1. Introduction
Lactate, a pivotal metabolic byproduct of anaerobic glycolysis, has garnered significant importance in clinical medicine, exercise physiology, and food science [
1]. Traditionally perceived as a metabolic waste associated with muscle fatigue [
2], recent research has unveiled lactate’s critical roles in both physiological and pathological cellular states. Approximately 1500 mM of lactate is produced daily from various tissues, including skeletal muscle, heart, and brain [
3]. It serves not only as an energy substrate, supplying energy [
4], but also participates in energy transfer processes within and between tissues. Converted into pyruvate, lactate contributes to oxidative phosphorylation and ATP generation through the tricarboxylic acid cycle [
5]. Beyond its role as an energy source, lactate functions as a signaling molecule, influencing processes such as tumor cell proliferation, immune evasion, and neuronal energy metabolism [
6].
Disruptions in lactate production and consumption can lead to various diseases, highlighting the critical importance of measuring blood lactate concentrations for diagnosis and treatment [
7]. In sports medicine, lactate serves as a signaling molecule that positively regulates metabolic processes during physical activity. Blood lactate concentration sensitively reflects changes in exercise intensity and duration, making it a vital metric for evaluating an athlete’s training level [
8]. Furthermore, during physical exertion, lactate serves as an alternate energy substrate for the brain, conserves glucose and stimulates the hypothalamus to regulate energy intake and neuronal activity, thereby playing a pivotal role in enhancing brain metabolism and overall function [
9]. In food analysis, monitoring lactate levels is crucial for evaluating the quality, freshness, and preservation stability of various food products [
10], including fruits, meats, alcoholic beverages [
11], and specific fermented dairy items [
12]. Given lactate’s association with inflammatory states, cancer, and other health issues, its precise control and detection within the food sector are paramount.
Tumor cells undergo aerobic glycolysis and produce lactate even in the presence of sufficient oxygen, a phenomenon known as the “Warburg effect.” [
13]. Lactate accumulation within the tumor microenvironment significantly impacts tumor progression by fostering cell invasion and angiogenesis [
14]. While lactate concentrations are tightly regulated at approximately 1.5–3 mM under normal physiological conditions [
15], they can escalate to 30 mM within tumor microenvironments, potentially influencing cellular functions [
16]. Tumor cells rapidly respond to metabolic signals in the environment and increase energy metabolism by regulating mitochondria-associated pathways and the TCA cycle, thereby promoting tumor growth and metastasis [
17]. Consequently, lactate serves not only as a crucial player in energy metabolism but also as a significant regulator of tumor development [
18]. Accurate and sensitive analysis of lactate secretion by tumor cells holds promise for early cancer diagnosis.
Due to the crucial regulatory role of lactate in tumor occurrence and development, accurate and reliable analysis and detection are essential. The main techniques for lactate detection include chromatographic analysis, fluorescence detection, luminescence methods, and electrochemical methods. Common chromatographic analysis methods used for lactate detection include high-performance liquid chromatography (HPLC), mass spectrometry (MS), gas chromatography (GC), and spectroscopic methods. These techniques quantitatively analyze lactate by separating and detecting the concentration of lactate present in the sample. Omar Kadi et al. proposed an LC-MS/MS method for concurrent detection and quantification of crucial metabolites in various cancer types like prostate cancer. This approach involves extracting and qualitatively and quantitatively analyzing glutamine, citrate, isocitrate, malate, succinate, fumarate, and lactate from body fluids, tissues, and human-derived cultured cell lines [
19]. Fluorescence methods are analytical techniques based on the interaction between the analyte and a fluorescent dye or label, producing a fluorescent signal. A study [
20] designed a glass capillary platform for fluorescence detection of lactate. The inner wall of the glass capillary is patterned with lipids and bovine serum albumin (BSA) modifications, utilizing electrostatic interactions to immobilize LDH on different regions of the lipid layers. It was found that the fluorescence intensity at the enzyme sites increases with the increasing L-lactate concentration, exhibiting a positive correlation. The established method has a detection limit of 4.9 μM for L-lactate. Electrochemical methods utilize the detection system to record and analyze changes in electrical signals, which are then converted into the concentration or activity of the target molecule. M. Briones et al. [
21] designed an electrochemical biosensing platform by modifying a gold electrode with undoped diamond nanoparticles and employing LOx as a model enzyme for lactate detection. This platform exhibits a linear concentration range of 0.05–0.7 mM, a sensitivity of 4.0 μA/mM, and a detection limit of 15 μM. Although accurate lactate detection can be provided based on the techniques described above, these techniques require a significant investment of time, complex preparation processes, and expensive equipment, which limits their widespread use, especially in point-of-care (POC) testing in less developed regions. Biosensors currently outperform traditional detection methods [
21], offering alternative solutions to circumvent the constraints of conventional techniques. Paper-based microfluidics is an emerging technology in the field of microfluidics, primarily utilizing porous materials like paper as the substrate for microfluidic chips, with capillary action driving the flow and diffusion of liquids within the paper. By designing specific hydrophobic/hydrophilic regions on the paper, the flow path and diffusion areas of the liquid can be controlled. Paper-based microfluidic chips can be used for various biochemical analyses, such as enzymatic reactions, immunoassays, and nucleic acid detection. By pre-immobilizing reagents in specific regions, the reaction can occur as the test liquid flows through, and the results can be read visually or using instruments. Paper-based chips can be stored for long periods of time due to their stabilizing properties. Due to its low cost, portability, and ease of operation, paper-based microfluidics has garnered significant attention in the field of lactate detection. Combining paper-based microfluidics with the catalytic reaction of lactate oxidase can enable the detection and analysis of lactate.
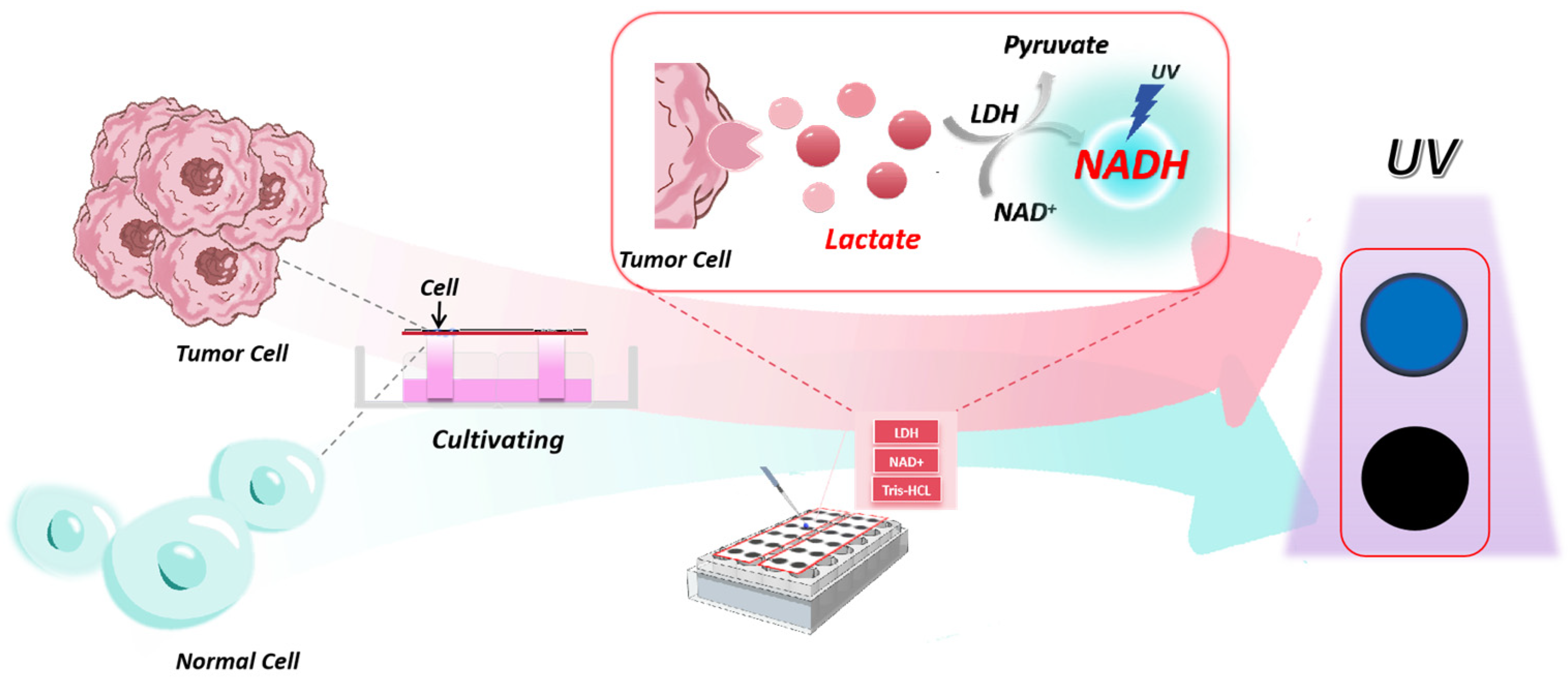
Based on the limitations of the above detection methods, this study combined the advantages of paper-based microfluidics to develop a paper-based microfluidic platform for lactate detection integrating the paper-based microarray previously developed by our team [
22]. Based on the chip’s ability to culture cells in three dimensions, the detection of exocrine lactate from living cells was realized. Although lactate lacks color characteristics or fluorescence absorption, its reaction with LDH and NAD
+ produces NADH through dehydrogenation, which can be detected through fluorescence [
23]. This method allows lactate detection within 30 min, providing a new avenue for early tumor diagnosis. Furthermore, we investigated the differences in lactate secretion levels between tumor and normal cells, which is crucial for early tumor detection. Additionally, this portable microdevice enables imaging and colorimetric analysis of drug screening results, showing potential applications in effective drug screening and biomedical research.
2. Materials and Methods
Lactate was obtained from Shanghai Titan Scientific Co., Ltd. (Shanghai, China), LDH was obtained from Ying Xin Laboratory Equipment Co., Ltd. (Shanghai, China), and NAD+ was obtained from Yi sheng Chemical Technology Co., Ltd. (Shanghai, China). The commercial lactate detection kit was provided by Elabscience Biotechnology Co., Ltd. (Wuhan, China), and paclitaxel was purchased from Dibai Biotechnology Co., Ltd. (Shanghai, China). Doxorubicin hydrochloride was obtained from Bide Pharmatech Ltd., (Shanghai, China). Collagen was purchased from Sigma Aldrich, Inc. (Saint Louis, MO, USA). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), and trypsin-EDTA were purchased from Thermos Fisher Scientific Inc. (Waltham, MA, USA). CCK-8 was obtained from TransGen Biotech Co., Ltd. (Shanghai, China). PMHS was provided by Darui Chemicals Co., Ltd. (Shanghai, China). All other chemicals were of analytical grade and used as received.
The filter paper of Grade 1 (particle retention in liquid: 11 µM; thickness: 180 µM) and Grade 4 (particle retention in liquid: 20 to 25 µM; thickness: 200 µM) were purchased from Whatman (London, UK). An inkjet printer (TS3150) was purchased from Cannon Co., Ltd. (Shanghai, China). A 24-well microplate (Corning Inc., Corning, NY, USA) served as the medium container in a paper-based microfluidic platform. Chromogenic results were imaged using a smartphone camera.
Human breast adenocarcinoma cell line (MCF-7), hepatocellular carcinoma cell line (HepG2), Metastatic Breast-231 cell line (MB-231), human embryonic kidney 293T cell line (HEK293T), HeLa cell line, and normal human fetal liver cells (L-02) were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured using a standard cell culture technique for 2 or 3 days, followed by digestion with 0.05% trypsin for 2 min and centrifugation at 8000× g for 2 min. The cell pellet was resuspended in the culture medium and saved for further use.
2.1. Production and Optimization of the Paper-Based Microfluidic Platform
This study utilized an inkjet printer to prepare paper-based chips for lactate detection. A hydrophobic ink was prepared by mixing polydimethylsiloxane (PMHS) and n-butanol in a 2:1 ratio and filtering through a 0.22 μm filter. The filtered ink was then loaded into a clean ink cartridge. The pattern design was created using Adobe Illustrator (Adobe Inc., San Jose, CA, USA) and subsequently printed onto the paper using an inkjet printer, with the printed area serving as the hydrophobic section. Following printing, the paper was subjected to a 65 °C oven to facilitate crosslinking of PMHS on the paper surface. The paper-based cell culture microfluidic platform was developed based on prior designs by our group, comprising two stacked layers of filter paper. A hydrophobic pattern was printed on the paper to delineate a closed hydrophilic zone conducive for cell growth. The two paper sheets were then bonded together using tape, aligning identical circular patterns. Subsequently, the hydrophilic channel was folded at a 90° angle to facilitate medium delivery. Finally, the paper device underwent overnight UV disinfection in preparation for subsequent experimental use.
To minimize the interference caused by the paper-based substrate’s background color in the fluorescence signal detection and facilitate direct visual detection of low-concentration target product fluorescence signals, an optimization of the paper-based background color was performed. For this purpose, red, yellow, purple, and black paper sheets were used for optimization experiments, with each sheet undergoing hydrophobic treatment. The experimental process involved applying NADH standard substance (10 mM, 20 mM, 30 mM, 40 mM, and 50 mM) onto the paper sheets, followed by detecting the fluorescence signals emitted by the experimental groups under a portable ultraviolet lamp. Fluorescence signals were photographed, and the images were processed using ImageJ to convert them into grayscale values. By analyzing the linear relationship between fluorescence signals on different color paper sheets, the optimal background color was determined. To ensure the reliability of the research results, consistent image acquisition conditions were maintained throughout all experiments, thereby improving the accuracy of the detection method.
2.2. Optimization of the Paper-Based Lactate Detection Platform
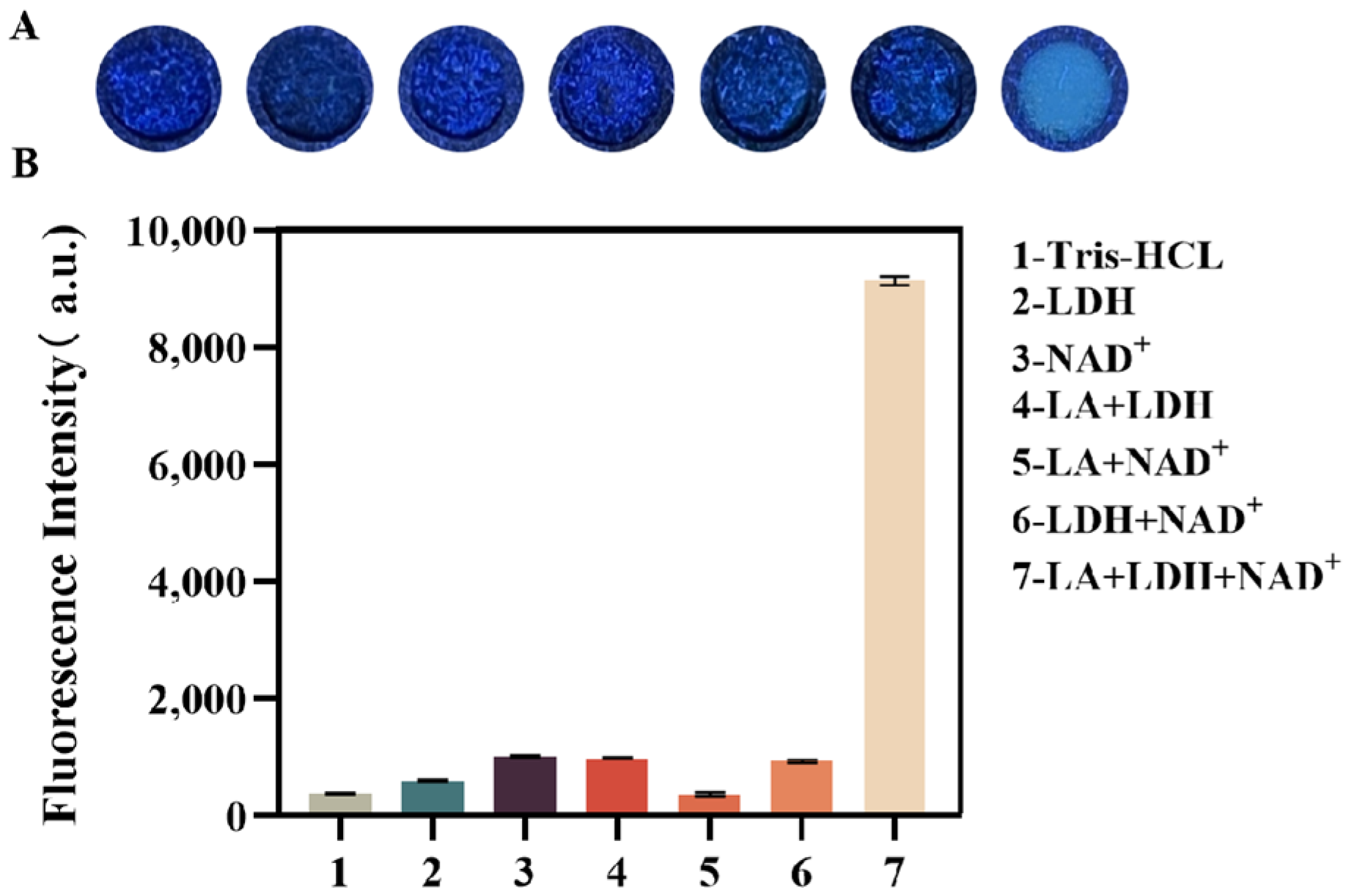
The feasibility of a fluorescence assay method for lactate detection was validated through fluorescence intensity measurements on a microplate reader. The following experimental groups were analyzed: (1) 100 μL Tris-HCl buffer (pH = 7); (2) 100 μL LDH; (3) 100 μL NAD+; (4) 50 μL LA + 50 μL LDH; (5) 50 μL LA + 50 μL NAD+; (6) 50 μL LDH + 50 μL NAD+; and (7) the positive control sample containing 50 μL LA + 25 μL LDH + 25 μL NAD+. All solutions were prepared in Tris-HCl buffer and added to the detection zone. The samples were then incubated at 37 °C for 30 min, exposed to a UV lamp, and the fluorescence of the reaction products was captured by photography. The gray-scale values of the images were analyzed using ImageJ (Ver. 1.50i) software.
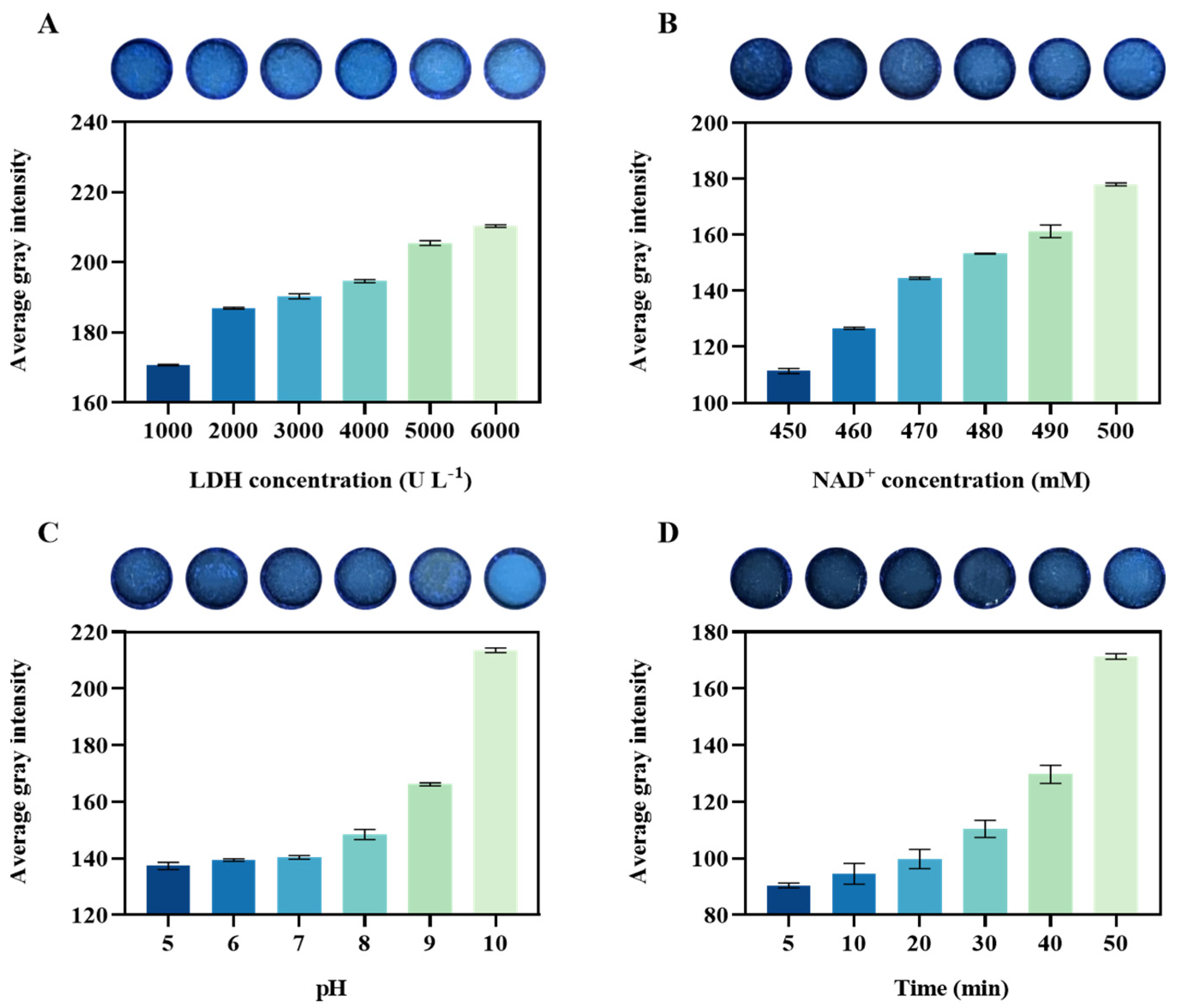
For the paper-based detection platform, we optimized the system based on four conditions, including the concentrations of LDH and NAD+, reaction pH, and reaction time. Initially, the lactate concentration was set at 100 mM, NAD+ concentration was set at 500 mM, and the pH of Tris-HCl buffer was set at 7. An LDH concentration gradient ranging from 1000 to 6000 U L−1 was established. The reaction mixture was added to the reaction zone of the paper chip and incubated at 37 °C for 30 min to facilitate the reaction. Subsequent observation and photography were conducted in a light-proof setting using a portable UV lamp for further analysis. Furthermore, optimization of NAD+ concentration (450–500 mM), buffer pH (7–10), and reaction time (5–30 min) was performed. During optimization, all parameters except the one under investigation were kept constant to ensure result reliability. A consistent photographic environment was maintained throughout all experiments to improve assay accuracy.
2.3. Establishment of the Paper-Based Lactate Detection Platform
To establish the relationship between fluorescence intensity and lactate concentration, quantitative analysis was performed using lactate standards at concentrations of 100 mM, 200 mM, 300 mM, 400 mM, 500 mM, and 600 mM. By capturing images and analyzing the fluorescence signals with specialized software, standard curves were generated, enabling precise quantification of lactate concentration based on fluorescence intensity. Furthermore, a commercial lactate assay kit was used to be compared with the paper-based lactate detection method. Lactate samples at various concentrations (100, 200, 300, 400, 500, and 600 mM) were tested to generate a calibration curve, following the manufacturer’s instructions. The kit uses NAD+ as a hydrogen acceptor, with LDH facilitating the conversion of lactate to pyruvate, thereby transforming NAD+ into NADH. In this process, N-methyl phenazine methyl sulfate transfers hydrogen to reduce nitro tetrazolium blue chloride to a purple colorant, which can be quantified by measuring the optical density.
2.4. Detection of Lactate Secreted by Tumor Cells Using the Paper-Based Platform
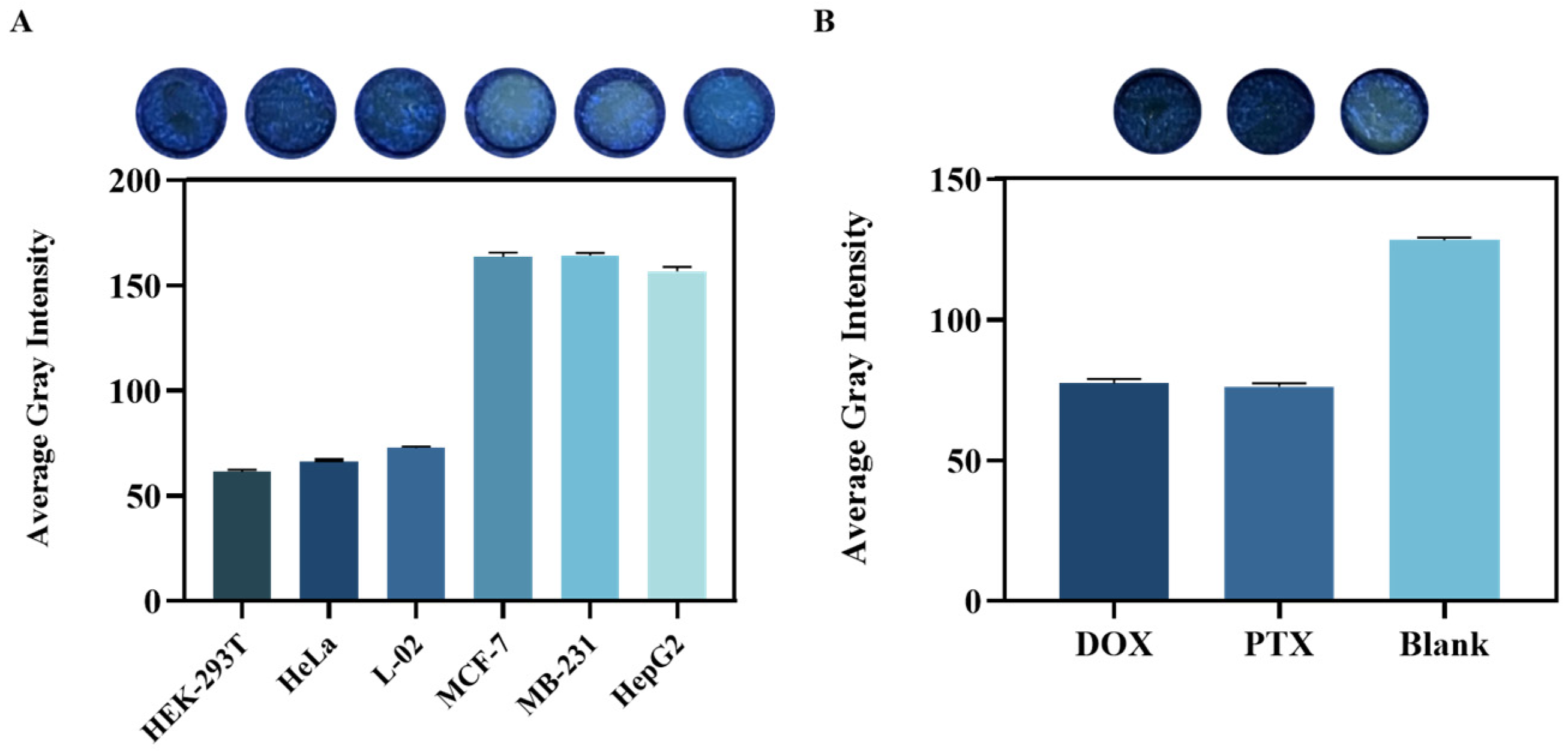
In this study, a 3D paper-based culture platform was developed for cell cultivation and the detection of cell-secreted lactate. Six different cell types (MCF-7, HepG2, MB231, HEK293T, HeLa, and L-02) were seeded onto the culture area and incubated at 37 °C with 5% CO2. The reaction system, which included 5000 U L−1 of LDH and 500 mM NAD+, was added to the culture zone after incubation. The mixture was then incubated at 37 °C, and images were collected and analyzed under light avoidance conditions. Cell viability in the culture area was evaluated using the CCK-8 assay, with color intensity representing cell viability and cytotoxicity.
2.5. Applications for the Paper-Based Lactate Detection Platform
Two anticancer drugs were prepared in DMEM following dissolution in DMSO, with DMSO concentrations ranging from 0.1% and 0.8%, a range deemed safe due to its toxicity being below 1%. The drug-containing medium was applied to the culture zone, saturating the paper-based chip, followed by the addition of MCF-7 cell suspension to each zone. The paper device was incubated at 37 °C for 24 h. After incubation, the lactate detection system was added to the incubated zones and further incubated at 37 °C for a predetermined time to facilitate reaction. Similarly, the CCK-8 re-agent was applied to the paper device to evaluate cell viability, providing a comprehensive assessment of the effects of the anticancer drugs on the cells.
Results were analyzed by one way analysis of variance (ANOVA) using GraphPad Prism 7 (GraphPad Software Inc., CA, USA). Data are presented as the mean ± standard deviation (SD) more than 3 independent experiments. Statistically significant was determined based on a p-value of less than 0.05 (* p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001; n = 3). It mathematically determines the difference between two experimental data set, and the baseline is not due to random chance.