Identification and Quantification of Extracellular Vesicles: Comparison of SDS-PAGE Analysis and Biosensor Analysis with QCM and IDT Chips
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method 1: SDS-PAGE Analysis
2.3. Method 2: Biosensor Measurements
2.3.1. Measurements by QCM
2.3.2. Measurements by Electroanalysis
3. Results and Discussion
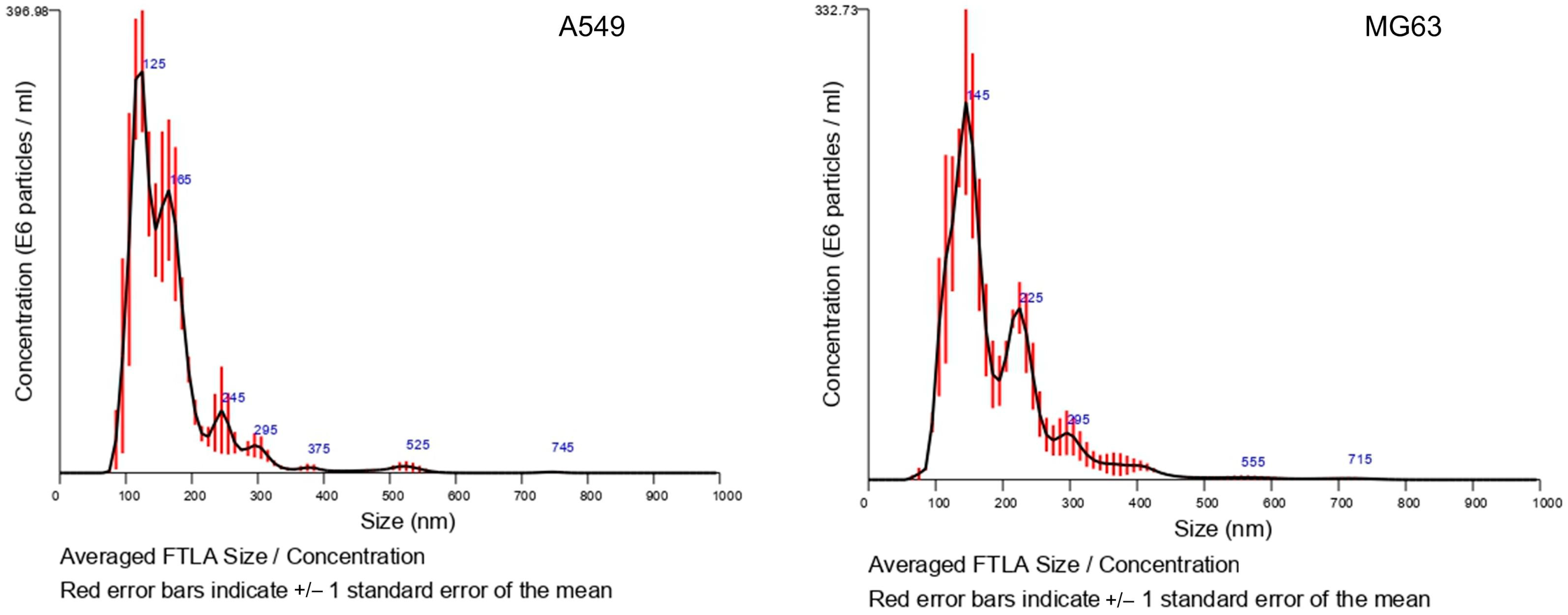
3.1. Analysis by NTA and ELISA Kit
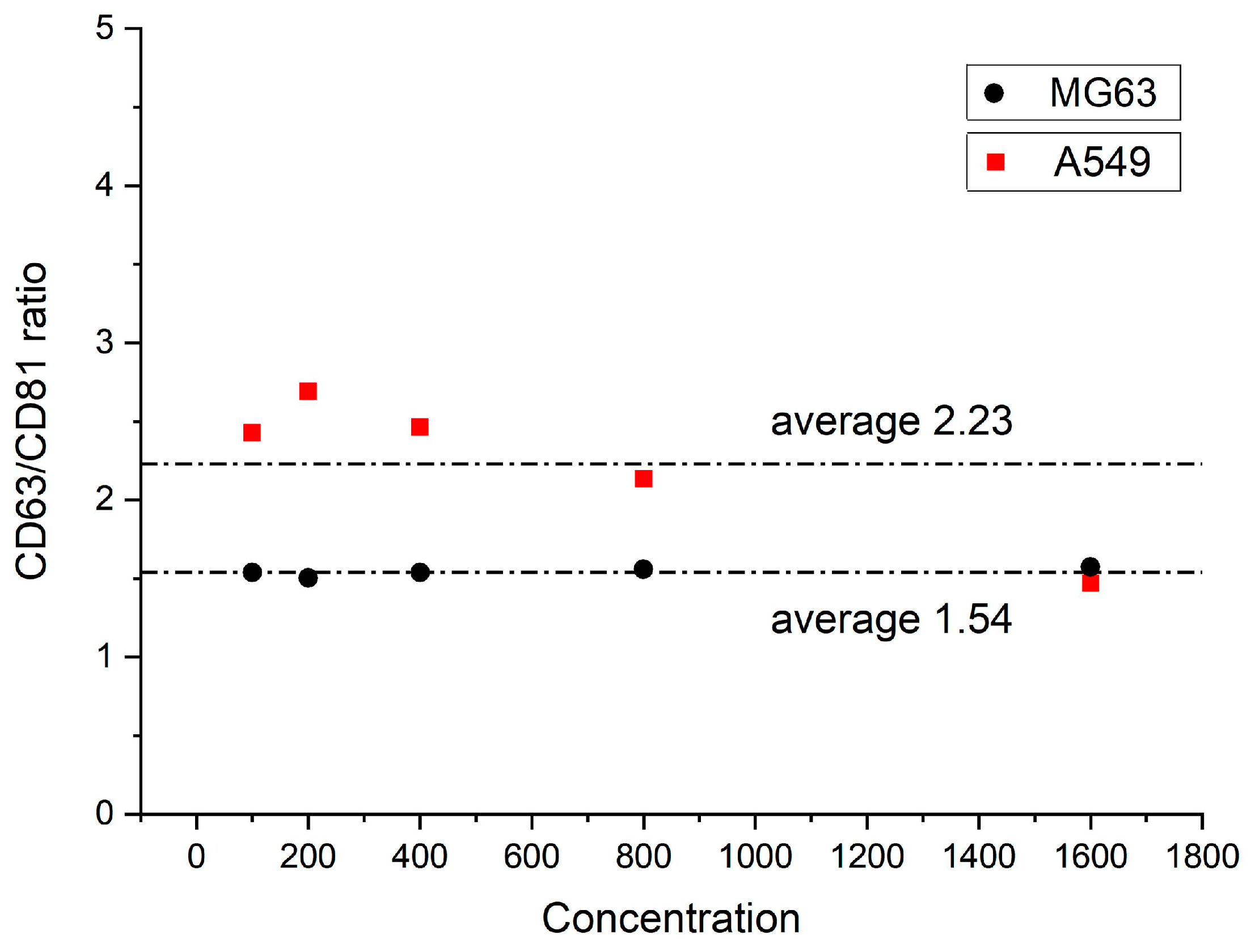
3.2. Method 1: SDS-PAGE Analysis
3.3. Method 2: Biosensor Measurements
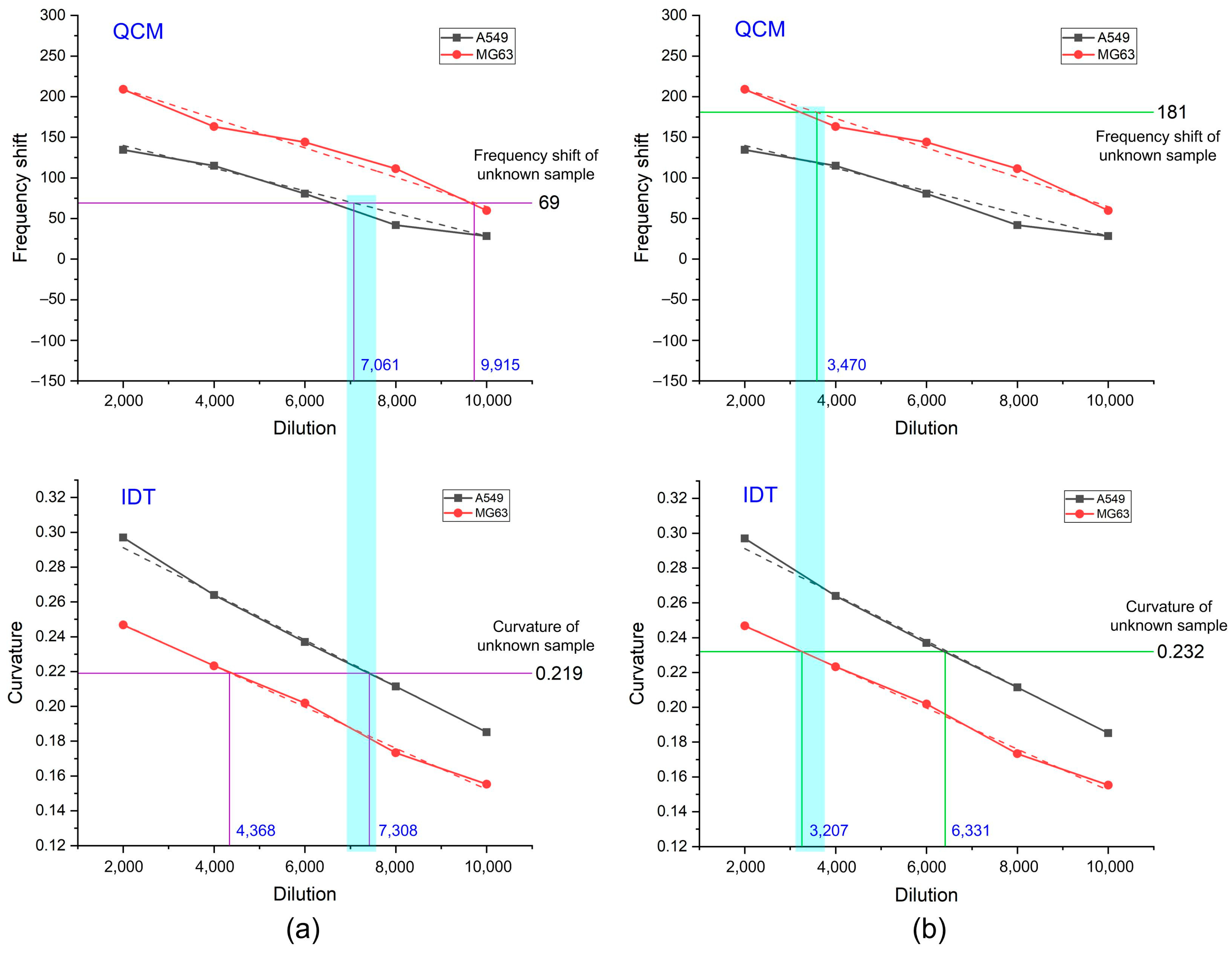
3.3.1. Measurements by QCM
3.3.2. Measurements by Electroanalysis
3.3.3. Identification of EVs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, W.A. Molecular Biology of Human Cancers; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Song, Q.; Merajver, S.D.; Li, J.Z. Cancer classification in the genomic era: Five contemporary problems. Hum. Genom. 2015, 9, 27. [Google Scholar] [CrossRef]
- Wang, C.-L.; Hsu, K.-H.; Chang, Y.-H.; Ho, C.-C.; Chiang, C.-J.; Chen, K.-C.; Cheung, Y.-C.; Huang, P.-C.; Chen, Y.-R.; Chen, C.-Y.; et al. Low-dose computed tomography screening in relatives with a family history of lung cancer. J. Thorac. Oncol. 2023, 18, 1492–1503. [Google Scholar] [CrossRef]
- Dhiman, B.; Kamboj, S.; Srivastava, V. Explainable AI based efficient ensemble model for breast cancer classification using optical coherence tomography. Biomed. Signal Process. Control 2024, 91, 106007. [Google Scholar] [CrossRef]
- Aristokli, N.; Polycarpou, I.; Themistocleous, S.C.; Sophocleous, D.; Mamais, I. Comparison of the diagnostic performance of Magnetic Resonance Imaging (MRI), ultrasound and mammography for detection of breast cancer based on tumor type, breast density and patient’s history: A review. Radiography 2022, 28, 848–856. [Google Scholar] [CrossRef]
- Byun, H.J.; Shin, T.J.; Jung, W.; Ha, J.Y.; Kim, B.H.; Kim, Y.H. The value of magnetic resonance imaging and ultrasonography (MRI/US)-fusion biopsy in clinically significant prostate cancer detection in patients with biopsy-naïve men according to PSA levels: A propensity score matching analysis. Prostate Int. 2022, 10, 45–49. [Google Scholar] [CrossRef]
- Song, P.; Zhang, L.; Bai, L.; Wang, Q.; Wang, Y. Diagnostic performance of ultrasound with computer-aided diagnostic system in detecting breast cancer. Heliyon 2023, 9, e20712. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Pérez-Vidal, J.R.; Tameish, S.; Chacón, E.; Manzour, N.; Mínguez, J.A. Ultrasound for assessing tumor spread in ovarian cancer. A systematic review of the literature and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 194–200. [Google Scholar] [CrossRef] [PubMed]
- de Rauglaudre, B.; Pioche, M.; Caillol, F.; Ratone, J.-P.; Pellat, A.; Coriat, R.; Rivory, J.; Lambin, T.; Dahan, L.; Giovanini, M.; et al. Follow-up after endoscopic resection for early gastric cancer in 3 French referral centers. iGIE 2022, 1, 49–56. [Google Scholar] [CrossRef]
- Chi, L.-M.; Hsiao, Y.-C.; Chien, K.-Y.; Chen, S.-F.; Chuang, Y.-N.; Lin, S.-Y.; Wang, W.-S.; Chang, I.Y.; Yang, C.; Chu, L.J.; et al. Assessment of candidate biomarkers in paired saliva and plasma samples from oral cancer patients by targeted mass spectrometry. J. Proteom. 2020, 211, 103571. [Google Scholar] [CrossRef]
- Shirkavand, A.; Babadi, M.; Fashtami, L.A.; Mohajerani, E. Application of optical spectroscopy in diagnosing and monitoring breast cancers: A technical review. Clin. Spectrosc. 2023, 5, 100027. [Google Scholar] [CrossRef]
- Sunidhi, C.R.; Jeyaprakash, M.R.; Rajeshkumar, R. Sonic Hedgehog gene as a potential target for the early prophylactic detection of cancer. Med. Hypotheses 2020, 137, 109534. [Google Scholar] [CrossRef]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular vesicles: New classification and tumor immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.A.; Kwon, M.H.; Kang, J.Y.; Rhee, W.J. In situ single step detection of exosome microRNA using molecular beacon. Biomaterials 2015, 54, 116–125. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, L.; Wang, Z.; Irfan, M.; Qu, F. Recent advances of aptasensors for exosomes detection. Biosens. Bioelectron. 2020, 160, 112213. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wen, Y.; Li, M. Emerging biosensing platforms for quantitative detection of exosomes as diagnostic biomarkers. Coord. Chem. Rev. 2021, 446, 214111. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, X.; Yu, J. Exosomes and organ-specific metastasis. Mol. Ther.-Methods Clin. Dev. 2021, 22, 133–147. [Google Scholar] [CrossRef]
- Gil, B.; Keshavarz, M.; Wales, D.; Darzi, A.; Yeatman, E. Orthogonal surface-enhanced Raman scattering/Field-Effect Transistor detection of breast and colorectal cancer-derived exosomes using graphene as a tag-free diagnostic template. Adv. NanoBiomed Res. 2023, 3, 2300055. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L.; Zhu, L.; Mei, X.; Xu, W. A sandwich-based evanescent wave fluorescent biosensor for simple, real-time exosome detection. Biosens. Bioelectron. 2022, 200, 113902. [Google Scholar] [CrossRef]
- Luo, S.; Wu, Y.; Pan, W.; Zhong, G.; Situ, B.; Li, B.; Ye, X.; Jiang, X.; Li, W.; Zhang, Y.; et al. An integrated magneto-fluorescent nanosensor for rapid and sensitive detection of tumor-derived exosomes. Sens. Actuator B-Chem. 2023, 374, 132792. [Google Scholar] [CrossRef]
- Chen, J.; Guo, J.; Hu, M.; Wang, Y.; Hua, F.; Meng, H.-M.; Jin, S. Accurate and portable tumor exosomes detection based on manganese dioxide and aptamer-functionalized fluorescent microspheres mediated dual-mode lateral flow assay. Sens. Actuator B-Chem. 2024, 409, 135614. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, Q.; Wang, F.; Liu, Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens. Bioelectron. 2019, 124, 184–190. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, P.; Zhang, Y.; Du, L.; Wang, Y.; Zhang, C.; Wang, C. Exosome detection via surface-enhanced Raman spectroscopy for cancer diagnosis. Acta Biomater. 2022, 144, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Shaw, I.; Chen, C. Application of microfluidic technology based on surface-enhanced Raman scattering in cancer biomarker detection: A review. J. Pharm. Anal. 2023, 13, 1429–1451. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct quantification of cancerous exosomes via surface plasmon resonance with dual gold nanoparticle-assisted signal amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Bae, S.; Lee, E.-M.; Cha, H.J.; Kim, K.; Yoon, Y.; Lee, H.; Kim, J.; Kim, Y.-J.; Lee, H.G.; Jeung, H.-K.; et al. Resveratrol alters microRNA expression profiles in A549 human non-small cell lung cancer cells. Mol. Cells 2011, 32, 243–249. [Google Scholar] [CrossRef]
- Xie, J.; Liu, J.; Liu, H.g.; Liang, S.; Lin, M.; Gu, Y.; Liu, T.; Wang, D.; Ge, H.; Mo, S.-L. The antitumor effect of tanshinone IIA on antiproliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm. Sin. B 2015, 5, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ju, Y.; Morita, Y.; Song, G. Effect of the nanostructure of porous alumina on growth behavior of MG63 osteoblast-like cells. J. Biosci. Bioeng. 2013, 116, 509–515. [Google Scholar] [CrossRef]
- Jafarkhani, S.; Amiri, E.; Moazzeni, S.; Zohoorian-Abootorabi, T.; Eftekhary, M.; Aminnezhad, S.; Khakbiz, M. Exploring the effects of micro-nano surface topography on MG63 osteoblast-like cell responses: An in vitro study. Colloid Surf. A-Physicochem. Eng. Asp. 2023, 675, 131872. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles 2013, 2, 20424. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Jakobsen, K.R.; Bæk, R.; Folkersen, B.H.; Rasmussen, T.R.; Meldgaard, P.; Varming, K.; Jørgensen, M.M.; Sorensen, B.S. Exosomal proteins as diagnostic biomarkers in lung cancer. J. Thorac. Oncol. 2016, 11, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.; An, H.; Jung, J.; Song, D. The prognostic significance of CD63 expressionin patients with non-small cell lung cancer. Pol. J. Pathol. 2019, 70, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, K.R.; Paulsen, B.S.; Bæk, R.; Varming, K.; Sorensen, B.S.; Jørgensen, M.M. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles 2015, 4, 26659. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Yang, W.-T.; Wu, J.-C. Isolation and detection of exosomes via AAO membrane and QCM measurement. Microelectron. Eng. 2019, 216, 111094. [Google Scholar] [CrossRef]
- Nakhlband, A.; Kholafazad-Kordasht, H.; Rahimi, M.; Mokhtarzadeh, A.; Soleymani, J. Applications of magnetic materials in the fabrication of microfluidic-based sensing systems: Recent advances. Microchem. J. 2022, 173, 107042. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Cinti, S. Electrochemical nano biosensors for the detection of extracellular vesicles exosomes: From the benchtop to everywhere? Biosens. Bioelectron. 2022, 216, 114635. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-J.; Yang, W.-T.; Lei, C.-H. Identification and Quantification of Extracellular Vesicles: Comparison of SDS-PAGE Analysis and Biosensor Analysis with QCM and IDT Chips. Biosensors 2024, 14, 366. https://doi.org/10.3390/bios14080366
Chang Y-J, Yang W-T, Lei C-H. Identification and Quantification of Extracellular Vesicles: Comparison of SDS-PAGE Analysis and Biosensor Analysis with QCM and IDT Chips. Biosensors. 2024; 14(8):366. https://doi.org/10.3390/bios14080366
Chicago/Turabian StyleChang, Yaw-Jen, Wen-Tung Yang, and Cheng-Hsuan Lei. 2024. "Identification and Quantification of Extracellular Vesicles: Comparison of SDS-PAGE Analysis and Biosensor Analysis with QCM and IDT Chips" Biosensors 14, no. 8: 366. https://doi.org/10.3390/bios14080366
APA StyleChang, Y.-J., Yang, W.-T., & Lei, C.-H. (2024). Identification and Quantification of Extracellular Vesicles: Comparison of SDS-PAGE Analysis and Biosensor Analysis with QCM and IDT Chips. Biosensors, 14(8), 366. https://doi.org/10.3390/bios14080366



