Abstract
Polluted air and the presence of numerous airborne pathogens affect our daily lives. The sensitive and fast detection of pollutants and pathogens is crucial for environmental monitoring and effective medical diagnostics. Compared to conventional detection methods (PCR, ELISA, metabolic tests, etc.), biosensors bring a very attractive possibility to detect chemicals and organic particles with the mentioned reliability and sensitivity in real time. Moreover, by integrating nanomaterials into the biosensor structure, it is possible to increase the sensitivity and specificity of the device significantly. However, air quality monitoring could be more problematic even with such devices. The greatest challenge with conservative and sensing methods for detecting organic matter such as bacteria is the need to use liquid samples, which slows down the detection procedure and makes it more difficult. In this work, we present the development of a polyacrylonitrile nanofiber bioreceptor functionalized with antibodies against bacterial antigens for the specific interception of bacterial cells directly from the air. We tested the presented novel nanofiber bioreceptor using a unique air filtration system we had previously created. The prepared antibody-functionalized nanofiber membranes for air filtration and pathogen detection (with model organisms E. coli and S. aureus) show a statistically significant increase in bacterial interception compared to unmodified nanofibers. Creating such a bioreceptor could lead to the development of an inexpensive, fast, sensitive, and incredibly selective bionanosensor for detecting bacterial polluted air in commercial premises or medical facilities.
1. Introduction
Nowadays, the rapid and eminent development of biomedicine and environmental monitoring is mainly due to the possibility of easy, fast, precise, and sensitive diagnostics and detection [1,2,3]. For such a development, sensors are the tools of great interest. In addition, combined with bioactive molecules (antibodies, enzymes, nucleic acids, etc.), (bio)sensors allow for the reliable detection of different biological and chemical markers. The main attractivity of biosensors stands especially on particular and sensitive biological interactions between analytes and the recognition bioactive element of the sensor (so-called bioreceptor) [1]. The most common biosensors commercially used are glucometers—sensors for glucose monitoring in blood [4,5]. However, in addition to monitoring and detecting glucose and other chemical analytes and biomarkers (hormones, enzymes, lipids, etc.), fast and so-called online detection of pathogens is also a significant priority.
Biosensors have become an exciting alternative to pathogen detection in microbiology and epidemiology. Today, the most common methods for determining bacteria and viruses are ELISA, PCR, and metabolic tests [6,7,8,9]. However, biosensors reduce costs (in some cases up to 96% [9], but on average, around 40% [10] and time (from hours with PCR to units to tens of minutes with biosensors [11]). Among other things, device sensitivity can be increased by incorporating nanomaterials [12,13,14,15,16,17,18] into the biosensor system, and it is possible to achieve LoD in fM concentration [12,13]. This increase in sensitivity is secured mainly using nanofibers. Their characteristic structure with the immense number of pores [19,20,21] creates an enormous active surface that can be modified, enriched, or functionalized [22,23].
Functionalization is a process of immobilizing bioactive molecules in the matrix structure [24]. Nanofibers modified by this process are the subject of recent studies. Whether it is functionalization with nucleic acids (such as DNA immobilization for the detection of Salmonella [25]) or antibodies (specific antibodies against Pseudomonas aeruginosa [26], Helicobacter pylori [27], or Streptococcus agalactiae [28]), low detection limits in the units of CFU·mL−1, high sensitivity, and fast response characterize these biosensors. These mentioned studies are dedicated to pathogen detection from liquid samples. However, many pathogens are transmitted through the air, and in addition to causing respiratory diseases, they also cause nosocomial infections. Pathogen detection directly from the air is becoming an attractive and desired method for the environmental monitoring of polluted air. Although many different biosensors exist, their use for detecting analytes from air faces challenges in bioreceptor preservation [29,30,31,32]. Nevertheless, pathogen monitoring in the air could help prevent respiratory disease epidemics or the emergence of nosocomial infections in operating rooms, intensive units, and hospitals in general.
The main goal of this work was to prepare antibody-functionalized nanofibers as bioreceptors for the interception and detection of selected bacterial organisms. In this work, we present the needleless electrospinning process of polyacrylonitrile nanofiber fabrication; the process of their functionalization; and finally, the evaluation of the bioreceptor’s bacterial interception effectiveness through optical density measurement. This work is directly linked to the conference paper from the EHB 2023 conference but expands the mentioned paper with more detailed methodology and new results (supplemented results of detecting E. coli and added new results of detecting S. aureus) [33].
2. Materials and Methods
Considering nanofibers’ characteristic structure, a mechanically and chemically durable synthetic polymer material with the possibility of functionalization had to be chosen to prepare desirable filtration membranes. The immobilization of proper bioactive molecules (antibodies) secured the functionalization of nanofiber membranes. For the required application, specific antibodies were selected as a biorecognition element for detecting the model bacteria (Escherichia coli and Staphylococcus aureus). After preparing and characterizing the prepared bioreceptor, functionalized nanofiber membranes were tested in the laboratory.
2.1. Materials
Polymer polyacrylonitrile (PAN) was purchased from Sigma-Aldrich (USA) to fabricate electrospun nanofibers. This polymer was chosen due to its mechanical and chemical endurance and the possibility of surface functionalization. The functionalization of PAN nanofibers was performed by the immobilization of specific antibodies. For the interception of Gram-negative model bacteria, Rabbit polyclonal IgG anti-Escherichia coli antibodies (4329–4906) were purchased from Bio-Rad (USA). Anti-Staphylococcus aureus LTA antibodies (SAB4200883-100UL) from Sigma-Aldrich (USA) were immobilized to nanofibers to detect the Gram-positive model bacteria Staphylococcus aureus.
The University of Chemistry and Technology, Prague, provided Gram-negative model bacteria Escherichia coli reference strains (O26:B6, E. coli DBM 3125—collection CCM 3988). The Institute of Medical Biochemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University in Prague, provided Gram-positive bacteria Staphylococcus aureus (STAV) strains.
2.2. Nanofiber Fabrication, Modification, and Characterization
For the biosensor matrix, polyacrylonitrile nanofibers were fabricated using the electrospinning method. Electrospinning uses the charge polymer solution under a high-voltage electric field to prepare ultrafine fibers with diameters of hundreds of nanometers [34]. Electrospun nanofibers are characterized by extremely high surface-to-volume ratio, high porosity, low weight, and excellent mechanical and chemical properties. Nevertheless, all the properties can be customized by adequately selecting a polymer solution and setting the process parameters of the fabrication method [19,35,36].
Polyacrylonitrile (PAN) polymer is suitable for preparing fine nanofibers with excellent mechanical and chemical stability. Fibers fabricated from polyacrylonitrile are ideal for filtration and the creation of biosensor matrices (mats). These fibers are also suited for surface functionalization by immobilizing bioactive molecules [37,38].
To fabricate suitable nanofibers, the powder of polyacrylonitrile was mixed with N, N-dimethylformamide (DMF) and homogenized for 2 h at 35 °C. Electrospun PAN nanofibers were fabricated (roller electrospinning—Figure 1) using Nanospider NS 1WS500U (Elmarco, Liberec, Czech Republic). The process parameters are shown in Table 1.
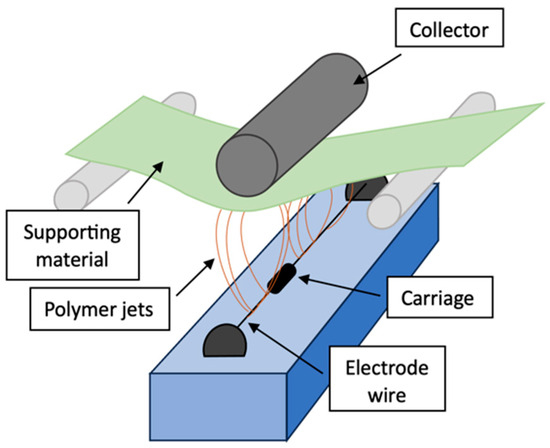
Figure 1.
Setup of Nanospider device for fabrication of electrospun nanofibers [33].

Table 1.
Set process parameters of the electrospinning (with the deviation given by the Nanospider NS 1WS500U device) [33].
After fabrication, samples of nanofibers were gilded and characterized through the scanning electron microscope Vega3 SB (Tescan, Brno, Czech Republic).
The created nanofibers were later modified and functionalized. PAN nanofibers’ surface modification (reduction) ensures the formation of functional groups suitable for bonding bioactive molecules [39]. Specific antibodies against bacteria E. coli and S. aureus were then covalently immobilized in the structure of PAN nanofibers. The concentration of bonded antibodies was determined by infrared spectroscopy IRAFfinity-1 (Shimadzu, Kyoto, Japan), and the absorbance of 1685 cm−1, characteristic of the peptide bond, was used. The calibration curve was determined using avidin and measuring the remaining protein in the solution after immobilization [40,41].
The functionalized nanofibers were prepared and preserved in a saline buffer with sodium azide. Samples preserved this way were stored in the fridge. Previous testing shows that preserved functionalized nanofiber membranes can be stored in the fridge for at least 2 months without changing the antibody activity.
2.3. Bacterial Cultivation
Both model organisms—E. coli and S. aureus—were cultured on a solid agar medium prepared from 2.5 g of yeast extract, 2.5 g of peptone, 1.125 g of NaCl, and 5 g of agar. Individual media components were mixed in 250 mL of distilled water, homogenized, heated, and sterilized before being poured into the Petri dishes.
From the reference strains, a single colony of bacteria was transferred to the agar medium using the streak plate method; passaged bacteria were cultured at 37 °C in the incubator (mini-incubator ICT 18, FALC Instruments, Treviglio BG, Italy). E. coli was incubated for 21 h and S. aureus for 24 h to achieve adequately grown bacterial colonies.
2.4. Testing of the Nanofiber Bioreceptor
A unique pump system was designed to test the detection effectivity of the functionalized nanofibers. The created system consists of a mechanical pump enabling the filtration of the air sample through the nanofiber membrane in a sealed chamber. A sample container with a volume of 1.5 l is connected directly to the sealed chamber. The whole pump system is closed and provided with filters and thus does not allow bacteria to escape from the experimental setup. Moreover, this unique pump system was designed to maintain suitable conditions for the immobilized antibodies by continually humidifying filtered air. The detailed layout (Figure 2) and function of the air filtration system are presented in the original paper from 2024 [42].
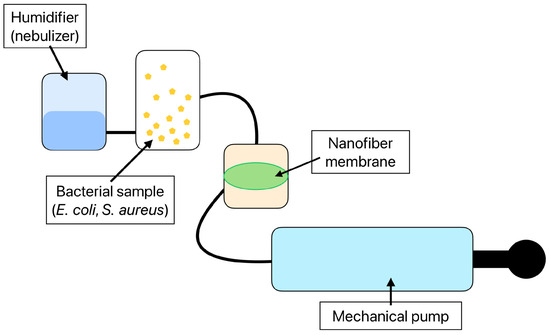
Figure 2.
The layout of the air filtration system consisting of a mechanical pump, a 1.5 l sample container, a sealed container with a nanofiber membrane, and a humidifier sustaining the proper environment for the antibody immobilized to the nanofiber structure [42].
Nanofiber membranes were tested as a bioreceptor for the interception of bacterial cells. Before use, membranes were washed in distilled water so the saline buffer and preservative residues would not affect the detection. After washing, the nanofiber membrane was evenly spread to the holder in the sealed chamber. The volume of contaminated air in the sample container was then filtered through the functionalized nanofiber membranes using the mechanical pump. After the filtration, membranes were cleansed for 10 s in 1× PBS buffer to wash out bacterial cells that did not bind to the antibodies.
The functionalized PAN nanofibers as bioreceptors were tested in different conditions, namely dry air filtration and air filtration with additional humidification of the nanofiber membranes.
Nanofiber membranes were transferred to the liquid growth medium and incubated at 37 °C for 21 h (E. coli) or 24 h (S. aureus). After the incubation, 1 mL of homogenized bacterial suspension was transferred to the spectrophotometric cuvette. The bacterial suspensions’ optical density (wavelength 600 nm) was measured through the spectrophotometer UV-3600 (Shimadzu, Kyoto, Japan) to evaluate the number of captured bacteria. The parameters of the used spectrophotometer are shown in Table 2.

Table 2.
Spectrophotometer hardware parameters [33].
2.5. Data Analysis and Evaluation of Bioreceptor Effectivity
The bioreceptor effectivity evaluation dataset consists of 144 measurements for E. coli and 90 measurements for S. aureus. For both model organisms, three types of samples were used: functionalized nanofibers FNn for humid air filtration, FNs for dry air filtration, and unmodified nanofibers NN for humid air filtration. Using a series of samples ensured the reproducibility and repeatability of the experiments. The individual series were compared with each other, and the comparison was evaluated.
For E. coli, 24 nanofiber membranes (8 for each type) were used. A series of 15 nanofiber membranes were tested through air filtration polluted by the model organism S. aureus. After the air filtration through the membranes and membrane incubation, bacterial suspensions were created, and the optical density (OD600) was measured (spectrophotometer UV-3600, Shimadzu, Kyoto, Japan).
The optical densities dataset consists of six measured values for each nanofiber sample, ranging from OD600 of 0.164 to 1.677 for E. coli and OD600 of 0.456 to 1.132 for S. aureus. From these values, the mean and the median were calculated and then compared for each type of nanofiber membrane (FNn, FNs, and NN). In addition, the statistical significance (p = 0.05) of the obtained data was determined through the t-test.
R software with an EZR plug-in was used to analyze the data and graphically represent the results [43].
3. Results
3.1. Preparation and Characterization of PAN Nanofibers
PAN nanofibers were prepared using the roller electrospinning method (needleless electrospinning) and functionalized by immobilizing the specific antibodies. Due to the high surface-to-volume ratio, even a small part of the functionalized nanofiber obtains many antibodies. The final concentration of antibodies was determined by IR spectroscopy to be 108 ± 12 µM/g.
The structure of PAN nanofibers was characterized through SEM. Predominantly regular fibers with a mean diameter between 500 and 900 nm were observed (Figure 3).

Figure 3.
Surface-modified (left) and anti-E. coli-functionalized (right) PAN nanofibers.
From the prepared nanofibers, circle membranes with a diameter of 1.5 cm were cut. Due to the use of a 3D-printed stand for the nanofiber membranes, the real functional diameter was limited to 1 cm (the part through which the air was filtered).
The nanofiber membranes were stored in a saline buffer, so the antibody was preserved. For longer preservation, sodium azide was added to the saline buffer. Before their use as filters, nanofiber membranes were washed from chemical residues and preservatives with distilled water.
3.2. The Detection of Bacteria and Evaluation of Bioreceptor Effectiveness
The effectiveness of the bacterial interception by nanofiber membrane was evaluated through the optical density of created bacterial suspensions. The obtained results are divided according to the detected model organisms in the following subsections:
3.2.1. Detection of Escherichia coli
To detect E. coli bacteria from sufficiently humid air (an average of 60%), unmodified and anti-E. coli PAN nanofiber targets were used and compared (Figure 4). In addition, filtration under different conditions was tested. To determine the extent of the proper environment, anti-E. coli PAN nanofibers were used to detect bacteria during humid air and dry air filtration, and the bacterial interception was compared (Figure 4). The measurements were divided into eight series always consisting of the three samples (FNn, FNs, and NN).
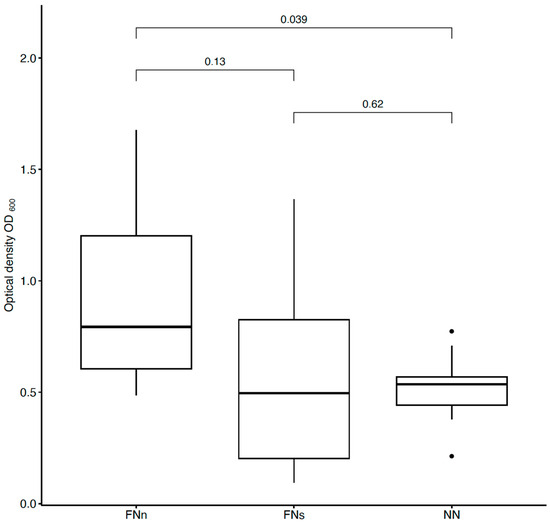
Figure 4.
Comparison of bacterial suspensions’ optical densities OD600 created from E. coli cells captured into the nanofiber structure during humid air and dry air filtration. In the figure, FNn (anti-E. coli PAN nanofibers) and NN (unmodified PAN nanofibers) show the data obtained during humid air filtration and FNs (anti-E. coli PAN nanofibers) during dry air filtration. The numbers above the boxplots show the p-values.
For better clarity, Figure 5 compares the filter effectiveness between unmodified and functionalized (FNn/NN and FNs/NN) nanofiber membranes and the two used filtration methods under different conditions (FNn/FNs).
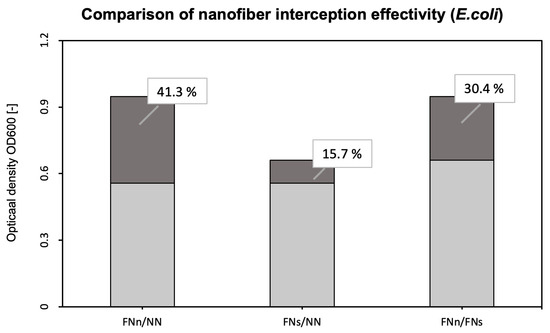
Figure 5.
Comparison of the interception effectivity for functionalized and unmodified nanofibers and two types of filtrations. The dark part and percentages show the increase in the effectivity of functionalized nanofibers (FNn and FNs) compared to unmodified nanofibers NN (FNn/NN for humid air filtration and FNs/NN for dry air filtration). The third column shows the increase in interception effectivity of functionalized nanofibers during humid air filtration (FNn) compared to dry air filtration (FNs).
3.2.2. Detection of Staphylococcus aureus
As explained previously for bacteria E. coli, two experiments were performed for Gram-positive bacteria Staphylococcus aureus. Functionalized anti-S. aureus PAN nanofibers (FNn) were compared to the unmodified ones (NN). In addition, a comparison of the bacterial interception of the functionalized nanofibers under different conditions was performed. The estimated optical densities of both experiments are shown in Figure 6. Five series consisting of the three nanofiber samples (FNn, FNs, and NN) were evaluated.
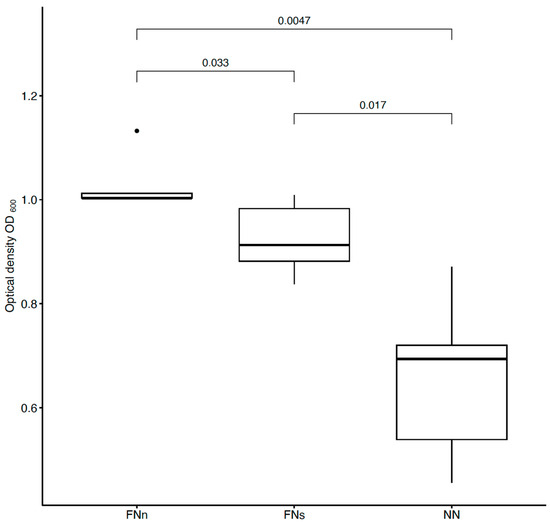
Figure 6.
Comparison of bacterial suspensions’ optical densities OD600 created from S. aureus cells captured into the nanofiber structure during humid air and dry air filtration. In the figure, FNn (anti-S. aureus PAN nanofibers) and NN (unmodified PAN nanofibers) show the data obtained during humid air filtration, and FNs (anti-S. aureus PAN nanofibers) during dry air filtration. The numbers above the boxplots show the corresponding p-values.
A more detailed comparison of the interception effectivity is shown in Figure 7.
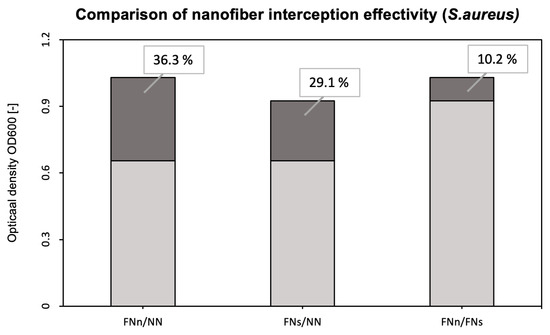
Figure 7.
Comparison of the interception effectivity for functionalized and unmodified nanofibers and two types of filtrations. FNn/NN shows the difference in the interception effectivity of the functionalized and unmodified nanofibers during humid air filtration. FNs/NN shows the same difference but during dry air filtration. The FNn/FNs column then shows the increase in interception effectivity of functionalized nanofibers during humid air filtration (FNn) compared to dry air filtration (FNs).
4. Discussion
This work presents the creation and the bacterial interception effectivity evaluation of a novel immunoreceptor based on antibody-functionalized PAN nanofibers. To detect airborne bacteria (E. coli and S. aureus) directly from the air, electrospun nanofibers with great mechanical and chemical durability were used as filtration membranes. PAN nanofibers were selected due to their exceptional filtration ability and the possibility of surface functionalization. Although electrospun PAN nanofiber membranes are capable of bacterial interception itself and with great effectivity (up to 99%) [44], antibody-functionalized nanofibers capture bacterial cells with specific biochemical bonds (antigen-antibody reaction). In the case of nanofiber bioreceptors, the mechanical interception of bacterial cells is undesirable due to the rapid clogging of the filtration membranes. In comparison with a previous study dealing with the filtration effectivity of PAN nanofibers [44], the area density of functionalized membranes for bacterial detection was reduced to 2.5 g/m2, so the mechanical interception was suppressed.
As mentioned earlier, PAN nanofibers were functionalized by immobilizing specific antibodies against E. coli and S. aureus. Bioactive molecules, such as antibodies, used as a biosensing layer of biosensors are dependent on stable and specific conditions (temperature, pH, humidity, and electrostatic repulsion). When detecting antigens directly from the air, humidity is the most challenging condition to maintain. Without additional moisturization, immobilized antibodies lose their bioactivity [45,46]. For this reason, bacterial detection, whether using conservative methods (ELISA, PCR, etc.) or (bio)sensors, is performed in liquid samples (water, body fluids, food, etc.) [26,29,30,47,48]. Airborne samples, thus, must undergo post-collection processing [31,49,50,51]. However, with the use of a previously designed air filtration system [42], the presented nanofiber bioreceptor was used and tested for the detection of bacterial cells directly from the air. This system humidifies nanofiber membranes during air filtration and protects immobilized antibodies from desiccations and, thus, inactivation (Figure 4 and Figure 6) [42].
To evaluate the bioreceptor effectiveness, bacterial interception through unmodified and functionalized nanofibers was compared. The increase in the optical density of bacterial suspensions (around 41 % for E. coli and 36 % for S. aureus, as seen in Figure 5 and Figure 7) belonging to the functionalized nanofiber membranes testing shows the effectivity of immobilized antibodies (the specific binding reaction of the bioreceptor). For both model organisms, the increase in interception effectivity due to the antibodies’ activity was found to be statistically significant at the significance level of p < 0.05. Thus, in comparison with other mentioned nanofiber biosensors for bacterial detection [26,27,28], the designed nanofiber bioreceptor combines both biosensing and filtration abilities. In further research, a combination of such a bioreceptor with a proper transducer could be a pioneering alternative for fast, sensitive, and continual environment monitoring presented in recent years [52,53,54,55].
As in other studies [56,57,58], PAN nanofibers have been proven to be membranes with extraordinary air filtration abilities. After enrichment by metal particles (TiO2, ZnO, Ag, etc.) [57] or bioactive molecules (enzymes and antibodies), PAN membranes show additional abilities, such as antibacterial [57] or biosensing activity, in relation to bacteria. Presented antibody-functionalized PAN nanofibers, thus, show great potential as a novel sensitive bioreceptor for detecting Gram-negative and Gram-positive bacteria such as E. coli and S. aureus.
5. Conclusions
Herein, we presented the preparation and use of the novel antibody-functionalized PAN nanofibers as bioreceptors for bacterial detection from the air. To detect model bacterial organisms E. coli and S. aureus, PAN nanofiber membranes were fabricated through the needleless electrospinning process and later functionalized by immobilizing corresponding antibodies. The specific structure of electrospun nanofibers enables the use of the membranes for air filtration. In addition, antibody functionalization significantly increases the bacterial interception effectivity of the membrane (on average about 40%) and facilitates the formation of special biochemical bonds with detected antigens (bacteria). In combination with the system for air filtration presented in previous work, the designed antibody-functionalized PAN nanofiber bioreceptor enables reliable, specific, and sensitive detection of Gram-negative and Gram-positive bacteria directly from the air and without inactivation and disintegration of the immobilized bioactive layer. Our finding opens the door for the development of a novel solution for continual environment monitoring. In addition, further studies will focus on combining the presented bioreceptor with a suitable electrode and the development of an ultrasensitive biosensor for bacterial detection.
Author Contributions
Methodology, L.V., B.S.; validation and data analysis, L.V. and B.S.; writing—original draft preparation, L.V.; writing—review and editing, L.V., B.S., P.K. and T.J.; visualization, L.V.; supervision, P.K. and T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Student Grant Competition of CTU (SGS22/199/OHK4/3T/17) provided by Czech Technical University in Prague, Czech Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request.
Acknowledgments
We thank the Department of Natural Sciences, FBME, CTU in Prague, and UCEEB, CTU in Prague, for providing the laboratories for our experiments. We would also like to acknowledge the doc. Dana Gášková and Tomáš Bartl from the Faculty of Mathematics and Physics, Charles University, and Evžen Amler from the Second Faculty of Medicine, Charles University, for the advice and all help on the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and their widespread impact on human health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Kim, E.R.; Joe, C.; Mitchell, R.J.; Gu, M.B. Biosensors for healthcare: Current and future perspectives. Trends Biotechnol. 2023, 41, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Murzin, D.; Mapps, D.J.; Levada, K.; Belyaev, V.; Omelyanchik, A.; Panina, L.; Rodionova, V. Ultrasensitive Magnetic Field Sensors for Biomedical Applications. Sensors 2020, 20, 1569. [Google Scholar] [CrossRef] [PubMed]
- Fiedorova, K.; Augustynek, M.; Kubicek, J.; Kudrna, P.; Bibbo, D. Review of present method of glucose from human blood and body fluids assessment. Biosens. Bioelectron. 2022, 211, 114348. [Google Scholar] [CrossRef]
- Yoon, J.-Y. Introduction to Biosensors: From Electric Circuits to Immunosensors, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-1-4419-6021-4. [Google Scholar]
- Yanagihara, K.; Kitagawa, Y.; Tomonaga, M.; Tsukasaki, K.; Kohno, S.; Seki, M.; Sugimoto, H.; Shimazu, T.; Tasaki, O.; Matsushima, A.; et al. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit. Care 2010, 14, 159. [Google Scholar] [CrossRef]
- Wolk, D.; Mitchell, S.; Patel, R. Principles Of Molecular Microbiology Testing Methods. Infect. Dis. Clin. N. Am. 2001, 15, 1157–1204. [Google Scholar] [CrossRef]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. R. Soc. Chem. 2017, 46, 4818–4832. [Google Scholar] [CrossRef] [PubMed]
- Alahi, M.E.; Mukhopadhyay, S.C. Detection Methodologies for Pathogen and Toxins: A Review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef]
- Myatt, C.J.; Delaney, M.; Todorof, K.; Heil, J. Low-Cost, Multiplexed Biosensor for Disease Diagnosis. In Proceedings of the SPIE Proceedings, Frontiers in Pathogen Detection: From Nanosensors to Systems, San Jose, CA, USA, 24–29 January 2009; Volume 1767, p. 716703. [Google Scholar] [CrossRef]
- Malhotra, S.; Pham, D.S.; Lau, M.P.H.; Nguyen, A.H.; Cao, H. A Low-Cost, 3D-Printed Biosensor for Rapid Detection of Escherichia coli. Sensors 2022, 22, 2382. [Google Scholar] [CrossRef]
- Fernando, L.M. Nanobiosensors for Detection of Pathogens. In Proceedings of the 16th Engineering Research and Development for Technology Conference, Pasay, Philippines, 25 October 2019. [Google Scholar]
- Song, M.; Yang, M.; Hao, J. Pathogenic Virus Detection by Optical Nanobiosensors. Cell Rep. Phys. Sci. 2021, 2, 100288. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Fang, F.; Li, L.; Yan, Z.; Zhang, L.; Sun, Q. Highly Sensitive and Miniature Microfiber-Based Ultrasound Sensor for Photoacoustic Tomography. Opto-Electron. Adv. 2022, 5, 200076. [Google Scholar] [CrossRef]
- Yu, W.; Yao, N.; Pan, J.; Fang, W.; Li, X.; Tong, L.; Zhang, L. Highly Sensitive and Fast Response Strain Sensor Based on Evanescently Coupled Micro/Nanofibers. Opto-Electron. Adv. 2022, 5, 210101. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Gorab, M.G.; Hashemi, S.M.; Javanshir, S.; Cohan, R.A.; et al. Applications of Carbon-Based Conductive Nanomaterials in Biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Štukovnik, Z.; Fuchs-Godec, R.; Bren, U. Nanomaterials and Their Recent Applications in Impedimetric Biosensing. Biosensors 2023, 13, 899. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Pires, N.M.M.; Yang, Z.; Jiang, Z. Advances in Electrochemical Biosensor Based on Nanomaterials for Protein Biomarker Detection in Saliva. Adv. Sci. 2023, 10, 2205429. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, E. Nanofibers: Production, Characterization, and Tissue Engineering Applications. In 21st Century Nanostructured Materials—Physics, Chemistry, Classification, and Emerging Application in Industry, Biomedicine, and Agriculture; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Chakrapani, G.; Ramakrishna, S.; Zare, M. Functionalization of electrospun nanofiber for biomedical application. J. Appl. Polym. Sci. 2023, 140, e53906. [Google Scholar] [CrossRef]
- Pashchenko, A.; Stuchlíková, S.; Varvařovská, L.; Firment, P.; Staňková, L.; Nečasová, A.; Filipejová, Z.; Urbanová, L.; Jarošíková, T.; Nečas, A.; et al. Smart Nanofibres For Specific And Ultrasensitive Nanobiosensors And Drug Delivery System. Acta Vet. Brno 2022, 91, 163–170. [Google Scholar] [CrossRef]
- Kulkarni, D.; Musale, S.; Panzade, P.; Paiva-Santos, A.C.; Sonwane, P.; Madibone, M.; Choundhe, P.; Giram, P.; Cavalu, S. Surface Functionalization of Nanofibers: The Multifaceted Approach for Advanced Biomedical Applications. Nanomaterials 2022, 12, 3899. [Google Scholar] [CrossRef]
- Gokce, Z.G.; Akalin, P.; Kok, F.N.; Sarac, A.S. Impedimetric DNA Biosensor Based On Polyurethane/Poly(M-Anthranilic Acid) Nanofibers. Sens. Actuators B Chem. 2018, 254, 719–726. [Google Scholar] [CrossRef]
- Sarabaegi, M.; Roushani, M.; Hosseini, H. Hollow Carbon Nanocapsules-Based Nitrogen-Doped Carbon Nanofibers With Rosary-Like Structure As A High Surface Substrate For Impedimetric Detection Of Pseudomonas Aeruginosa. Talanta 2021, 223, 121700. [Google Scholar] [CrossRef]
- Sarabaegi, M.; Roushani, M.; Hosseini, H.; Saedi, Z.; Lemraski, E.G. A Novel Ultrasensitive Biosensor Based On Nico-Mof Nanostructure And Confined To Flexible Carbon Nanofibers With High-Surface Skeleton To Rapidly Detect Helicobacter Pylori. Mater. Sci. Semicond. Process. 2021, 139, 106351. [Google Scholar] [CrossRef]
- Ghasemi, R.; Mirahmadi-Zare, S.Z.; Allafchian, A.; Behmanesh, M. Fast fluorescent screening assay and dual electrochemical sensing of bacterial infection agent (streptococcus agalactiae) based on fluorescent-immune nanofibers. Sens. Actuators B. Chem. 2022, 352, 130968. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Yoon Lee, N. Recent advances in airborne pathogen detection using optical and electrochemical biosensors. Anal. Chim. Acta 2022, 1234, 340297. [Google Scholar] [CrossRef]
- Al-Taie, A.; Han, X.; Williams, C.M.; Abdulwhhab, M.; Abbott, A.P.; Goddard, A.; Wegrzyn, M.; Garton, N.J.; Barer, M.R.; Pan, J. 3-D printed polyvinyl alcohol matrix for detection of airborne pathogens in respiratory bacterial infections. Microbiol. Res. 2020, 241, 126587. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Bhardwaj, N.; Kumar, V.; Bhatt, D.; Azzouz, A.; Bhaumik, J.; Kim, K.-H.; Deep, A. Recent progress in nanomaterial-based sensing of airborne viral and bacterial pathogens. Environ. Int. 2021, 146, 106183. [Google Scholar] [CrossRef]
- Triadó-Margarit, X.; Cáliz, J.; Casamayor, E.O. A long-term atmospheric baseline for intercontinental exchange of airborne pathogens. Environ. Int. 2022, 158, 106916. [Google Scholar] [CrossRef] [PubMed]
- Varvařovská, L.; Kudrna, P.; Jarošíková, T. The development of a specific nanofiber bioreceptor for bacterial detection. In Advances in Digital Health and Medical Bioengineering, Proceedings of the 11th International Conference on E-Health and Bioengineering (EHB-2023), Bucharest, Romania, 9–10 November 2023; Springer Nature Publishing AG: Cham, Switzerland, 2024. [Google Scholar]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Hackensack, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Lim, C.T. Nanofiber Technology: Current Status and Emerging Developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Pereira, T.S.; Migliorini, F.L.; dos Santos, D.M.; Correa, D.S. Nanofibers interfaces for biosensing: Design and applications. Sens. Actuators Rep. 2021, 3, 100048. [Google Scholar] [CrossRef]
- Lasenko, I.; Grauda, D.; Butkauskas, D.; Sanchaniya, J.V.; Viluma-Gudmona, A.; Lusis, V. Testing the physical and mechanical properties of polyacrylonitrile nanofibers reinforced with succinite and silicon dioxide nanoparticles. Textiles 2022, 2, 162–173. [Google Scholar] [CrossRef]
- Sanchaniya, J.V.; Kanukuntla, K. Morphology and mechanical properties of PAN nanofiber Mat. J. Phys. Conf. Ser. 2022, 2423, 012018. [Google Scholar] [CrossRef]
- Senthil, R.; Sumathi, V.; Tamilselvi, A.; Kavukcu, S.B.; Aruni, A.W. Functionalized electrospun nanofibers for high efficiency removal of particulate matter. Sci. Rep. 2022, 12, 8411. [Google Scholar] [CrossRef] [PubMed]
- Haris, P.I. Infrared Spectroscopy of Protein Structure. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-16712-6. [Google Scholar]
- Tatulian, S.A. FTIR Analysis of Proteins and Protein-Membrane Interactions. Methods Mol. Biol. 2019, 2003, 281–325. [Google Scholar] [CrossRef]
- Varvařovská, L.; Sopko, B.; Gášková, D.; Bartl, T.; Amler, E.; Jarošíková, T. Surface-Functionalized PAN Nanofiber Membranes for the Sensitive Detection of Airborne Specific Markers. PeerJ 2024. accepted. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Varvařovská, L.; Sopko, B.; Divín, R.; Pashschenko, A.; Fedačko, J.; Sabo, J.; Nečas, A.; Amler, E.; Jarošíková, T. Bacteria trapping effectivity on nanofibre membrane in liquids is exponentially dependent on the surface density. Acta Vet. Brno 2023, 92, 435–441. [Google Scholar] [CrossRef]
- Wang, J.; Yiu, B.; Obermeyer, J.; Filipe, C.D.M.; Brennan, J.D.; Pelton, R. Effects of Temperature and Relative Humidity on the Stability of Paper-Immobilized Antibodies. Biomacromolecules 2012, 13, 559–564. [Google Scholar] [CrossRef]
- Slocik, J.M.; Dennis, P.B.; Kuang, Z.; Pelton, A.; Naik, R.R. Creation of stable water-free antibody based protein liquids. Commun. Mater. 2021, 2, 118. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Ventura, B.D.; Cennamo, M.; Minopoli, A.; Campanile, R.; Censi, S.B.; Terracciano, D.; Portella, G.; Velotta, R. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sens. 2020, 5, 3043–3048. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Bianco, A.; Kalantar-Zadeh, K. Two-dimensional material-based biosensors for virus detection. ACS Sens. 2020, 5, 3739–3769. [Google Scholar] [CrossRef]
- Prieto-Simón, B.; Bandaru, N.M.; Saint, C.; Voelcker, N.H. Tailored carbon nanotube immunosensors for the detection of microbial contamination. Biosens. Bioelectron. 2015, 67, 642–648. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Yao, Y.; Jing, W.; Liu, S.; Sui, G. A novel microfluidic module for rapid detection of airborne waterborne pathogens. Sens. Actuators B Chem. 2018, 258, 1138–1145. [Google Scholar] [CrossRef]
- Eltzov, E.; Pavluchov, V.; Burstin, M.; Marks, R.S. Creation of fiber optic based biosensor for air toxicity monitoring. Sens. Actuators B Chem. 2011, 155, 859–867. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, S.J.; Park, C.S.; Le, T.-H.; Lee, S.H.; Ha, T.H.; Kim, H.-I.; Kim, J.; Lee, C.-S.; Yoon, H.; et al. Surface-modified polymer nanofiber membrane for high-efficiency microdust capturing. Chem. Eng. J. 2018, 339, 204–213. [Google Scholar] [CrossRef]
- Shuvo, S.N.; Gomez, A.M.U.; Mishra, A.; Chen, W.Y.; Dongare, A.M.; Stanciu, L.A. Sulfur-doped titanium carbide MXenes for room-temperature gas sensing. ACS Sens. 2020, 5, 2915–2924. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, T.; Cui, J.; Samal, S.K.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building block for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 119623. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun nanofiber membranes for effective air filtration. Macromol. Mater. Eng. 2016, 302, 1600353. [Google Scholar] [CrossRef]
- Bortolassi, A.C.C.; Guerra, V.G.; Aguiar, M.L.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Composites Based on Nanoparticle and Pan Electrospun Nanofiber Membranes for Air Filtration and Bacterial Removal. Nanomaterials 2019, 9, 1740. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).