Solid-Phase Electrochemiluminescence Enzyme Electrodes Based on Nanocage Arrays for Highly Sensitive Detection of Cholesterol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurements and Instrumentations
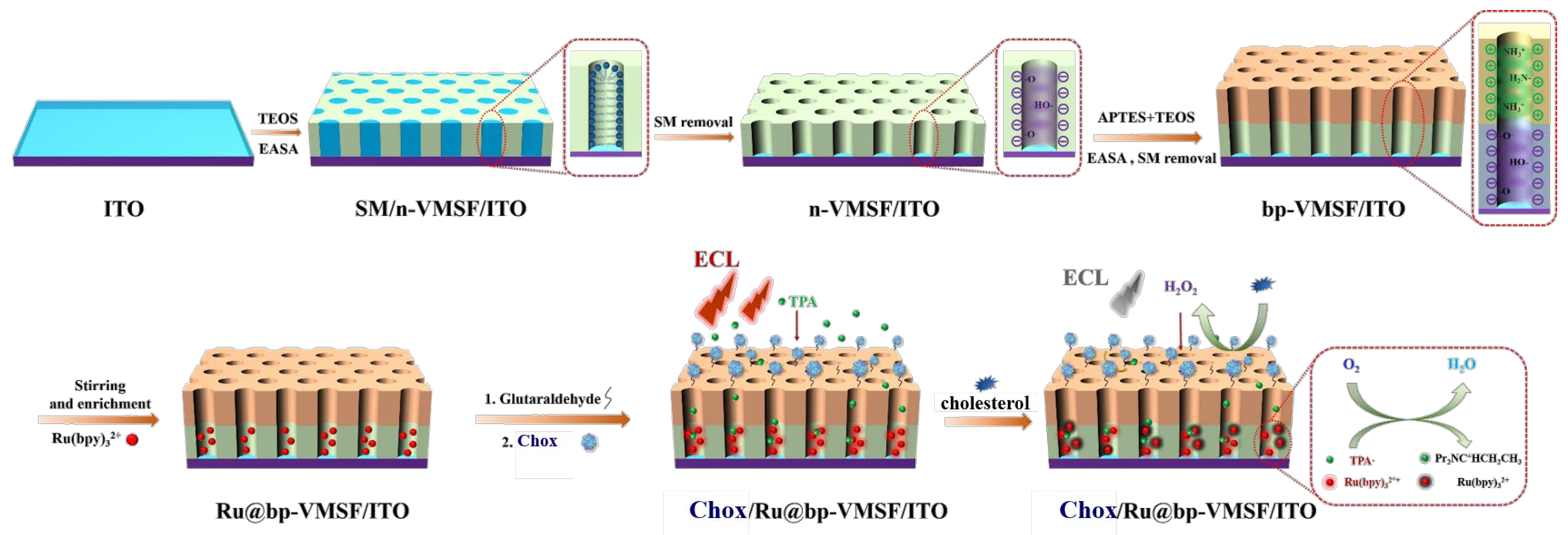
2.3. Preparation of bp-VMSF-Modified Electrode
2.4. Preparation of Solid-Phase ECL Enzyme-Sensing Platform
2.5. ECL Detection of Cholesterol
3. Results and Discussion
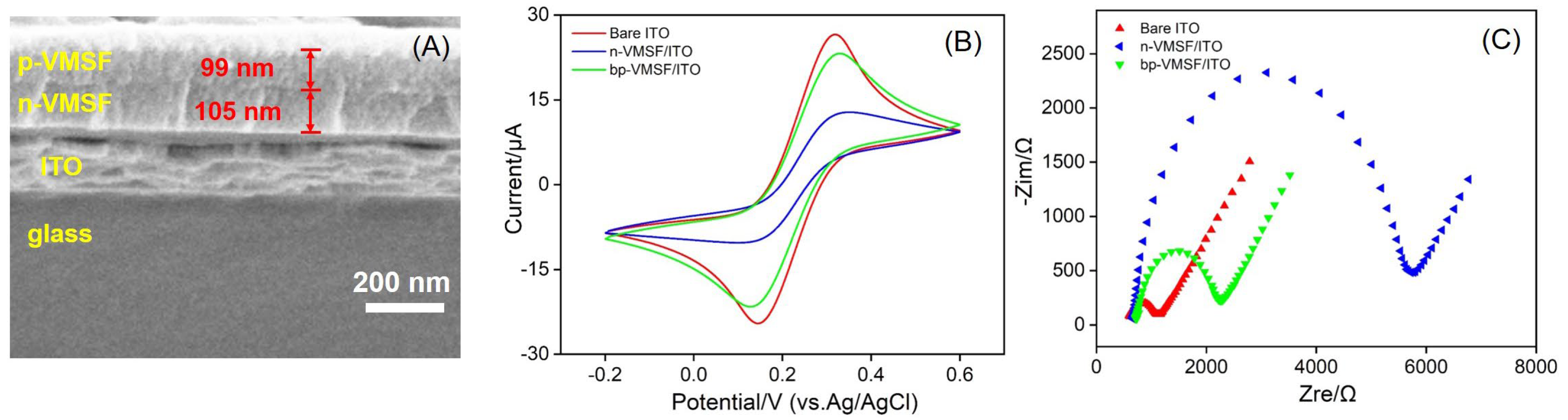
3.1. Characterization of bp-VMSF
3.2. Stability of Ru(bpy)32+ Fixed in bp-VMSF
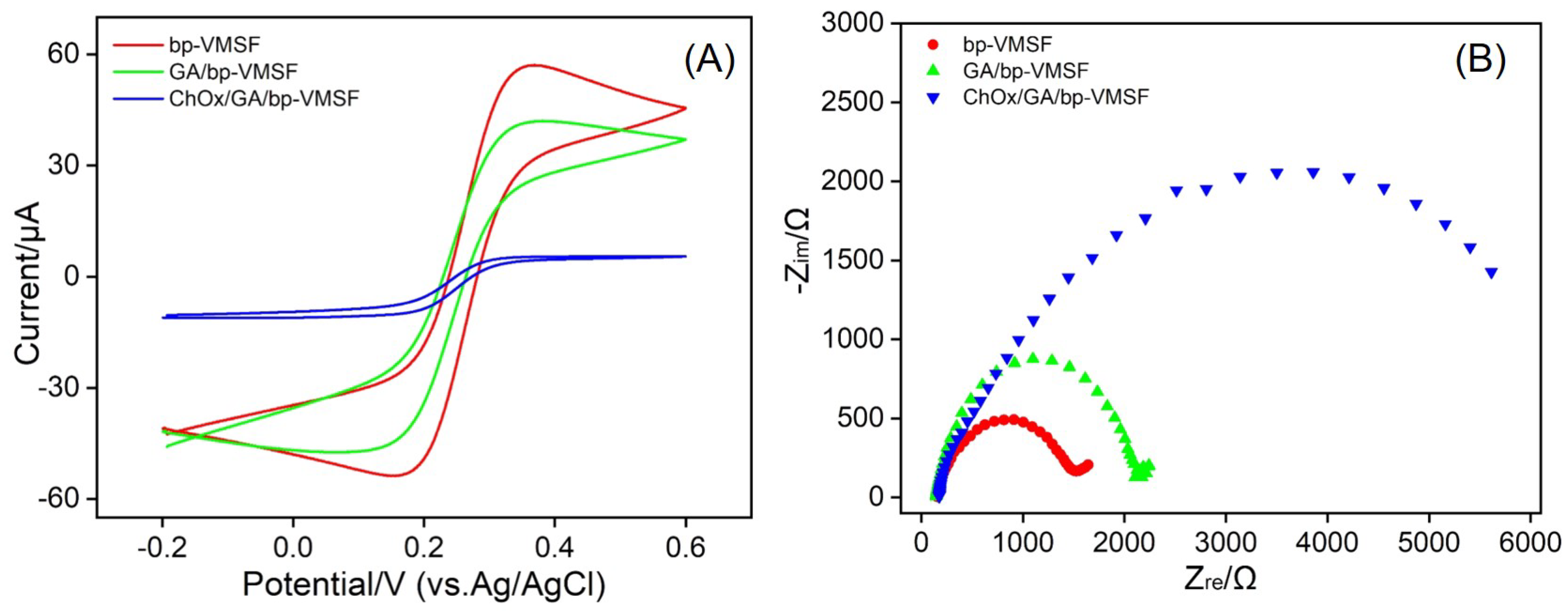
3.3. Feasibility for the Fabrication of Enzyme-Immobilized Electrodes
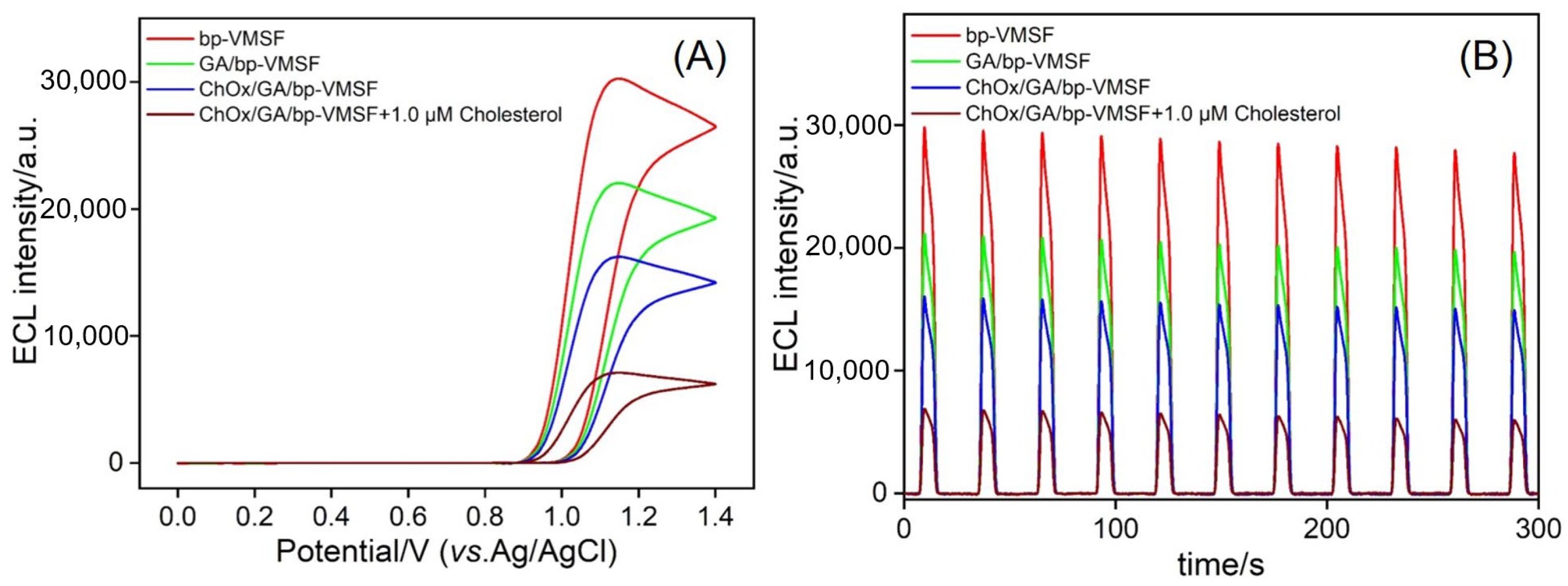
3.4. Feasibility of ECL Detection of Cholesterol Using Enzyme-Immobilized Electrode
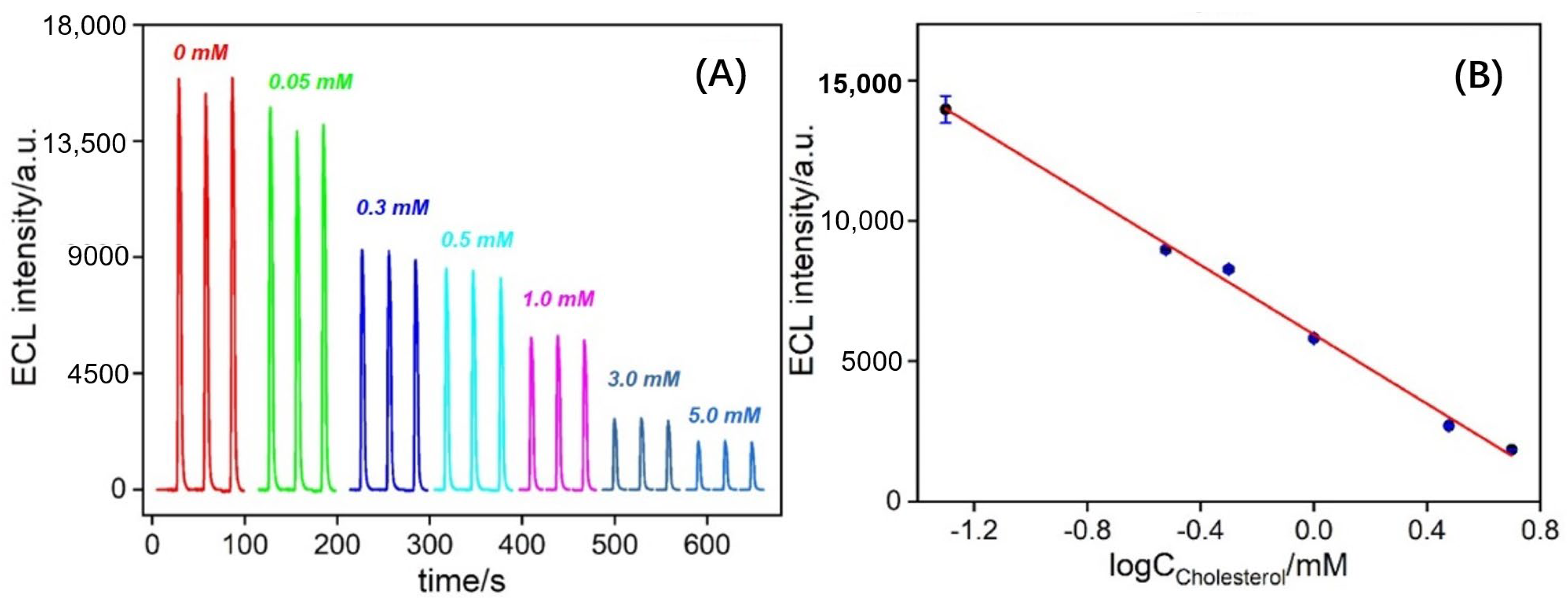
3.5. Performance for ECL Detection of Cholesterol
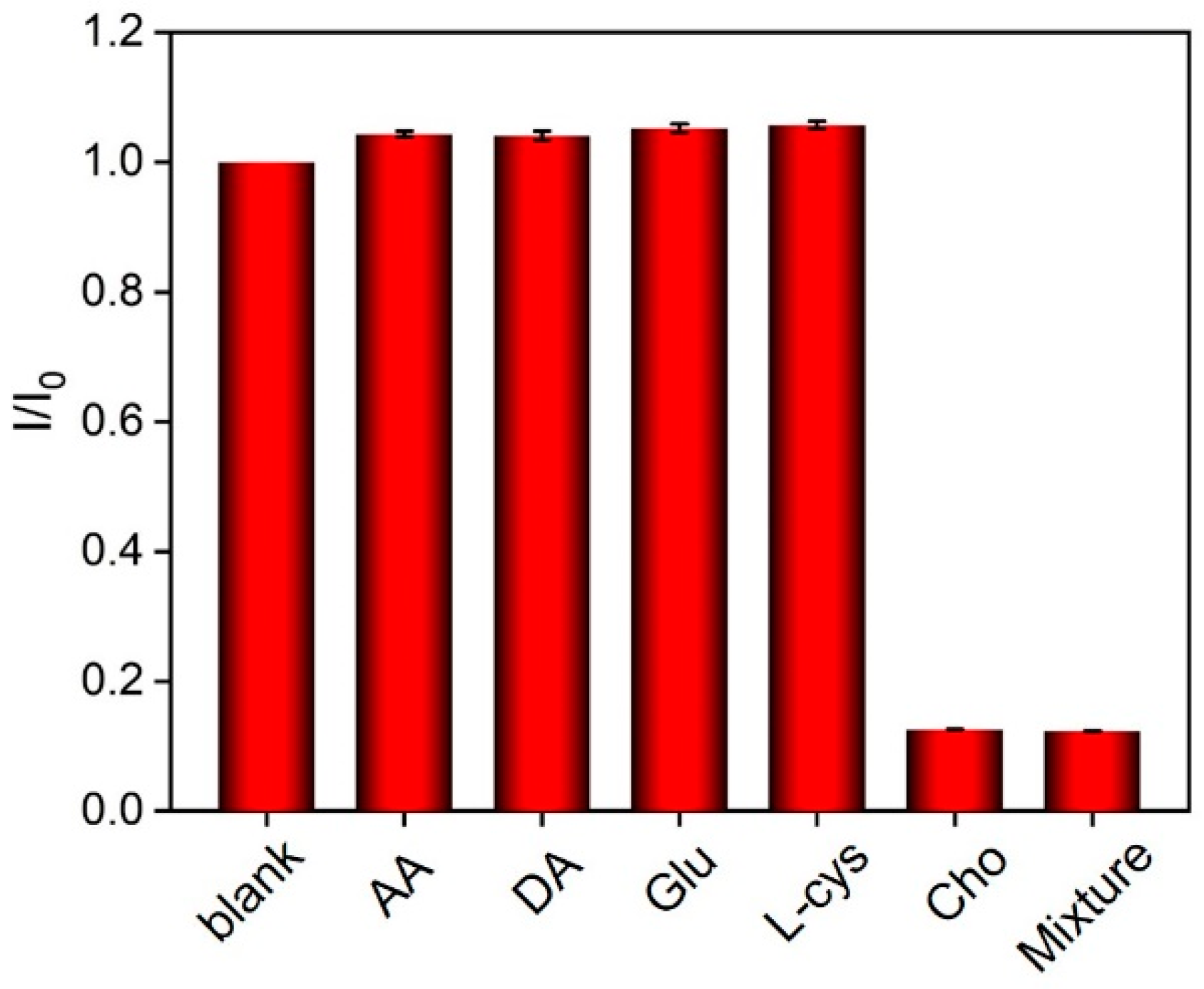
3.6. Selectivity of the Fabricated Sensors
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, L.; Grover, P.; Naved, T.; Mukherjee, D. Metabolite detection and profiling using analytical methods. Curr. Pharm. Anal. 2021, 17, 2–9. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, S.; Zhou, X.; Yan, F.; Hu, W. Silica nanochannel array on co-electrodeposited graphene-carbon nanotubes 3D composite film for antifouling detection of uric acid in human serum and urine samples. Microchem. J. 2023, 190, 108632. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J. Magnetic nanozyme based on loading nitrogen-doped carbon dots on mesoporous Fe3O4 nanoparticles for the colorimetric detection of glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kumar, M.; Bhalla, V. Enzyme-/metal-free quinoxaline assemblies: Direct light-up detection of cholesterol in human serum. Chem. Commun. 2023, 59, 1501–1504. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Y.; Chen, Q.; Tang, L.; Liu, J.; Sun, Y.; Liu, Q.; Zhang, Y.; Zhang, G.; Chen, S. Pt,P-codoped carbon nitride nanoenzymes for fluorescence and colorimetric dual-mode detection of cholesterol. Anal. Chim. Acta 2024, 1297, 342351. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Electrochemical cholesterol sensor based on cholesterol oxidase and MoS2-AuNPs modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 233, 100–106. [Google Scholar] [CrossRef]
- Wilson, R.; Clavering, C.; Hutchinson, A. Electrochemiluminescence enzyme immunoassays for TNT and pentaerythritol tetranitrate. Anal. Chem. 2003, 75, 4244–4249. [Google Scholar] [CrossRef]
- Zhao, Y.; Bouffier, L.; Xu, G.; Loget, G.; Sojic, N. Electrochemiluminescence with semiconductor (nano)materials. Chem. Sci. 2022, 13, 2528–2550. [Google Scholar] [CrossRef]
- Meng, C.; Knežević, S.; Du, F.; Guan, Y.; Kanoufi, F.; Sojic, N.; Xu, G. Recent advances in electrochemiluminescence imaging analysis. eScience 2022, 2, 591–605. [Google Scholar] [CrossRef]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Han, D.; Yang, K.; Sun, S.; Wen, J. Signal amplification strategies in electrochemiluminescence biosensors. Chem. Eng. J. 2023, 476, 146688. [Google Scholar] [CrossRef]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Knight, A.W.; Greenway, G.M. Relationship between structural attributes and observed electrogenerated chemiluminescence (ECL) activity of tertiary amines as potential analytes for the tris(2,2-bipyridine)ruthenium(II) ECL reaction. A review. Analyst 1996, 121, 101R–106R. [Google Scholar] [CrossRef]
- Dai, H.; Wu, X.; Xu, H.; Wang, Y.; Chi, Y.; Chen, G. A highly performing electrochemiluminescent biosensor for glucose based on a polyelectrolyte-chitosan modified electrode. Electrochim. Acta 2009, 54, 4582–4586. [Google Scholar] [CrossRef]
- Ying, X.; Zhou, L.; Fu, W.; Wang, Y.; Su, B. Electrochemiluminescence devices for point-of-care testing. Sens. Diagn. 2023, 2, 480–491. [Google Scholar] [CrossRef]
- Bezuneh, T.T.; Fereja, T.H.; Kitte, S.A.; Li, H.; Jin, Y. Gold nanoparticle-based signal amplified electrochemiluminescence for biosensing applications. Talanta 2022, 248, 123611. [Google Scholar] [CrossRef]
- Ding, H.; Guo, W.; Zhou, P.; Su, B. Nanocage-confined electrochemiluminescence for the detection of dopamine released from living cells. Chem. Commun. 2020, 56, 8249–8252. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wu, J.; Zhang, T.; Liu, J. Electrochemical/electrochemiluminescence sensors based on vertically-ordered mesoporous silica films for biomedical analytical applications. ChemBioChem 2024, e202400320. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, Z.; Li, H.; Li, F. Directionally in situ self-assembled iridium(III)-polyimine complex-encapsulated metal–organic framework two-dimensional nanosheet electrode to boost electrochemiluminescence sensing. Anal. Chem. 2023, 95, 12024–12031. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Wang, Z.; Li, F. Iridium complex with specific intercalation in the G-quadruplex: A phosphorescence and electrochemiluminescence dual-mode homogeneous biosensor for enzyme-free and label-free detection of microRNA. ACS Sens. 2023, 8, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gu, X.; Zhang, S.; Zou, Y.; Yan, F. Magnetic graphene oxide and vertically-ordered mesoporous silica film for universal and sensitive homogeneous electrochemiluminescence aptasensor platform. Microchem. J. 2024, 200, 110315. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ru, H.; Yan, F.; Liu, J. Silica nanochannels as nanoreactors for the confined synthesis of Ag NPs to boost electrochemical stripping chemiluminescence of the luminol-O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef]
- Wei, X.; Luo, X.; Xu, S.; Xi, F.; Zhao, T. A flexible electrochemiluminescence sensor equipped with vertically ordered mesoporous silica nanochannel film for sensitive detection of clindamycin. Front. Chem. 2022, 10, 872582. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, J.; Han, Q.; Hu, W.; Yan, F.; Liu, J. Nanogold amplified electrochemiluminescence/electrochemistry in bipolar silica nanochannel array for ultrasensitive detection of SARS-CoV-2 pseudoviruses. Talanta 2024, 277, 126319. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, Z.; Xu, H.; Zhang, A. Sensitive electrochemiluminescence resonance energy transfer (ECL-RET) between Ru(bpy)32+ and Au nanorod for hydrogen peroxide detection. Sci. China Chem. 2016, 60, 410–414. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Wang, Y.; Wang, A.; Yu, X.; Ding, L. A flexible electrochemical sensor for paracetamol based on porous honeycomb-like NiCo-MOF nanosheets. Rare Met. 2023, 42, 3311–3317. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Li, Q.; Zhang, L.; Wang, A. A highly selective electrochemical impedimetric sensor for imidacloprid determination based on WO3/MoS2 nanosheets/molecularly imprinted polymer composite. Rare Met. 2023, 43, 1309–1315. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Liu, X.; Zhang, L.; Guo, W.; Zhang, J.; Ding, L. 2D Co metal-organic framework nanosheet as an oxidase-like nanozyme for sensitive biomolecule monitoring. Rare Met. 2022, 42, 797–805. [Google Scholar] [CrossRef]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef]
- Guo, Q.; Fan, X.; Yan, F.; Wang, Y. Highly sensitive electrochemical immunosensor based on electrodeposited platinum nanostructures confined in silica nanochannels for the detection of the carcinoembryonic antigen. Front. Chem. 2023, 11, 1271556. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Jin, H.; Wang, J.; Yan, F. Copper nanoparticles confined in a silica nanochannel film for the electrochemical detection of nitrate ions in water samples. Molecules 2023, 28, 7515. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Gu, X.; He, L.; Xi, F. A highly sensitive immunosensor based on nanochannel-confined nano-gold enhanced electrochemiluminescence for procalcitonin detection. Front. Chem. 2023, 11, 1274424. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023, 33, 2308183. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, S.; Ma, X.; Yan, F.; Tang, W. Highly sensitive electrochemical determination of butylated hydroxyanisole in food samples using electrochemical-pretreated three-dimensional graphene electrode modified with silica nanochannel film. Nanomaterials 2024, 14, 569. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fan, X.; Yan, F.; Liu, J. Vertical silica nanochannels and o-phenanthroline chelator for the detection of trace Fe(II). ACS Appl. Nano Mater. 2024, 7, 7743–7752. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Z.; Liu, J.; Luo, T.; Xi, F.; Zeng, Q. Simple fabrication of electrochemical sensor based on integration of dual signal amplification by the supporting electrode and modified nanochannel array for direct and sensitive detection of vitamin B(2). Front. Nutr. 2024, 11, 1352938. [Google Scholar] [CrossRef]
- Li, F.; Han, Q.; Xi, F. The fabrication of a probe-integrated electrochemiluminescence aptasensor based on double-layered nanochannel array with opposite charges for the sensitive determination of C-reactive protein. Molecules 2023, 28, 7867. [Google Scholar] [CrossRef]
- Zheng, H.; Zu, Y. Emission of tris(2,2′-bipyridine)ruthenium(II) by coreactant electrogenerated chemiluminescence: from O2-insensitive to highly O2-sensitive. J. Phy. Chem. B 2005, 109, 12049–12053. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-ordered mesoporous silica films for electrochemical detection of Hg(II) ion in pharmaceuticals and soil samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, X.; Yan, F.; Mou, Y. Electrostatic nanocage-confined probe for electrochemical detection of CA19-9 in human serum. ACS Omega 2023, 8, 48491–48498. [Google Scholar] [CrossRef]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Xi, F.; Lu, C. Sensitive electrochemical detection of carcinoembryonic antigen based on biofunctionalized nanochannel modified carbonaceous electrode. Molecules 2024, 29, 858. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Wang, H.; Zhang, S. Donor–acceptor covalent organic frameworks-confined ultrafine bimetallic Pt-based nanoclusters for enhanced photocatalytic H2 generation. Nano Res. 2024, 17, 5835–5844. [Google Scholar] [CrossRef]
- Aidli, W.; Pifferi, V.; Mars, A.; Marinotto, D.; Longhi, M.; Manfredi, A.; Hamzaoui, A.H.; Falciola, L. Β-cyclodextrin based platform for bimodal detection of o-toluidine and cholesterol: Electrochemical and fluorescence sensing. Electrochim. Acta 2023, 464, 142936. [Google Scholar] [CrossRef]
- Bhaiyya, M.L.; Srivastava, S.K.; Pattnaik, P.K.; Goel, S. Closed-bipolar mini electrochemiluminescence sensor to detect various biomarkers: A machine learning approach. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Canbay, E.; Yaşa, İ.; Akyilmaz, E. Development an amperometric microbial-enzyme hybrid cholesterol biosensor based on ionic liquid MWCNT carbon paste electrode. Electroanalysis 2021, 33, 2381–2391. [Google Scholar] [CrossRef]
- Yin, H.; Lei, M.; Liu, H.; Dong, Y. Dual-potential electrochemiluminescence from black phosphorus and graphitic carbon nitrides for label-free enzymatic biosensing. Analyst 2021, 146, 6281–6287. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, H.; Zhang, T.; Zhang, Z.; Zhao, G.; Wang, C. Electrocatalytic generation of cathodic luminol electrochemiluminescence with carbonized polydopamine nanotubes at a low positive potential. ACS Sustain. Chem. Eng. 2022, 10, 10361–10368. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Shang, K.; Gao, X.; Wang, X. Sensitive electrochemical detection of cholesterol using a portable paper sensor based on the synergistic effect of cholesterol oxidase and nanoporous gold. Int. J. Biol. Macromol. 2021, 189, 356–362. [Google Scholar] [CrossRef]
- Wu, S.; Sui, C.; Wang, C.; Wang, Y.; He, D.; Sun, Y.; Zhang, Y.; Meng, Q.; Ma, T.; Song, X. Gold nanoparticles/single-stranded DNA-reduced graphene oxide nanocomposites based electrochemical biosensor for highly sensitive detection of cholesterol. Front. Chem. Sci. Eng. 2021, 15, 1572–1582. [Google Scholar] [CrossRef]

| Analyte | Added (mM) | Detected (mM) | RSD (%, n = 3) | Recovery (%) |
|---|---|---|---|---|
| cholesterol | 0.50 | 0.55 | 2.1 | 110.0 |
| 1.00 | 1.09 | 1.2 | 109.0 | |
| 4.50 | 4.47 | 0.2 | 99.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, Z.; Zheng, Y.; Liu, J. Solid-Phase Electrochemiluminescence Enzyme Electrodes Based on Nanocage Arrays for Highly Sensitive Detection of Cholesterol. Biosensors 2024, 14, 403. https://doi.org/10.3390/bios14080403
Ma X, Zhang Z, Zheng Y, Liu J. Solid-Phase Electrochemiluminescence Enzyme Electrodes Based on Nanocage Arrays for Highly Sensitive Detection of Cholesterol. Biosensors. 2024; 14(8):403. https://doi.org/10.3390/bios14080403
Chicago/Turabian StyleMa, Xinying, Zhe Zhang, Yanyan Zheng, and Jiyang Liu. 2024. "Solid-Phase Electrochemiluminescence Enzyme Electrodes Based on Nanocage Arrays for Highly Sensitive Detection of Cholesterol" Biosensors 14, no. 8: 403. https://doi.org/10.3390/bios14080403
APA StyleMa, X., Zhang, Z., Zheng, Y., & Liu, J. (2024). Solid-Phase Electrochemiluminescence Enzyme Electrodes Based on Nanocage Arrays for Highly Sensitive Detection of Cholesterol. Biosensors, 14(8), 403. https://doi.org/10.3390/bios14080403





