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms. J. Muscle Res. Cell Motil. 1995, 16, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Saadaoui, M.; Dunsing, V.; Kerridge, S.; Da Silva, E.; Philippe, J.-M.; Maurange, C.; Lecuit, T. Serotonin signaling regulates actomyosin contractility during morphogenesis in evolutionarily divergent lineages. Nat. Commun. 2023, 14, 5547. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.; Chen, Y.; Brenner, B.; Gordon, A.M.; Kraft, T.; Martyn, D.A.; Regnier, M.; Rivera, A.J.; Wang, C.-K.; Chase, P.B. Familial hypertrophic cardiomyopathy mutations in troponin I (K183Δ, G203S, K206Q) enhance filament sliding. Physiol. Genom. 2003, 14, 117–128. [Google Scholar] [CrossRef]
- Barrick, S.K.; Greenberg, L.; Greenberg, M.J. A Troponin T Variant Linked with Pediatric Dilated Cardiomyopathy Reduces the Coupling of Thin Filament Activation to Myosin and Calcium Binding. Mol. Biol. Cell 2021, 32, 1677–1689. [Google Scholar] [CrossRef]
- Bing, W.; Knott, A.; Redwood, C.; Esposito, G.; Purcell, I.; Watkins, H.; Marston, S. Effect of hypertrophic cardiomyopathy mutations in human cardiac muscle α-tropomyosin (Asp175Asn and Glu180Gly) on the regulatory properties of human cardiac troponin determined by in vitro motility assay. J. Mol. Cell. Cardiol. 2000, 32, 1489–1498. [Google Scholar] [CrossRef]
- Bing, W.; Redwood, C.S.; Purcell, I.F.; Esposito, G.; Watkins, H.; Marston, S.B. Effects of two hypertrophic cardiomyopathy mutations in α-tropomyosin, Asp175Asn and Glu180Gly, on Ca2+ regulation of thin filament motility. Biochem. Biophys. Res. Commun. 1997, 236, 760–764. [Google Scholar] [CrossRef]
- Brunet, N.M.; Chase, P.B.; Mihajlović, G.; Schoffstall, B. Ca2+-regulatory function of the inhibitory peptide region of cardiac troponin I is aided by the C-terminus of cardiac troponin T: Effects of familial hypertrophic cardiomyopathy mutations cTnI R145G and cTnT R278C, alone and in combination, on filament sliding. Arch. Biochem. Biophys. 2014, 552–553, 11–20. [Google Scholar] [CrossRef]
- Burton, D.; Abdulrazzak, H.; Knott, A.; Elliott, K.; Redwood, C.; Watkins, H.; Marston, S.; Ashley, C. Two mutations in troponin I that cause hypertrophic cardiomyopathy have contrasting effects on cardiac muscle contractility. Biochem. J. 2002, 362 Pt 2, 443–451. [Google Scholar] [CrossRef]
- Fraser, I.D.C.; Marston, S.B. In vitro motility analysis of actin-tropomyosin regulation by troponin and calcium: The thin filament is switched as a single cooperative unit. J. Biol. Chem. 1995, 270, 7836–7841. [Google Scholar] [CrossRef]
- Redwood, C.; Lohmann, K.; Bing, W.; Esposito, G.M.; Elliott, K.; Abdulrazzak, H.; Knott, A.; Purcell, I.; Marston, S.; Watkins, H. Investigation of a truncated cardiac troponin T that causes familial hypertrophic cardiomyopathy: Ca2+ regulatory properties of reconstituted thin filaments depend on the ratio of mutant to wild-type protein. Circ. Res. 2000, 86, 1146–1152. [Google Scholar] [CrossRef]
- Wang, F.; Brunet, N.M.; Grubich, J.R.; Bienkiewicz, E.; Asbury, T.M.; Compton, L.A.; Mihajlović, G.; Miller, V.F.; Chase, P.B. Facilitated cross-bridge interactions with thin filaments by familial hypertrophic cardiomyopathy mutations in α-tropomyosin. J. Biomed. Biotechnol. 2011, 2011, 435271. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Martyn, D.A.; Chase, P.B. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys. J. 1998, 74, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Teitgen, A.E.; Hock, M.T.; McCabe, K.J.; Childers, M.C.; Huber, G.A.; Marzban, B.; Beard, D.A.; McCammon, J.A.; Regnier, M.; McCulloch, A.D. Multiscale modeling shows how 2′-deoxy-ATP rescues ventricular function in heart failure. Proc. Natl. Acad. Sci. USA 2024, 121, e2322077121. [Google Scholar] [CrossRef] [PubMed]
- Månsson, A.; Usaj, M.; Moretto, L.; Matusovsky, O.; Velayuthan, L.P.; Friedman, R.; Rassier, D.E. New paradigms in actomyosin energy transduction: Critical evaluation of non-traditional models for orthophosphate release. Bioessays 2023, 45, e2300040. [Google Scholar] [CrossRef]
- Månsson, A. Theoretical treatment of tension transients in muscle following sudden changes in orthophosphate concentration: Implications for energy transduction. J. Muscle Res. Cell Motil. 2025, 46, 193–213. [Google Scholar] [CrossRef]
- Månsson, A. Mechanistic insights into effects of the cardiac myosin activator omecamtiv mecarbil from mechanokinetic modelling. Front. Physiol. 2025, 16, 1576245. [Google Scholar] [CrossRef]
- Chase, P.B.; Brunet, N.M.; Mihajlović, G.; Xiong, P.; von Molnár, S. Molecular motor-based assays for altered nanomechanical function of Ca2+-regulatory proteins in cardiomyopathies. Mater. Res. Soc. Symp. Proc. 2008, 1096, 1096-FF02. [Google Scholar] [CrossRef]
- Robinson, P.; Griffiths, P.J.; Watkins, H.; Redwood, C.S. Dilated and hypertrophic cardiomyopathy mutations in troponin and a-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ. Res. 2007, 101, 1266–1273. [Google Scholar] [CrossRef]
- Donkervoort, S.; Papadaki, M.; de Winter, J.M.; Neu, M.B.; Kirschner, J.; Bolduc, V.; Yang, M.L.; Gibbons, M.A.; Hu, Y.; Dastgir, J.; et al. TPM3 deletions cause a hypercontractile congenital muscle stiffness phenotype. Ann. Neurol. 2015, 78, 982–994. [Google Scholar] [CrossRef]
- Song, W.; Dyer, E.; Stuckey, D.J.; Copeland, O.; Leung, M.C.; Bayliss, C.; Messer, A.; Wilkinson, R.; Tremoleda, J.L.; Schneider, M.D.; et al. Molecular mechanism of the E99K mutation in cardiac actin (ACTC Gene) that causes apical hypertrophy in man and mouse. J. Biol. Chem. 2011, 286, 27582–27593. [Google Scholar] [CrossRef]
- Harada, Y.; Noguchi, A.; Kishino, A.; Yanagida, T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature 1987, 326, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Sata, M.; Sugiura, S.; Momomura, S.; Serizawa, T.; Iizuka, M. ADP inhibits the sliding velocity of fluorescent actin filaments on cardiac and skeletal myosins. Circ. Res. 1994, 74, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Brunet, N.M.; Mihajlović, G.; Aledealat, K.; Wang, F.; Xiong, P.; von Molnár, S.; Chase, P.B. Micromechanical thermal assays of Ca2+-regulated thin-filament function and modulation by hypertrophic cardiomyopathy mutants of human cardiac troponin. J. Biomed. Biotechnol. 2012, 2012, 657523. [Google Scholar] [CrossRef]
- Kato, H.; Nishizaka, T.; Iga, T.; Kinosita, K., Jr.; Ishiwata, S. Imaging of thermal activation of actomyosin motors. Proc. Natl. Acad. Sci. USA 1999, 96, 9602–9606. [Google Scholar] [CrossRef]
- Rossi, R.; Maffei, M.; Bottinelli, R.; Canepari, M. Temperature dependence of speed of actin filaments propelled by slow and fast skeletal myosin isoforms. J. Appl. Physiol. 2005, 99, 2239–2245. [Google Scholar] [CrossRef][Green Version]
- Ishii, S.; Oyama, K.; Arai, T.; Itoh, H.; Shintani, S.A.; Suzuki, M.; Kobirumaki-Shimozawa, F.; Terui, T.; Fukuda, N.; Ishiwata, S. Microscopic heat pulses activate cardiac thin filaments. J. Gen. Physiol. 2019, 151, 860–869. [Google Scholar] [CrossRef]
- Ishii, S.; Oyama, K.; Kobirumaki-Shimozawa, F.; Nakanishi, T.; Nakahara, N.; Suzuki, M.; Ishiwata, S.; Fukuda, N. Myosin and tropomyosin-troponin complementarily regulate thermal activation of muscles. J. Gen. Physiol. 2023, 155, e202313414. [Google Scholar] [CrossRef]
- Homsher, E.; Lee, D.M.; Morris, C.; Pavlov, D.; Tobacman, L.S. Regulation of force and unloaded sliding speed in single thin filaments: Effects of regulatory proteins and calcium. J. Physiol. 2000, 524 Pt 1, 233–243. [Google Scholar] [CrossRef]
- Tobacman, L.S. Thin filament-mediated regulation of cardiac contraction. Annu. Rev. Physiol. 1996, 58, 447–481. [Google Scholar] [CrossRef]
- Melbacke, A.; Salhotra, A.; Ušaj, M.; Månsson, A. Improved longevity of actomyosin in vitro motility assays for sustainable lab-on-a-chip applications. Sci. Rep. 2024, 14, 22768. [Google Scholar] [CrossRef]
- Stewart, T.J.; Murthy, V.; Dugan, S.P.; Baker, J.E. Velocity of myosin-based actin sliding depends on attachment and detachment kinetics and reaches a maximum when myosin-binding sites on actin saturate. J. Biol. Chem. 2021, 297, 101178. [Google Scholar] [CrossRef]
- Vemula, V.; Huber, T.; Ušaj, M.; Bugyi, B.; Månsson, A. Myosin and gelsolin cooperate in actin filament severing and actomyosin motor activity. J. Biol. Chem. 2021, 296, 100181. [Google Scholar] [CrossRef]
- Riveline, D.; Ott, A.; Jülicher, F.; Winkelmann, D.A.; Cardoso, O.; Lacapère, J.J.; Magnúsdóttir, S.; Viovy, J.L.; Gorre-Talini, L.; Prost, J. Acting on actin: The electric motility assay. Eur. Biophys. J. 1998, 27, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, O.M.; Ziebert, F.; Schwarz, U.S. Optogenetic control of migration of contractile cells predicted by an active gel model. Commun. Phys. 2023, 6, 158. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miura, H.; Ishida, M.; Mii, Y.; Kinoshita, N.; Takada, S.; Ueno, N.; Sawai, S.; Kondo, Y.; Aoki, K. Optogenetic relaxation of actomyosin contractility uncovers mechanistic roles of cortical tension during cytokinesis. Nat. Commun. 2021, 12, 7145. [Google Scholar] [CrossRef] [PubMed]
- Ganji, E.; Chan, C.S.; Ward, C.W.; Killian, M.L. Optogenetic activation of muscle contraction in vivo. Connect. Tissue Res. 2021, 62, 15–23. [Google Scholar] [CrossRef]
- Matsubayashi, H.T.; Razavi, S.; Rock, T.W.; Nakajima, D.; Nakamura, H.; Kramer, D.A.; Matsuura, T.; Chen, B.; Murata, S.; Nomura, S.M.; et al. Light-guided actin polymerization drives directed motility in protocells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Yumerefendi, H.; Dickinson, D.J.; Wang, H.; Zimmerman, S.P.; Bear, J.E.; Goldstein, B.; Hahn, K.; Kuhlman, B. Control of Protein Activity and Cell Fate Specification via Light-Mediated Nuclear Translocation. PLoS ONE 2015, 10, e0128443. [Google Scholar] [CrossRef]
- Hiratsuka, Y.; Tada, T.; Oiwa, K.; Kanayama, T.; Uyeda, T.Q.P. Controlling the direction of kinesin-driven microtubule movements along microlithographic tracks. Biophys. J. 2001, 81, 1555–1561. [Google Scholar] [CrossRef]
- Sundberg, M.; Balaz, M.; Bunk, R.; Rosengren-Holmberg, J.P.; Montelius, L.; Nicholls, I.A.; Omling, P.; Tågerud, S.; Månsson, A. Selective spatial localization of actomyosin motor function by chemical surface patterning. Langmuir 2006, 22, 7302–7312. [Google Scholar] [CrossRef] [PubMed]
- Engin, S.; Fichtner, D.; Wedlich, D.; Fruk, L. SNAP-tag as a tool for surface immobilization. Curr. Pharm. Des. 2013, 19, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Sakamoto, R.; Miyazaki, M.; Maeda, Y.T. Myosin-driven advection and actin reorganization control the geometry of confined actomyosin gel. arXiv 2025, arXiv:2505.01717. [Google Scholar] [CrossRef]
- Murrell, M.; Oakes, P.W.; Lenz, M.; Gardel, M.L. Forcing cells into shape: The mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 2015, 16, 486–498. [Google Scholar] [CrossRef]
- Agarwal, P.; Zaidel-Bar, R. Principles of Actomyosin Regulation In Vivo. Trends Cell Biol. 2019, 29, 150–163. [Google Scholar] [CrossRef]
- Okeyo, K.O.; Adachi, T.; Sunaga, J.; Hojo, M. Actomyosin contractility spatiotemporally regulates actin network dynamics in migrating cells. J. Biomech. 2009, 42, 2540–2548. [Google Scholar] [CrossRef]
- Semenova, I.; Burakov, A.; Berardone, N.; Zaliapin, I.; Slepchenko, B.; Svitkina, T.; Kashina, A.; Rodionov, V. Actin dynamics is essential for myosin-based transport of membrane organelles. Curr. Biol. 2008, 18, 1581–1586. [Google Scholar] [CrossRef]
- Titus, M.A. Myosin-Driven Intracellular Transport. Cold Spring Harb. Perspect. Biol. 2018, 10, a021972. [Google Scholar] [CrossRef]
- Martin, A.C.; Kaschube, M.; Wieschaus, E.F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009, 457, 495–499. [Google Scholar] [CrossRef]
- Green, R.A.; Paluch, E.; Oegema, K. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 2012, 28, 29–58. [Google Scholar] [CrossRef]
- Fang, N.N.; Ng, A.H.; Measday, V.; Mayor, T. Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins. Nat. Cell Biol. 2011, 13, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Rzadzinska, A.K.; Schneider, M.E.; Davies, C.; Riordan, G.P.; Kachar, B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J. Cell Biol. 2004, 164, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Rahman, M.A.; Ruijgrok, P.V.; Meinecke, C.R.; Ušaj, M.; Zemsky, S.; Lindberg, F.W.; Surendiran, P.; Lyttleton, R.W.; Linke, H.; et al. Exploitation of Engineered Light-Switchable Myosin XI for Nanotechnological Applications. ACS Nano 2023, 17, 17233–17244. [Google Scholar] [CrossRef]
- Agarwal, A.; Hess, H. Biomolecular motors at the intersection of nanotechnology and polymer science. Prog. Polym. Sci. 2010, 35, 252–277. [Google Scholar] [CrossRef]
- Persson, M.; Gullberg, M.; Tolf, C.; Lindberg, A.M.; Månsson, A.; Kocer, A. Transportation of nanoscale cargoes by myosin propelled actin filaments. PLoS ONE 2013, 8, e55931. [Google Scholar] [CrossRef]
- Lard, M.; ten Siethoff, L.; Kumar, S.; Persson, M.; te Kronnie, G.; Linke, H.; Månsson, A. Ultrafast molecular motor driven nanoseparation and biosensing. Biosens. Bioelectron. 2013, 48, 145–152. [Google Scholar] [CrossRef]
- Sundberg, M.; Bunk, R.; Albet-Torres, N.; Kvennefors, A.; Persson, F.; Montelius, L.; Nicholls, I.A.; Ghatnekar-Nilsson, S.; Omling, P.; Tågerud, S.; et al. Actin filament guidance on a chip: Toward high-throughput assays and lab-on-a-chip applications. Langmuir 2006, 22, 7286–7295. [Google Scholar] [CrossRef]
- Miranda, M.S.; Lyttleton, R.; Siu, P.H.; Diez, S.; Linke, H.; Micolich, A.P. Prospects for single-molecule electrostatic detection in molecular motor gliding motility assays. New J. Phys. 2021, 23, 065003. [Google Scholar] [CrossRef]
- Yoshida, K.; Kohno, K.; Hiratsuka, Y.; Onoe, H. Macroscale Collagen-Actomyosin Hybrid Actuator Built from Bioderived Materials. Adv. Funct. Mater. 2023, 33, 2307766. [Google Scholar] [CrossRef]
- Heisser, R.H.; Bawa, M.; Shah, J.; Bu, A.; Raman, R. Soft Biological Actuators for Meter-Scale Homeostatic Biohybrid Robots. Chem. Rev. 2025, 125, 3976–4007. [Google Scholar] [CrossRef]
- Shi, M.; Yeatman, E.M. A comparative review of artificial muscles for microsystem applications. Microsyst. Nanoeng. 2021, 7, 95. [Google Scholar] [CrossRef]
- Mihajlović, G.; Brunet, N.M.; Trbović, J.; Xiong, P.; von Molnár, S.; Chase, P.B. All-electrical switching and control mechanism for actomyosin-powered nanoactuators. Appl. Phys. Lett. 2004, 85, 1060–1062. [Google Scholar] [CrossRef]
- Jaber, J.A.; Chase, P.B.; Schlenoff, J.B. Actomyosin driven motility on patterned polyelectrolyte mono- and multilayers. Nano Lett. 2003, 3, 1505–1509. [Google Scholar] [CrossRef]
- Huang, L.; Manandhar, P.; Byun, K.-E.; Chase, P.B.; Hong, S. Selective assembly and alignment of actin filaments with desired polarity on solid substrates. Langmuir 2006, 22, 8635–8638. [Google Scholar] [CrossRef] [PubMed]
- Albet-Torres, N.; O’Mahony, J.; Charlton, C.; Balaz, M.; Lisboa, P.; Aastrup, T.; Månsson, A.; Nicholls, I.A. Mode of heavy meromyosin adsorption and motor function correlated with surface hydrophobicity and charge. Langmuir 2007, 23, 11147–11156. [Google Scholar] [CrossRef]
- Bunk, R.; Klinth, J.; Montelius, L.; Nicholls, I.A.; Omling, P.; Tågerud, S.; Månsson, A. Actomyosin motility on nanostructured surfaces. Biochem. Biophys. Res. Commun. 2003, 301, 783–788. [Google Scholar] [CrossRef]
- Bunk, R.; Sundberg, M.; Månsson, A.; Nicholls, I.A.; Omling, P.; Tågerud, S.; Montelius, L. Guiding motor-propelled molecules with nanoscale precision through silanized bi-channel structures. Nanotechnology 2005, 16, 710–717. [Google Scholar] [CrossRef]
- Sundberg, M.; Rosengren, J.P.; Bunk, R.; Lindahl, J.; Nicholls, I.A.; Tågerud, S.; Omling, P.; Montelius, L.; Månsson, A. Silanized surfaces for in vitro studies of actomyosin function and nanotechnology applications. Anal. Biochem. 2003, 323, 127–138. [Google Scholar] [CrossRef]
- Lindberg, F.W.; Norrby, M.; Rahman, M.A.; Salhotra, A.; Takatsuki, H.; Jeppesen, S.; Linke, H.; Månsson, A. Controlled Surface Silanization for Actin-Myosin Based Nanodevices and Biocompatibility of New Polymer Resists. Langmuir 2018, 34, 8777–8784. [Google Scholar] [CrossRef]
- Grove, T.J.; Puckett, K.A.; Brunet, N.M.; Mihajlović, G.; McFadden, L.A.; Xiong, P.; von Molnár, S.; Moerland, T.S.; Chase, P.B. Packaging actomyosin-based biomolecular motor-driven devices for nanoactuator applications. IEEE Trans. Adv. Packag. 2005, 28, 556–563. [Google Scholar] [CrossRef]
- Debold, E.P.; Beck, S.E.; Warshaw, D.M. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am. J. Physiol. Cell Physiol. 2008, 295, C173–C179. [Google Scholar] [CrossRef]
- Sich, N.M.; O’Donnell, T.J.; Coulter, S.A.; John, O.A.; Carter, M.S.; Cremo, C.R.; Baker, J.E. Effects of actin-myosin kinetics on the calcium sensitivity of regulated thin filaments. J. Biol. Chem. 2010, 285, 39150–39159. [Google Scholar] [CrossRef][Green Version]
- Rahman, M.A.; Ušaj, M.; Rassier, D.E.; Månsson, A. Blebbistatin Effects Expose Hidden Secrets in the Force-Generating Cycle of Actin and Myosin. Biophys. J. 2018, 115, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Turney, S.G.; Ahmed, M.; Chandrasekar, I.; Wysolmerski, R.B.; Goeckeler, Z.M.; Rioux, R.M.; Whitesides, G.M.; Bridgman, P.C. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Mol. Biol. Cell 2016, 27, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Homsher, E.; Kim, B.; Bobkova, A.; Tobacman, L.S. Calcium regulation of thin filament movement in an in vitro motility assay. Biophys. J. 1996, 70, 1881–1892. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lard, M.; ten Siethoff, L.; Månsson, A.; Linke, H. Tracking actomyosin at fluorescence check points. Sci. Rep. 2013, 3, 1092. [Google Scholar] [CrossRef]
- Kekic, M.; Hanson, K.L.; Perumal, A.S.; Solana, G.; Rajendran, K.; Dash, S.; Nicolau, D.V., Jr.; Dobroiu, S.; Dos Remedios, C.G.; Nicolau, D.V. Biosensing using antibody-modulated motility of actin filaments on myosin-coated surfaces. Biosens. Bioelectron. 2024, 246, 115879. [Google Scholar] [CrossRef]
- Mihajlović, G.; Xiong, P.; von Molnár, S.; Ohtani, K.; Ohno, H.; Field, M.; Sullivan, G.J. Detection of single magnetic bead for biological applications using an InAs quantum well micro-Hall sensor. Appl. Phys. Lett. 2005, 87, 112502. [Google Scholar] [CrossRef]
- Aledealat, K.; Mihajlović, G.; Chen, K.-S.; Field, M.; Sullivan, G.J.; Xiong, P.; Chase, P.B.; von Molnár, S. Dynamic micro-Hall detection of superparamagnetic beads in a microfluidic channel. J. Magn. Magn. Mater. 2010, 322, L69–L72. [Google Scholar] [CrossRef][Green Version]
- Hira, S.M.; Aledealat, K.; Chen, K.-S.; Field, M.; Sullivan, G.J.; Chase, P.B.; Xiong, P.; von Molnár, S.; Strouse, G.F. Detection of target ssDNA using a micro-fabricated Hall magnetometer with correlated optical readout. J. Biomed. Biotechnol. 2012, 2012, 492730. [Google Scholar] [CrossRef]
- Manandhar, P.; Chen, K.-S.; Aladealat, K.; Mihajlović, G.; Yun, C.S.; Field, M.; Sullivan, G.J.; Strouse, G.F.; Chase, P.B.; von Molnár, S.; et al. The detection of specific biomolecular interactions with micro-Hall magnetic sensors. Nanotechnology 2009, 20, 355501. [Google Scholar] [CrossRef]
- Labugger, R.; Organ, L.; Collier, C.; Atar, D.; Van Eyk, J.E. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation 2000, 102, 1221–1226. [Google Scholar] [CrossRef]
- McGrath, S.; Alaour, B.; Kampourakis, T.; Marber, M. Cardiac Troponin: Fragments of the Future? JACC Adv. 2025, 4, 101695. [Google Scholar] [CrossRef]
- Salonen, S.M.; Tuominen, T.J.K.; Raiko, K.I.S.; Vasankari, T.; Aalto, R.; Hellman, T.A.; Lahtinen, S.E.; Soukka, T.; Juhani Airaksinen, K.E.; Wittfooth, S.T. Highly Sensitive Immunoassay for Long Forms of Cardiac Troponin T Using Upconversion Luminescence. Clin. Chem. 2024, 70, 1037–1045. [Google Scholar] [CrossRef]
- Wu, A.H.B.; Feng, Y.-J.; Moore, R.; Apple, F.S.; McPherson, P.H.; Buechler, K.F.; Bodor, G. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin. Chem. 1998, 44 Pt 1, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, K.-S.; Meyer, N.L.; Yuan, J.; Hirst, L.S.; Chase, P.B.; Xiong, P. Functionalized SnO2 nanobelt field-effect transistor sensors for label-free detection of cardiac troponin. Biosens. Bioelectron. 2011, 26, 4538–4544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Yang, S.-G.; Zhang, C.; Hu, M.-W.; Qian, J.; Ma, J.-J.; Zhang, Y.; Yang, B.-B.; Weng, Y.-L.; Ming, G.-L.; et al. Knocking Out Non-muscle Myosin II in Retinal Ganglion Cells Promotes Long-Distance Optic Nerve Regeneration. Cell Rep. 2020, 31, 107537. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, K.; Maurer, S.J.; Weber, C.; Bücher, J.E.H.; Schoenit, A.; D’Este, E.; Cavalcanti-Adam, E.A.; Göpfrich, K. Actomyosin-Assisted Pulling of Lipid Nanotubes from Lipid Vesicles and Cells. Nano Lett. 2022, 22, 1145–1150. [Google Scholar] [CrossRef]
- Talaat, F.M.; Ali, Z.H.; Mostafa, R.R.; El-Rashidy, N. Real-time facial emotion recognition model based on kernel autoencoder and convolutional neural network for autism children. Soft. Comput. 2024, 28, 6695–6708. [Google Scholar] [CrossRef]
- Talaat, M.; Tayseer, M.; Farahat, M.A.; Song, D. Artificial intelligence strategies for simulating the integrated energy systems. Artif. Intell. Rev. 2024, 57, 106. [Google Scholar] [CrossRef]
- Hosney, R.; Talaat, F.M.; El-Gendy, E.M.; Saafan, M.M. AutYOLO-ATT: An attention-based YOLOv8 algorithm for early autism diagnosis through facial expression recognition. Neural. Comput. Appl. 2024, 36, 17199–17219. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Iost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chase, P.B.; Kushmerick, M.J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys. J. 1988, 53, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Pate, E.; Bhimani, M.; Franks-Skiba, K.; Cooke, R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: Implications for fatigue. J. Physiol. 1995, 486, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Pate, E.; Cooke, R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflügers Arch. 1989, 414, 73–81. [Google Scholar] [CrossRef]
- Cooke, R.; Franks, K.; Luciani, G.B.; Pate, E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J. Physiol. 1988, 395, 77–97. [Google Scholar] [CrossRef]
- Wiseman, R.W.; Brown, C.M.; Beck, T.W.; Brault, J.J.; Reinoso, T.R.; Shi, Y.; Chase, P.B. Creatine Kinase Equilibration and ΔGATP Over an Extended Range of Physiological Conditions: Implications for Cellular Energetics, Signaling and Muscle Performance. Int. J. Mol. Sci. 2023, 24, 13244. [Google Scholar] [CrossRef]
- Godt, R.E.; Nosek, T.M. Changes in intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J. Physiol. 1989, 412, 155–180. [Google Scholar] [CrossRef]
- Nosek, T.M.; Leal-Cardoso, J.H.; McLaughlin, M.; Godt, R.E. Inhibitory influence of phosphate and arsenate on contraction of skinned skeletal and cardiac muscle. Am. J. Physiol. 1990, 259 Pt 1, C933–C939. [Google Scholar] [CrossRef]
- Nosek, T.M.; Fender, K.Y.; Godt, R.E. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 1987, 236, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Debold, E.P.; Romatowski, J.G.; Fitts, R.H. The depressive effect of Pi on the force-calcium relationship in skinned single muscle fibers is temperature dependent. Am. J. Physiol. Cell Physiol. 2006, 290, C1041–C1050. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.; Debold, E.P. Acidosis and Phosphate Directly Reduce Myosin’s Force-Generating Capacity Through Distinct Molecular Mechanisms. Front. Physiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Reisler, E. Sulfhydryl modification and labeling of myosin. Methods Enzymol. 1982, 85, 84–93. [Google Scholar] [CrossRef]
- Barakat, J.M.; Modica, K.J.; Lu, L.; Anujarerat, S.; Choi, K.H.; Takatori, S.C. Surface Topography Induces and Orients Nematic Swarms of Active Filaments: Considerations for Lab-On-A-Chip Devices. ACS Appl. Nano Mater. 2024, 7, 12142–12152. [Google Scholar] [CrossRef]
- Ishigure, Y.; Nitta, T. Simulating an Actomyosin in Vitro Motility Assay: Toward the Rational Design of Actomyosin-Based Microtransporters. IEEE Trans. Nanobioscience 2015, 14, 641–648. [Google Scholar] [CrossRef]
- Webb, M.R. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc. Natl. Acad. Sci. USA 1992, 89, 4884–4887. [Google Scholar] [CrossRef]
- Pate, E.; Franks-Skiba, K.; Cooke, R. Depletion of phosphate in active muscle fibers probes actomyosin states within the powerstroke. Biophys. J. 1998, 74, 369–380. [Google Scholar] [CrossRef][Green Version]
| Class | Example(s) | Actuation Trigger | Potential Biomedical Applications |
|---|---|---|---|
| Natural Protein Motors | Myosin, Kinesin, Dynein | ATP, GTP | Targeted transport, neural interfaces, synthetic muscles |
| Rotary Enzymes | F1-ATPase, bacterial flagella | Proton gradient, ATP hydrolysis | Bioelectronic coupling, molecular stirring/mixing |
| DNA-Based Walkers | DNA walkers, spiders | Strand displacement, enzyme fuel | Molecular diagnostics, intracellular tracking |
| Synthetic Molecular Motors | Feringa motors, rotaxanes, catenanes | Light, redox, pH | Drug release, chemical computation |
| Stimuli-Responsive Polymers | PNIPAM, polyacrylamide derivatives | Heat, pH, light | Drug delivery, dynamic scaffolds |
| Nanoparticle Systems | Magnetic NPs, gold NPs, quantum dots | Magnetic field, light | Imaging, hyperthermia, theranostics |
| Motor Protein | Track | Directionality * | Typical Step Size | Velocity | Primary Function | Nanotech Applications |
|---|---|---|---|---|---|---|
| Myosin | Actin filaments | + end (varies by isoform); − end (e.g., Myosin VI) | 5–36 nm | ~0.1–5 µm/s | Muscle contraction, short-range intracellular transport | Bioactuators, contractile scaffolds, responsive biosensors |
| Kinesin | Microtubules | + end (most isoforms); − end (e.g., Kinesin-14) | 8 nm | ~0.5–2 µm/s | Anterograde transport (e.g., vesicles, organelles) | Cargo transport on microtubule tracks, molecular sorters |
| Dynein | Microtubules | – end | 8–32 nm | ~1–14 µm/s | Retrograde transport, mitosis, ciliary and flagellar motion | Directional switches, retrograde cargo delivery, motile microsensors |
| Control Method | Precision | Reversibility | Biocompatibility | Scalability | Notes |
|---|---|---|---|---|---|
| ATP/Ca2+ Regulation | Moderate | High | High | High | Direct physiological mimicry |
| Thermal Control | High | High | Moderate | Moderate | Strong for MEMS * integration |
| Electrical Stimulation | High | Moderate | Moderate | High | Interface-ready but risk of side effects |
| Optical/Photothermal | Very High | High | High (with care) | Moderate | Allows contact-free, localized control |
| Surface Patterning | Moderate | Low | High | High | Ideal for guidance, not activation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunet, N.M.; Xiong, P.; Chase, P.B. Actomyosin-Based Nanodevices for Sensing and Actuation: Bridging Biology and Bioengineering. Biosensors 2025, 15, 672. https://doi.org/10.3390/bios15100672
Brunet NM, Xiong P, Chase PB. Actomyosin-Based Nanodevices for Sensing and Actuation: Bridging Biology and Bioengineering. Biosensors. 2025; 15(10):672. https://doi.org/10.3390/bios15100672
Chicago/Turabian StyleBrunet, Nicolas M., Peng Xiong, and Prescott Bryant Chase. 2025. "Actomyosin-Based Nanodevices for Sensing and Actuation: Bridging Biology and Bioengineering" Biosensors 15, no. 10: 672. https://doi.org/10.3390/bios15100672
APA StyleBrunet, N. M., Xiong, P., & Chase, P. B. (2025). Actomyosin-Based Nanodevices for Sensing and Actuation: Bridging Biology and Bioengineering. Biosensors, 15(10), 672. https://doi.org/10.3390/bios15100672





