Exhaled Aldehydes and Ketones as Biomarkers of Lung Cancer and Diabetes: Review of Sensor Technologies for Early Disease Diagnosis
Abstract
1. Introduction
2. Potential Volatile Biomarkers in Exhaled Breath
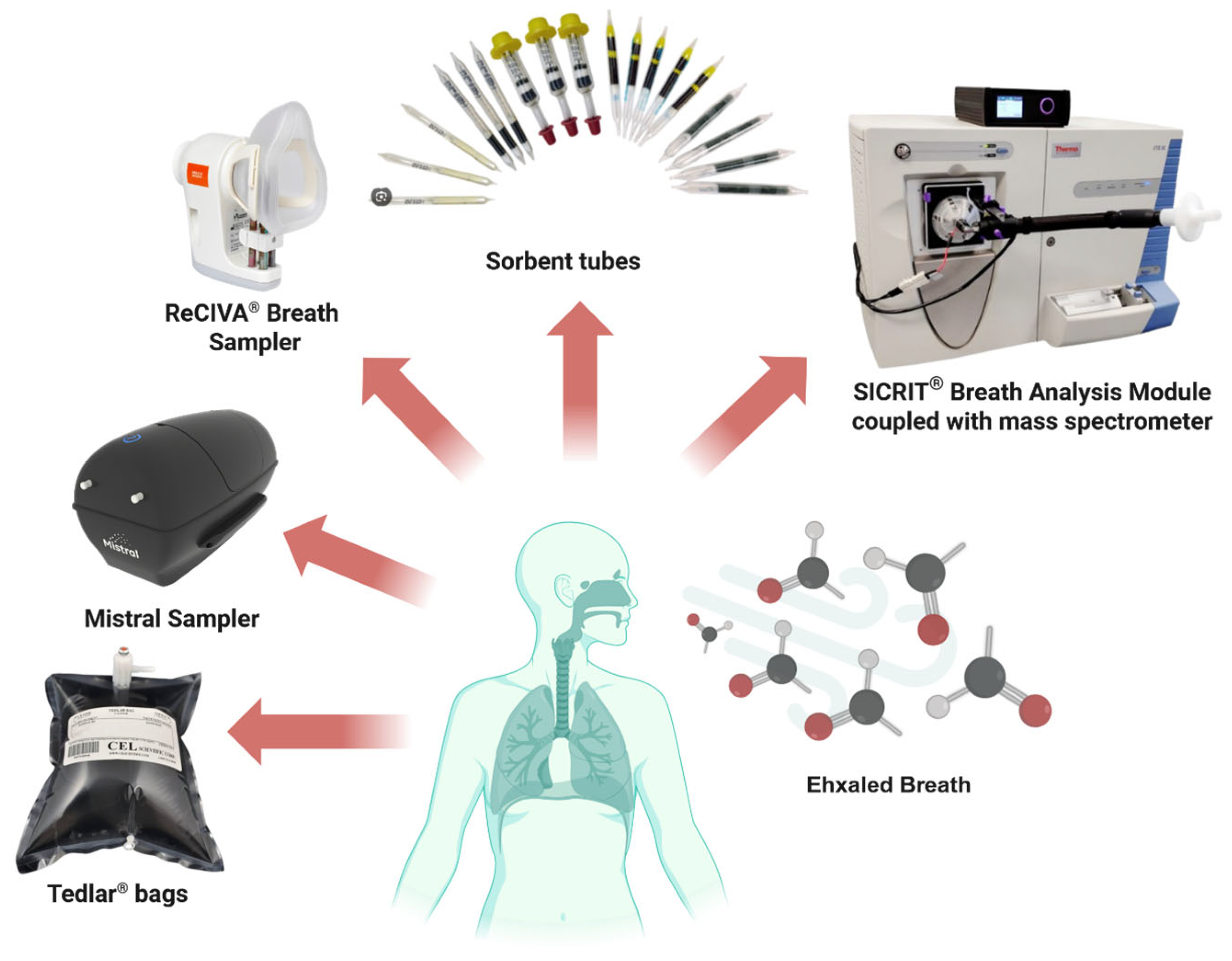
2.1. Sampling Methodologies for Exhaled Air
2.2. Aldehydes and Ketones as Key Biomarkers Present in Exhaled Breath
2.3. Current Limitations and Challenges in Disease Diagnosis Via Exhaled VOCs
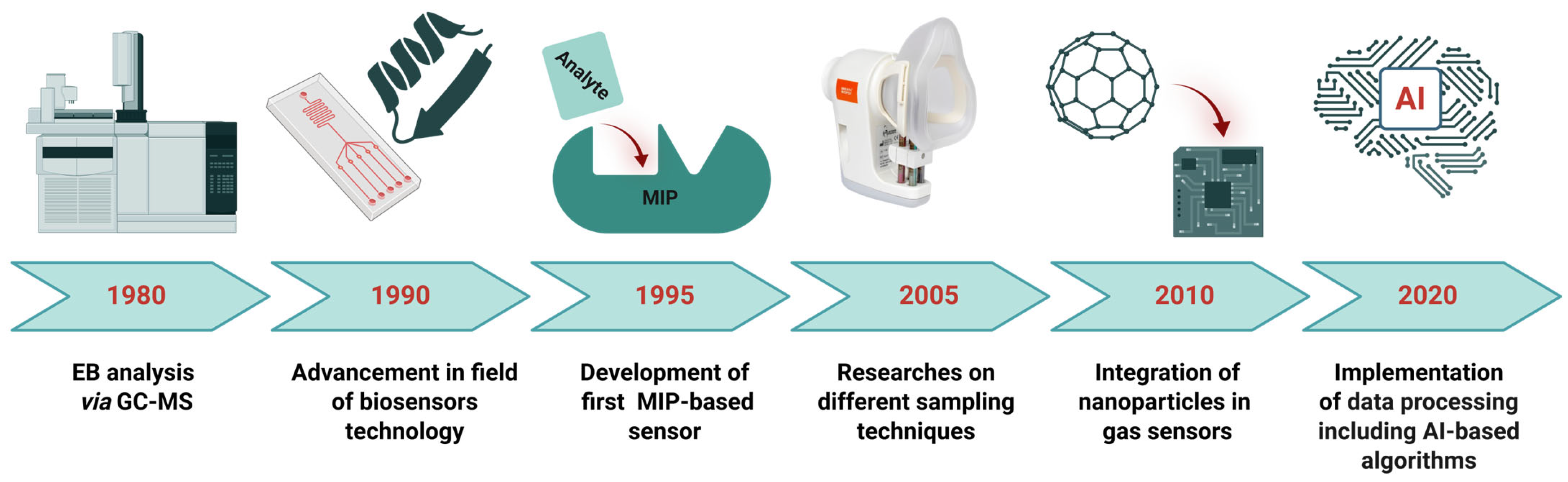
3. Conventional Approaches for VOCs Analysis
4. Gas Sensors for the Detection of Aldehydes and Ketones Present in EB
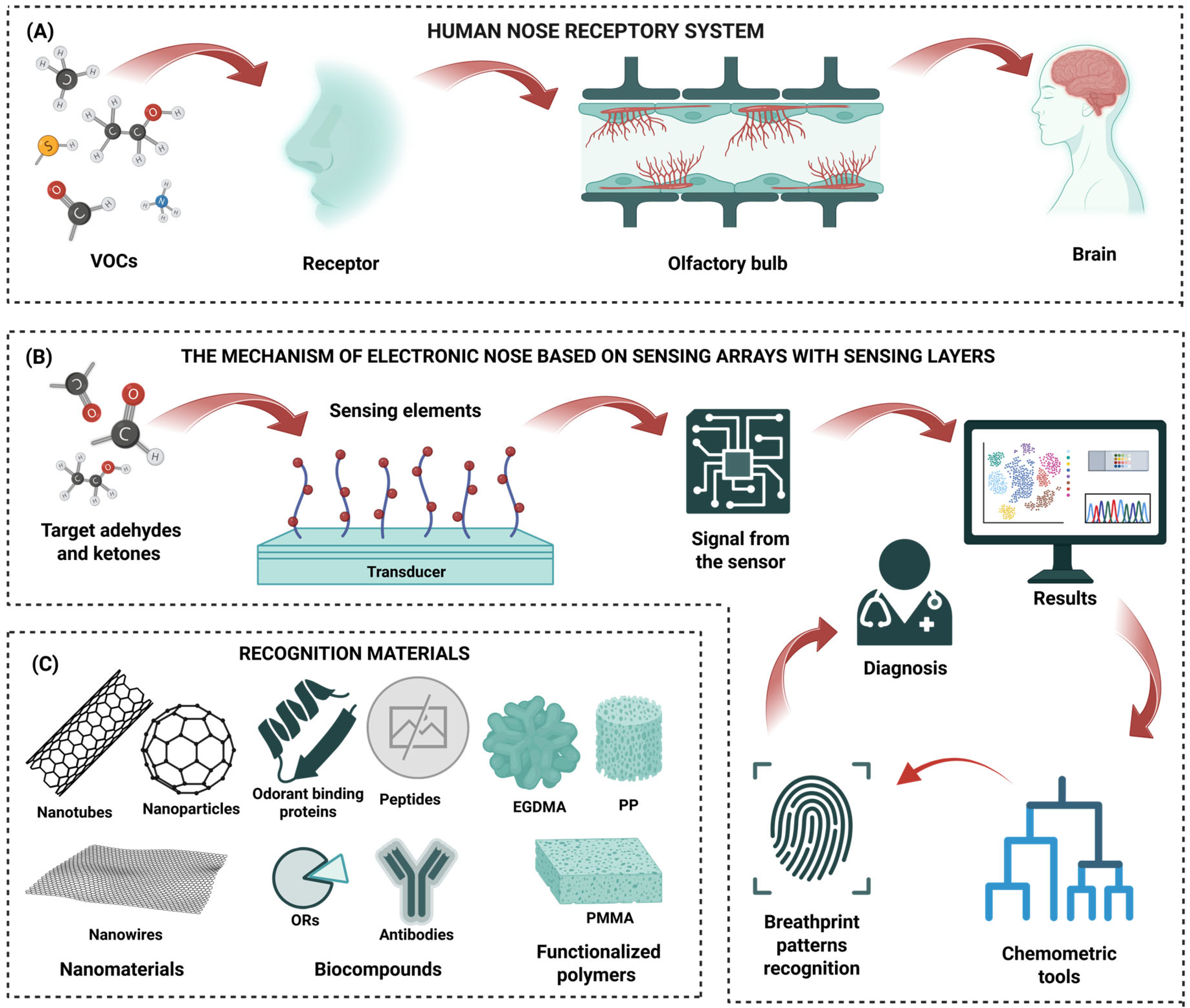
4.1. Electronic Nose Devices
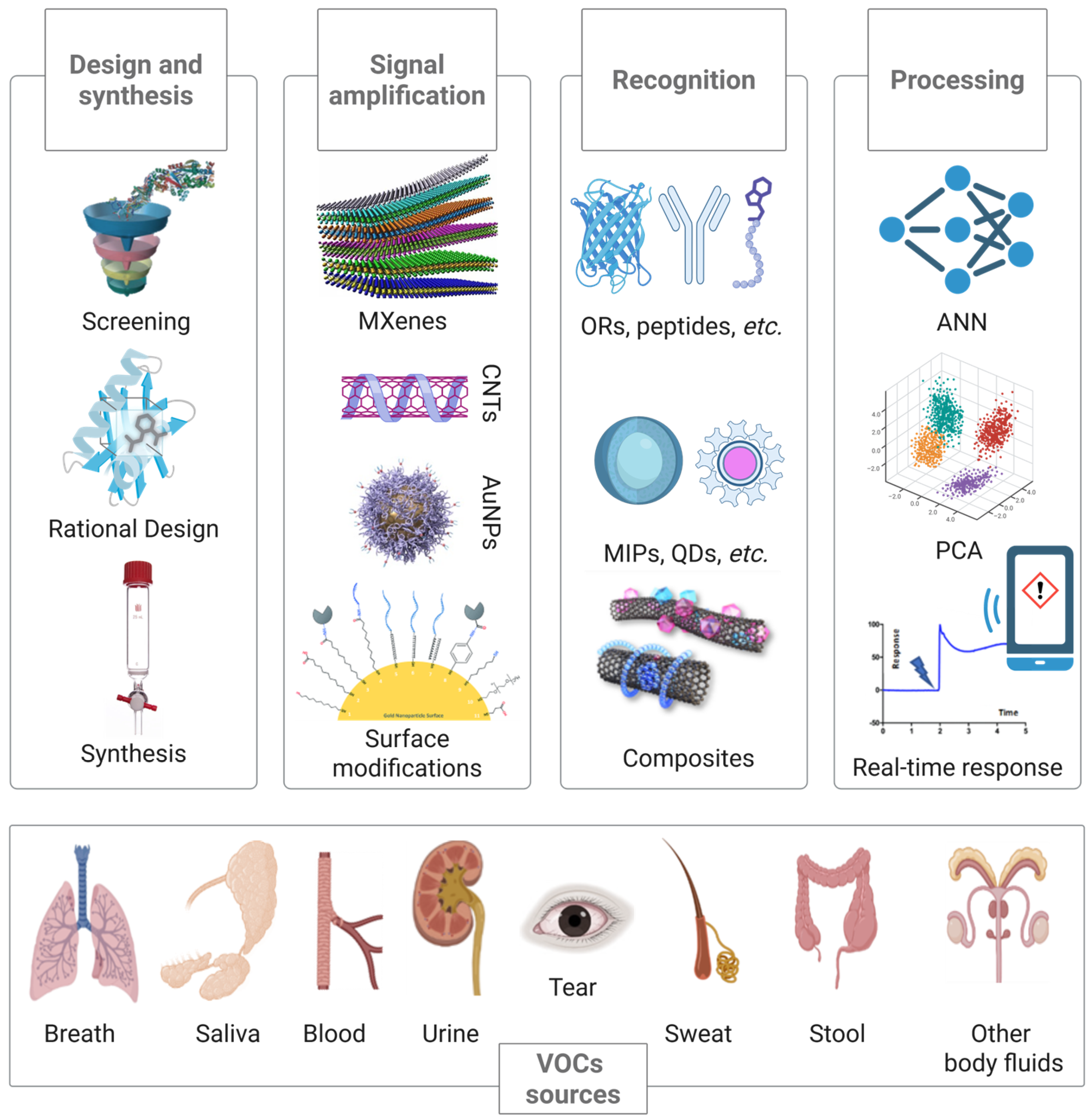
4.2. The Role of Nanostructured Recognition Materials
4.3. Bioreceptors
4.4. Molecularly Imprinted Polymers
4.5. Overview of Functional Materials as Sensors for EB Analysis
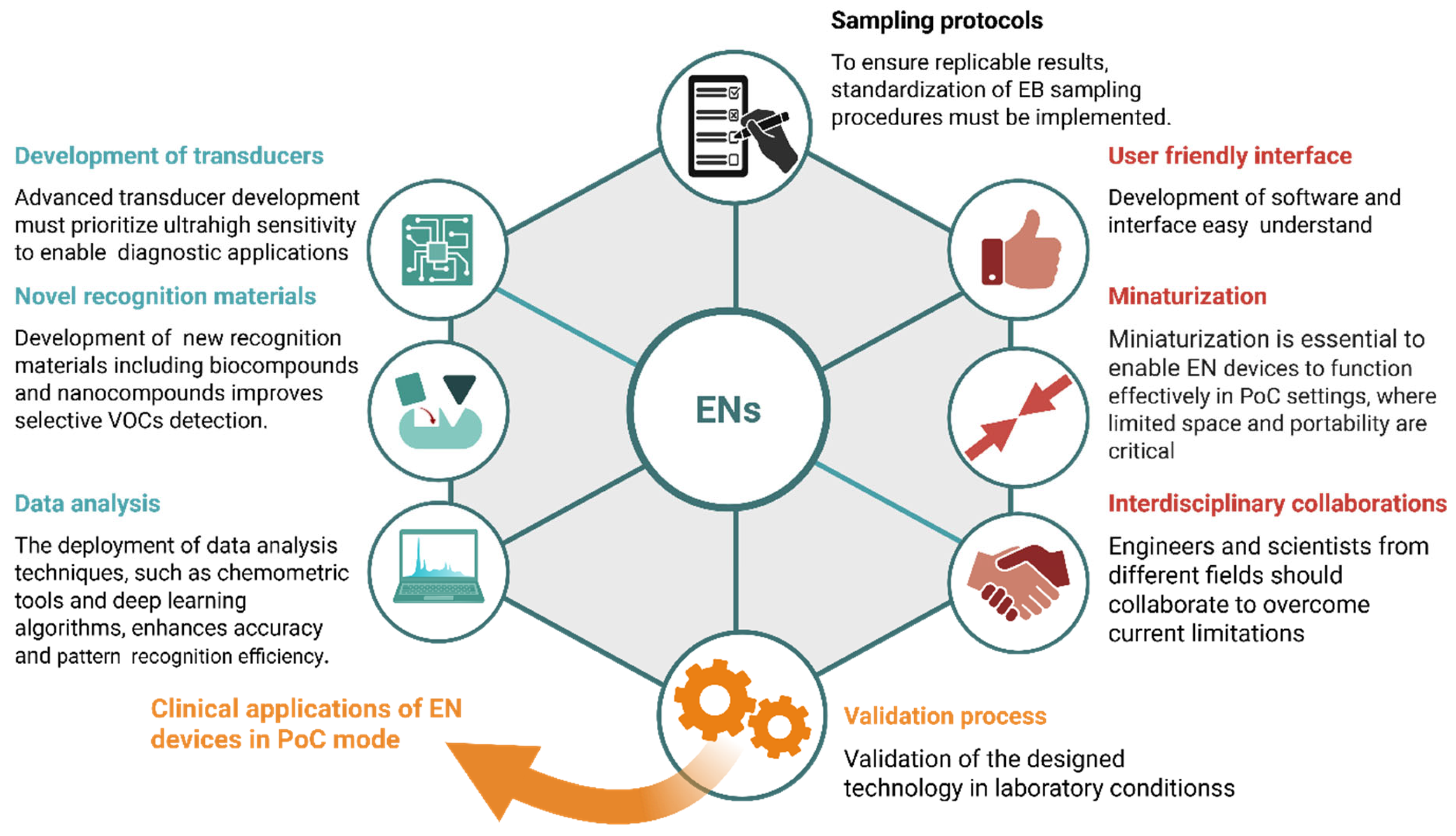
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| EB | Exhaled Breath |
| EN | Electronic Nose |
| FET | Field-Effect Transistor |
| GC-IMS | Gas Chromatography–Ion Mobility Spectrometry |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| LC | Lung Cancer |
| LOD | Limit of Detection |
| MIPs | Molecularly Imprinted Polymers |
| ML | Machine Learning |
| MOS | Metal–Oxide Semiconductor |
| NPs | Nanoparticles |
| OBP | Odorant-Binding Protein |
| OBPP | Odorant-Binding Protein-Derived Peptide |
| PoC | Point-of-Care |
| PPB | Parts per Billion |
| PPM | Parts per Million |
| PTR-MS | Proton Transfer Reaction–Mass Spectrometry |
| QCM | Quartz Crystal Microbalance |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SIFT-MS | Selected Ion Flow Tube–Mass Spectrometry |
| VOCs | Volatile Organic Compounds |
References
- Bag, A.; Lee, N.E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Aina, O.E.; Zine, N.; Raffin, G.; Jaffrezic-Renault, N.; Elaissari, A.; Errachid, A. Integrated Breath Analysis Technologies: Current Advances and Future Prospects. TrAC Trends Anal. Chem. 2024, 181, 118048. [Google Scholar] [CrossRef]
- Rowan, D.D. Volatile Metabolites. Metabolites 2011, 1, 41. [Google Scholar] [CrossRef]
- Käser, T.; Giannoukos, S.; Zenobi, R. Challenges in the Identification and Quantitation in On-Line Breath Analysis. J. Breath. Res. 2025, 19, 036002. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Chen, Q.; Pan, Z.; Chen, J.; Sun, M.; Wang, J.; Li, Y.; Ye, Q. Development and Validation of a Screening Model for Lung Cancer Using Machine Learning: A Large-Scale, Multi-Center Study of Biomarkers in Breath. Front. Oncol. 2022, 12, 975563. [Google Scholar] [CrossRef]
- Feng, Z.; Pepe, M.S. Adding Rigor to Biomarker Evaluations-EDRN Experience. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2575–2582. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Aligayev, A.; Jiang, H.; Muhammad; Wang, S.; Yu, X.; Tao, J.; Wang, J.; Jastrzębska, A.; et al. Dual-Mode SERS and Colorimetric Sensor for Lung Cancer VOC-Biomarker Detection Using Hydrogel Patches. Chem. Eng. J. 2025, 523, 168343. [Google Scholar] [CrossRef]
- Levart, A.; Veber, M. Determination of Aldehydes and Ketones in Air Samples Using Cryotrapping Sampling. Chemosphere 2001, 44, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rodríguez, M.; Ruiz-Montoya, M.; Giraldez, I.; López, R.; Madejón, E.; Díaz, M.J. Use of Electronic Nose and GC-MS in Detection and Monitoring Some VOC. Atmos. Environ. 2012, 51, 278–285. [Google Scholar] [CrossRef]
- American Lung Association. 2020: The Year We Lost Our Breath. Available online: https://www.lung.org/blog/2020-breath (accessed on 15 July 2025).
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath Gas Aldehydes as Biomarkers of Lung Cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yang, X. Volatile Organic Compound Emissions from the Human Body: Decoupling and Comparison between Whole-Body Skin and Breath Emissions. Build. Environ. 2022, 226, 109713. [Google Scholar] [CrossRef]
- Floss, M.A.; Fink, T.; Maurer, F.; Volk, T.; Kreuer, S.; Müller-Wirtz, L.M. Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review. Molecules 2022, 27, 5258. [Google Scholar] [CrossRef]
- WHO. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. WHO. 9 December 2020. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 30 April 2025).
- WHO. The Top 10 Causes of Death. WHO. 7 August 2024. Available online: https://www.who.int/news-Room/fact-Sheets/detail/the-Top-10-Causes-of-Death (accessed on 11 April 2025).
- Chaudhary, V.; Taha, B.A.; Lucky, N.; Rustagi, S.; Khosla, A.; Papakonstantinou, P.; Bhalla, N. Nose-on-Chip Nanobiosensors for Early Detection of Lung Cancer Breath Biomarkers. ACS Sens. 2024, 9, 4469–4494. [Google Scholar] [CrossRef]
- Bousamra, M.; Schumer, E.; Li, M.; Knipp, R.J.; Nantz, M.H.; Van Berkel, V.; Fu, X.A. Quantitative Analysis of Exhaled Carbonyl Compounds Distinguishes Benign from Malignant Pulmonary Disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 1074–1081. [Google Scholar] [CrossRef]
- Campanella, A.; De Summa, S.; Tommasi, S. Exhaled Breath Condensate Biomarkers for Lung Cancer. J. Breath. Res. 2019, 13, 044002. [Google Scholar] [CrossRef]
- Swargiary, K.; Thaneerat, S.; Kongsawang, N.; Pathak, A.K.; Viphavakit, C. Highly Sensitive and Real-Time Detection of Acetone Biomarker for Diabetes Using a ZnO-Coated Optical Fiber Sensor. Biosens. Bioelectron. 2025, 271, 117061. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.D.; Vernick, S. The Emergence of Insect Odorant Receptor-Based Biosensors. Biosensors 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Baroni, S.; Urbani, A.; Greco, V. Mapping of Urinary Volatile Organic Compounds by a Rapid Analytical Method Using Gas Chromatography Coupled to Ion Mobility Spectrometry (GC–IMS). Metabolites 2022, 12, 1072. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Y.; Song, X.; Liu, Y.; Xie, Y.; Xu, L.; Yu, J.; Qiu, L.; Wang, X.; Lin, J. Application and Development of SERS Technology in Detection of VOC Gases. Mater. Chem. Front. 2025, 9, 349–366. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Shi, W.; Xie, Y.; Liu, A.; Xu, L.; Qiu, L.; Song, X.; Zhang, M.; Zhang, J.; et al. Single-Atom Cu Anchored on a UiO-66 Surface-Enhanced Raman Scattering Sensor for Trace and Rapid Detection of Volatile Organic Compounds. Research 2025, 8, 0841. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as Complementary Techniques for Analysis of Volatiles: A Tutorial Review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. Quantification of Volatile Metabolites in Exhaled Breath by Selected Ion Flow Tube Mass Spectrometry, SIFT-MS. Clin. Mass Spectrom. 2020, 16, 18–24. [Google Scholar] [CrossRef]
- Suzuki, R.; Emura, T.; Tokura, Y.; Kawamura, N.; Hori, Y. A Quartz Crystal Microbalance Based Portable Gas Sensing Platform for On-Demand Human Breath Monitoring. IEEE Access 2020, 8, 146166–146171. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- van der Sar, I.G.; Wijbenga, N.; Nakshbandi, G.; Aerts, J.G.J.V.; Manintveld, O.C.; Wijsenbeek, M.S.; Hellemons, M.E.; Moor, C.C. The Smell of Lung Disease: A Review of the Current Status of Electronic Nose Technology. Respir. Res. 2021, 22, 246. [Google Scholar] [CrossRef]
- Viccione, G.; Zarra, T.; Giuliani, S.; Naddeo, V.; Belgiorno, V. Performance Study of E-Nose Measurement Chamber for Environmental Odour Monitoring. Chem. Eng. Trans. 2012, 30, 109–114. [Google Scholar] [CrossRef]
- Rodríguez-Torres, M.; Altuzar, V.; Mendoza-Barrera, C.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J.; Muñoz-Aguirre, S. Acetone Detection and Classification as Biomarker of Diabetes Mellitus Using a Quartz Crystal Microbalance Gas Sensor Array. Sensors 2023, 23, 9823. [Google Scholar] [CrossRef]
- Chaudhary, V.; Rustagi, S.; Kaushik, A. Bio-Derived Smart Nanostructures for Efficient Biosensors. Curr. Opin. Green. Sustain. Chem. 2023, 42, 100817. [Google Scholar] [CrossRef]
- Xie, X.; Yu, W.; Wang, L.; Yang, J.; Tu, X.; Liu, X.; Liu, S.; Zhou, H.; Chi, R.; Huang, Y. SERS-Based AI Diagnosis of Lung and Gastric Cancer via Exhaled Breath. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 314, 124181. [Google Scholar] [CrossRef]
- Pham, Y.L.; Beauchamp, J. Breath Biomarkers in Diagnostic Applications. Molecules 2021, 26, 5514. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Malpartida, M.G. Breath Biopsy and Discovery of Exclusive Volatile Organic Compounds for Diagnosis of Infectious Diseases. Front. Cell Infect. Microbiol. 2021, 10, 564194. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Chuang, M.C.; Chen, I.P. Why People Do Not Attend Health Screenings: Factors That Influence Willingness to Participate in Health Screenings for Chronic Diseases. Int. J. Environ. Res. Public Health 2020, 17, 3495. [Google Scholar] [CrossRef]
- Yadav, H.; Shah, D.; Sayed, S.; Horton, S.; Schroeder, L.F. Availability of Essential Diagnostics in Ten Low-Income and Middle-Income Countries: Results from National Health Facility Surveys. Lancet Glob. Health 2021, 9, e1553–e1560. [Google Scholar] [CrossRef]
- Aydin, I.; Aydin, F.N.; Agilli, M. The Association of Red Cell Distribution Width and Morbid Obesity. Clin. Biochem. 2014, 47, 1349. [Google Scholar] [CrossRef]
- Sarkar, S.; Bhattacharya, G.; Bhattacharjee, S.; Banerjee, D. A Drop of Hydrogen Peroxide Can Differentiate Exudative Pleural Effusion from Transudate—Development of a Bedside Screening Test. Clin. Chim. Acta 2009, 405, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Hofman, P. Pros: Can Tissue Biopsy Be Replaced by Liquid Biopsy? Transl. Lung Cancer Res. 2016, 5, 420. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The Benefits and Harms of Breast Cancer Screening: An Independent Review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-Analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef]
- Wilson, S.A. The American Society for Gastrointestinal Endoscopy First-Year Fellows’ Endoscopy Course: See One, Do One, Teach One? Gastrointest. Endosc. 2008, 67, 513–514. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The Human Volatilome: Volatile Organic Compounds (VOCs) in Exhaled Breath, Skin Emanations, Urine, Feces and Saliva. J. Breath. Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Mazzone, P.J. Beyond the Usual Suspects. J. Thorac. Oncol. 2012, 7, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer-A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Varadwaj, P. Smelling the Disease: Diagnostic Potential of Breath Analysis. Mol. Diagn. Ther. 2023, 27, 321–347. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Liu, H.; Niu, Y.; Wang, S.; Zhang, F.; Liu, H. Simultaneous Determination of Seven Endogenous Aldehydes in Human Blood by Headspace Gas Chromatography-Mass Spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2019, 1118–1119, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Monedeiro, F.; Dos Reis, R.B.; Peria, F.M.; Sares, C.T.G.; De Martinis, B.S. Investigation of Sweat VOC Profiles in Assessment of Cancer Biomarkers Using HS-GC-MS. J. Breath. Res. 2020, 14, 026009. [Google Scholar] [CrossRef]
- Serrano, M.; Gallego, M.; Silva, M. Analysis of Endogenous Aldehydes in Human Urine by Static Headspace Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2016, 1437, 241–246. [Google Scholar] [CrossRef]
- Annovazzi, L.; Cattaneo, V.; Viglio, S.; Perani, E.; Zanone, C.; Rota, C.; Pecora, F.; Cetta, G.; Silvestri, M.; Iadarola, P. High-Performance Liquid Chromatography and Capillary Electrophoresis: Methodological Challenges for the Determination of Biologically Relevant Low-Aliphatic Aldehydes in Human Saliva. Electrophoresis 2004, 25, 1255–1263. [Google Scholar] [CrossRef]
- Ramdzan, A.N.; Almeida, M.I.G.S.; McCullough, M.J.; Kolev, S.D. Development of a Microfluidic Paper-Based Analytical Device for the Determination of Salivary Aldehydes. Anal. Chim. Acta 2016, 919, 47–54. [Google Scholar] [CrossRef]
- Qu, D.; Liu, T.; Cheng, Y.; Du, T.; Cheng, B.; Zhang, Y.; Su, C.; Zheng, Y.; Xu, X.; Wang, G.; et al. Volatilomics in Diseases Odour and Electronic Nose Diagnosis. TrAC Trends Anal. Chem. 2025, 193, 118440. [Google Scholar] [CrossRef]
- Sachan, A.; Castro, M.; Feller, J. Volatolomics for Anticipated Diagnosis of Cancers with Chemoresistive Vapour Sensors: A Review. Chemosensors 2025, 13, 15. [Google Scholar] [CrossRef]
- Wilson, A.D. Developments of Recent Applications for Early Diagnosis of Diseases Using Electronic-Nose and Other VOC-Detection Devices. Sensors 2023, 23, 7885. [Google Scholar] [CrossRef]
- Khorshed, A.A.; Lamontagne, H.R.; Healy, L.; Shuhendler, A.J.; Lessard, B.H. Monitoring Ketoacidosis and Ketosis through Electrochemical Sensing of Acetone and Acetoacetate in Biological Fluids after Dilution. Sens. Actuators B Chem. 2025, 433, 137557. [Google Scholar] [CrossRef]
- Semwal, K.; Das, A.K. Recent Progress in Fluorescent Chemosensors for Selective Aldehyde Detection. RSC Adv. 2025, 15, 10005–10021. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Watanabe, S.; Osada, M.; Tajima, K.; Karashima, A.; Maruo, Y.Y. Small Acetone Sensor with a Porous Colorimetric Chip for Breath Acetone Detection Using the Flow–Stop Method. Chemosensors 2025, 13, 136. [Google Scholar] [CrossRef]
- Czippelová, B.; Nováková, S.; Šarlinová, M.; Baranovičová, E.; Urbanová, A.; Turianiková, Z.; Krohová, J.Č.; Halašová, E.; Škovierová, H. Impact of Breath Sample Collection Method and Length of Storage of Breath Samples in Tedlar Bags on the Level of Selected Volatiles Assessed Using Gas Chromatography-Ion Mobility Spectrometry (GC-IMS). J. Breath. Res. 2024, 18, 036004. [Google Scholar] [CrossRef]
- Doran, S.L.F.; Romano, A.; Hanna, G.B. Optimisation of Sampling Parameters for Standardised Exhaled Breath Sampling. J. Breath. Res. 2018, 12, 016007. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, E.M.; Lappas, A.S.; Tzortzi, A.S.; Behrakis, P.K. Exhaled Breath Condensate: Technical and Diagnostic Aspects. Sci. World J. 2015, 2015, 435160. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the Use of Tedlar® Bags for Breath-Gas Sampling and Analysis. J. Breath. Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.L.; Oxbøl, A.; Hansen, M.J.; Feilberg, A. Mechanisms of Loss of Agricultural Odorous Compounds in Sample Bags of Nalophan, Tedlar, and PTFE. J. Environ. Qual. 2018, 47, 246–253. [Google Scholar] [CrossRef]
- Mistral Sampler. Available online: https://www.mistral-breath.it/en/mistral-sampler/ (accessed on 30 April 2025).
- ReCIVA Breath Sampler. Available online: https://www.owlstonemedical.com/products/reciva/ (accessed on 30 April 2025).
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath Analysis: Comparison among Methodological Approaches for Breath Sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-State Gas Sensors for Breath Analysis: A Review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schee, M.P.; Fens, N.; Brinkman, P.; Bos, L.D.J.; Angelo, M.D.; Nijsen, T.M.E.; Raabe, R.; Knobel, H.H.; Vink, T.J.; Sterk, P.J. Effect of Transportation and Storage Using Sorbent Tubes of Exhaled Breath Samples on Diagnostic Accuracy of Electronic Nose Analysis. J. Breath. Res. 2013, 7, 016002. [Google Scholar] [CrossRef]
- Lippi, G.; Heaney, L.M. The “Olfactory Fingerprint”: Can Diagnostics Be Improved by Combining Canine and Digital Noses? Clin. Chem. Lab. Med. 2020, 58, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; He, X.; Yang, P.; Zong, T.; Sun, P.; Sun, R.; Yu, T.; Jiang, Z. The Cellular Function and Molecular Mechanism of Formaldehyde in Cardiovascular Disease and Heart Development. J. Cell Mol. Med. 2021, 25, 5358. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Zhou, Y.; Wang, J.; You, R. Research Progress of Electronic Nose Technology in Exhaled Breath Disease Analysis. Microsyst. Nanoeng. 2023, 9, 129. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Wex, K.; Sabel, B.; Schubert, J.K.; Miekisch, W. Electrochemical Sensor System for Breath Analysis of Aldehydes, CO and NO. J. Breath. Res. 2015, 9, 016008. [Google Scholar] [CrossRef]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F.; Dababneh, L.; Grove, D.; Alkhouri, N.; Lopez, R.; Dweik, R.A. The Breathprints in Patients With Liver Disease Identify Novel Breath Biomarkers in Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging Deeper into Volatile Organic Compounds Associated with Cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Guan, H.; Wang, T.; Liang, X.; Liu, F.; Liu, F.; Zhang, C.; Lu, G. Mixed Potential Type Acetone Sensor Based on GDC Used for Breath Analysis. Sens. Actuators B Chem. 2021, 326, 128846. [Google Scholar] [CrossRef]
- Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors 2021, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chen, Z.; Gong, Z.; Zhao, X.; Jiang, C.; Yuan, Y.; Wang, Z.; Li, Y.; Wang, C. Determination of Breath Acetone in 149 Type 2 Diabetic Patients Using a Ringdown Breath-Acetone Analyzer. Anal. Bioanal. Chem. 2015, 407, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath Volatile Organic Compounds (VOCs) as Biomarkers for the Diagnosis of Pathological Conditions: A Review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of Potential Breath Biomarkers for the Early Diagnosis of Breast Cancer Using Gas Chromatography-Mass Spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Tietje, O.; Wong, C. Prediction of Breast Cancer Using Volatile Biomarkers in the Breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef]
- Xie, Z.; Morris, J.D.; Pan, J.; Cooke, E.A.; Sutaria, S.R.; Balcom, D.; Marimuthu, S.; Parrish, L.W.; Aliesky, H.; Huang, J.J.; et al. Detection of COVID-19 by Quantitative Analysis of Carbonyl Compounds in Exhaled Breath. Sci. Rep. 2024, 14, 14568. [Google Scholar] [CrossRef]
- Berna, A.Z.; Odom John, A.R. Breath Metabolites to Diagnose Infection. Clin. Chem. 2021, 68, 43. [Google Scholar] [CrossRef]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath Biomarkers in Non-Carcinogenic Diseases. Clin. Chim. Acta 2024, 552, 117692. [Google Scholar] [CrossRef]
- Dryahina, K.; Sovová, K.; Nemec, A.; Španěl, P. Differentiation of Pulmonary Bacterial Pathogens in Cystic Fibrosis by Volatile Metabolites Emitted by Their in Vitro Cultures: Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia Complex. J. Breath. Res. 2016, 10, 037102. [Google Scholar] [CrossRef]
- Robroeks, C.M.H.H.T.; Van Berkel, J.J.B.N.; Dallinga, J.W.; Jöbsis, Q.; Zimmermann, L.J.I.; Hendriks, H.J.E.; Wouters, M.F.M.; Van Der Grinten, C.P.M.; Van De Kant, K.D.G.; Van Schooten, F.J.; et al. Metabolomics of Volatile Organic Compounds in Cystic Fibrosis Patients and Controls. Pediatr. Res. 2010, 68, 75–80. [Google Scholar] [CrossRef]
- Casimirri, E.; Stendardo, M.; Bonci, M.; Andreoli, R.; Bottazzi, B.; Leone, R.; Schito, M.; Vaccari, A.; Papi, A.; Contoli, M.; et al. Biomarkers of Oxidative-Stress and Inflammation in Exhaled Breath Condensate from Hospital Cleaners. Biomarkers 2016, 21, 115–122. [Google Scholar] [CrossRef]
- Corradi, M.; Folesani, G.; Andreoli, R.; Manini, P.; Bodini, A.; Piacentini, G.; Carraro, S.; Zanconato, S.; Baraldi, E. Aldehydes and Glutathione in Exhaled Breath Condensate of Children with Asthma Exacerbation. Am. J. Respir. Crit. Care Med. 2003, 167, 395–399. [Google Scholar] [CrossRef]
- Reese, K.L.; Rasley, A.; Avila, J.R.; Jones, A.D.; Frank, M. Metabolic Profiling of Volatile Organic Compounds (VOCs) Emitted by the Pathogens Francisella tularensis and Bacillus anthracis in Liquid Culture. Sci. Rep. 2020, 10, 9333. [Google Scholar] [CrossRef]
- Li, C.; Lei, S.H.; Ding, L.; Xu, Y.; Wu, X.N.; Wang, H.; Zhang, Z.J.; Gao, T.; Zhang, Y.Q.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, J.; Ngo, J.; Blake, D.; Meinardi, S.; Pontello, A.M.; Newcomb, R.; Galassetti, P.R. Improved Predictive Models for Plasma Glucose Estimation from Multi-Linear Regression Analysis of Exhaled Volatile Organic Compounds. J. Appl. Physiol. 2009, 107, 155–160. [Google Scholar] [CrossRef]
- Tiele, A.; Wicaksono, A.; Ayyala, S.K.; Covington, J.A. Development of a Compact, IoT-Enabled Electronic Nose for Breath Analysis. Electronics 2020, 9, 84. [Google Scholar] [CrossRef]
- Casella, G.; Ingravalle, F.; Ingravalle, A.; Monti, C.; Bonetti, F.; Limonta, A. COVID Emergency: An Opportunity to Increase the Interaction between Hepatologist and Primary Care Physician. Minerva Gastroenterol. Dietol. 2020, 66, 328–330. [Google Scholar] [CrossRef]
- Xie, Z.; Morris, J.D.; Mattingly, S.J.; Sutaria, S.R.; Huang, J.; Nantz, M.H.; Fu, X.-A. Analysis of a Broad Range of Carbonyl Metabolites in Exhaled Breath by UHPLC-MS. Anal. Chem. 2023, 95, 4344–4352. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, Q.; Meng, S.; Mu, T.; Liu, Z.; He, M.; Li, Q.; Zhao, S.; Wang, S.; Qiu, M. Identification of Lung Cancer Breath Biomarkers Based on Perioperative Breathomics Testing: A Prospective Observational Study. EClinicalMedicine 2022, 47, 101384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, M.; Wang, Z.; Chen, Z.; Zhao, X.; Yuan, Y.; Li, Y.; Wang, C. A Portable Real-Time Ringdown Breath Acetone Analyzer: Toward Potential Diabetic Screening and Management. Sensors 2016, 16, 1199. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of Aldehydes in Exhaled Breath of Patients with Lung Cancer by Means of On-Fiber-Derivatisation SPME–GC/MS. J. Chromatogr. B 2010, 878, 2643–2651. [Google Scholar] [CrossRef]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Ibrahim, W.; Natarajan, S.; Wilde, M.; Cordell, R.; Monks, P.S.; Greening, N.; Brightling, C.E.; Evans, R.; Siddiqui, S. A Systematic Review of the Diagnostic Accuracy of Volatile Organic Compounds in Airway Diseases and Their Relation to Markers of Type-2 Inflammation. ERJ Open Res. 2021, 7, 00030–02021. [Google Scholar] [CrossRef]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of Exhaled Breath Composition on Exogenous Factors, Smoking Habits and Exposure to Air Pollutants. J. Breath. Res. 2012, 6, 036008. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Advances in Electronic-Nose Technologies Developed for Biomedical Applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef]
- Alkhalifah, Y.; Phillips, I.; Soltoggio, A.; Darnley, K.; Nailon, W.H.; McLaren, D.; Eddleston, M.; Thomas, C.L.P.; Salman, D. VOCCluster: Untargeted Metabolomics Feature Clustering Approach for Clinical Breath Gas Chromatography/Mass Spectrometry Data. Anal. Chem. 2020, 92, 2937–2945. [Google Scholar] [CrossRef]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath Acetone Concentration Decreases with Blood Glucose Concentration in Type I Diabetes Mellitus Patients during Hypoglycaemic Clamps. J. Breath. Res. 2009, 3, 046004. [Google Scholar] [CrossRef]
- Sutaria, S.R.; Morris, J.D.; Xie, Z.; Cooke, E.A.; Silvers, S.M.; Long, G.A.; Balcom, D.; Marimuthu, S.; Parrish, L.W.; Aliesky, H.; et al. A Feasibility Study on Exhaled Breath Analysis Using UV Spectroscopy to Detect COVID-19. J. Breath. Res. 2023, 18, 016004. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of Acetone in Human Breath by Gas Chromatography-Mass Spectrometry and Solid-Phase Microextraction with on-Fiber Derivatization. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Karakaya, D.; Ulucan, O.; Turkan, M. Electronic Nose and Its Applications: A Survey. Int. J. Autom. Comput. 2020, 17, 179–209. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, H.W.; Kim, S.S.; Neri, G. Nanostructured Semiconducting Metal Oxide Gas Sensors for Acetaldehyde Detection. Chemosensors 2019, 7, 56. [Google Scholar] [CrossRef]
- Gancarz, M.; Nawrocka, A.; Rusinek, R. Identification of Volatile Organic Compounds and Their Concentrations Using a Novel Method Analysis of MOS Sensors Signal. J. Food Sci. 2019, 84, 2077–2085. [Google Scholar] [CrossRef]
- Xia, Z.; Li, D.; Deng, W. Identification and Detection of Volatile Aldehydes as Lung Cancer Biomarkers by Vapor Generation Combined with Paper-Based Thin-Film Microextraction. Anal. Chem. 2021, 93, 4924–4931. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Nguyen, C.M.; Huynh, M.A.; Vu, H.H.; Nguyen, T.K.; Nguyen, N.T. Field Effect Transistor Based Wearable Biosensors for Healthcare Monitoring. J. Nanobiotechnol. 2023, 21, 411. [Google Scholar] [CrossRef]
- Tiwary, A.; Kumar, J.; Behera, B. Analysis of CNT-Based SAW Sensor for the Detection of Volatile Organic Compounds. Phys. B Condens. Matter 2023, 669, 415279. [Google Scholar] [CrossRef]
- Ozsandikcioglu, U.; Atasoy, A.; Guney, S. Enhanced Lung Cancer Classification Accuracy via Hybrid Sensor Integration and Optimized Fuzzy Logic-Based Electronic Nose. Sensors 2025, 25, 5271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ren, W.; Xiao, F.; Li, J.; Zu, B.; Dou, X. Engineered Olfactory System for in Vitro Artificial Nose. Eng. Regen. 2022, 3, 427–439. [Google Scholar] [CrossRef]
- Steenhuis, E.G.M.; Asmara, O.D.; Kort, S.; Papenhuijzen, M.H.G.; Veeger, N.J.G.M.; Van den Heuvel, M.M.; Van Geffen, W.H. The Electronic Nose in Lung Cancer Diagnostics: A Systematic Review and Meta-Analysis. ERJ Open Res. 2025, 11, 00723–02024. [Google Scholar] [CrossRef] [PubMed]
- Ozsandikcioglu, U.; Atasoy, A.; Sevim, Y. Lung Cancer Detection Utilizing Mixed Sensor Based Electronic Nose. IEEE Access 2025, 13, 45400–45414. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath Sensors for Health Monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A Study of an Electronic Nose for Detection of Lung Cancer Based on a Virtual SAW Gas Sensors Array and Imaging Recognition Method. Meas. Sci. Technol. 2005, 16, 1535. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic Nose: A Non-Invasive Technology for Breath Analysis of Diabetes and Lung Cancer Patients. J. Breath. Res. 2019, 13, 024001. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen Zur Wägung Dünner Schichten Und Zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Licht, J.C.; Grasemann, H. Potential of the Electronic Nose for the Detection of Respiratory Diseases with and without Infection. Int. J. Mol. Sci. 2020, 21, 9416. [Google Scholar] [CrossRef]
- Fasola, S.; Ferrante, G.; Sabatini, A.; Santonico, M.; Zompanti, A.; Grasso, S.; Antonelli Incalzi, R.; La Grutta, S. Repeatability of Exhaled Breath Fingerprint Collected by a Modern Sampling System in Asthmatic and Healthy Children. J. Breath. Res. 2019, 13, 036007. [Google Scholar] [CrossRef]
- De Vries, R.; Brinkman, P.; Van Der Schee, M.P.; Fens, N.; Dijkers, E.; Bootsma, S.K.; De Jongh, F.H.C.; Sterk, P.J. Integration of Electronic Nose Technology with Spirometry: Validation of a New Approach for Exhaled Breath Analysis. J. Breath. Res. 2015, 9, 046001. [Google Scholar] [CrossRef]
- Krauss, E.; Haberer, J.; Barreto, G.; Degen, M.; Seeger, W.; Guenther, A. Recognition of Breathprints of Lung Cancer and Chronic Obstructive Pulmonary Disease Using the Aeonose®electronic Nose. J. Breath Res. 2020, 14, 046004. [Google Scholar] [CrossRef]
- Wang, X.R.; Lizier, J.T.; Berna, A.Z.; Bravo, F.G.; Trowell, S.C. Human Breath-Print Identification by E-Nose, Using Information-Theoretic Feature Selection Prior to Classification. Sens. Actuators B Chem. 2015, 217, 165–174. [Google Scholar] [CrossRef]
- Bikov, A.; Lázár, Z.; Horvath, I. Established Methodological Issues in Electronic Nose Research: How Far Are We from Using These Instruments in Clinical Settings of Breath Analysis? J. Breath. Res. 2015, 9, 034001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.H.; Xu, H. Graphene/Polyaniline Electrodeposited Needle Trap Device for the Determination of Volatile Organic Compounds in Human Exhaled Breath Vapor and A549 Cell. RSC Adv. 2017, 7, 11959–11968. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Lin, Y.; Sai, L.; Chang, Q.; Shi, W.; Chen, Q. Fully Gravure-Printed WO3/Pt-Decorated RGO Nanosheets Composite Film for Detection of Acetone. Sens. Actuators B Chem. 2018, 255, 1482–1490. [Google Scholar] [CrossRef]
- Nugent, P.; Giannopoulou, E.G.; Burd, S.D.; Elemento, O.; Giannopoulou, E.G.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous Materials with Optimal Adsorption Thermodynamics and Kinetics for CO2 Separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Homayoonnia, S.; Zeinali, S. Design and Fabrication of Capacitive Nanosensor Based on MOF Nanoparticles as Sensing Layer for VOCs Detection. Sens. Actuators B Chem. 2016, 237, 776–786. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath Analysis by Nanostructured Metal Oxides as Chemo-Resistive Gas Sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Pité, H.; Morais-Almeida, M.; Rocha, S.M. Metabolomics in Asthma: Where Do We Stand? Curr. Opin. Pulm. Med. 2018, 24, 94–103. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Haick, H. Nanomaterial-Based Sensors for Detection of Disease by Volatile Organic Compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-Based Gas Sensors Used for Breath Diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Characteristics of Surface Acoustic Wave Sensors with Nanoparticles Embedded in Polymer Sensitive Layers for VOC Detection. Sensors 2018, 18, 2401. [Google Scholar] [CrossRef]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing Lung Cancer in Exhaled Breath Using Gold Nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Al-Nasser, L.F.; Shan, S.; Li, J.; Skeete, Z.; Kang, N.; Luo, J.; Lu, S.; Zhong, C.-J.; Grausgruber, C.J.; et al. Detection of Mixed Volatile Organic Compounds and Lung Cancer Breaths Using Chemiresistor Arrays with Crosslinked Nanoparticle Thin Films. Sens. Actuators B Chem. 2016, 232, 292–299. [Google Scholar] [CrossRef]
- Masuda, Y.; Kato, K.; Kida, M.; Otsuka, J. Selective Nonanal Molecular Recognition with SnO2 Nanosheets for Lung Cancer Sensor. Int. J. Appl. Ceram. Technol. 2019, 16, 1807–1811. [Google Scholar] [CrossRef]
- Fu, X.A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive Detection of Lung Cancer Using Exhaled Breath. Clin. Cancer Res. 2013, 19, 1822–1832. [Google Scholar] [CrossRef]
- Jia, Z.; Thavasi, V.; Venkatesan, T.; Lee, P. Breath Analysis for Lung Cancer Early Detection—A Clinical Study. Metabolites 2023, 13, 1197. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, S.; Hao, X.; Wang, Y.; Gong, C.; Cheng, P. Gas Sensor for Efficient Acetone Detection and Application Based on Au-Modified ZnO Porous Nanofoam. Sensors 2024, 24, 8100. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Murali, G.; Kim, T.H.; Kim, J.H.; Lim, Y.J.; Kim, B.S.; Sahay, P.P.; Lee, S.H. Nanocube In 2 O 3 @RGO Heterostructure Based Gas Sensor for Acetone and Formaldehyde Detection. RSC Adv. 2017, 7, 38714–38724. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, T.; Shin, W.; Kato, K. SnO2 Nanosheet/Nanoparticle Detector for the Sensing of 1-Nonanal Gas Produced by Lung Cancer. Sci. Rep. 2015, 5, 10122. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nakashima, T.; Akamatsu, T.; Izu, N.; Shin, W. Nonanal Gas Sensing Properties of Platinum, Palladium, and Gold-Loaded Tin Oxide VOCs Sensors. Sens. Actuators B Chem. 2013, 187, 135–141. [Google Scholar] [CrossRef]
- Zhang, S.; Pu, Y.; Cao, S.; Zhu, D. SnO2 Nanoparticles Derived from Metal-Organic Precursors as an Acetaldehyde Gas Sensor with Ppb-Level Detection Limit. ACS Appl. Nano Mater. 2023, 6, 13177–13187. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Z.; Yuan, F.; Xie, C. Fabrication and Hexanal Gas Sensing Property of Nano-SnO2 Flat-Type Coplanar Gas Sensor Arrays at Ppb Level. Curr. Nanosci. 2013, 9, 357–362. [Google Scholar] [CrossRef]
- Paul, S.; Mendoza, E.R.; To, D.T.H.; Stahovich, T.F.; Schaefer, J.; Myung, N.V. Enhancing Room-Temperature Gas Sensing Performance of Metal Oxide Semiconductor Chemiresistors through 400 Nm UV Photoexcitation. Sens. Actuators Rep. 2024, 7, 100194. [Google Scholar] [CrossRef]
- Peng, C.; Sui, Y.; Fang, C.; Sun, H.; Liu, W.; Li, X.; Qu, C.; Li, W.; Liu, J.; Wu, C. Highly Sensitive and Selective Electrochemical Biosensor Using Odorant-Binding Protein to Detect Aldehydes. Anal. Chim. Acta 2024, 1318, 342932. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.J.M.; Oliveira, A.R.; Roque, A.C.A. Protein- and Peptide-Based Biosensors in Artificial Olfaction. Trends Biotechnol. 2018, 36, 1244. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Bioelectronic Nose: Current Status and Perspectives. Biosens. Bioelectron. 2017, 87, 480–494. [Google Scholar] [CrossRef]
- Deng, H.; Chen, Z.; Feng, P.; Tian, L.; Zong, H.; Nakamoto, T. Recent Advances and Applications of Odor Biosensors. Electronics 2025, 14, 1852. [Google Scholar] [CrossRef]
- Wasilewski, T.; Szulczyński, B.; Kamysz, W.; Gębicki, J.; Namieśnik, J. Evaluation of Three Peptide Immobilization Techniques on a QCM Surface Related to Acetaldehyde Responses in the Gas Phase. Sensors 2018, 18, 3942. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. Determination of Long-Chain Aldehydes Using a Novel Quartz Crystal Microbalance Sensor Based on a Biomimetic Peptide. Microchem. J. 2020, 154, 104509. [Google Scholar] [CrossRef]
- Wasilewski, T.; Neubauer, D.; Wojciechowski, M.; Szulczyński, B.; Gębicki, J.; Kamysz, W. Evaluation of Linkers’ Influence on Peptide-Based Piezoelectric Biosensors’ Sensitivity to Aldehydes in the Gas Phase. Int. J. Mol. Sci. 2023, 24, 10610. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, S.; Luo, J.Y.; Cui, J.J.; Ma, Y.; Dong, S.L. Two Minus-C Odorant Binding Proteins from Helicoverpa Armigera Display Higher Ligand Binding Affinity at Acidic PH than Neutral PH. J. Insect. Physiol. 2013, 59, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. Investigation of Different Materials as Acetone Sensors for Application in Type-1 Diabetes Diagnosis. Biomed. J. Sci. Tech. Res. 2019, 14, 10940–10945. [Google Scholar] [CrossRef]
- Huang, Q.; Han, L.; Ma, H.; Lan, W.; Tu, K.; Peng, J.; Su, J.; Pan, L. An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam. Foods 2025, 14, 182. [Google Scholar] [CrossRef]
- Ozdemir, A.; Algül, Y.; Tirgil, N.Y. Reusable Turn-on Fluorescent Biosensor for Cardiac Biomarker Troponin I Detection Using QDs-SPION-Aptamer. J. Fluoresc. 2025. [Google Scholar] [CrossRef] [PubMed]
- de March, C.A.; Fukutani, Y.; Vihani, A.; Kida, H.; Matsunami, H. Real-Time in Vitro Monitoring of Odorant Receptor Activation by an Odorant in the Vapor Phase. J. Vis. Exp. 2019, 2019, e594462019. [Google Scholar] [CrossRef]
- Ye, M.; Chien, P.J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An Acetone Bio-Sniffer (Gas Phase Biosensor) Enabling Assessment of Lipid Metabolism from Exhaled Breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. A Highly Selective Biosensor Based on Peptide Directly Derived from the HarmOBP7 Aldehyde Binding Site. Sensors 2019, 19, 4284. [Google Scholar] [CrossRef]
- Iitani, K.; Chien, P.J.; Suzuki, T.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Fiber-Optic Bio-Sniffer (Biochemical Gas Sensor) Using Reverse Reaction of Alcohol Dehydrogenase for Exhaled Acetaldehyde. ACS Sens. 2018, 3, 425–431. [Google Scholar] [CrossRef]
- Iitani, K.; Mori, H.; Ichikawa, K.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Gas-Phase Biosensors (Bio-Sniffers) for Measurement of 2-Nonenal, the Causative Volatile Molecule of Human Aging-Related Body Odor. Sensors 2023, 23, 5857. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Wasilewski, T.; Orbay, S.; Brito, N.F.; Sikora, K.; Melo, A.C.A.; Melendez, M.E.; Szulczyński, B.; Sanyal, A.; Kamysz, W.; Gębicki, J. Molecularly Imprinted Polymers for the Detection of Volatile Biomarkers. TrAC—Trends Anal. Chem. 2024, 177, 106988. [Google Scholar] [CrossRef]
- Zare, A.; Babamiri, B.; Hassani, M.; Khalghollah, M.; Mohammadi, M.; Haghjooy Javanmard, S.; Sanati Nezhad, A. CapSense-MIP: Self-Operating Molecularly Imprinted Polymer (MIP) Biosensor for Point-of-Care Diagnostics. Biosens. Bioelectron. 2025, 286, 117599. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, Y.; Kim, J.; Jung, H.G.; Jang, J.W.; Kim, H.; Hwang, K.S.; Lee, D.; Lee, S.W.; Lee, J.H.; et al. Recent Advances in Molecularly Imprinted Polymers toward Biomedical Healthcare Devices. Biosens. Bioelectron. 2025, 287, 117637. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Raskin, J.P.; Lahem, D.; Krumpmann, A.; Decroly, A.; Debliquy, M. A Formaldehyde Sensor Based on Molecularly-Imprinted Polymer on a TiO2 Nanotube Array. Sensors 2017, 17, 675. [Google Scholar] [CrossRef] [PubMed]
- Chul Yang, J.; Won Hong, S.; Jeon, S.; Ik Park, W.; Byun, M.; Park, J. Molecular Imprinting of Hemispherical Pore-Structured Thin Films via Colloidal Lithography for Gaseous Formaldehyde Gravimetric Sensing. Appl. Surf. Sci. 2021, 570, 151161. [Google Scholar] [CrossRef]
- Jha, S.K.; Liu, C.; Hayashi, K. Molecular Imprinted Polyacrylic Acids Based QCM Sensor Array for Recognition of Organic Acids in Body Odor. Sens. Actuators B Chem. 2014, 204, 74–87. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M. Fabrication of Chemiresistive Nanosensor Using Molecularly Imprinted Polymers for Acetone Detection in Gaseous State. Iran. Polym. J. 2022, 31, 883–891. [Google Scholar] [CrossRef]
- Debliquy, M.; Dony, N.; Lahem, D.; Tang, X.; Zhang, C.; Raskin, J.P.; Olivier, M.G. Acetaldehyde Chemical Sensor Based on Molecularly Imprinted Polymer Polypyrrole. Procedia Eng. 2016, 168, 569. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Molecularly Imprinted Polymer-Based Chemiresistive Sensor for Detection of Nonanal as a Cancer Related Biomarker. Microchem. J. 2022, 173, 106988. [Google Scholar] [CrossRef]
- Janfaza, S.; Banan Nojavani, M.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A Selective Chemiresistive Sensor for the Cancer-Related Volatile Organic Compound Hexanal by Using Molecularly Imprinted Polymers and Multiwalled Carbon Nanotubes. Microchim. Acta 2019, 186, 137. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Kotova, K.; Lieberzeit, P.A. Molecularly Imprinted Polymer Nanoparticles for Formaldehyde Sensing with QCM. Sensors 2016, 16, 1011. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Shivashankar, S.; Chhetri, K.B.; Kumar, A.; Rao, B.B.; Aravind, J.; Swami, D.V. Enhancing Food Authentication through E-Nose and E-Tongue Technologies: Current Trends and Future Directions. Trends Food Sci. Technol. 2024, 150, 104574. [Google Scholar] [CrossRef]
- Iragorri, N.; Spackman, E. Assessing the Value of Screening Tools: Reviewing the Challenges and Opportunities of Cost-Effectiveness Analysis. Public. Health Rev. 2018, 39, 17. [Google Scholar] [CrossRef]
- Itoh, T.; Akamatsu, T.; Tsuruta, A.; Shin, W. Selective Detection of Target Volatile Organic Compounds in Contaminated Humid Air Using a Sensor Array with Principal Component Analysis. Sensors 2017, 17, 1662. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in Volatile Organic Compounds in the Breath of Normal Humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Mortazavi, S.; Makouei, S.; Abbasian, K.; Danishvar, S. Exhaled Breath Analysis (EBA): A Comprehensive Review of Non-Invasive Diagnostic Techniques for Disease Detection. Photonics 2025, 12, 848. [Google Scholar] [CrossRef]
- Ravizza, A.; De Maria, C.; Di Pietro, L.; Sternini, F.; Audenino, A.L.; Bignardi, C. Comprehensive Review on Current and Future Regulatory Requirements on Wearable Sensors in Preclinical and Clinical Testing. Front. Bioeng. Biotechnol. 2019, 7, 313. [Google Scholar] [CrossRef]
- Ansermino, J.M.; Dumont, G.A.; Ginsburg, A.S. Measurement Uncertainty in Clinical Validation Studies of Sensors. Sensors 2023, 23, 2900. [Google Scholar] [CrossRef]
- Chen, K.C.; Kuo, S.W.; Shie, R.H.; Yang, H.Y. Advancing Accuracy in Breath Testing for Lung Cancer: Strategies for Improving Diagnostic Precision in Imbalanced Data. Respir. Res. 2024, 25, 32. [Google Scholar] [CrossRef] [PubMed]
- Vinhas, M.; Leitão, P.M.; Raimundo, B.S.; Gil, N.; Vaz, P.D.; Luis-Ferreira, F. AI Applied to Volatile Organic Compound (VOC) Profiles from Exhaled Breath Air for Early Detection of Lung Cancer. Cancers 2024, 16, 2200. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, J.; Lee, J.O.; Hwang, Y.; Bahn, H.K.; Park, I.; Jheon, S.; Lee, D.S. Breath Analysis System with Convolutional Neural Network (CNN) for Early Detection of Lung Cancer. Sens. Actuators B Chem. 2024, 409, 135578. [Google Scholar] [CrossRef]
- Pargoletti, E. Rethinking Biosensors for Exhaled Breath: A Perspective on Gas and Condensate Detection. Adv. Sens. Res. 2025, e00086. [Google Scholar] [CrossRef]
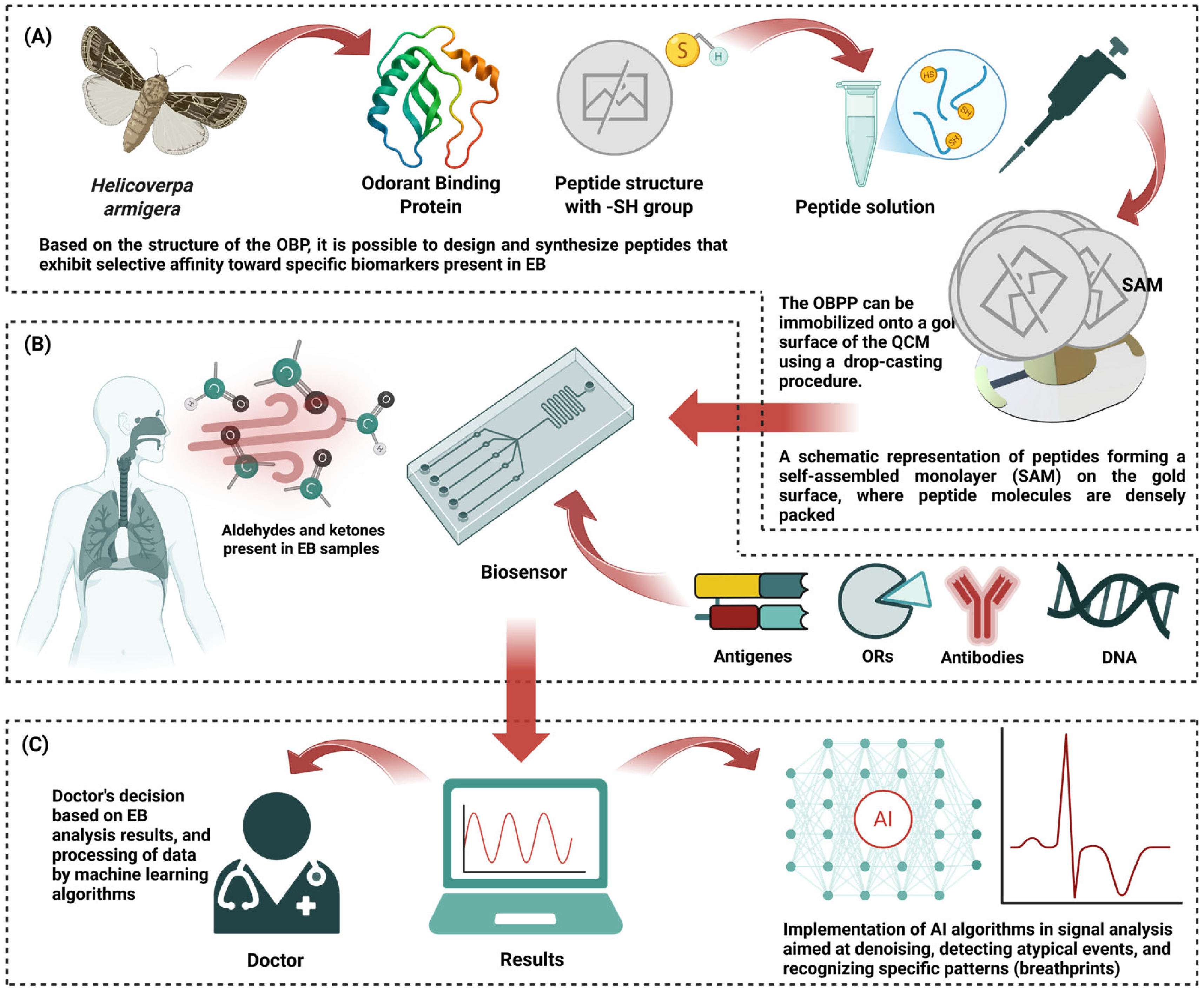
| Diagnostic Method | Advantage | Disadvantage | Refs. |
|---|---|---|---|
| Blood sampling | Venipuncture is a minimally invasive, rapid, and cost-effective method for acquiring diagnostic samples, widely used in routine clinical testing. | Even routine phlebotomy can cause patient discomfort and is associated with local complications, such as hematoma formation. | [37,38] |
| Tissue biopsy | Tissue biopsy provides a source of fresh tumor material for direct histopathological and molecular analyses and remains the gold-standard diagnostic method in clinical oncology. | An invasive procedure, it carries inherent risks including bleeding, infection, and patient discomfort, which limit its repeatability in clinical practice. The procedure requires trained personnel. | [39] |
| Mammography | Reduces breast-cancer mortality through early detection of asymptomatic lesions. | Employs low-dose ionizing radiation and is associated with a relatively high false-positive rate, resulting in overdiagnosis and subsequent unnecessary follow-up diagnostic tests. | [40,41] |
| Gastroscopy | Provides direct visualization of the upper gastrointestinal tract while permitting simultaneous tissue sampling via biopsy. | Associated with significant patient discomfort and anxiety. The procedure requires trained personnel. | [42] |
| Breath sampling | Completely non-invasive and quick method, offering potential for PoC disease screening applications. | Lack of standardized sampling and analysis protocols leads to high variability and limited reproducibility in clinical conditions. | [43] |
| Exhaled VOCs | Potentially Diseases | Refs. |
|---|---|---|
| Formaldehyde, acetaldehyde, pentanal, hexanal, heptanal, octanal, nonanal, undecane | Lung cancer | [11,13,46,72,74] |
| Acetone | Non-alcoholic liver disease | [73] |
| Acetone | Diabetes | [46,75,76,77] |
| Acetophenone, formaldehyde, heptanal | Breast cancer | [78,79,80] |
| Acetaldehyde, acetone | COVID-19 | [81,82] |
| Benzaldehyde | Chronic obstructive pulmonary disease | [83] |
| Tolualdehyde Malondialdehyde | Cystic fibrosis Oxidative stress and inflammation | [84,85,86] |
| Glutathione Malondialdehyde | Asthma | [87] |
| 2-Heptanone 2-Nonanone 2-Undecanone | Francisella tularensis infection | [88] |
| Compound | Disease | Concentration of VOCs in EB | Refs. |
|---|---|---|---|
| Acetone | T1D | 4.9 ppm | [96] |
| Acetone | T2D | 1.5 ppm | [96] |
| Nonanal | LC | 44.0 pM | [97] |
| Octanal | LC | 23.0 pM | [97] |
| Hexanal | LC | 37.3 pM | [97] |
| Pentanal | LC | 19.1 pM | [97] |
| Propanal | LC | 53.6 pM | [97] |
| Technology | Advantages | Disadvantages |
|---|---|---|
| GC-MS | High sensitivity, specificity, and the ability to quantify compounds, and relatively fast analysis. The capability to detect compound mixtures. | Requires sample pre-concentration. High equipment and operating costs, along with complex operations requiring trained operators. |
| SIFT-MS | Provides high sensitivity. Enables real-time analysis of EB. There is no need for preconcentration of analytes. | Methods involve expensive instrumentation, technical complexity, and typically require skilled operators. |
| ENs | The ability to perform rapid and non-invasive analysis, enabling real-time detection without the need for complex sample preparation. Can be miniaturized and adapted for use in PoC. Easy to operate. | Characterized by a relatively low specificity towards individual chemical compounds, it can be significantly affected by various environmental factors, particularly fluctuations in humidity, which may interfere with its accuracy and reliability. |
| PTR-MS | Requires no sample preparation. Quick response time enables real-time detection of VOCs. Compact design allows for space-saving or portable configurations and simple operation. High resolution and sensitivity. | Difficulties in determining complex mixtures with undefined composition. The inability to detect compounds with an affinity for protons is lower than that of water. High costs associated with measurement equipment. |
| Raman Spectroscopy | Non-destructive, requires minimal sample preparation, and offers high specificity. Possibility of real-time analysis. | Limited sensitivity for some compounds. High equipment cost. Data interpretation complexity, especially in complex VOC mixtures. |
| Quantitative Nuclear Magnetic Resonance (qNMR) | Enables the determination of the compound’s structure as well as the quantification of VOCs. | Very expensive equipment requires skilled operators. Relatively low sensitivity compared to GC-MS. Due to the size of the device, it is unsuitable for use in PoC mode. |
| Chemiluminescence Detection | Offers high sensitivity and capability for specific detection. | Applicable only to chemiluminescent analytes, susceptible to interference, and may require optimized conditions. |
| Fluorescence Spectroscopy | High sensitivity and the capability to selectively detect specific compounds through the use of fluorophores. | Limited to VOCs exhibiting fluorescence properties. Background fluorescence may interfere with signal interpretation and often requires optimization of experimental conditions. |
| EN Devices | Type of Transducer Technology | Refs. |
|---|---|---|
| BIONOTE (Bionote, Big Lake, MN, USA) | QCM sensors with anthocyanin-coated gold electrodes | [123,124] |
| SpiroNose (Breathomix, Leiden, The Netherlands) | Sensor arrays, each composed of MOS sensors | [123,125] |
| Aeonose (The eNose Company, Zutphen, The Netherlands) | Micro hotplate MOS | [123,126] |
| DiagNose (Figaro Engineering, Osaka, Japan) | MOS sensors | [127,128] |
| Cyranose 320 (Sensigent, Baldwin Park, CA, USA) | Carbon black–polymer composite chemiresistor | [123,128] |
| Owlstone Lonestar (Owlstone Medical, Cambridge, UK) | Field asymmetric ion mobility spectrometry | [123] |
| Material/Structure | Analyte | Sensor Response Range/LOD [ppm] | Working Temperature | Refs. |
|---|---|---|---|---|
| Au-modified ZnO nanofoam | Acetone | 20–100/– | 275 °C | [143] |
| In2O nanocube | Formaldehyde | –/25 | 225 °C | [144] |
| SnO2 nanosheet with nanoparticle + noble metal catalyst | Nonanal | 1–10/– | 300 °C | [145] |
| Au/SnO2 | Nonanal | –/9.5 | 250 °C | [146] |
| SnO2 nanoparticles | Acetaldehyde | –/40 | 100 °C | [147] |
| Nano-SnO2 powders | Hexanal | –/0.05 | 200 °C | [148] |
| Biosensor | Method | Analytes | LOD [ppm]/ Sensor Response Range [ppm] | Refs. |
|---|---|---|---|---|
| OBPP1 | QCM | Acetaldehyde Octanal | 243/– | [164] |
| 455/– | ||||
| OBPP3 | QCM | Acetaldehyde Octanal | 571/– | [164] |
| 49/– | ||||
| OBPP4 | QCM | Acetaldehyde | 327/981–3988 | [156] |
| Hexanal | 186/558–1826 | |||
| Octanal | 114/342–1437 | |||
| Nonanal | 14/42–1303 | |||
| OBPP4-GSGSGS | QCM | Nonanal | 2/– | [157] |
| S-ADH/NADH | Fluorescence (fiber-optic) | Acetone | –/0.02–5.3 | [163] |
| ADH/NADH (reverse reaction) | Fluorescence (fiber-optic) | Acetaldehyde | –/0.02–10 | [165] |
| LEKKKKDC-NH2 | QCM | Acetaldehyde | 1/– | [154] |
| Aldehyde dehydrogenase | Fluorescence | Trans-2-nonenal | 0.23/0.4–7.5 | [166] |
| Sensor | Analyte | Template | Functional Monomers | Polymerization Method | Refs. |
|---|---|---|---|---|---|
| MIP titanium dioxide nanotube array | Formaldehyde | Formaldehyde | Pyrrole | Electropolymerization | [173] |
| MIP-coated QCM | Formaldehyde | Formaldehyde | 2-(Trifluoromethyl) acrylic acid, ethylene glycol dimethacrylate, 1-hydroxycyclohexyl phenyl ketone | Photopolymerization | [174] |
| MIP-coated QCM | Decanal | Decanal | Methacrylic acid, ethylene glycol dimethacrylate, 2,2′-azobis-isobutyronitrile | Free radical polymerization | [175] |
| MIP and AuNP chemiresistive sensor | Acetone | Acetone | Methyl methacrylate | Free radical polymerization | [176] |
| MIP interdigitated in gold electrodes | Acetaldehyde | Acetaldehyde | Pyrrole | Electropolymerization | [177] |
| Sensor | Analyte | LOD [ppm] | Sensor Response Range [ppm] | Refs. |
|---|---|---|---|---|
| MIP interdigitated in gold electrodes | Acetaldehyde | 500 | – | [177] |
| MIP-AuNPs | Nonanal | 4.5 | 2.5–100 | [178] |
| MIP-MWCNTs | Hexanal | 10 | 10–200 | [179] |
| MIP-AuNPs | Acetone | 66 | 50–300 | [176] |
| IP NPs | Formaldehyde | 0.5 | – | [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiejzik, R.; Wasilewski, T.; Kamysz, W. Exhaled Aldehydes and Ketones as Biomarkers of Lung Cancer and Diabetes: Review of Sensor Technologies for Early Disease Diagnosis. Biosensors 2025, 15, 668. https://doi.org/10.3390/bios15100668
Kiejzik R, Wasilewski T, Kamysz W. Exhaled Aldehydes and Ketones as Biomarkers of Lung Cancer and Diabetes: Review of Sensor Technologies for Early Disease Diagnosis. Biosensors. 2025; 15(10):668. https://doi.org/10.3390/bios15100668
Chicago/Turabian StyleKiejzik, Rafał, Tomasz Wasilewski, and Wojciech Kamysz. 2025. "Exhaled Aldehydes and Ketones as Biomarkers of Lung Cancer and Diabetes: Review of Sensor Technologies for Early Disease Diagnosis" Biosensors 15, no. 10: 668. https://doi.org/10.3390/bios15100668
APA StyleKiejzik, R., Wasilewski, T., & Kamysz, W. (2025). Exhaled Aldehydes and Ketones as Biomarkers of Lung Cancer and Diabetes: Review of Sensor Technologies for Early Disease Diagnosis. Biosensors, 15(10), 668. https://doi.org/10.3390/bios15100668






