A Flexible Electrochemical Sensor Based on Porous Ceria Hollow Microspheres Nanozyme for Sensitive Detection of H2O2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
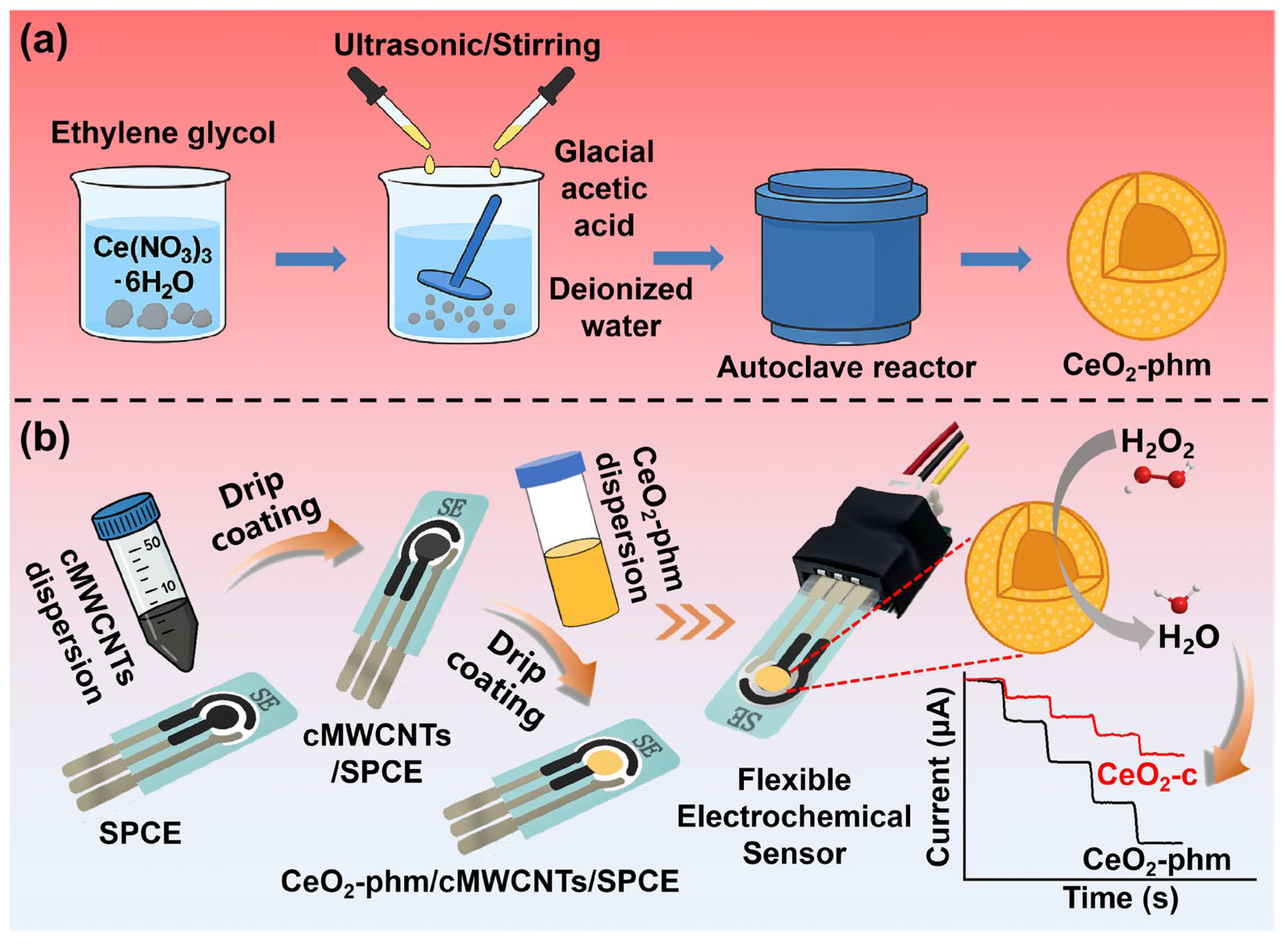
2.2. Synthesis of CeO2-phm
2.3. Characterization
2.4. Fabrication of the Flexible Electrochemical Biosensor
2.5. Electrochemical Measurements
3. Results
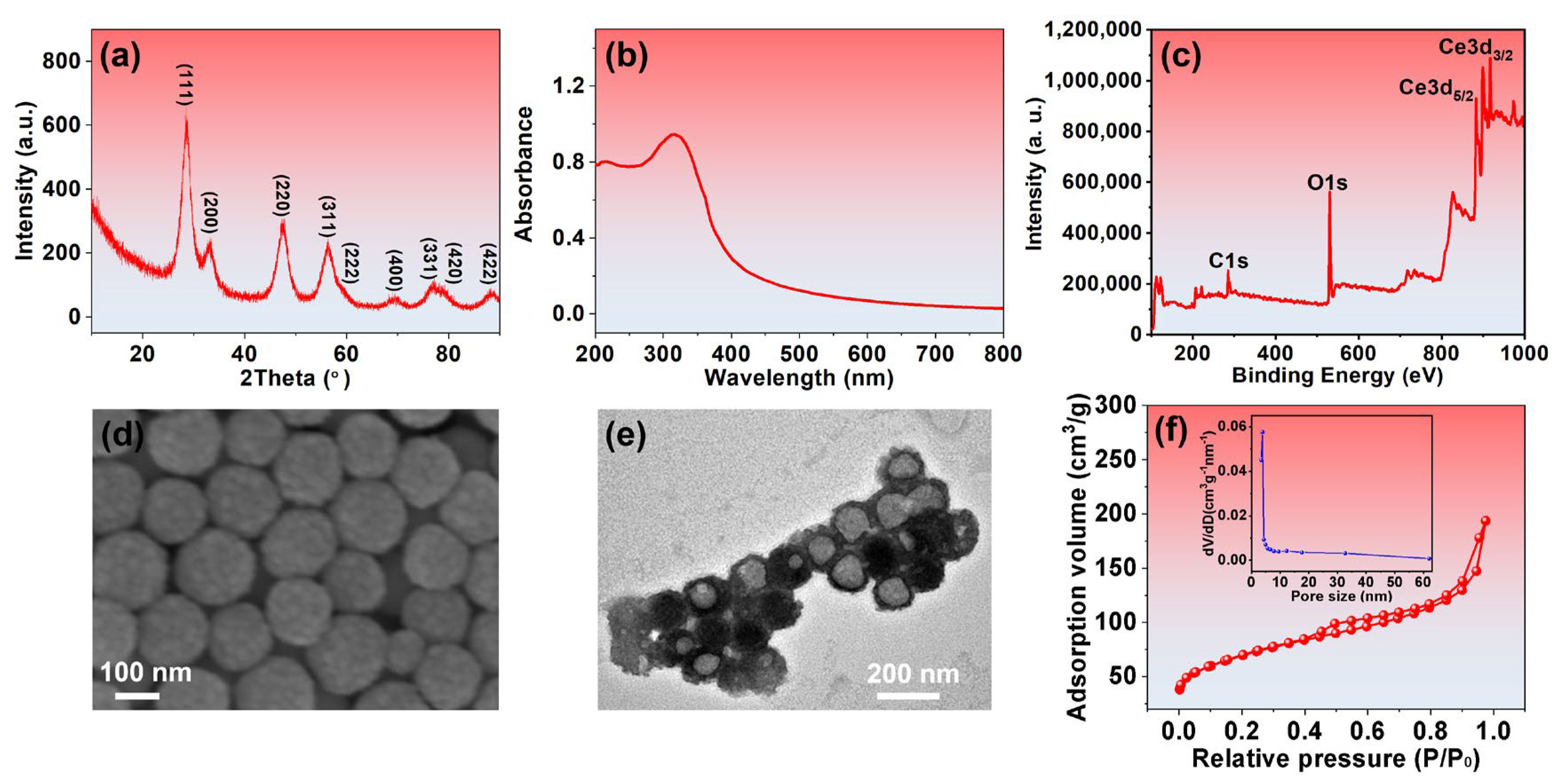
3.1. Preparation and Characterization of CeO2-phm
3.2. Electrochemical Characterization of Modified Electrodes
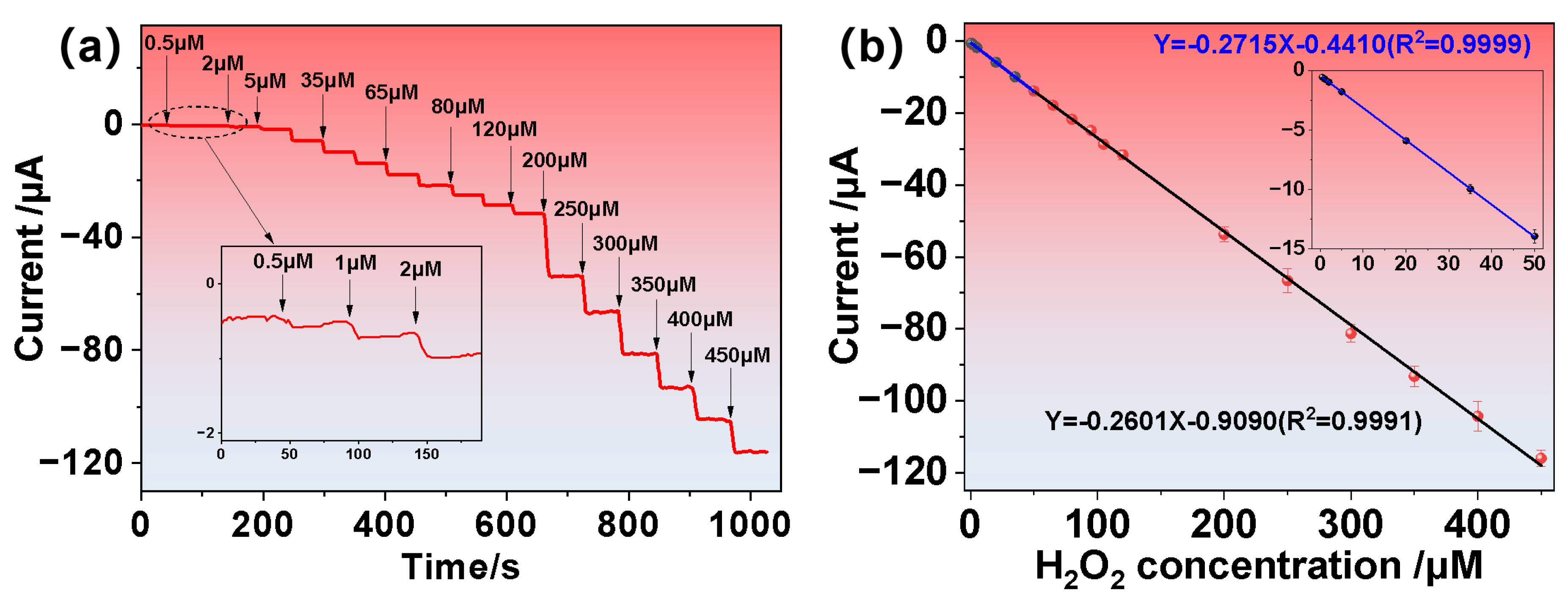
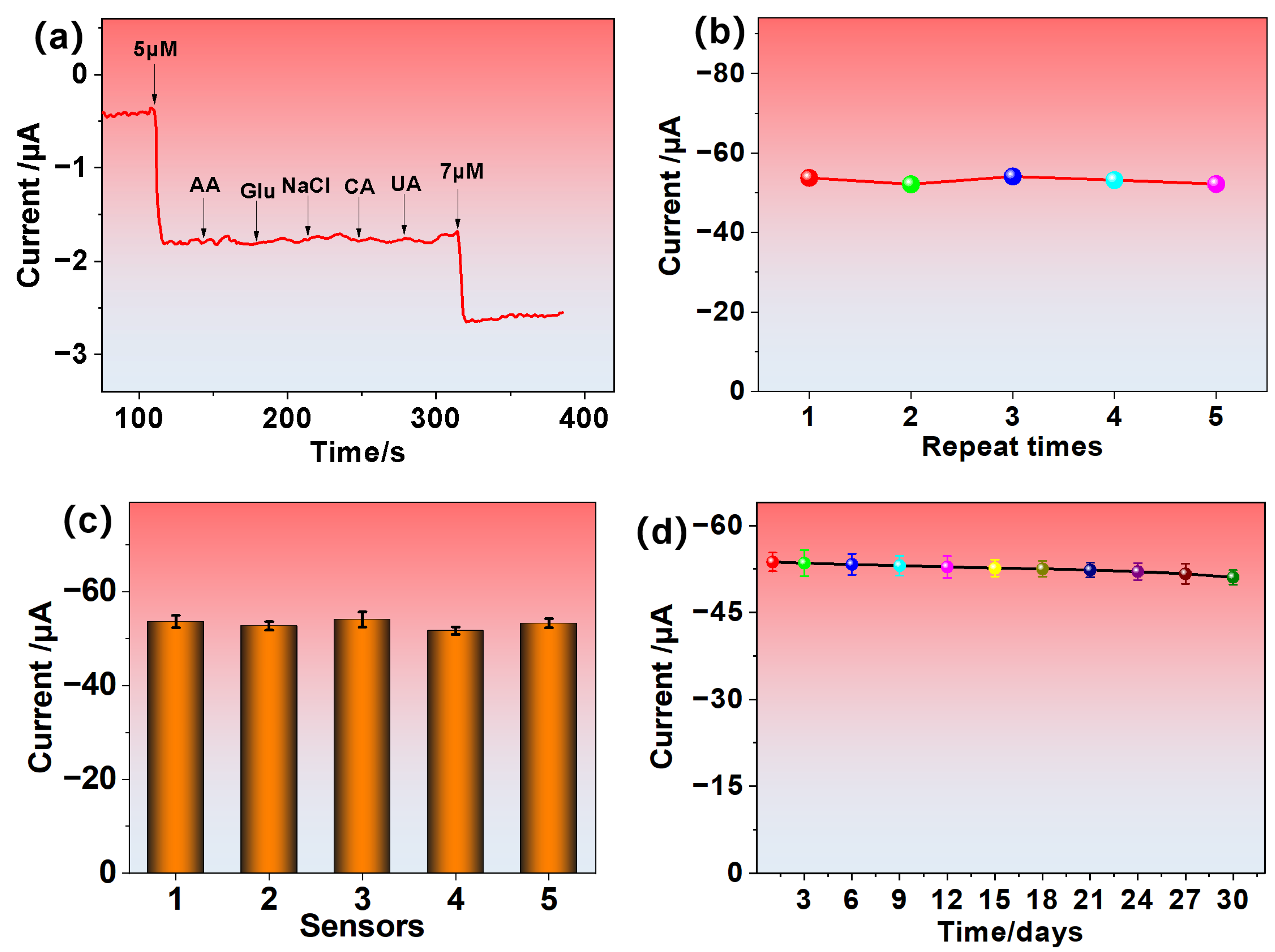
3.3. Electrochemical Sensing Performance of H2O2
3.4. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CeO2-phm | porous ceria hollow microspheres |
| CeO2-c | commercial CeO2 nanospheres with solid cores |
| SPCE | screen-printed carbon electrode |
| ROS | reactive oxygen species |
| CeO2 | cerium dioxide |
| cMWCNTs | carboxylated multi-walled carbon nanotubes |
| Ce(NO3)3·6H2O | Cerium nitrate hexahydrate |
| EG | ethylene glycol |
| NHS | N-hydroxysuccinimide |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| AA | ascorbic acid |
| Glu | glucose |
| CA | citric acid |
| UA | uric acid |
| NaCl | sodium chloride |
| PBS | Phosphate-buffered saline |
| CeO2-c | commercial CeO2 nanospheres |
| DLS | dynamic light scattering |
| PDI | polydispersity index |
| FBS | Fetal Bovine Serum |
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Mu, Y.; Zhou, J.; Wang, C.; Fang, Z.; Liu, Y.; Liu, S.; Zhai, Q.; Zhang, C. Ferroptosis is involved in trophoblast cells cytotoxicity induced by black phosphorus nanoparticles. Toxicology 2024, 505, 153810. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and reactive oxygen species in aging and age-related diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Prasad, V.; Siddiqui, L.; Mishra, P.K.; Ekielski, A.; Talegaonkar, S. Recent advancements in lignin valorization and biomedical applications: A patent review. Recent Pat. Nanotechnol. 2022, 16, 107–127. [Google Scholar] [CrossRef]
- Devi, A.; Saran, C.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Mulla, S.I.; Bharagava, R.N. Sustainable Algal Industrial Wastewater Treatment: Applications and Challenges. Microbes Based Approaches Manag. Hazard. Contam. 2024, 12, 190–205. [Google Scholar]
- Wang, F.; Van Halem, D.; Van der Hoek, J. The fate of H2O2 during managed aquifer recharge: A residual from advanced oxidation processes for drinking water production. Chemosphere 2016, 148, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Lo, W.H. Removal of refractory compounds from stabilized landfill leachate using an integrated H2O2 oxidation and granular activated carbon (GAC) adsorption treatment. Water Res. 2009, 43, 4079–4091. [Google Scholar] [CrossRef]
- Ullah, M.; Kanjariya, P.; Rekha, M.M.; Kundlas, M.; Prasad, G.V.S.; Chahar, M.; Algahtani, A.; Tirth, V.; Zhengxin, L. Correction to: Fabrication of an electrochemical sensor based on g-C3N4–NiO nanocomposite for sensitive and selective detection of hydrogen peroxide. J. Mater. Sci. Mater. Electron. 2025, 36, 1342. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, A.; Halim, S.A.; Uddin, J.; Khan, A.; El Deeb, S.; Al-Harrasi, A. Recent advances in electrochemical sensing of hydrogen peroxide (H2O2) released from cancer cells. Nanomaterials 2022, 12, 1475. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Narwal, V. Quantitative analysis of hydrogen peroxide with special emphasis on biosensors. Bioprocess Biosyst. Eng. 2018, 41, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, S.; Chen, F.; Lv, Y.; Fu, L. Enhancing sensitivity and selectivity: Current trends in electrochemical immunosensors for organophosphate analysis. Biosensors 2024, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Thatikayala, D.; Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.J.; Al-Ali, A.K.; Malik, R.A.; Min, B. Progress of advanced nanomaterials in the non-enzymatic electrochemical sensing of glucose and H2O2. Biosensors 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.; Liu, P.; Zhao, K.; Ye, S.; Liang, G. Rapid, ultrasensitive and non-enzyme electrochemiluminescence detection of hydrogen peroxide in food based on the ssDNA/g-C3N4 nanosheets hybrid. Food Chem. 2021, 357, 129753. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Cai, Y.; Ren, W.; Du, C.; Zhang, J.; Zhao, Y.; Yu, J.; Du, H.; Kang, H.; Ge, X.; Lu, M.; et al. Cerium Oxide Nanozymes: Exploring Synthesis, Catalytic Activity, and Biomedical Potential. Small 2025, 21, 2504436. [Google Scholar] [CrossRef]
- Wang, R.; Du, Y.; Fu, Y.; Guo, Y.; Gao, X.; Guo, X.; Wei, J.; Yang, Y. Ceria-based nanozymes in point-of-care diagnosis: An emerging futuristic approach for biosensing. ACS Sens. 2023, 8, 4442–4467. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 2020, 121, 649–735. [Google Scholar] [CrossRef]
- Wu, T.; Han, M.-Y.; Xu, Z.J. Size effects of electrocatalysts: More than a variation of surface area. ACS Nano 2022, 16, 8531–8539. [Google Scholar] [CrossRef]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Jiang, J.; Liu, R.; Zhu, B.; Xu, M.; Wang, Y.; Cao, J.; Li, M.; Yuan, Z.; et al. Porous ceria hollow microspheres: Synthesis and characterization. Micropor. Mesopor. Mater. 2009, 123, 349–353. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Dai, Q.; Wang, Y.; Guo, Y.; Guo, Y. Hierarchical Organization and Catalytic Activity of High-Surface-Area Mesoporous Ceria Microspheres Prepared Via Hydrothermal Routes. ACS Appl. Mater. Interfaces 2010, 2, 838–846. [Google Scholar] [CrossRef]
- Khan, A.; DeVoe, E.; Andreescu, S. Carbon-based electrochemical biosensors as diagnostic platforms for connected decentralized healthcare. Sens. Diagn. 2023, 2, 529–558. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, W.; Richardson-Barlow, C. Navigating ecological civilisation: Polycentric environmental governance and policy regulatory framework in China. Energy Res. Soc. Sci. 2025, 128, 104347. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Lv, L.; Yu, Z.; Yao, W.; Cheng, H.; Niu, W.; Wang, J.; Zhang, J.; Qi, H. Design and Verification of a Wearable Micro-Capacitance Test System for POC Biosensing. IEEE Trans. Instrum. Meas. 2025, 74, 1–11. [Google Scholar]
- Jeerapan, I.; Poorahong, S. Flexible and stretchable electrochemical sensing systems: Materials, energy sources, and integrations. J. Electrochem. Soc. 2020, 167, 037573. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, Z.; Huang, W.; Liu, L.; Li, J.; Li, J.; Wu, Q. Redox enzyme-mimicking activities of CeO2 nanostructures: Intrinsic influence of exposed facets. Sci. Rep. 2016, 6, 35344. [Google Scholar] [CrossRef] [PubMed]
- Culica, M.E.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. [Google Scholar] [CrossRef] [PubMed]
- Atisme, T.B.; Yu, C.-Y.; Tseng, E.N.; Chen, Y.-C.; Shu, P.-K.; Chen, S.Y. Interface Interactions in Conjugated Polymer Composite with Metal Oxide Nanoparticles. Nanomaterials 2019, 9, 1534. [Google Scholar] [CrossRef]
- Qin, J.; Feng, Y.; Cheng, D.; Liu, B.; Wang, Z.; Zhao, Y.; Wei, J. Construction of a Mesoporous Ceria Hollow Sphere/Enzyme Nanoreactor for Enhanced Cascade Catalytic Antibacterial Therapy. ACS Appl. Mater. Interfaces 2021, 13, 40302–40314. [Google Scholar] [CrossRef]
- Bortamuly, R.; Konwar, G.; Boruah, P.K.; Das, M.R.; Mahanta, D.; Saikia, P. CeO2-PANI-HCl and CeO2-PANI-PTSA composites: Synthesis, characterization, and utilization as supercapacitor electrode materials. Ionics 2020, 26, 5747–5756. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Bao, N.H.; Anh, N.P.; Phuong, P.H.; Nguyen, T.-T.; Tri, N. Green-synthesized silver nanoparticles decorated on ceria nanorods for room-temperature p-nitrophenol hydrogenation. Green Chem. Lett. Rev. 2022, 15, 449–459. [Google Scholar] [CrossRef]
- Zhou, C.; Richardson-Barlow, C.; Fan, L.; Cai, H.; Zhang, W.; Zhang, Z. Towards organic collaborative governance for a more sustainable environment: Evolutionary game analysis within the policy implementation of China’s net-zero emissions goals. J. Environ. Manage. 2025, 373, 123765. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Foroutan, Z.; Afshari, A.R.; Sabouri, Z.; Mostafapour, A.; Far, B.F.; Jalili-Nik, M.; Darroudi, M. Plant-based synthesis of cerium oxide nanoparticles as a drug delivery system in improving the anticancer effects of free temozolomide in glioblastoma (U87) cells. Ceram. Int. 2022, 48, 30441–30450. [Google Scholar] [CrossRef]
- Gao, J.; Gong, L.; Fan, X.; Yu, K.; Zheng, Z.; Zhou, B. {P2W18O62}-Encapsulated Potassium-Ion Nanotubes Intercalated in Copper Biimidazole Frameworks for Supercapacitors and Hydrogen Peroxide Sensing. ACS Appl. Nano Mater. 2020, 3, 1497–1507. [Google Scholar] [CrossRef]
- Rajendran, S.; Manoj, D.; Suresh, R.; Vasseghian, Y.; Ghfar, A.A.; Sharma, G.; Soto-Moscoso, M. Electrochemical detection of hydrogen peroxide using micro and nanoporous CeO2 catalysts. Environ. Res. 2022, 214, 113961. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Ding, F.; Chen, D.; Wang, Y.; Jin, X.; Zhu, X. Rational design of electroactive redox enzyme nanocapsules for high-performance biosensors and enzymatic biofuel cell. Biosens. Bioelectron. 2021, 174, 112805. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, H.; Wu, J.J.; Mao, X.; Zhang, H.; Amin, N.; Xu, F.; Dong, C.; Wang, C.; Wang, P.; et al. Disposable peptidoglycan-specific biosensor for noninvasive real-time detection of broad-spectrum Gram-positive bacteria in exhaled breath condensates. Anal. Chem. 2024, 96, 9817–9825. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Ipekci, H.H. The use of CeO2-modified Pt/C catalyst inks for the construction of high-performance enzyme-free H2O2 sensors. J. Electroanal. Chem. 2019, 848, 113302. [Google Scholar] [CrossRef]
- Jha, S.K.; Kumar, C.N.; Raj, R.P.; Jha, N.S.; Mohan, S. Synthesis of 3D porous CeO2/reduced graphene oxide xerogel composite and low level detection of H2O2. Electrochim. Acta 2014, 120, 308–313. [Google Scholar] [CrossRef]
- Remani, K.C.; Binitha, N.N. Cobalt doped ceria catalysts for the oxidative abatement of gaseous pollutants and colorimetric detection of H2O2. Mater. Res. Bull. 2021, 139, 111253. [Google Scholar] [CrossRef]
- Dong, W.; Huang, Y. CeO2/C nanowire derived from a cerium(III) based organic framework as a peroxidase mimic for colorimetric sensing of hydrogen peroxide and for enzymatic sensing of glucose. Microchim. Acta 2019, 187, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Liu, Q.; Zhang, X. Colorimetric and ultrasensitive detection of H2O2 based on Au/Co3O4-CeOx nanocomposites with enhanced peroxidase-like performance. Sens. Actuators, B 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, Y.; Yang, Y.; Zhang, L.; Sun, L.; Chen, P.; Gao, C. Enhanced peroxidase-like activity of porphyrin functionalized ceria nanorods for sensitive and selective colorimetric detection of glucose. Mater. Sci. Eng. C 2016, 59, 445–453. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Song, L.; Chi, M.; Li, M.; Wang, C.; Lu, X. Encapsulation of Co3O4 Nanoparticles Inside CeO2 Nanotubes: An Efficient Biocatalyst for the Ultrasensitive Detection of Ascorbic Acid. Part. Part. Syst. Char. 2018, 35, 1800049. [Google Scholar] [CrossRef]
- Rahman, M.M.; Adeosun, W.A.; Asiri, A.M. Fabrication of selective and sensitive chemical sensor development based on flower-flake La2ZnO4 nanocomposite for effective non-enzymatic sensing of hydrogen peroxide by electrochemical method. Microchem. J. 2020, 159, 105536. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Devendrachari, M.C.; Kotresh, H.M.N.; Khan, F. Phthalocyanine pendented polyaniline via amide linkage for an electrochemical sensing of H2O2. Microchem. J. 2021, 161, 105781. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF modified glassy carbon electrode as enzyme-free electrochemical sensor detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Song, W.; Xu, W.; Jiao, L.; Luo, X.; Wu, N.; Yan, H.; Wang, X.; Gu, W.; et al. Fe3C-Assisted Single Atomic Fe Sites for Sensitive Electrochemical Biosensing. Anal. Chem. 2021, 93, 5334–5342. [Google Scholar] [CrossRef]
- Othmani, A.; Derbali, M.; Kalfat, R.; Touati, F.; Dhaouadi, H. A novel 1D/2D Bi2S3/g-C3N4 core–shell nanocomposite as a highly performing H2O2 non-enzymatic electrochemical sensor. J. Mater. Res. Technol. 2021, 15, 5762–5775. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Ghasemi, S.; Ghaffari-Rad, H. Ag-doped zeolitic imidazolate framework-8 nanoparticles modified CPE for efficient electrocatalytic reduction of H2O2. Electrochim. Acta 2015, 163, 280–287. [Google Scholar] [CrossRef]
- Lee, P.K.; Nia, P.M.; Woi, P.M. Facile self-assembled Prussian blue-polypyrrole nanocomposites on glassy carbon: Comparative synthesis methods and its electrocatalytic reduction towards H2O2. Electrochim. Acta 2017, 246, 841–852. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Guo, X.; Sun, D. Self-supporting electrochemical sensors for monitoring of cell-released H2O2 based on metal nanoparticle/MOF nanozymes. Microchem. J. 2022, 181, 107715. [Google Scholar] [CrossRef]
- Riaz, M.A.; Yuan, Z.; Mahmood, A.; Liu, F.; Sui, X.; Chen, J.; Huang, Q.; Liao, X.; Wei, L.; Chen, Y. Hierarchically porous carbon nanofibers embedded with cobalt nanoparticles for efficient H2O2 detection on multiple sensor platforms. Sens. Actuators, B 2020, 319, 128243. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, S.; Sun, C. Ni3Mo3N coupled with nitrogen-rich carbon microspheres as an efficient hydrogen evolution reaction catalyst and electrochemical sensor for H2O2 detection. Int. J. Hydrog. Energy 2022, 47, 14906–14915. [Google Scholar] [CrossRef]
- Zhou, J.; Min, M.; Liu, Y.; Tang, J.; Tang, W. Layered assembly of NiMn-layered double hydroxide on graphene oxide for enhanced non-enzymatic sugars and hydrogen peroxide detection. Sens. Actuators, B 2018, 260, 408–417. [Google Scholar] [CrossRef]
- Woo, S.; Kim, Y.-R.; Chung, T.D.; Piao, Y.; Kim, H. Synthesis of a graphene–carbon nanotube composite and its electrochemical sensing of hydrogen peroxide. Electrochim. Acta 2012, 59, 509–514. [Google Scholar] [CrossRef]
- Temur, E.; Eryiğit, M.; Kurt Urhan, B.; Demir, Ü.; Öznülüer Özer, T. Cu/Electrochemically reduced graphene oxide layered nanocomposite for non-enzymatic H2O2 sensor. Mater. Today Proc. 2021, 46, 6971–6975. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Vishnu, N.; Badhulika, S. Cuprous oxide nanocubes decorated reduced graphene oxide nanosheets embedded in chitosan matrix: A versatile electrode material for stable supercapacitor and sensing applications. J. Electroanal. Chem. 2019, 834, 187–195. [Google Scholar] [CrossRef]
- Jia, W.; Guo, M.; Zheng, Z.; Yu, T.; Rodriguez, E.G.; Wang, Y.; Lei, Y. Electrocatalytic oxidation and reduction of H2O2 on vertically aligned Co3O4 nanowalls electrode: Toward H2O2 detection. J. Electroanal. Chem. 2009, 625, 27–32. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Zhao, P.; Chen, Y.; Lei, J.; Hou, J.; Hou, C.; Huo, D. An electrochemical sensor based on FeCo bimetallic single-atom nanozyme for sensitive detection of H2O2. Anal. Chim. Acta 2023, 1281, 341867. [Google Scholar] [CrossRef]
- Li, T.; Bao, Y.; Qiu, H.; Tong, W. Boosted peroxidase-like activity of metal-organic framework nanoparticles with single atom Fe(Ⅲ) sites at low substrate concentration. Anal. Chim. Acta 2021, 1152, 338299. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, W.; Gao, R.; Wang, Y.; Bai, Y.; Xu, Z.; Bao, S.-J. MIL-47(V) catalytic conversion of H2O2 for sensitive H2O2 detection and tumor cell inhibition. Sens. Actuators, B 2022, 354, 131201. [Google Scholar] [CrossRef]
- Bhangoji, J.C.; Sahoo, S.; Satpati, A.K.; Shendage, S.S. Facile and green synthesis of silver nanoparticle-reduced graphene oxide composite and its application as nonenzymatic electrochemical sensor for hydrogen peroxide. Curr. Chem. Lett. 2021, 10, 295–308. [Google Scholar] [CrossRef]

| Sensor Material | Operating Potential (V, vs. Ag/AgCl)) | Linear Range | Sensitivity | Detection Limit | Reference |
|---|---|---|---|---|---|
| CeO2-phm/cMWCNTs | −0.55 | 0.5–50 μM; 50–450 μM | 2161.6 μA·mM−1·cm−2; 2070.9 μA·mM−1·cm−2 | 0.017 μM | This work |
| CeO2/Pt/C | −0.4 | 0.01–30 mM | 185.4 ± 6.5 μA mM−1 cm−2 | 2 μM | [41] |
| CeO2/rGO xerogel | −0.3 | 60.7 nM–3.0 μM | 1.978 × 10−1 μA mM−1 | 30.40 nM | [42] |
| CeO2 | −0.3 | 91.88 μM–2.0 mM | 2.9346 × 10−5 μA mM−1 | 31.29 μM | [42] |
| Co/CeO2 | - | 3.33–100 μM; 100–1166 μM; 1166–5000 μM | - | 3.33 μM | [43] |
| CeO2/C nanowires | - | 0.5–100 μM | - | 0.42 μM | [44] |
| porphyrin functionalized CeO2 | - | 10–100 μM | - | 5.29 μM | [45] |
| porphyrin functionalized CeO2 nanorods | - | 10–100 μM | - | 6.1 μM | [46] |
| Co3O4 nanoparticles inside CeO2 nanotubes | - | 2–80 μM | - | 1.2 μM | [47] |
| La2ZnO4 | - | 3.0–85.0 μM | 25,000 μA mM−1 cm−2 | 0.04 μM | [48] |
| Phthalocyanine pendented polyaniline | 0.015 | 0.2–52 μM | 2317.5 μA mM−1 cm−2 | 0.15 μM | [49] |
| AuNPs-NH2/Cu-MOF | −0.15 | 5–850 μM | 1710 μA mM−1 cm−2 | 1.2 μM | [50] |
| Au/ZnO | 0.05 | 1 μM–3.0 mM | 1336.7 μA mM−1 cm−2 | 0.1 μM | [48] |
| Fe3C@C/Fe-N-C | - | 1–6000 μM | 1225 μA mM−1 cm−2 | 0.26 μM | [51] |
| Bi2S3/g-C3N4 | +0.26 | 0.5–950 μM | 1011 μA mM−1 cm−2 | 78 nM | [52] |
| Ag/ZIF-8 | −0.6 | 20 μM–5 mM; 5.5–10 mM | 398.47 and 145.21 μA mM−1 cm−2 | 6.2 μM | [53] |
| Prussian blue-polypyrrole composite | −0.1 | 0–3.5 mM | 377.43 μA mM−1 cm−2 | - | [54] |
| Ag/2D Zn-MOFs | −0.55 | 5.0 μM–70 mM | 358.7 μA mM−1 cm−2 | 1.67 μM | [55] |
| Co-NC/CNF | −0.5 | 10–5000 μM | 300 μA mM−1 cm−2 | 10 μM | [56] |
| Ni3Mo3N/NC MSs | −0.60 | 5 μM–40 mM | 120.3 μA mM−1 cm−2 | 1 μM | [57] |
| NiMn-LDH/GO | −0.45 | 20–5860 μM | 96.82 μA mM−1 cm−2 | 4.4 μM | [58] |
| Graphene-MWCNT | −0.4 | 20–2000 μM | 32.91 μA mM−1 cm−2 | 9.4 μM | [59] |
| Cu nanoparticles/ERGO | −0.2 | 0.01–1 mM | 20 μA mM−1 cm−2 | 1.87 × 10−9 M | [60] |
| CNC-rGO | −0.2 | 20–160 μM | 0.333 μA mM−1 cm−2 | 5.28 μM | [61] |
| Co3O4 nanowalls | +0.8 | 0–1.4 mM | 100.3 μA mM−1 | 2.8 μM | [62] |
| Co3O4 nanowalls | −0.2 | 0–5.35 mM | 4.844 μA mM−1 | 10 μM | [62] |
| Fe SAs/Co CNs | - | 1–400 μM | - | 0.36 μM | [63] |
| Fe–HCl–NH2-UiO-66 | - | 3.125–100 μM | - | 1.0 μM | [64] |
| MIL-47(V)-OH | - | 4.38–43.97 μM; 50.33–2240 μM | - | 5.84 μM | [65] |
| Sample | Added (μM) | Relative Standard Deviation (%, n = 3) | Measured (μM) | Recovery (%) |
|---|---|---|---|---|
| Groundwater | 5.0 | 2.46 | 5.13 | 102.6% |
| Commercial drinking water | 2.0 | 1.34 | 2.01 | 100.5% |
| Milk | 1.0 | 0.96 | 1.01 | 101.0% |
| Fetal Bovine Serum | 10 | 1.02 | 10.13 | 101.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; He, X.; Zou, S.; Ling, K.; Zhu, H.; Jiang, Q.; Zhang, Y.; Feng, Z.; Wang, P.; Duan, X.; et al. A Flexible Electrochemical Sensor Based on Porous Ceria Hollow Microspheres Nanozyme for Sensitive Detection of H2O2. Biosensors 2025, 15, 664. https://doi.org/10.3390/bios15100664
Huang J, He X, Zou S, Ling K, Zhu H, Jiang Q, Zhang Y, Feng Z, Wang P, Duan X, et al. A Flexible Electrochemical Sensor Based on Porous Ceria Hollow Microspheres Nanozyme for Sensitive Detection of H2O2. Biosensors. 2025; 15(10):664. https://doi.org/10.3390/bios15100664
Chicago/Turabian StyleHuang, Jie, Xuanda He, Shuang Zou, Keying Ling, Hongying Zhu, Qijia Jiang, Yuxuan Zhang, Zijian Feng, Penghui Wang, Xiaofei Duan, and et al. 2025. "A Flexible Electrochemical Sensor Based on Porous Ceria Hollow Microspheres Nanozyme for Sensitive Detection of H2O2" Biosensors 15, no. 10: 664. https://doi.org/10.3390/bios15100664
APA StyleHuang, J., He, X., Zou, S., Ling, K., Zhu, H., Jiang, Q., Zhang, Y., Feng, Z., Wang, P., Duan, X., Liao, H., Yuan, Z., Liu, Y., & Tan, J. (2025). A Flexible Electrochemical Sensor Based on Porous Ceria Hollow Microspheres Nanozyme for Sensitive Detection of H2O2. Biosensors, 15(10), 664. https://doi.org/10.3390/bios15100664






