Mapping Surface Potential in DNA Aptamer–Neurochemical and Membrane–Ion Interactions on the SOS Substrate Using Terahertz Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. SOS Substrate and Terahertz Chemical Microscope Optical Setup
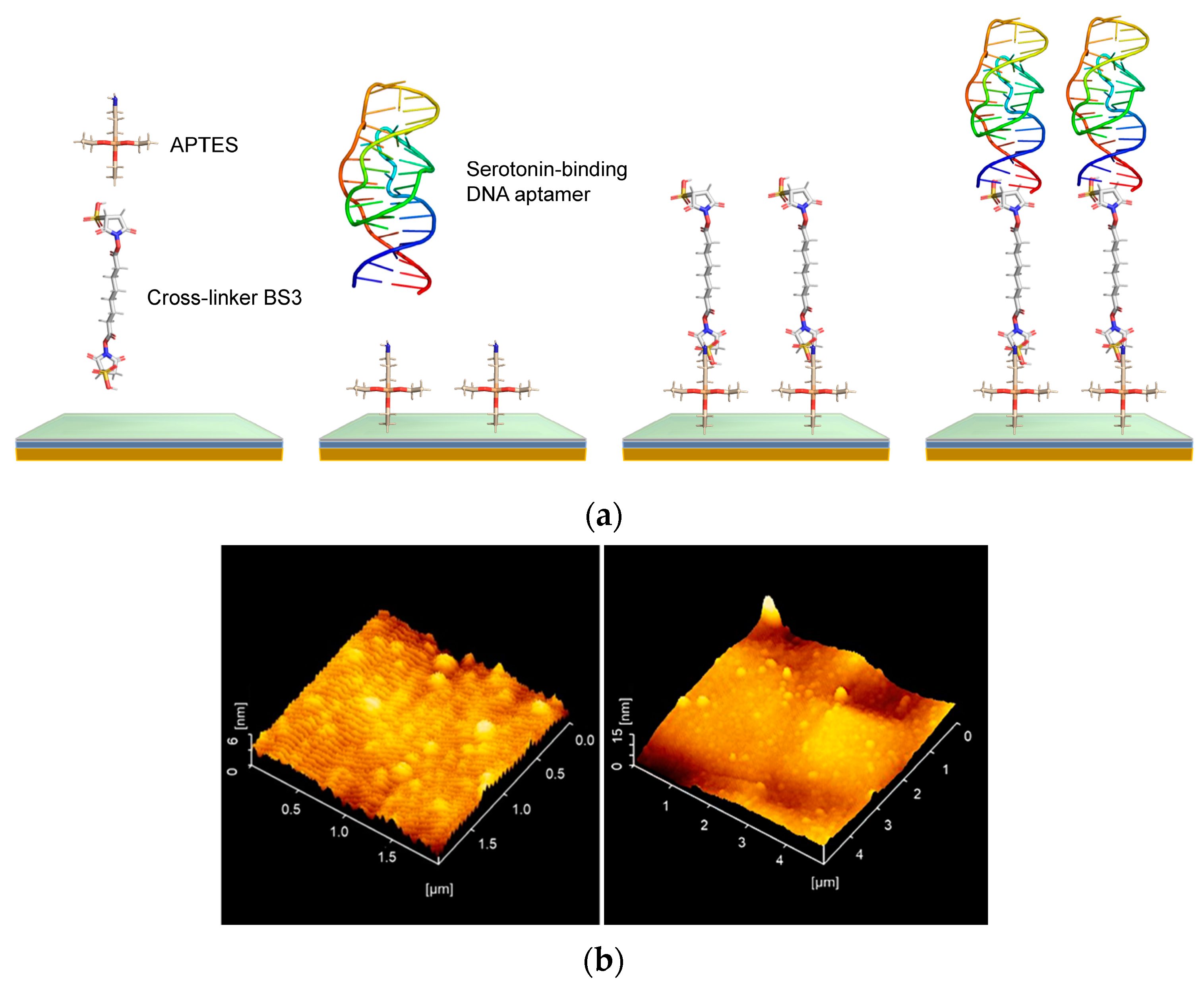
2.3. Substrate Cleaning and Aptamer Modification
2.4. For Surface Potential Mapping and Real-Time Aptamer–Serotonin Interaction Monitoring
2.5. 3D-Printed PDMS Microwell for Calcium Ion Measurements
3. Results and Discussion
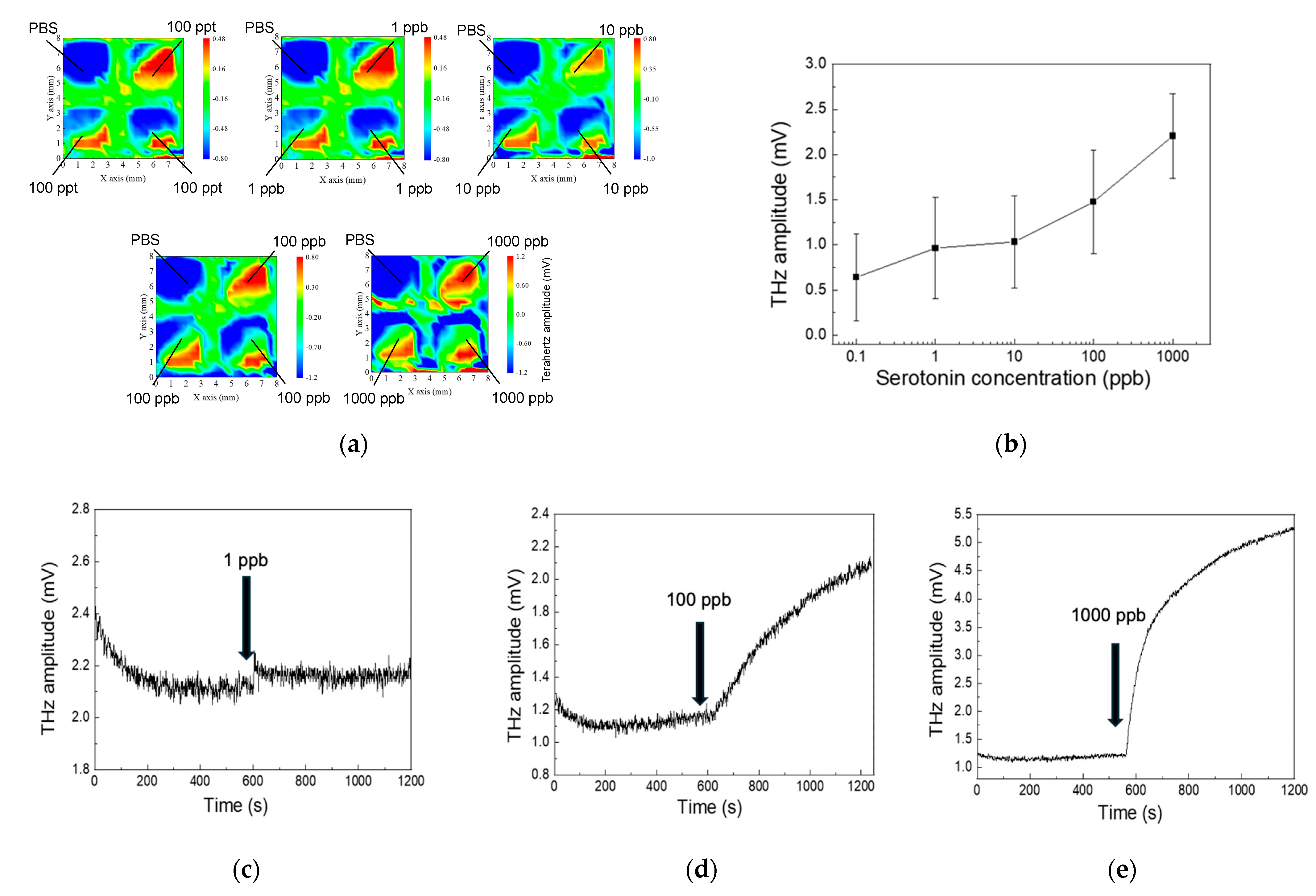
3.1. Mapping of the Surface Potential of DNA Aptamer–Serotonin Complexes
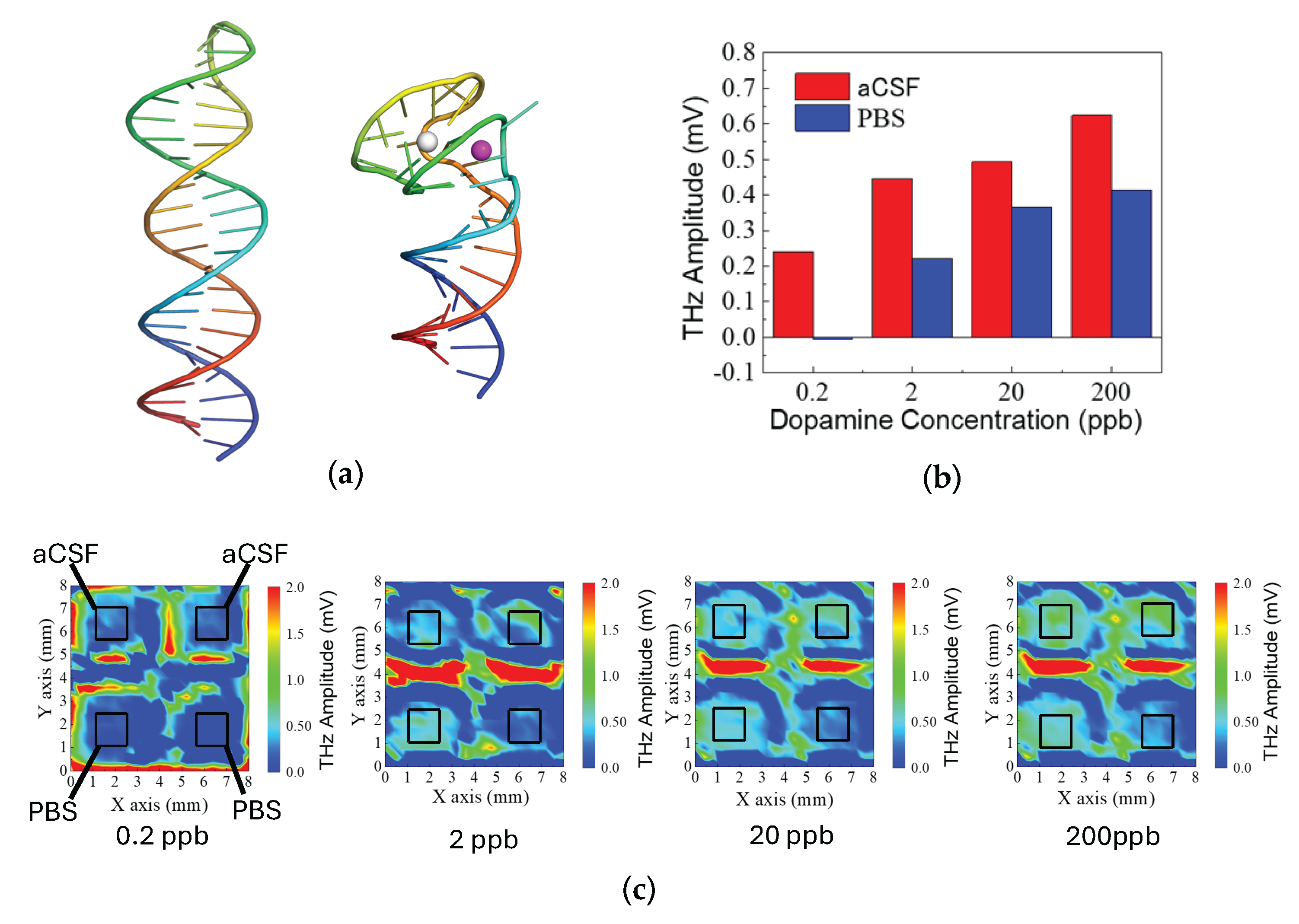
3.2. Comparative Study of DNA Aptamer Conformational Changes and Terahertz Responses Changes in PBS and aCSF
3.3. PDMS Microwell-Based Membrane–Calcium Ion Interaction and Potential Mapping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kathmann, S.M.; Kuo, I.F.W.; Mundy, C.J.; Schenter, G.K. Understanding the surface potential of water. J. Phys. Chem. B 2011, 115, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, H.; Yang, J. Surface potential/charge sensing techniques and applications. Sensors 2020, 20, 1690. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Nakahara, H.; Phan, C.M. Surface potential of the air/water interface. J. Phys. Chem. 2020, 69, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, W.; Lee, J.H.; Yoon, D.S. Surface potential analysis of nanoscale biomaterials and devices using Kelvin probe force microscopy. J. Nanomater. 2016, 2016, 4209130. [Google Scholar] [CrossRef]
- Brown, M.A.; Abbas, Z.; Kleibert, A.; Green, R.G.; Goel, A.; May, S.; Squires, T.M. Determination of surface potential and electrical double-layer structure at the aqueous electrolyte-nanoparticle interface. Phys. Rev. X 2016, 6, 011007. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Y.; Wang, J.; Li, L.; Zhang, Y. Recent progress in regulating surface potential for high-efficiency perovskite solar cells. ACS Energy Lett. 2024, 9, 1674–1681. [Google Scholar] [CrossRef]
- Fu, W.; Jiang, L.; Van Geest, E.P.; Schneider, G.F.; Schneider, G.F. Sensing at the Surface of Graphene Field-Effect Transistors. Adv. Mater. 2016, 29, 1603610. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Zhan, K.; Sun, D.; Hou, X. Chemiresistive nanosensors with convex/concave structures. Nano Today 2018, 20, 84–100. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The Role of Polarization in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 10061–10073. [Google Scholar] [CrossRef]
- Takadoum, J. The influence of potential on the tribocorrosion of nickel and iron in sulfuric acid solution. Corros. Sci. 1996, 38, 643–654. [Google Scholar] [CrossRef]
- Nordt, F.J. Alterations in surface charge density versus changes in surface charge topography in aging red blood cells. Ann. Hematol. 1980, 40, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.; Wheeler, A.R.; Zare, R.N.; Fruetel, J.A.; Shepodd, T.J. Programmable modification of cell adhesion and zeta potential in silica microchips. Lab Chip 2003, 3, 5–10. [Google Scholar] [CrossRef]
- Ruiz-Mateos Serrano, R.; Farina, D.; Malliaras, G.G. Body surface potential mapping: A perspective on high-density cutaneous electrophysiology. Adv. Sci. 2024, 2411087. [Google Scholar] [CrossRef]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Yoshimi, Y. Development of deep brain stimulation control method for Parkinson’s disease therapy using molecularly imprinted polymeric sensors. Life 2023, 13, 78. [Google Scholar]
- Audhya, T.; Adams, J.B.; Johansen, L. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim. Biophys. Acta 2012, 1820, 1496–1501. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, C.; Chen, M.; Liu, Y.; Zhao, Z.; Wu, F.; Li, Z.; Weiss, P.S.; Andrews, A.M.; Zhou, C. Flexible multiplexed In2O3 nanoribbon aptamer-field-effect transistors for biosensing. iScience 2020, 23, 101469. [Google Scholar] [CrossRef]
- Keene, S.T.; Fogarty, D.; Cooke, R.; Casadevall, C.D.; Salleo, A.; Parlak, O. Wearable organic electrochemical transistor patch for multiplexed sensing of calcium and ammonium ions from human perspiration. Adv. Mater. 2019, 31, 1901321. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.-A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Andrews, A.M. Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Chen, F.; Wu, C.; Krause, S.; Wang, J.; Zhang, D.-W. Light-addressable electrochemical sensors toward spatially resolved biosensing and imaging applications. ACS Sens. 2022, 7, 1791–1807. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Jiang, S.; Kunze, J.; Schmuki, P.; Krause, S. High-resolution LAPS and SPIM. Electrochem. Commun. 2010, 12, 758–760. [Google Scholar] [CrossRef]
- Zhao, S.; Piatkevich, K.D. Techniques for in vivo serotonin detection in the brain: State of the art. J. Neurochem. 2023, 166, 453–480. [Google Scholar] [CrossRef] [PubMed]
- Yoshinobu, T.; Iwasaki, H.; Ui, Y.; Furuichi, K.; Ermolenko, Y.; Mourzina, Y.; Wagner, T.; Nather, N.; Schoning, M.J. The light-addressable potentiometric sensor for multi-ion sensing and imaging. Methods 2005, 37, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hafeman, D.; Parce, J.; McConnell, H. Light-addressable potentiometric sensor for biochemical systems. Science 1988, 240, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Rantataro, S.; Parkkinen, I.; Airavaara, M.; Laurila, T. Real-time selective detection of dopamine and serotonin at nanomolar concentration from complex in vitro systems. Biosens. Bioelectron. 2023, 241, 115579. [Google Scholar] [CrossRef] [PubMed]
- Kiwa, T.; Kamiya, T.; Morimoto, T.; Fujiwara, K.; Maeno, Y.; Akiwa, Y.; Iida, M.; Kuroda, T.; Sakai, K.; Nose, H.; et al. Imaging of chemical reactions using a terahertz chemical microscope. Photonics 2019, 6, 10. [Google Scholar] [CrossRef]
- Wang, J.; Ando, M.; Nagata, H.; Yoshida, S.; Sakai, K.; Kiwa, T. Multifunctional terahertz microscopy for biochemical and chemical imaging and sensing. Biosens. Bioelectron. 2023, 220, 114901. [Google Scholar] [CrossRef]
- Kiwa, T.; Tenma, A.; Takahashi, S.; Sakai, K.; Tsukada, K. Label-free immune assay using terahertz chemical microscope. Sens. Actuators B Chem. 2013, 187, 8–11. [Google Scholar] [CrossRef]
- Kiwa, T.; Tsukada, K.; Suzuki, M.; Tonouchi, M.; Migitaka, S.; Yokosawa, K. Laser terahertz emission system to investigate hydrogen gas sensors. Appl. Phys. Lett. 2005, 86, 261102. [Google Scholar] [CrossRef]
- Kiwa, T.; Tonouchi, M.; Yamashita, M.; Kawase, K. Laser terahertz-emission microscope for inspecting electrical faults in integrated circuits. Opt. Lett. 2003, 28, 2058–2060. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Röhrig, U.F.; Goullieux, M.; Bugnon, M.; Zoete, V. Attracting Cavities 2.0: Improving the flexibility and robustness for small-molecule docking. J. Chem. Inf. Model. 2023, 63, 3925–3940. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.; Lafaye, C.; Wang, S.; Fernandez-Sanchez, C.; Saubade, M.; Liu, S.-C.; Jimenez-Jorquera, C. Analytical assessment of sodium ISFET-based sensors for sweat analysis. Sens. Actuators B Chem. 2023, 393, 134135. [Google Scholar] [CrossRef]
- You, X.; Pak, J. Graphene-based field-effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, H.J.; Kim, C.Y.; Oh, B.K.; Rho, K.L.; Nam, H.; Cha, G.S. ISFET-based differential pCO2 sensors employing a low-resistance gas-permeable membrane. Anal. Chem. 1996, 68, 3166–3172. [Google Scholar] [CrossRef]



| Polyvinyl chloride (Base Polymer) | 33 wt% |
| Bis(2-ethylhexyl) sebacate (Plasticizer) | 65.5 wt% |
| N,N,N′,N′-Tetra(cyclohexyl)diglycolic acid diamide (Ionophore) | 1 wt% |
| Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl] borate (Additive) | 0.5 wt% |
| Tetrahydrofuran (Solvent) | 588 μL |
| PBS | aCSF | |
|---|---|---|
| Composition | Concentration (mM) | |
| NaCl | 137 | 147 |
| KCl | 2.7 | 3.5 |
| Na2HPO4 | 10 | 10 |
| KH2PO4 | 1.8 | 2.5 |
| MgCl2 | - | 1.2 |
| CaCl2 | - | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, K.; Mitsuda, Y.; Yoshida, S.; Kiwa, T.; Wang, J. Mapping Surface Potential in DNA Aptamer–Neurochemical and Membrane–Ion Interactions on the SOS Substrate Using Terahertz Microscopy. Biosensors 2025, 15, 46. https://doi.org/10.3390/bios15010046
Morita K, Mitsuda Y, Yoshida S, Kiwa T, Wang J. Mapping Surface Potential in DNA Aptamer–Neurochemical and Membrane–Ion Interactions on the SOS Substrate Using Terahertz Microscopy. Biosensors. 2025; 15(1):46. https://doi.org/10.3390/bios15010046
Chicago/Turabian StyleMorita, Kosei, Yuta Mitsuda, Sota Yoshida, Toshihiko Kiwa, and Jin Wang. 2025. "Mapping Surface Potential in DNA Aptamer–Neurochemical and Membrane–Ion Interactions on the SOS Substrate Using Terahertz Microscopy" Biosensors 15, no. 1: 46. https://doi.org/10.3390/bios15010046
APA StyleMorita, K., Mitsuda, Y., Yoshida, S., Kiwa, T., & Wang, J. (2025). Mapping Surface Potential in DNA Aptamer–Neurochemical and Membrane–Ion Interactions on the SOS Substrate Using Terahertz Microscopy. Biosensors, 15(1), 46. https://doi.org/10.3390/bios15010046







