Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
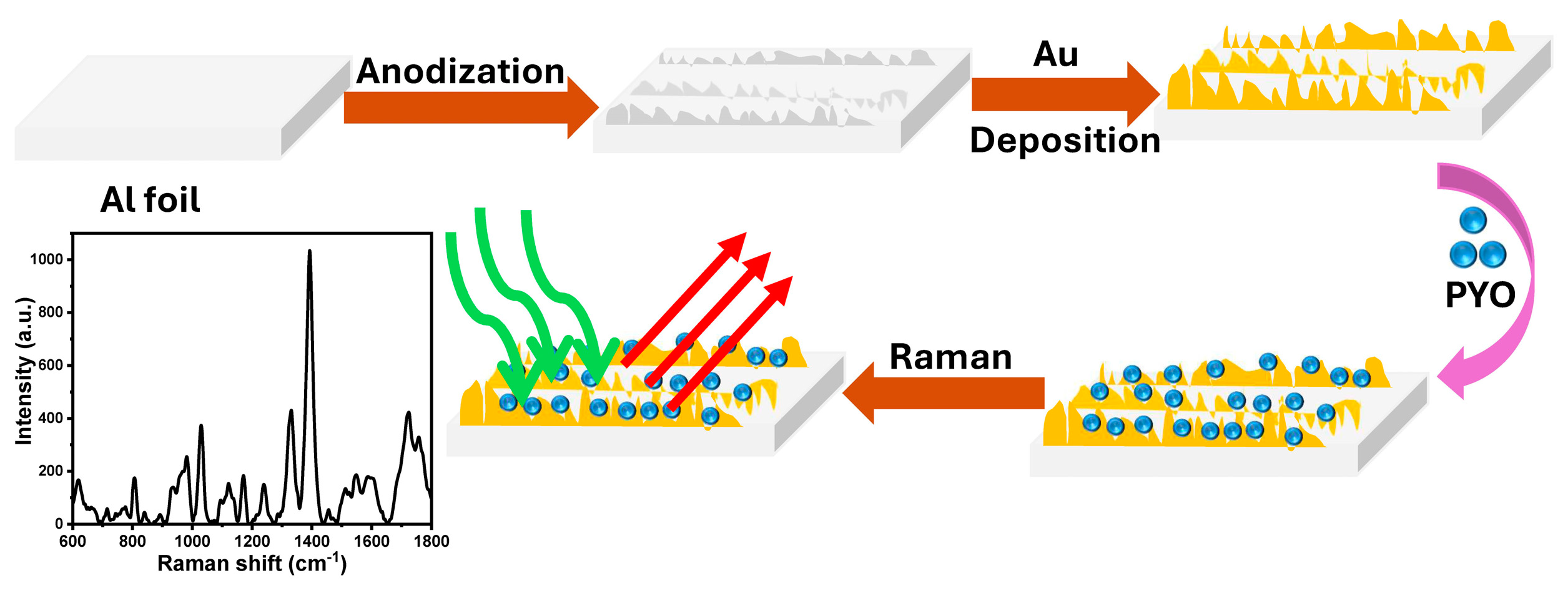
2.3. Fabrication of SERS-Active Surfaces
2.4. Investigate the Substrates’ Roughness
2.5. Pseudomonas aeruginosa Clinical Isolates
2.6. PYO Sensing in Pseudomonas aeruginosa Cultures Based on SERS Technique
3. Results and Discussion
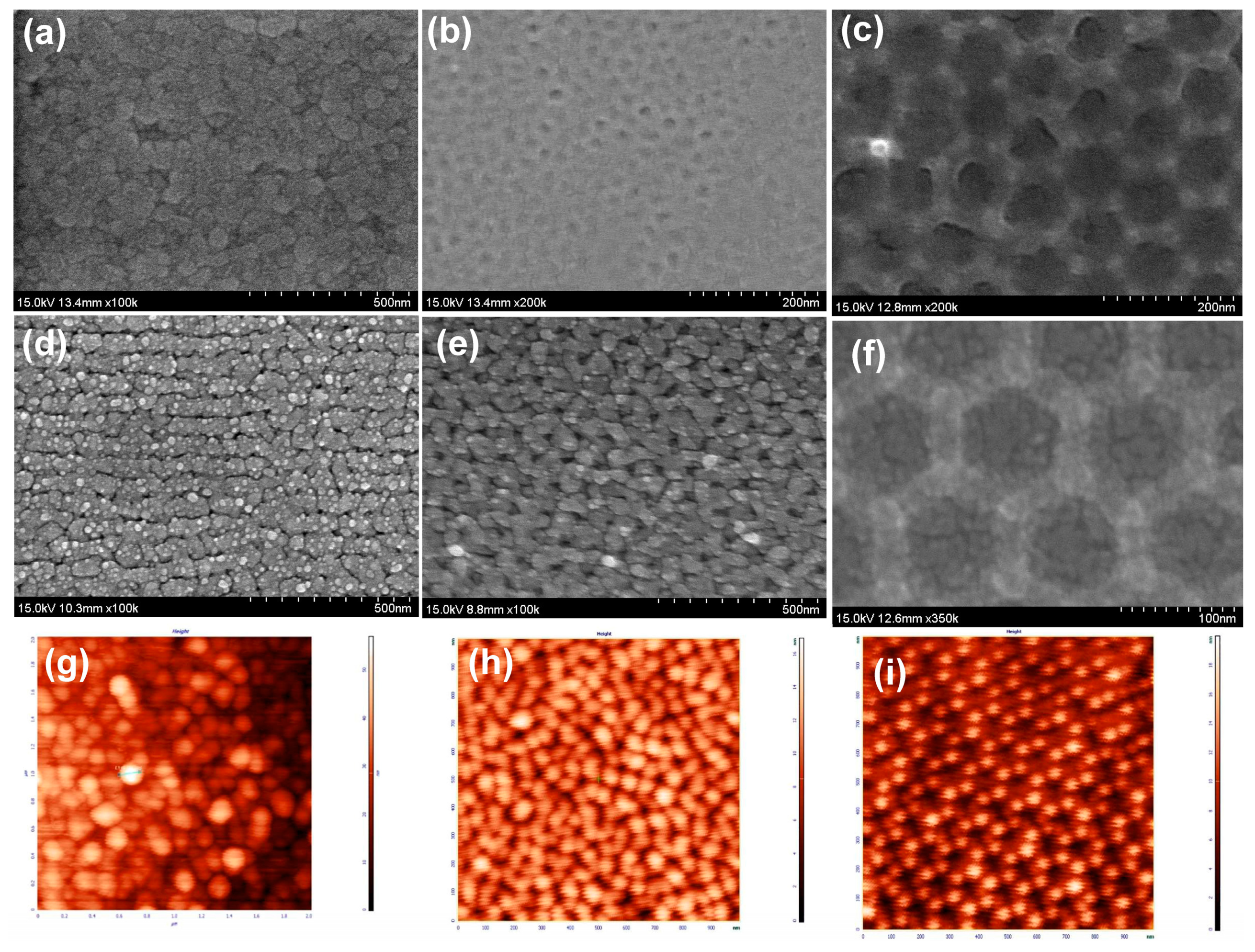
3.1. The Rough Substrate Fabrication
3.2. AFM Analysis of the Surface Roughness
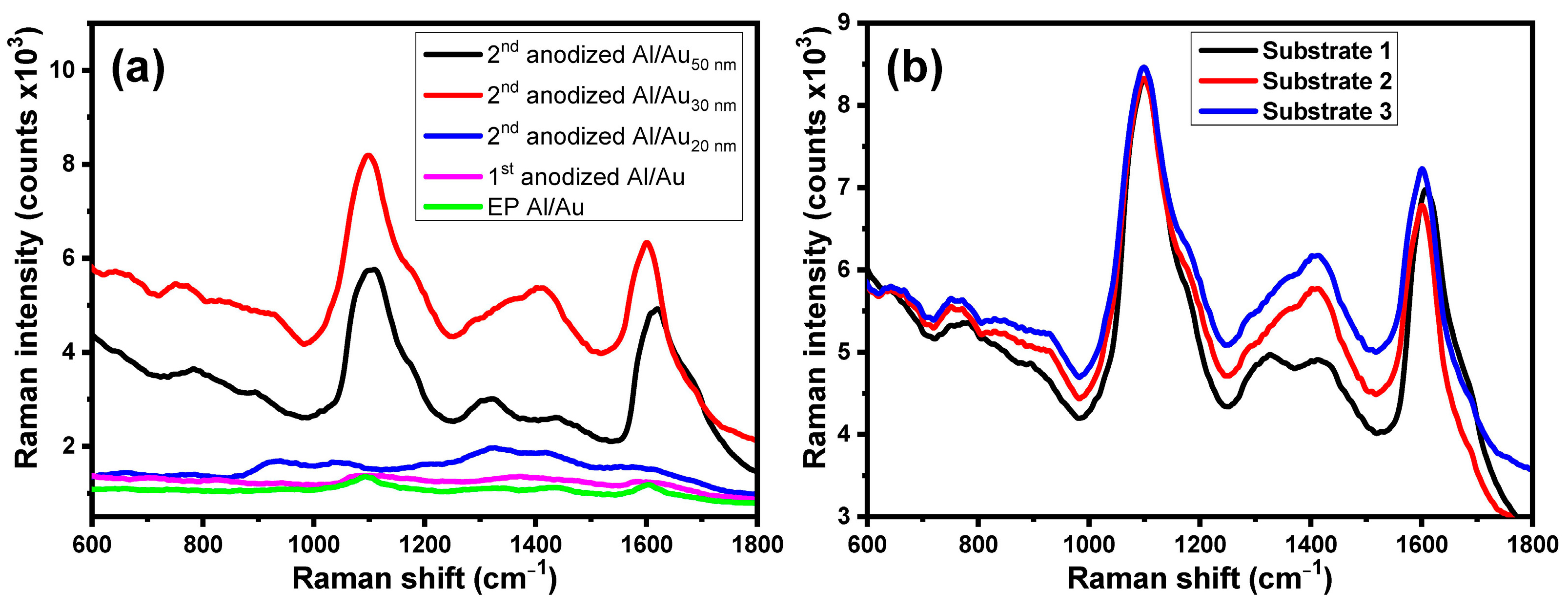
3.3. Effect of the Surface Roughness on the Raman Enhancement of the Different Substrates
3.4. SERS of PYO at the Designed Substrates
3.5. Sensing of PYO Secreted from Pseudomonas aeruginosa Based on SERS Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu, E.A.; Su, S.; Sallans, L.; Boissy, R.E.; Greatens, A.; Heineman, W.R.; Daniel, J.H. Cyclic voltammetric, fluorescence and biological analysis of purified aeruginosin A, a secreted red pigment of Pseudomonas aeruginosa PAO1. Microbiology 2013, 159, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; O’Loughlin, C.T.; Zhang, Z.; Siryaporn, A.; Silpe, J.E.; Bassler, B.L.; Martin, F.S. Development of Potent Inhibitors of Pyocyanin Production in Pseudomonas aeruginosa. J. Med. Chem. 2015, 58, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Damkiær, S.; Yang, L.; Molin, S.; Jelsbak, L. Evolutionary remodeling of global regulatory networks during long-term bacterial adaptation to human hosts. Proc. Natl. Acad. Sci. USA 2013, 110, 7766–7771. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Søren, M. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Bren, L. Battle of the bugs: Fighting antibiotic resistance. FDA Consum. 2002, 36, 28–34. [Google Scholar]
- O’Neill, J. Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics. Review on Antimicrobial Resistance. Wellcome Trust (London, England). 2015. Available online: https://wellcomecollection.org/works/gcrdafjx (accessed on 1 October 2015).
- Webster, T.A.; Sismaet, H.J.; Conte, J.L.; Chan, I.J.; Goluch, E.D. Electrochemical detection of Pseudomonas aeruginosa in human fluid samples via pyocyanin. Biosens. Bioelectron. 2014, 60, 265–270. [Google Scholar] [CrossRef]
- Elkhawaga, A.A.; Khalifa, M.M.; El-badawy, O.; Hassan, M.A.; El-Said, W.A. Rapid and highly sensitive detection of pyocyanin biomarker in different Pseudomonas aeruginosa infections using gold nanoparticles modified sensor. PLoS ONE 2019, 14, e0216438. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Elkhawaga, A.A.; Hassan, M.A.; Zahran, A.M.; Fathalla, A.M.; El-Said, W.A.; El-Badawy, O. Highly specific Electrochemical Sensing of Pseudomonas aeruginosa in patients suffering from corneal ulcers: A comparative study. Sci. Rep. 2019, 9, 18320. [Google Scholar] [CrossRef]
- Do, H.; Kwon, S.-R.; Fu, K.; Morales-Soto, N.; Shrout, J.D.; Bohn, P.W. Electrochemical Surface-Enhanced Raman Spectroscopy of Pyocyanin Secreted by Pseudomonas aeruginosa Communities. Langmuir 2019, 35, 7043–7049. [Google Scholar] [CrossRef]
- Abdul-Hussein, Z.R.; Atia, S.S. Antimicrobial Effect of Pyocyanin Extracted from Pseudomonas aeruginosa. Eur. J. Exp. Biol. 2016, 6, 1–4. [Google Scholar]
- Yang, X.; Dang, Y.; Lou, J.; Shao, H.; Jiang, X. D-alanyl-D-alanine-Modified Gold Nanoparticles Form a Broad-Spectrum Sensor for Bacteria. Theranostics 2018, 8, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, S.; Baig, N.F.; Morales-Soto, N.; Shrout, J.D.; Bohn, P.W. Spatial Mapping of Pyocyanin in Pseudomonas aeruginosa Bacterial Communities Using Surface Enhanced Raman Scattering. Appl. Spectrosc. 2017, 71, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.F.; Greenhagen, B.T.; Shi, K.; Calabrese, K.; Robinson, H.; Ladner, J.E. Structural and Functional Analysis of the Pyocyanin Biosynthetic Protein PhzM from Pseudomonas aeruginosa. Biochemistry 2007, 46, 1821–1828. [Google Scholar] [CrossRef]
- Sismaeta, H.J.; Pintob, A.J.; Goluch, E.D. Electrochemical sensors for identifying pyocyanin production in clinical Pseudomonas aeruginosa isolates. Biosens. Bioelectron. 2017, 97, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- Bodelón, G.; Montes-García, V.; Pérez-Juste, J.; Pastoriza-Santos, I. Surface-Enhanced Raman Scattering Spectroscopy for Label-Free Analysis of P. aeruginosa Quorum Sensing. Front. Cell. Infect. Microbiol. 2018, 8, 143. [Google Scholar] [CrossRef]
- Žukovskaja, O.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Detection of Pseudomonas aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique. Sensors 2017, 17, 1704. [Google Scholar] [CrossRef]
- Noto, M.J.; Burns, W.J.; Beavers, W.N.; Skaar, E.P. Mechanisms of pyocyanin toxicity and genetic determinants of resistance in Staphylococcus aureus. J. Bacteriol. 2017, 199, e00221-17. [Google Scholar] [CrossRef]
- Meirelles, L.A.; Newman, D.K. Both toxic and beneficial effects of pyocyanin contribute to the lifecycle of Pseudomonas aeruginosa. Mol. Microbiol. 2018, 110, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Yea, C.-H.; Choi, J.-W.; Kwon, I.l.-K. Ultrathin polyaniline film coated on an indium–tin oxide cell-based chip for study of anticancer effect. Thin Solid Films 2009, 518, 661–667. [Google Scholar] [CrossRef]
- Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chern. Phys. 1978, 69, 4159–4161. [Google Scholar] [CrossRef]
- Weaver, M.J.; Zou, S.; Chan, H.Y.H. The new interfacial ubiquity of surface-enhanced Raman spectroscopy, Anal. Chem. 2000, 72, 38A–47A. [Google Scholar]
- Choi, J.H.; El-Said, W.A.; Choi, J.-W. Highly sensitive surface-enhanced Raman spectroscopy (SERS) platform using core/double shell (Ag/polymer/Ag) nanohorn for proteolytic biosensor. Appl. Surf. Sci. 2020, 506, 144669. [Google Scholar] [CrossRef]
- Jensen, T.R.; Malinsky, M.D.; Haynes, C.L.; Van Duyne, R.P. Nanosphere Lithography: Tunable Localized Surface Plasmon Resonance Spectra of Silver Nanoparticles. J. Phys. Chem. B 2000, 104, 10549–10556. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasar, R.R.; Feld, M.S. Ultrasensitive Chemical Analysis by Raman Spectroscopy. Chem. Rev. 1999, 99, 2957–2975. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; El-Said, W.A.; Choi, J.-W. Fabrication of gold nanodot arrays on a transparent substrate as a nanobioplatform for label-free visualization of living cells. Nanotechnology 2011, 22, 235304. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Yea, C.-H.; Jung, M.; Kim, H.; Choi, J.-W. Analysis of effect of nanoporous alumina substrate coated with polypyrrole nanowire on cell morphology based on AFM topography. Ultramicroscopy 2010, 110, 676–681. [Google Scholar] [CrossRef]
- Altunbek, M.; Kelestemur, S.; Culha, M. In situ monitoring of biomolecular processes in living systems using surface-enhanced Raman scattering. Biophotonics Jpn. 2015, 2015, 979206. [Google Scholar]
- Bodelón, G.; Montes-García, V.; López-Puente, V.; Hill, E.H.; Hamon, C.; Sanz-Ortiz, M.N.; Rodal-Cedeira, S.; Costas, C.; Celiksoy, S.; Pérez-Juste, I.; et al. Detection and imaging of quorum sensing in Pseudomonas aeruginosa biofilm communities by surface-enhanced resonance Raman scattering. Nat. Mater. 2016, 15, 1203. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Li, X.; Zhao, Y.; Zughaier, S.M. Culture-free diagnostics of Pseudomonas aeruginosa infection by silver nanorod array based SERS from clinical sputum samples. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, A.; Zhang, D.; Tam, F.; Halas, N.J. Surface-enhanced Raman spectroscopy of DNA. J. Am. Chem. Soc. 2008, 130, 5523–5529. [Google Scholar] [CrossRef]
- Alatraktchi, F.; Breum, A.S.; Krogh, J.H.; Molin, S.; Svendsen, W.J.S. Fast selective detection of pyocyanin using cyclic voltammetry. Sensors 2016, 16, 408. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Simoska, O.; Karasik, S.; Shear, J.B.; Stevenson, K.J. Transparent Carbon Ultramicroelectrode Arrays for the Electrochemical Detection of a Bacterial Warfare Toxin, Pyocyanin. Anal. Chem. 2017, 89, 6285–6289. [Google Scholar] [CrossRef]
- Ciui, B.; Tertiş, M.; Cernat, A.; Săndulescu, R.; Wang, J.; Cristea, C. Finger-Based Printed Sensors Integrated on a Glove for On-Site Screening of Pseudomonas aeruginosa Virulence Factors. Anal. Chem. 2018, 90, 7761–7768. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.; Gladstone, P.; Smith, R.B.; Forsythe, S.; Davis, J. Approaching intelligent infection diagnostics: Carbon fibre sensor for electrochemical pyocyanin detection. Bioelectrochemistry 2010, 77, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Alatraktchi, F.A.; Noori, J.S.; Tanev, G.P.; Mortensen, J.; Dimaki, M.; Johansen, H.K.; Madsen, J.; Molin, S.; Svendsen, W.E. Paper-based sensors for rapid detection of virulence factor produced by Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0194157. [Google Scholar] [CrossRef] [PubMed]
- Buzid, A.; Shang, F.; Reen, F.J.; Muimhneacháin, E.Ó.; Clarke, S.L.; Zhou, L.; Luong JHTO’Gara, F.; McGlacken, G.P.; Glennon, J.D. Molecular Signature of Pseudomonas aeruginosa with Simultaneous Nanomolar Detection of Quorum Sensing Signaling Molecules at a Boron-Doped Diamond Electrode. Sci. Rep. 2016, 6, 30001. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Johansen, H.K.; Molin, S.; Svendsen, W.E. Electrochemical sensing of biomarker for diagnostics of bacteria-specific infections. Nanomedicine 2016, 11, 2185–2195. [Google Scholar] [CrossRef]

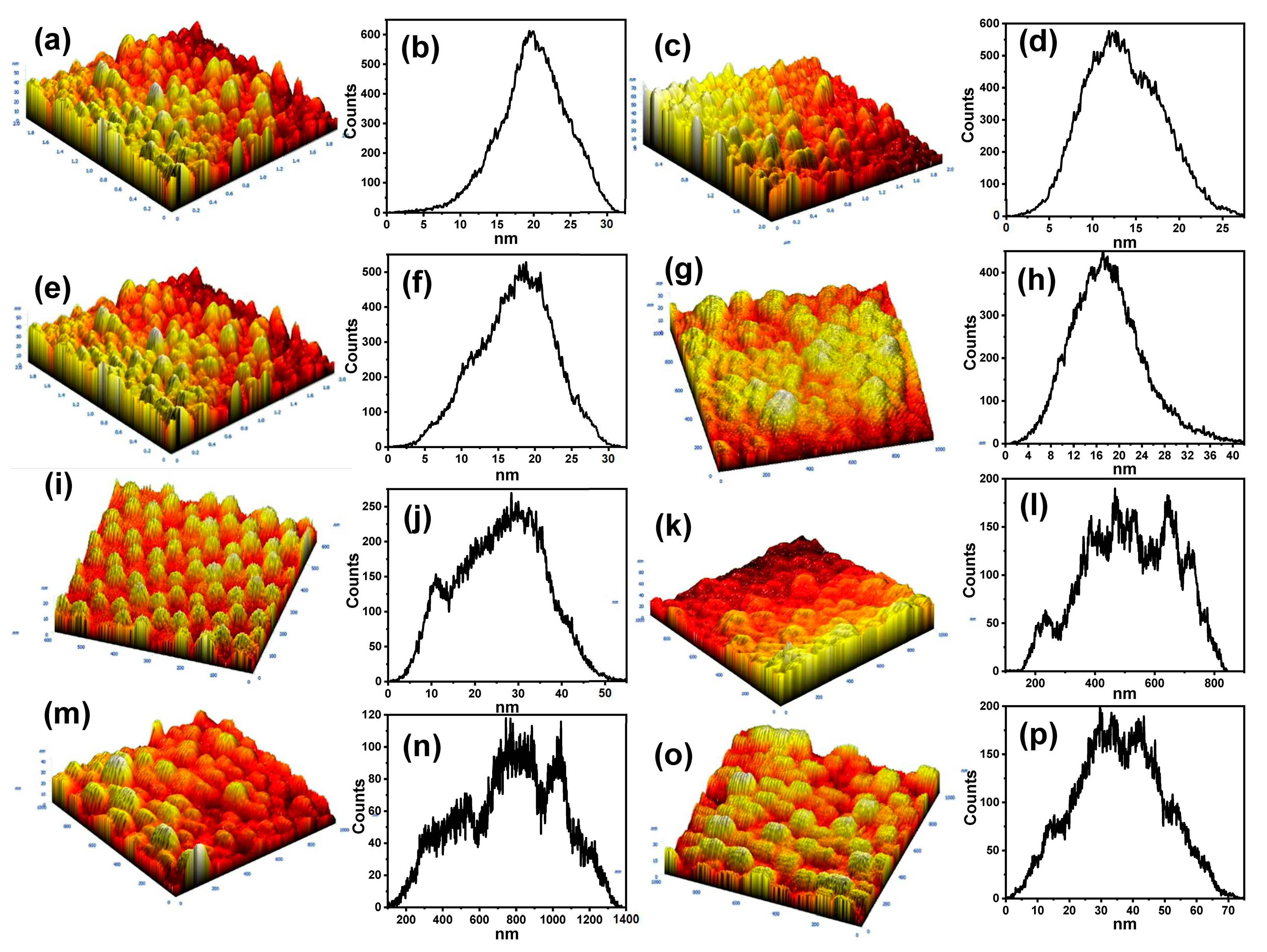

| EP Al | EP Al/Au | 1st Al | 1st Al/Au | 2nd Al | 2nd Al/Au20 | 2nd Al/Au30 | 2nd Al/Au50 | |
|---|---|---|---|---|---|---|---|---|
| Sy (nm) | 23.42 | 27.55 | 32.35 | 43.00 | 56.83 | 80.74 | 131.71 | 75.55 |
| Sz (nm) | 11.61 | 13.92 | 16.16 | 21.87 | 28.637 | 39.11 | 65.54 | 37.93 |
| Average (nm) | 11.14 | 13.54 | 17.31 | 17.98 | 25.88 | 31.22 | 58.43 | 35.67 |
| Sa (nm) | 3.01 | 3.61 | 4.16 | 5.01 | 8.15 | 12.02 | 21.26 | 11.13 |
| Sq (nm) | 3.66 | 4.43 | 5.15 | 6.40 | 9.86 | 14.38 | 25.74 | 13.59 |
| Ssk | 0.27 | 0.21 | −0.20 | 0.53 | −0.04 | 0.17 | 0.21 | 0.02 |
| Ska | −0.48 | −0.35 | −0.34 | 0.44 | −0.62 | −0.76 | −0.73 | −0.54 |
| Second moment | 11.73 | 14.24 | 18.06 | 19.09 | 27.69 | 34.37 | 63.84 | 38.17 |
| Entropy | 7.25 | 7.55 | 7.77 | 8.07 | 8.69 | 9.18 | 10.03 | 9.16 |
| Redundance | −0.61 | −0.60 | −0.56 | −0.50 | −0.50 | −0.45 | −0.43 | −0.47 |
| Technique | LOD (µM) | Reference |
|---|---|---|
| Cyclic voltammetry | 0.5 | [9] |
| Cyclic voltammetry | 2 | [34] |
| Square wave voltammetry | 0.51 | [35] |
| Square wave voltammetry | 1 | [35] |
| Square wave voltammetry | 1.6 | [35] |
| Square wave voltammetry | 0.003 | [36] |
| Square wave voltammetry | 0.03 | [37] |
| Square wave voltammetry | 0.95 | [38] |
| Differential plus voltammetry | 0.05 | [39] |
| Amperometry | 0.125 | [40] |
| Raman spectroscopy | 0.5 | [19] |
| Raman spectroscopy | 0.096 | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Said, W.A.; Saleh, T.S.; Al-Bogami, A.S.; Wani, M.Y.; Choi, J.-w. Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa. Biosensors 2024, 14, 399. https://doi.org/10.3390/bios14080399
El-Said WA, Saleh TS, Al-Bogami AS, Wani MY, Choi J-w. Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa. Biosensors. 2024; 14(8):399. https://doi.org/10.3390/bios14080399
Chicago/Turabian StyleEl-Said, Waleed A., Tamer S. Saleh, Abdullah Saad Al-Bogami, Mohmmad Younus Wani, and Jeong-woo Choi. 2024. "Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa" Biosensors 14, no. 8: 399. https://doi.org/10.3390/bios14080399
APA StyleEl-Said, W. A., Saleh, T. S., Al-Bogami, A. S., Wani, M. Y., & Choi, J.-w. (2024). Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa. Biosensors, 14(8), 399. https://doi.org/10.3390/bios14080399








