1. Introduction
Sesame,
Sesamum indicum, is a seed used in many dishes, particularly in Middle Eastern cuisine, in different ways such as in paste (tahini), a sweet (halva), a topping (like in hamburger buns), or a cooking oil [
1], among others. Furthermore, sesame is also a common food allergen, and reports of sesame allergies have been increasing worldwide [
2]. Sesame can induce adverse reactions in 0.1% to 0.2% of the general population. Some seeds, particularly their oils, may cause delayed hypersensitivity (T-cell-mediated), often manifesting as allergic contact dermatitis. Although ingestion is the primary mode of exposure, inhalation and skin contact, especially in occupational settings, can also trigger reactions. Immunoglobulin E (IgE)-mediated sesame food allergy typically presents with clinical symptoms early in life (around 2 years of age) and may be persistent. This allergy can cause a range of adverse effects, including mucocutaneous reactions such as atopic dermatitis and urticaria, gastrointestinal symptoms, respiratory issues, and life-threatening anaphylaxis. Sesame allergies have been connected with at least five different proteins, with Ses i 1, Ses i 2, and Ses i 8 being the most representative. Ses i 1 is a 2S albumin with a low concentration of sulfur and 40% homology to Brazilian nut, sunflower seed, and castor bean allergens. Ses i 2 and Ses i 8 is a 2S and 8S albumin with rich sulfur.
Currently, there are several methods for sesame detection including enzyme-linked immunoassay (ELISA) [
3], polymerase chain reaction (PCR) [
4,
5,
6], and targeted proteomics based on liquid chromatography coupled with mass spectrometry (LC-MS) [
7,
8]. Although these methods are highly sensitive and selective, most of them require complicated sample pretreatment, which makes on-site field measurement difficult or impossible [
9,
10], which is primarily needed for allergen determination in food. Additionally, the conventional method application typically requires specialized and expensive laboratory equipment, as well as trained personnel to interpret the results [
11]. Today, there is a need to develop new analytical methodologies that allow for the on-site and simple screening of allergens in food using simple instrumentation and small volumes of samples and reagents. Furthermore, the use of biosensors in chemical analysis offers significant cost benefits. As mentioned earlier, biosensors require fewer reagents and specialized equipment, thereby reducing costs. In addition, biosensors enable faster and simpler analyses, reducing both labor costs and analysis time. In contrast, conventional methods such as PCR, while highly accurate and sensitive, are more expensive due to the need for expensive reagents, equipment, and specialized technical personnel, which significantly increases the overall cost of the analysis [
12,
13,
14]. The detection of sesame seed DNA targets in food could allow for an accurate identification of its presence in food products, and quantitative PCR is required. Several methods have been developed for the detection of sesame DNA in food products, many of them based on the PCR technique [
15]. Alternatively, a combination of inorganic nanoparticles with MNAzymes for the amplified detection of nucleic acids appears as a powerful strategy for developing simple and highly sensitive bioassays for DNA detection [
5,
16].
Gold nanoparticles have unique optoelectronic properties that make them ideal for biosensor development. Also, AuNPs can be easily functionalized with different biomolecules increasing their applicability in the development of optical biosensors. These biomolecules act as recognition elements, binding selectively to target analytes such as specific proteins or nucleic acids, among others. This interaction induces changes in the nanoparticle’s optical properties, including shifts in their surface plasmon resonance, which can be detected optically. The ability to precisely control the bioconjugation of gold nanoparticles with DNA enhances the specificity and sensitivity of biosensors, enabling them to detect and quantify target molecules with high accuracy and efficiency. Thus, the combination of gold nanoparticles’ optoelectronic properties with bioconjugation techniques forms the foundation for advanced biosensing technologies with diverse applications in clinical diagnostics [
12]. Also, their size-dependent optical properties, in particular their surface plasmon resonance, enable sensitive detection mechanisms. The interaction of light with these nanoparticles can cause them to change color, providing a visual indication of molecular interactions or changes in their environment. This phenomenon is due to the ability of gold nanoparticles to exhibit plasmonic effects, where the collective oscillation of free electrons on their surface results in enhanced light absorption and scattering. The optoelectronic properties of gold nanoparticles offer the possibility of observing a change in color as a result of changes in the distance between them, making them invaluable in the development of highly sensitive biosensors capable of detecting minute molecular interactions with precision and reliability [
4].
A different DNA detection technique that has a lot of potential for fast and easy assays is the use of two gold nanoparticle sets bioconjugated with different DNA probes which recognize the target sequence inducing aggregation of AuNP sets and color changes due to the interparticle plasmon resonance from red (dispersed AuNPs) to purple or blue (aggregated AuNPs) [
17,
18,
19]. The simplest way to do so is the use of gold nanoparticles that have been bioconjugated to DNA/RNA chains that are each complementary to half of the target analyte, so in their presence, they come together and aggregate [
20]. This results in a change in color, from red to blue, and can be detected visually. However, this simple method is usually not sensitive enough to achieve limits of detection low enough for many DNAs, needing a previous amplification step. One such way is the use of MNAzymes, a synthetic DNA chain that acts as an enzyme [
21].
MNAzymes, a class of nucleic acid enzymes have emerged as powerful tools [
18,
20] for the optical detection of genetic biomarkers. These enzymes are composed of single-stranded sequences of either DNA or RNA organized into a catalytic core, sensor arms that are complementary to the target sequence, and substrate-binding arms complementary to another sequence known as the DNA linker [
22]. The linker contains two DNA bases where cleavage occurs. This cleavage only happens in the presence of a target, upon hybridization with the sensor arms of the MNAzyme. In contrast, in the absence of the target, the linker sequence remains intact. However, in the presence of the target, the linker is cleaved into two shorter strands. Thus, the catalytic activity of MNAzymes can be modulated by the presence of genetic biomarkers, making them ideal candidates for developing highly specific bioanalytical strategies [
23]. Specifically, when coupled with gold nanoparticles (AuNPs) as detection tags, MNAzymes can be used for the direct detection of nucleic acids in a protein, isothermal, and optical format allowing for signal amplification [
24]. MNAzyme amplification makes use of a linker single-stranded DNA chain that is complementary to two nucleotide chains that are bioconjugated to the gold nanoparticles. The presence of the linker produces aggregation of the gold nanoparticles, and it is cleaved when the MNAzymes become active if the DNA target is present in the solution [
25]. The use of MNAzymes presents several advantages, and signal amplification increases from the probe molecule hybridized to the target nucleic acid sequence. Also, the advantages of MNAZymes amplification include specific detection, a wide dynamic range, ease of use, and reproducibility.
Following this procedure, in this work, we have established a novel colorimetric method based on bioconjugated gold nanoparticles, combining MNAzyme amplification and free color image software-assisted tools to achieve low detection limits for the determination of sesame. The image software tool has been utilized for chemical analysis based on color analysis, offering a powerful, fast, and cost-effective method to detect target analytes based on the color changes in digital images captured by cameras [
26,
27]. A digital camera has been used as an optical sensing element to acquire chemical information through the image processing technology of a TLC plate with different sample spots [
28,
29]. Image color analysis significantly enhances the sensitivity of colorimetric assays in chemical analysis by eliminating the inherent subjectivity of human visual perception [
30]. By using digital cameras to capture images of samples on the TLC plate, a precise digital representation of the colors is obtained. These photographs are processed with advanced software, allowing for the accurate and semiquantitative detection of color changes that are difficult to discern with the naked eye [
31]. This method not only improves the sensitivity of the assay but also ensures more reproducible and reliable results, regardless of the observer’s subjectivity and varying ambient light conditions. Consequently, the proposed approach enables a sensitive, rapid, robust, low-cost, and user-friendly method for detecting sesame in food products. The colorimetric sensor developed here demonstrates good sensitivity, specificity, precision, and accuracy for detecting sesame as an allergen.
3. Results
3.1. Principle of Proposed Colorimetric Method
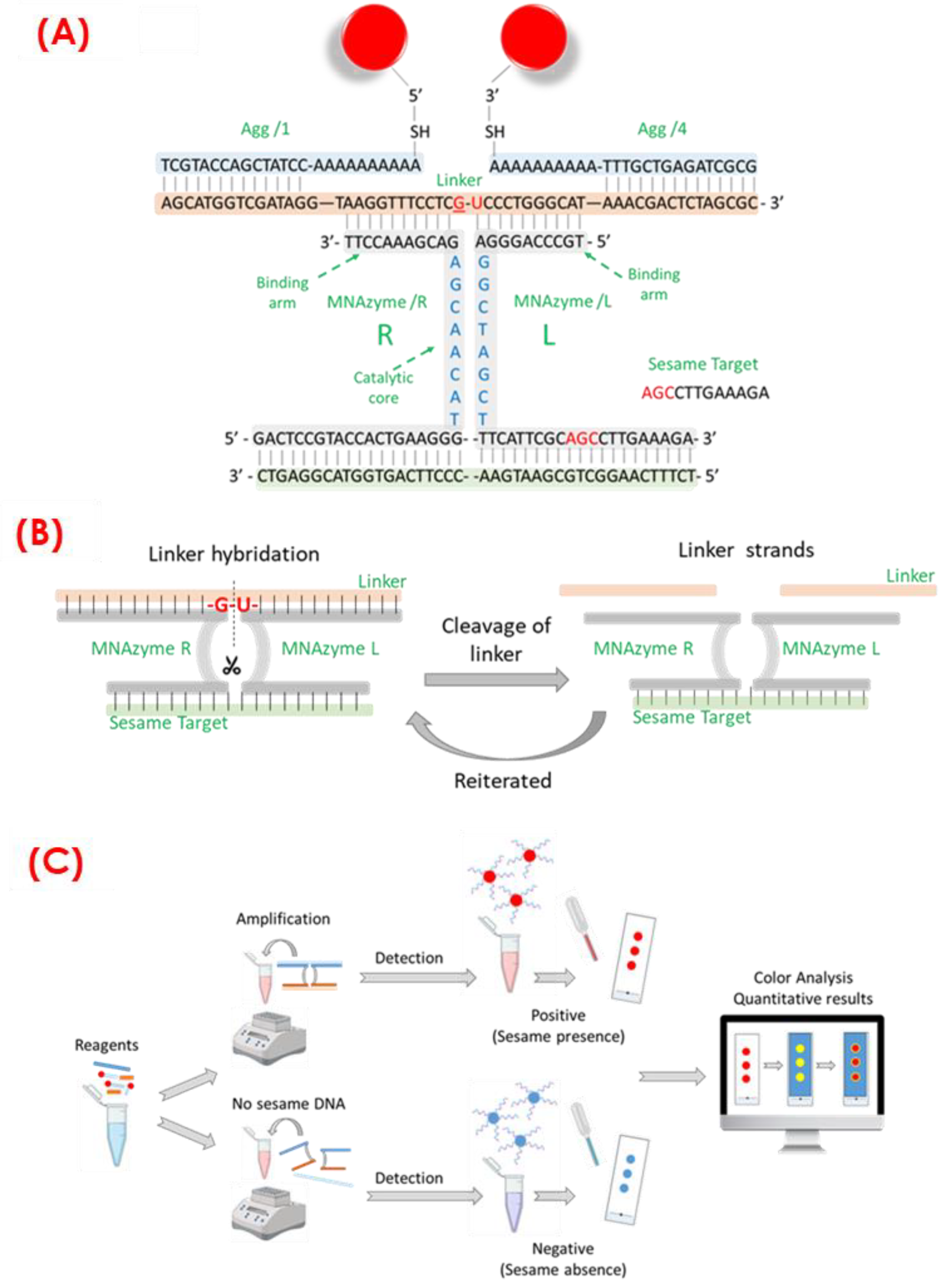
The proposed colorimetric assay for sesame DNA detection, based on AuNPs bioconjugated with the two DNA strands selected (Agg1 and Agg4) and MNAzyme amplification, is illustrated in
Scheme 1A. MNAzyme amplification makes use of a “linker” single-stranded DNA chain (Linker in
Scheme 1B) that is complementary to half of the two chains that are bioconjugated to the gold nanoparticles and to both arms of the MNAzyme system (see
Scheme 1A, MNAzyme/R, and MNAzyme/L).
The MNAzyme amplification process starts with the activation of the catalytic core of the MNAazyme by the target, thus bringing together MNAzymes R and L. Then, the linker may subsequently be recognized by the active MNAzyme complex, which irreversibly cleaves it into two DNA nucleotides. Subsequently, the pieces of the linker dehydrate, allowing for the active MNAzyme complex to sequentially cleave many linkers (amplification). The concentration of the DNA target in the sample determines the quantity of cleaved linkers, and it is associated with the amount of sesame in the sample. Finally, visual quantification of the linkers or the cleavage products is achieved by labeling both ends with DNA-functionalized AuNPs (detection). The higher the concentration of DNA in the sample, the higher the percentage of free AuNPs and the closer the solution’s color is to bare AuNPs (red color). Conversely, if the DNA concentration is lower, AuNP aggregates are more prevalent, and the color of the test shifts to blue values.
The color change of the colloidal dispersion due to the presence of the target can be detected by placing a 3 µL drop onto a TCL plate; as a result, the evolution of the colors while changing the DNA target concentration can be distinguished by the naked eye. Thanks to the strong signal amplification achieved by the use of the described genetic amplification approach, this assay allows for the visual detection of low concentration levels of the targets by the naked eye. In order to enhance the sensitivity of this methodology and avoid errors related to human-eye color interpretation, image processing software (Ilastik) has been employed (see
Scheme 1C). In this case, using image processing software aims to improve sensitivity in visual detection.
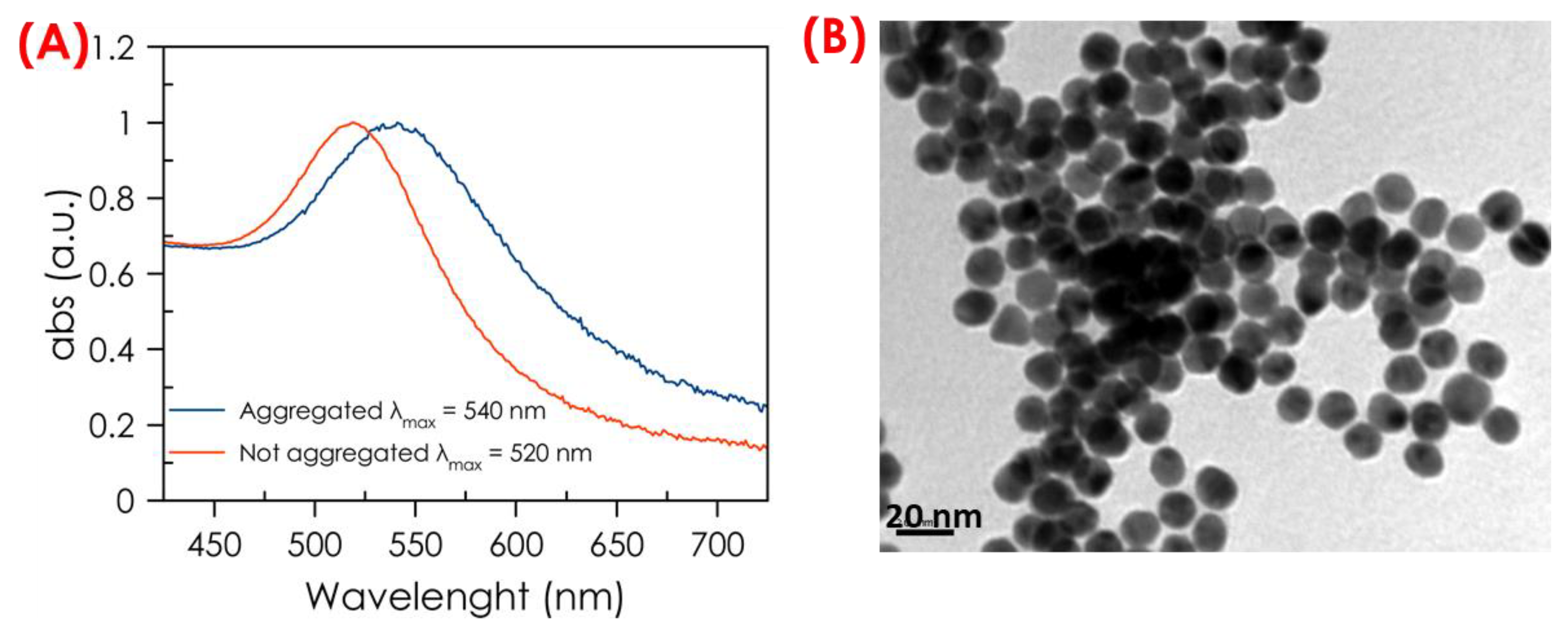
These color changes can be visualized by the naked eye and by the UV-Vis absorption spectra of AuNPs. There is a displacement of the maximum absorbance wavelength from blue-purple for aggregated AuNPs (approx. 540 nm) to red for dispersed AuNPs (approx. 520 nm), and it can be measured using UV-Vis spectroscopy. The optical properties of AuNPs change depending on the distance between them. A shorter linker single-stranded DNA chain results in a larger absorption wavelength shift of the AuNPs because the distance between them is smaller. In contrast, longer linker single-stranded DNA chains generate greater separation between the nanoparticles, leading to smaller wavelength shifts. In this work, a linker chain of 54 base pairs was used, which, as mentioned above, produced a blue shift of 20 nm.
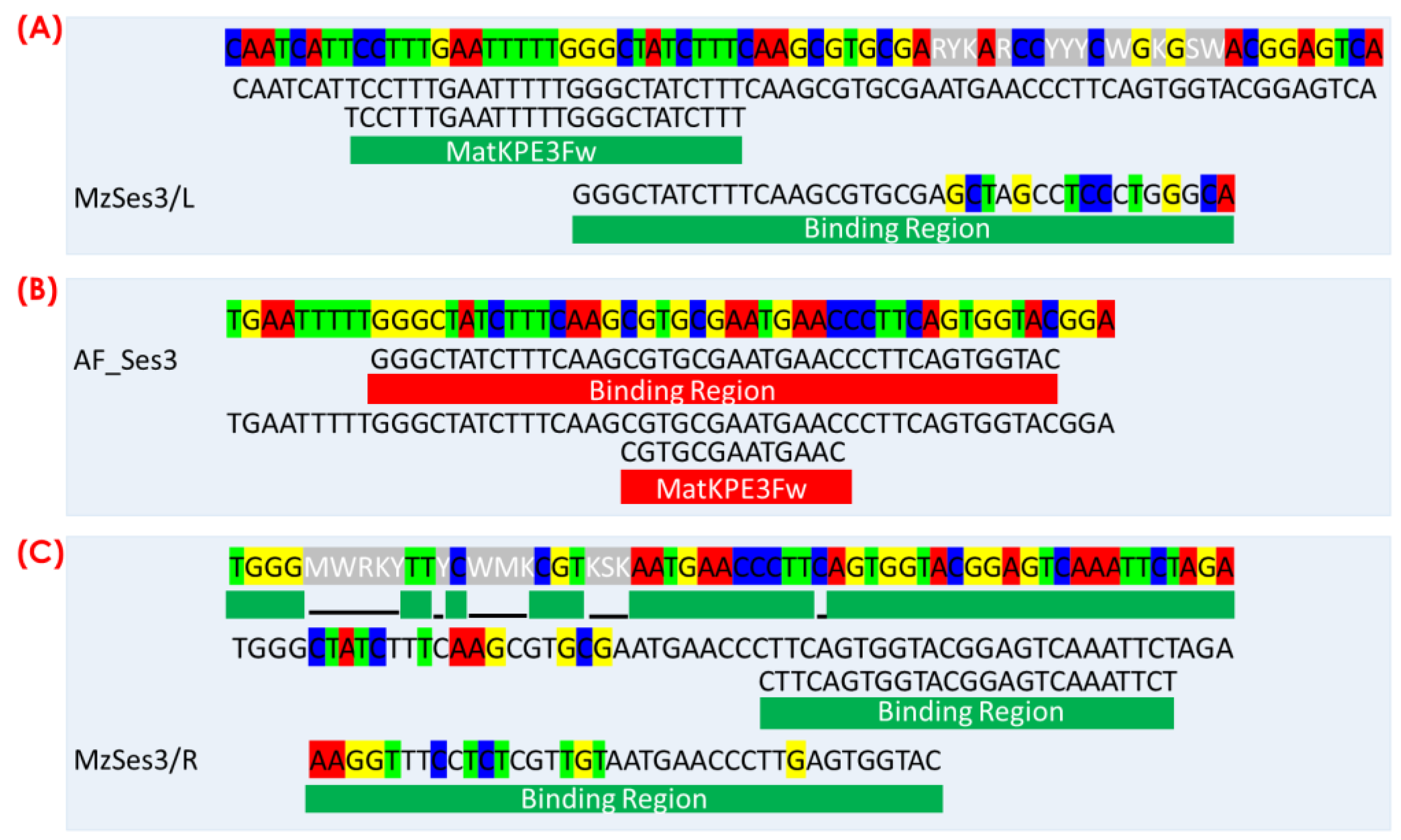
MNAzyme structures contain a catalytic core flanked by two binding arms (R and L in
Scheme 1A). Also, the linker strand contains two DNA bases where cleavage occurs when it is hybridized to the MNAzyme in the presence of the target (
Scheme 1B). The catalytic core is responsible for the cleavage of the DNA linker, whereas the binding arms are used to hybridize to the linker through Watson–Crick base pairing [
35]. The sequences of the binding arms in these MNAzymes have little effect on the catalytic activity. The cleavage site of the linker is a dinucleotide junction located between the two binding arm domains within the linker. The cleavage mechanism involves attacking the adjacent phosphodiester linkage via the 2′-hydroxil group of the upstream nucleotide within the cleavage junction [
35,
36]. In the absence of sesame DNA, the MNAzyme arms (R and L) do not undergo hybridization, and the catalytic core does not cleave the DNA linker. As a result, genetic amplification does not take place.
3.2. Characterization of Synthesized and Bioconjugated AuNPS
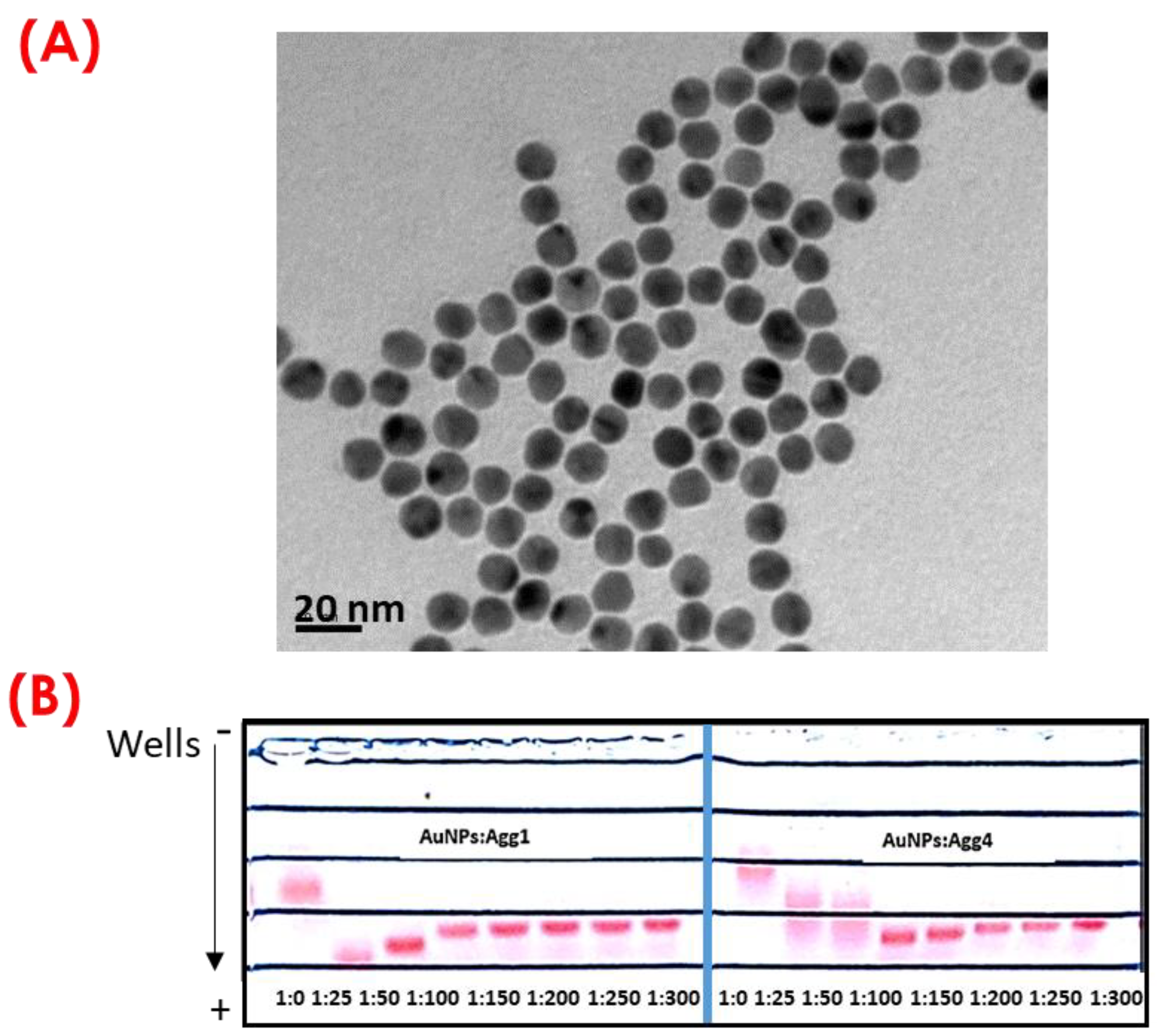
The size of synthesized AuNPs was confirmed using dynamic light scattering (DLS) and transmission electron microscopy (TEM). The size of AuNPs was found to be 13.6 ± 1.6 nm using DLS analysis and 15.2 ± 1.2 nm using TEM, see
Figure 2A. After synthesizing AuNPs, these were bioconjugated with Agg1 and Agg4 DNA sequences using the procedure described in
Section 2.5. AuNP bioconjugation. Different molar ratios of AuNPs: Agg1 and AuNPs: Agg4 were tested in the bioconjugation reaction to optimize the AuNP bioconjugates: 1:0, 1:25, 1:50, 1:100, 1:150, 1:200, and 1:300. All the AuNPs bioconjugated were analyzed via gel electrophoresis, using agarose gels at 1% (in agarose) and applying an electric potential of 100 V for 35 min. As shown in
Figure 2B, when the surface of the AuNPs is not completely functionalized with DNA, the bioconjugate migrates at a faster speed, and the bands generated are broader. However, for a given AuNPs: Agg1 and AuNPs: Agg2 molar ratio, it is observed that the migration speed of the bioconjugates formed remains constant and the bands are narrow, which indicates the formation of homogeneous bioconjugates with a surface of the bioconjugated nanoparticles with DNA strands. The electrophoresis gels shown in
Figure 2B indicate that the adequate molar ratios of AuNPs: Agg1 and AuNPs: Agg4 required for an efficient bioconjugation of gold nanoparticles are 1:100 and 1:150, respectively. Thus, the molar ratios were selected for further experiments.
In the development of the sensor presented here, 15 nm nanoparticles have been used for various reasons. Firstly, there is a trade-off between the size of the nanoparticles and the length of the DNA chain used for bioconjugation. If the nanoparticles are too small and the DNA chain is long, aggregation after linker hybridization with the bioconjugated gold nanoparticles becomes difficult because a minimum distance is needed for the nanoparticles to aggregate. Conversely, if the nanoparticles are too large and the DNA chain is short, the nanoparticles will be close enough to aggregate, hindering the recognition of sesame DNA during MNAzyme amplification. Furthermore, using larger nanoparticles requires more DNA sequences for biofunctionalization. For this reason, in the presented work, a 15 nm value for gold nanoparticles was used [
18,
19].
3.3. Optimization of MNAzyme Amplification
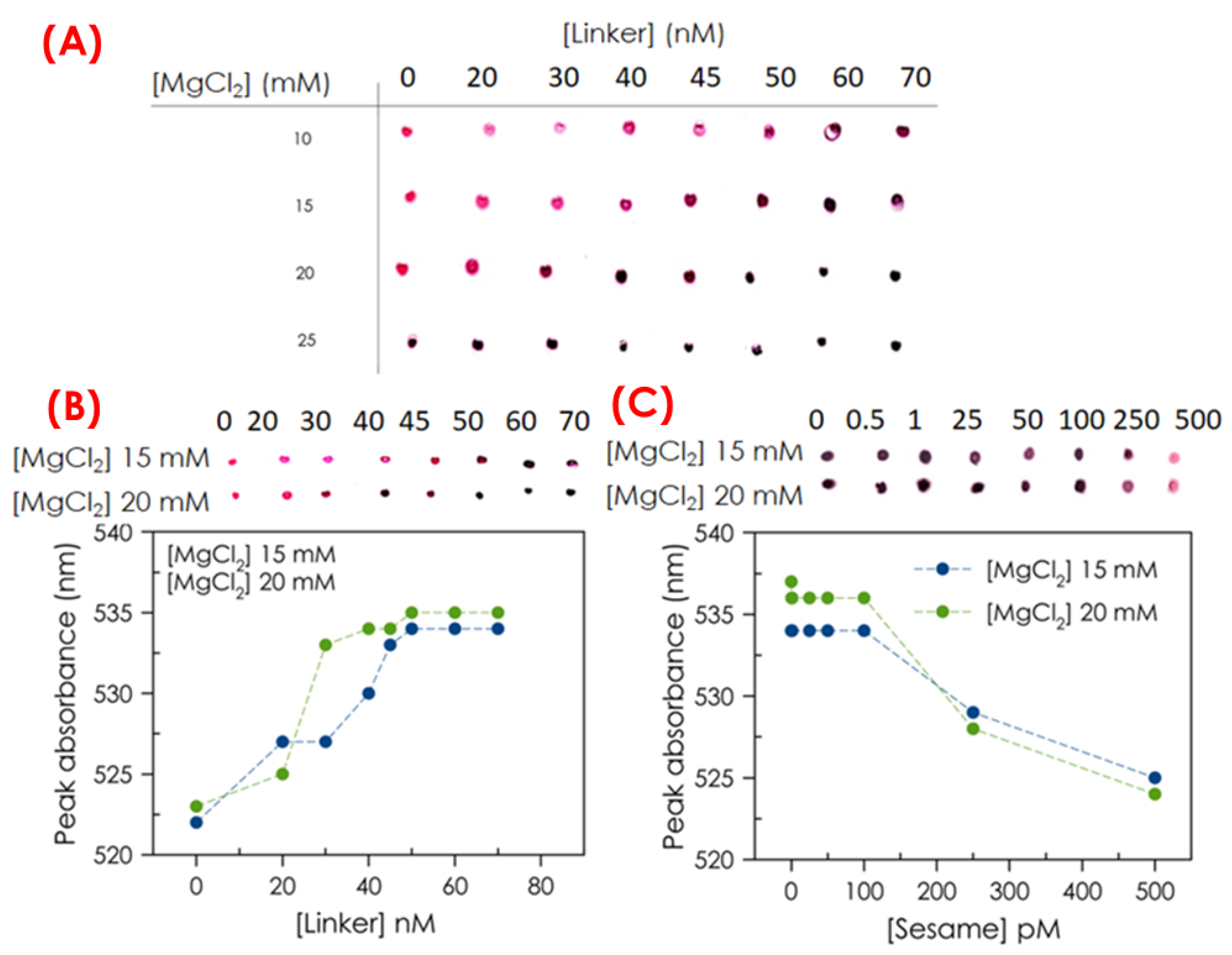
In a MNAzyme assay, several parameters can be optimized to achieve the best analytical performance in terms of sensitivity and reproducibility. Some of these key parameters were here optimized, including the magnesium ions’ concentration, which can enhance the efficient cleavage by the MNAzymes. Also, the DNA linker concentration used in the assay can impact its performance as it ensures efficient hybridization and stability of the MNAzyme complex. Therefore, here, the concentration of MgCl2 and the linker used in the assay has been evaluated to ensure the aggregation of AuNPs. For this purpose, different molar concentrations of the linker and MgCl2 were studied.
The MgCl
2 concentrations ranging from 10 mM to 25 mM and from 0 nM to 70 nM were prepared for the linker, see
Figure 3A,B. The degree of the displacement of the plasmon peak wavelength was the parameter used to select the best experimental conditions for signal amplification. The optimum concentration of MgCl
2 was found to be between 15 to 20 mM because no aggregation of AuNPs is observed in the absence of a linker. In addition, at this MgCl
2 concentration, the catalytic efficiency of MNAzymes is guaranteed when the aggregation of AuNPs is observed. A range of concentrations between 50 and 70 nM was tested to optimize linker concentration. As shown in
Figure 3A,B, by using linker concentrations higher than 50 nm, AuNP aggregation occurs because the color changes from red (dispersed AuNPs) to blue color (aggregated AuNPs), and the absorption wavelength is shifted to 535 nm. A linker concentration of 50 nM was selected as optimum since it corresponds to the minimum concentration at which there is a higher aggregation of AuNPs.
On the other hand, the concentration of MgCl
2 (15 and 20 mM) employed was also optimized, fixing the linker concentration of 50 nM in the presence of sesame DNA at concentrations of 0.5, 1, 25, 50, 100, and 500 pM, see
Figure 3C. The color change and the displacement of the surface plasmon resonance (SPR) wavelength were established from 100 to 500 pM of sesame DNA in both conditions. According to our results, we decided to use an optimum concentration of MgCl
2 of 15 mM because it is the lowest concentration in which no aggregation of AuNPs is observed in the presence of the target. Reaction times were also investigated. Longer incubation times may improve sensitivity, but excessively long times were found for non-specific cleavage. The optimum time in the assay was found to be 60 min.
Furthermore, the absorption wavelengths of the AuNPs were studied using a UV–Vis spectrophotometer.
Figure 4A shows the UV–Vis absorbance spectra of non-aggregated (red color) AuNPs and when the NPs are aggregated (blue color). As observed, the wavelength corresponding to the absolute maximum absorbance in the spectrum curve, the plasmon peak λ max, shifts towards higher values as aggregation occurs. In the developed assay strategy, the AuNPs are aggregated when the sample does not contain the sesame DNA target. However, when sesame DNA is present in the sample, the AuNPs are dispersed into the solution. So, the 540 nm peak (aggregated AuNPs) blue-shifts gradually when the concentration of sesame DNA increases.
Figure 4B shows a TEM picture of the assay mixture containing the DNA-surface-modified AuNPs after adding a sample without sesame DNA.
3.4. Analytical Characterization
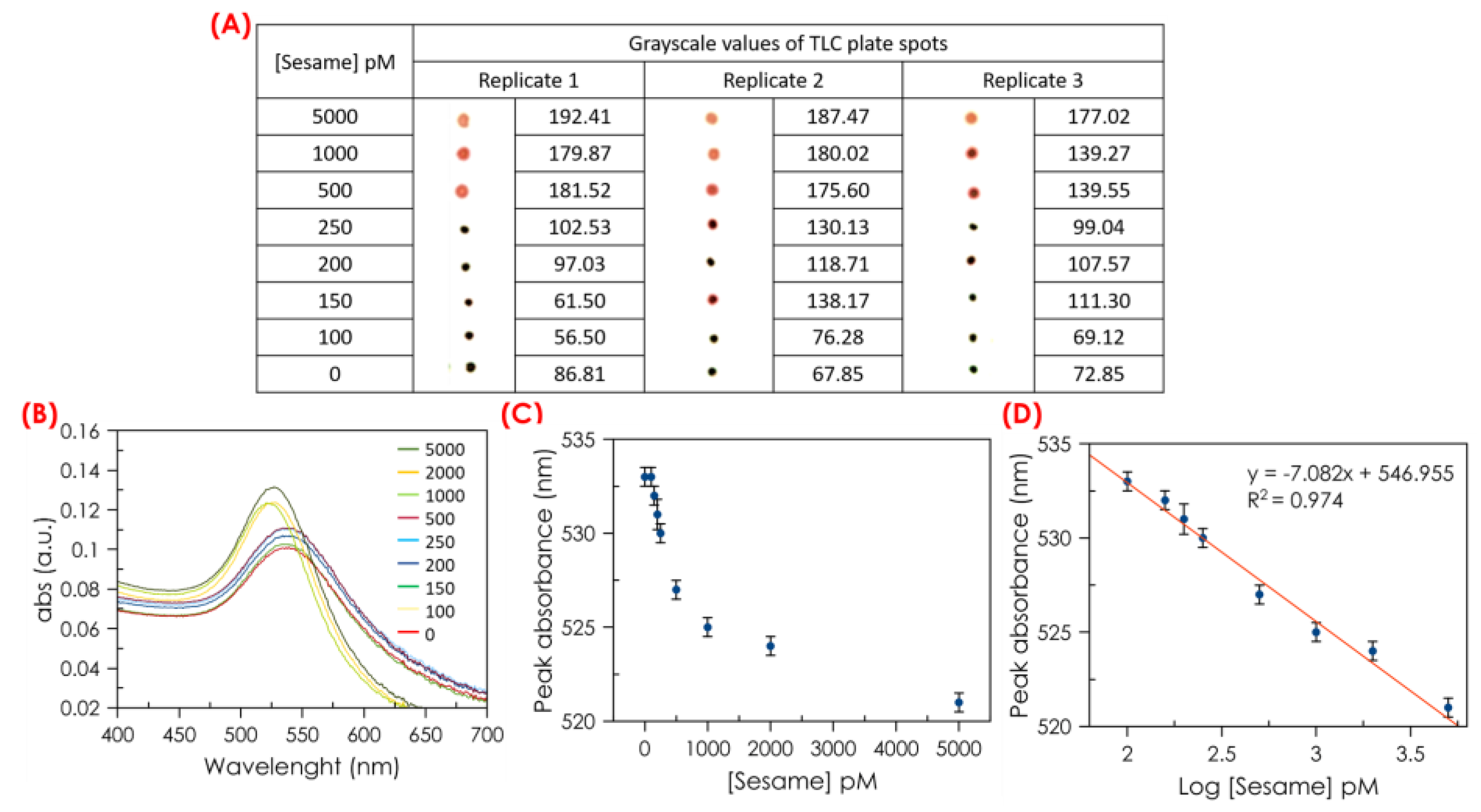
Under the optimal conditions, the detection of the linear dynamic range and sensitivity of the colorimetric method for sesame DNA detection were evaluated. The response of the method for sesame DNA standard solutions, in a range of sesame concentration from 0 to 5000 pM, was studied. As shown in
Figure 5A, the color of the spots in the TLC indicates the presence (red) and absence (purple or black) of the sesame DNA. The images were adjusted for contrast and brightness. Also, the digitalization of the TLC plate was carried out to obtain the grayscale values for each sample. The grayscale values were calculated using software and were summarized in
Figure 5A. Color analysis utilizing image processing software (Ilastik) facilitates the conversion of color into numerical data, enabling a straightforward classification of the samples in the presence of sesame. Meanwhile, in the UV-vis absorption spectra shown in
Figure 5B, the absorbance at 541 nm gradually displaced to 520 nm with the increasing sesame DNA concentration. The spectroscopic data showed a shift from 533 nm to 520 nm for sesame DNA concentrations between 100 and 5000 pM. Both spectroscopic measurements and spots in TLC were carried out in triplicate. It must be noted that the presence of sesame DNA even at a concentration above 100 pM does not cause aggregation of the gold nanoparticles, see
Figure 5C,D. We observed through color analysis that grayscale values increased with the concentration of sesame DNA. This correlation aligns with expectations in color analysis, considering that in grayscale, dark colors are represented by low-intensity values, thus high-intensity values represent light colors. Typically, image software assigns grayscale values ranging from 0 to 255, with 0 representing black and 255 representing white. All shades of gray therefore fall within this range. This difference in grayscale allows us to distinguish small color changes in samples that would be indistinguishable to the human eye.
Although it may seem at first glance that the absorbance maximum also decreases in samples with low concentrations of sesame DNA, the effect of the wavelength shift is more significant. This shift enables us to differentiate between samples containing sesame DNA and those without. This can be seen in
Figure 5C,D. Thus, the detection limit was calculated to be 140 pM. This LOD was determined by considering the mean value minus three standard deviations for the blank sample, which was 531.7 nM. Using the equation included in
Figure 5D, the corresponding value of log [sesame] was found to be 2.15, yielding a sesame concentration of approximately 140 pM. However,
Figure 5C,D show that only contents around or higher than 150 pM can be quantified. The high sensitivity of the proposed method was derived from the amplification of AuNPs-DNA induced by sesame DNA.
The performance data for the colorimetric detection of sesame allergen using DNA−functionalized gold nanoparticles are presented in
Table 2, alongside data from independent studies [
3,
12,
37,
38]. These data suggest that integrating biosensor chemistry with color analysis software offers a versatile and powerful platform for the sensitive, on-site, real-time detection of sesame DNA in different samples. Particularly, for allergen detection, highly sensitive detection systems are essential. The effect of allergens on the body depends not only on their concentration. It also depends on the individual’s tolerance to exposure. Therefore, it is necessary to determine the presence of the allergen quickly and with high sensitivity.
For allergen detection, high-detection systems are essential. The effect of allergens on the body depends not only on their concentration. It also depends on the individual’s tolerance to exposure. Therefore, it is necessary to determine the presence of the allergen quickly and with high sensitivity. Consequently, the proposed biosensor here meets these requirements for sesame DNA detection achieving sensitivity levels as low as 140 pM, which is indeed significant.
3.5. Sesame DNA Detection in Food Samples
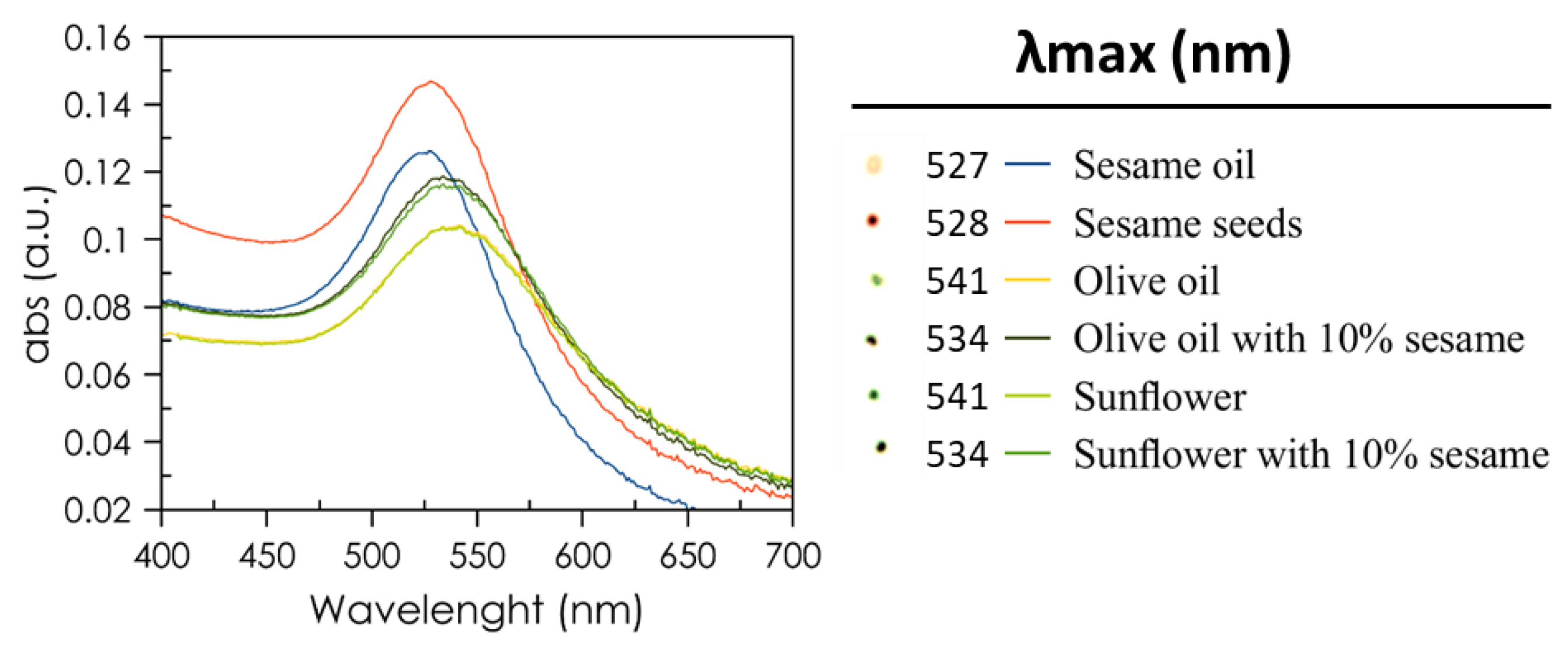
The developed procedure was tested to detect sesame DNA in different food samples including sesame oil, sesame seeds, olive oil, and sunflower oil. For olive and sunflower oils, 10% sesame oil was added, simulating olive oil adulteration. As can be seen in
Figure 6, the results are skewed towards aggregation when compared with the DNA standards, but there is a clear difference in maximum absorbance wavelength between the sesame-containing samples (~527 nm) and the ones without sesame (541 nm). To verify the potential of the developed method for the quantitative determination of sesame DNA in real food samples, sesame oils and oil samples spiked with sesame DNA were analyzed. The DNA extraction protocol was carried out as described in
Section 2.6. Extracting DNA from Food Samples. Once the sesame DNA was extracted, the detection assay was carried out using experimental conditions. Assuming a 70% yield of the DNA extraction [
34], the concentration at which a change in color is undoubtedly observed is 78 pM, which agrees with the LOD obtained from this method. Hence, it can be considered that this method can be applied to the analysis of sesame DNA in real samples. Also, the spots in the TLC presented different colors for sesame-containing samples and those without sesame which can be observed with the naked eye.
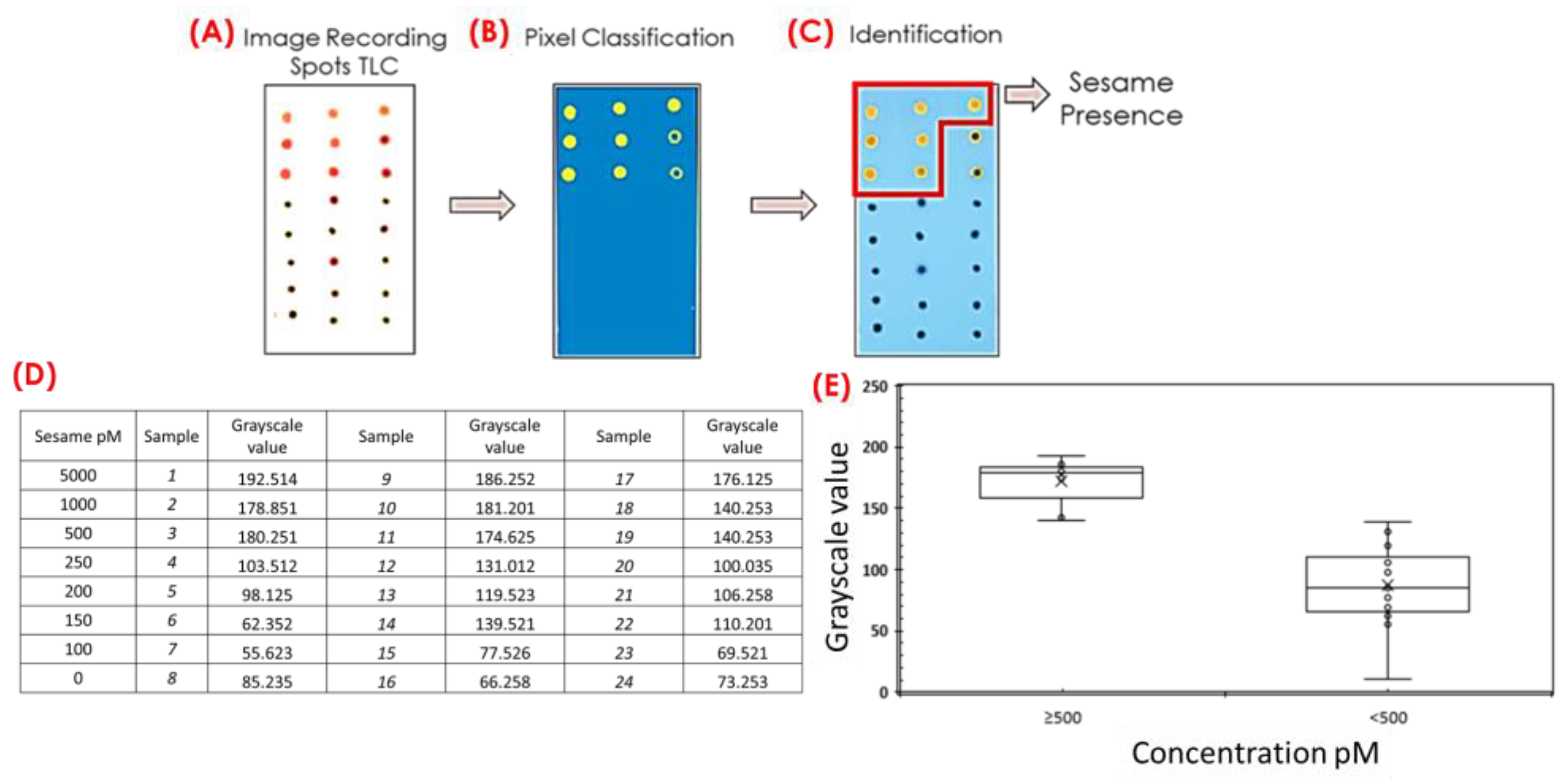
An interesting alternative to ensure an unbiased visual color-change detection and at the same time to improve the sensitivity and reliability of the assay is to combine MNAzyme amplification and color processing procedures. The use of processing software to discriminate between the aggregation of gold nanoparticles (blue-purple) and non-aggregation (red) was evaluated for the development of a simple, precise, easy-to-handle, and portable analytical method. For such purposes, 3 µL of the assay solution was deposited onto a TLC plate to facilitate the visual differentiation between aggregated and non-aggregated AuNPs. Then, images of TLC captured with a digital camera were used for pattern recognition analysis based on their color values, see
Figure 7A.
Color values were calculated using Ilastik, an open-source image analysis software that converts, through segmentation and classification, the color of an image into grayscale values. The general process is based on the loading of an image in jpg or png formats into the program. The software accesses the color values of each pixel, and the image is then automatically segmented and classified by pixel. Different regions of the image are identified and labeled. During this process, the software analyzes the characteristics of each pixel and classifies them based on color intensity (referred to as grayscale). The software then assigns grayscale values to each pixel once the image has been segmented and classified. For the analysis of the spots in the TLC plate, a circular area of the TLC point was selected to reduce noise in the data caused by edge effects and shadows,
Figure 7B. The “Pixel Classification” application allows us to use different parameters, including color, and classify each point in the image as not added (presence of sesame) or added (no presence of sesame), which is useful for semiqualitative analysis. The software identifies the red and blue points on the TLC plate. Then, it highlights the pixels (
Figure 7C) in yellow for non-aggregated AuNPs and marks the pixels in blue for aggregated AuNPs. These grayscale values are taken directly from the original image and are used to visually represent the different segmented regions. The software supports the exportation of the color analysis results in various formats for further interpretation.
A comparison of the images before and after Ilastik color analysis is shown in
Figure 7C, showing marked differences between samples. The software classifies samples based on their numerical conversion (
Figure 7B,D). Visually, samples deposited on the TLC plate can be distinguished as either having the presence of sesame DNA (red dots) or no presence of sesame DNA (blue dots); however, some samples are not easily identifiable through visual inspection alone. Color analysis utilizing image processing software facilitates the conversion of color into numerical data (see
Figure 7D), enabling a straightforward classification of the samples in the presence of sesame (see
Figure 7C). This represents a significant utility of the image processing software employed in this study, enhancing assay sensitivity. A total of 24 samples with concentrations between 100 and 5000 pM of sesame DNA and without sesame were analyzed using the proposed procedure, as can be seen in
Figure 7D. Color analysis using Ilastik software enabled us to classify the samples into two groups: samples with a sesame DNA concentration ≥500 pM and samples with a concentration <500 pM, see
Figure 7E.
4. Conclusions
This work reports a new colorimetric bioanalytical method for simple, efficient, specific, and highly sensitive detection of trace levels of sesame DNA in food samples. It has been demonstrated that the combination of DNA-surface-modified AuNPs with a signal amplification strategy based on MNAzymes allows for the detection of the DNA target with a high selectivity providing very low detection limits using spectrophotometric measurements (140 pM). Also, spotting onto a TLC plate of a few microliters of the assay solution, in combination with the use of open-source software for color signal processing, greatly facilitates the visualization of the color differences, minimizing the effects derived from differences in the perception of the color due to ambient light. The assay was successfully tested for the determination of sesame in contaminated olive and sunflower oils and in sesame seeds.
In brief, this methodology can be used in a wide range of laboratories for food safety control because it is easy to use, specific, and highly sensitive. Considering that the analytical signal is only a color change, there is no need for trained personnel to perform the analysis. Moreover, the developed assay could be applied to the detection of some other different genetic material characteristic of a wide variety of toxins, adulterants, or contaminants eventually present in food samples, due to the inherent properties of MNAzymes. The future of the groundbreaking colorimetric bioanalytical method for detecting sesame DNA in food samples is highly promising. This versatile method can be adapted to identify various genetic materials from different contaminants, enhancing food safety across diverse products. This work also paves the way for the use of digital cameras and open-source color signal processing software, enabling rapid and accurate analysis. This makes the method accessible in resource-limited settings. Future advancements may integrate the assay with portable devices for on-site testing, automate high-throughput screening, and improve sensitivity and specificity. Additionally, potential applications in food and health monitoring highlight its broad utility and potential impact on public health and environmental protection.