Enzyme Immobilization by Inkjet Printing on Reagentless Biosensors for Electrochemical Phosphate Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Functionalization of the MWCNTs
2.3. Ink Formulation
2.4. Settings of Printing Parameters
2.5. Voltammetric Experiments Using the Proposed Biosensor
3. Results and Discussion
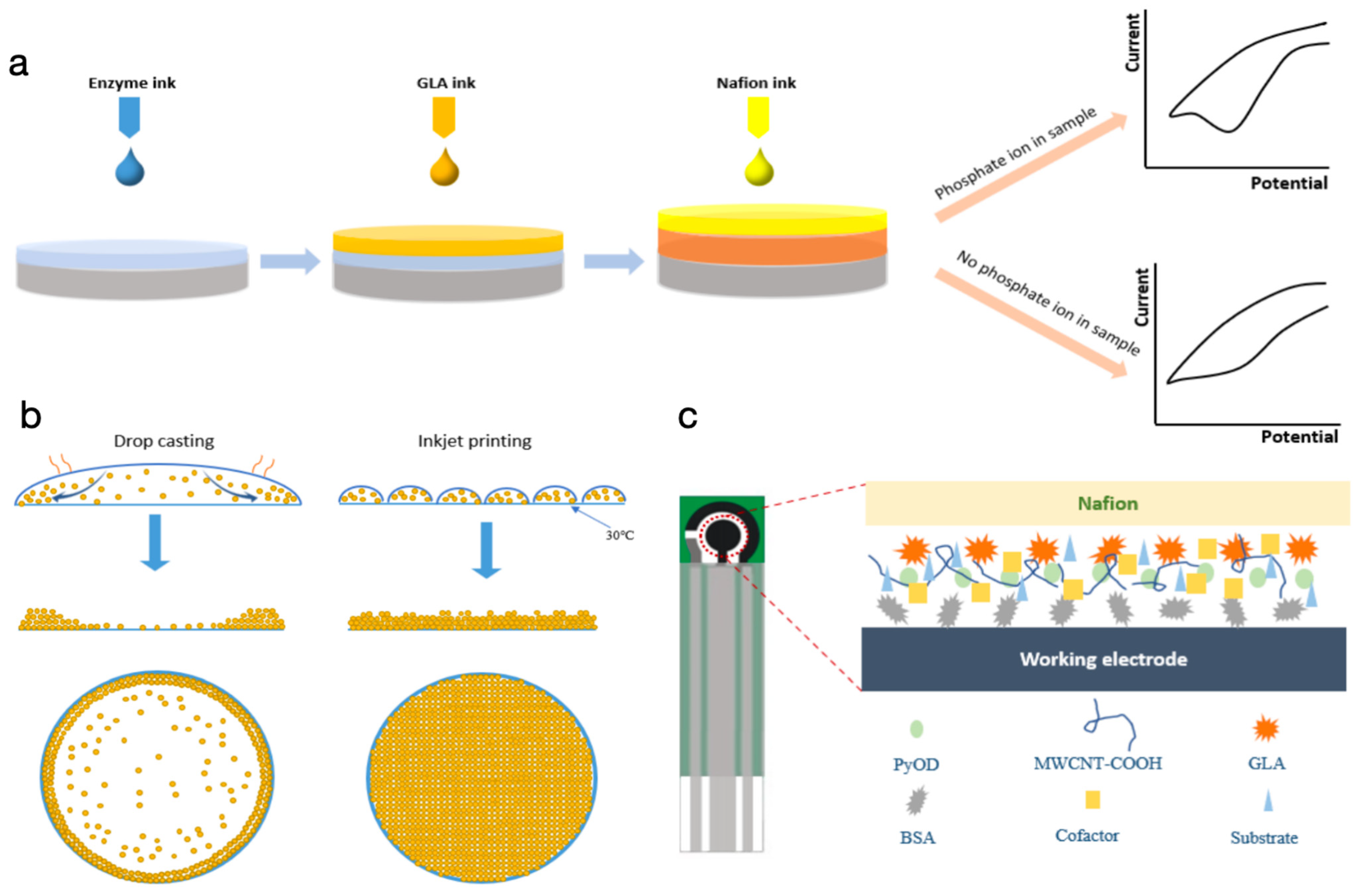
3.1. Mechanism for the Biosensor through the Proposed Printing Strategy
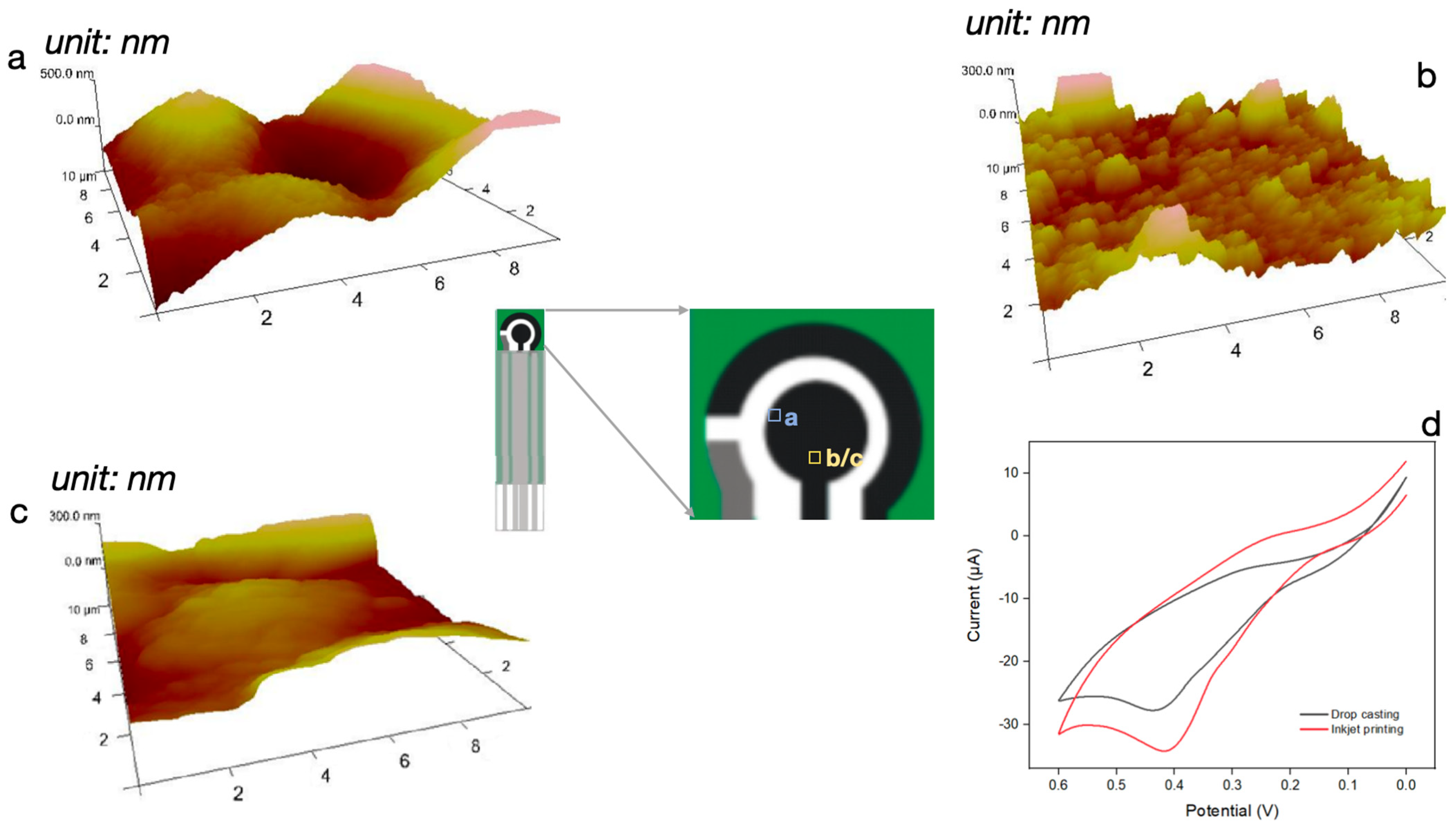
3.2. Comparison of Surface Functionalization Performance between Drop-Casting and Inkjet-Printing Methods
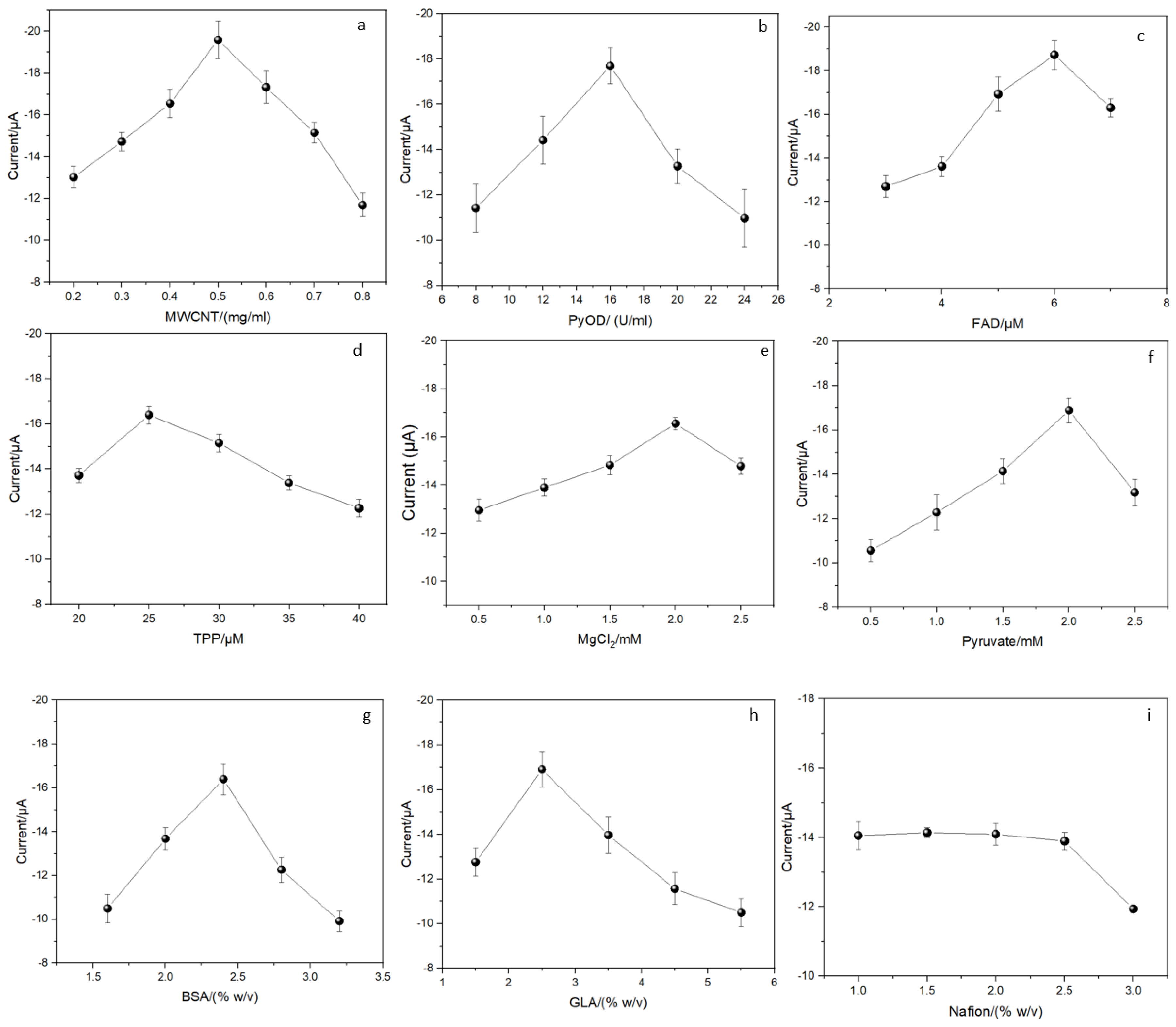
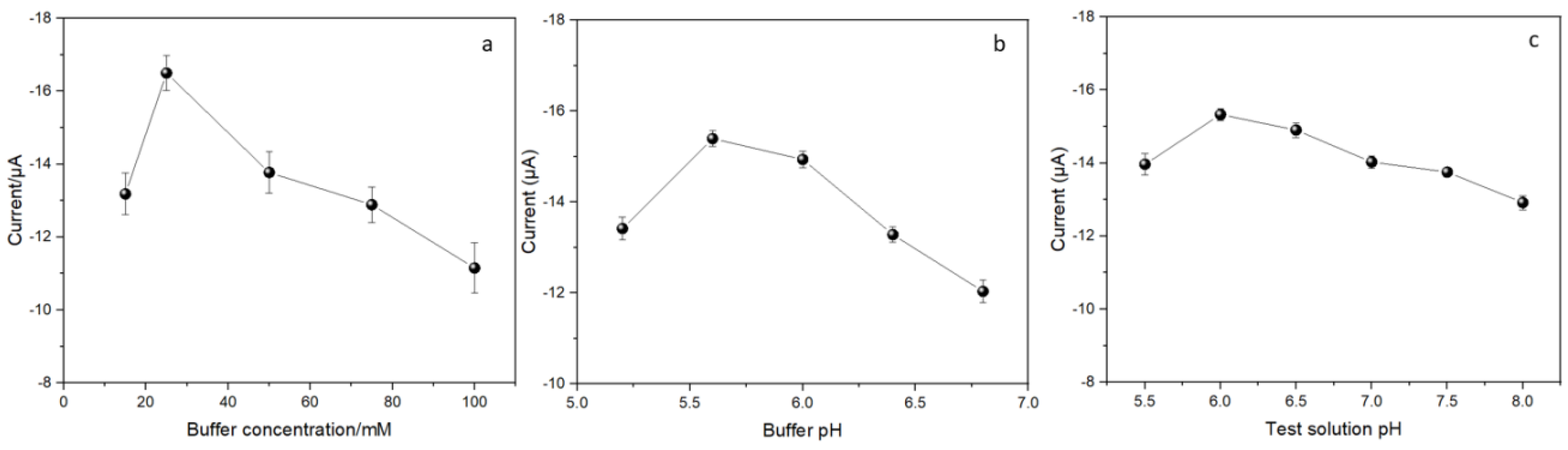
3.3. Optimization of the Experimental Conditions
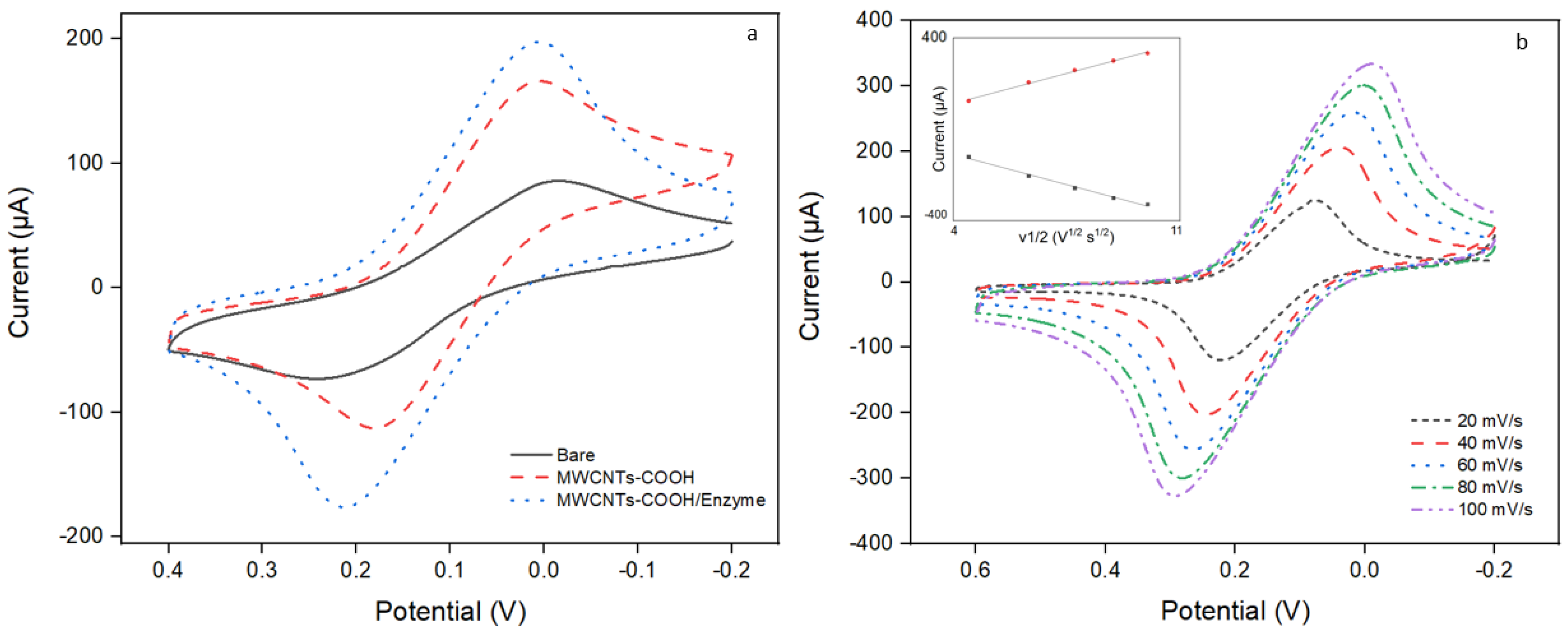
3.4. Evaluation of the Functionalized Working Electrode
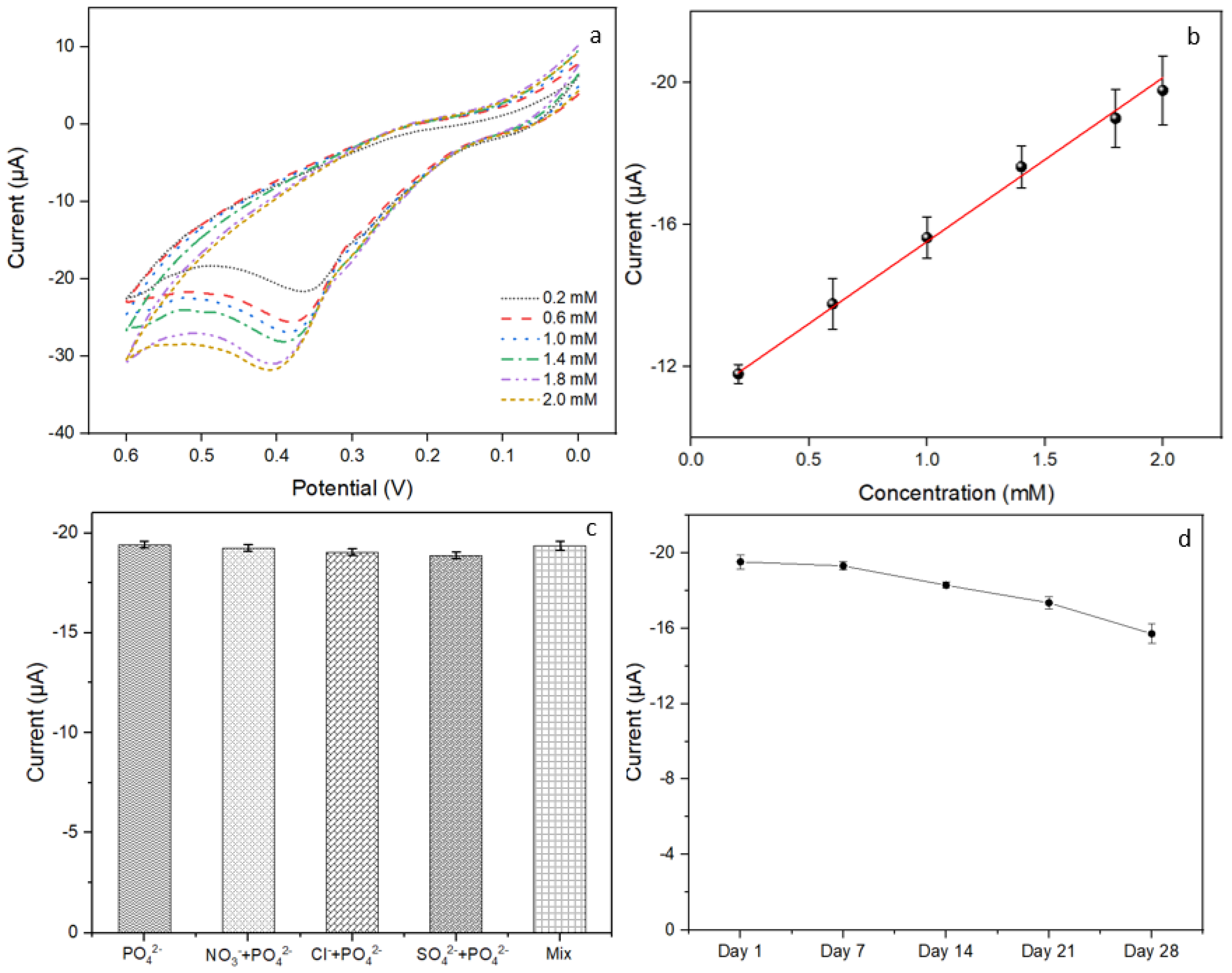
3.5. Evaluation of the Constructed Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Bollella, P. Enzyme-based amperometric biosensors: 60 years later… Quo Vadis? Anal. Chim. Acta 2022, 1234, 340517. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Podsiadły, B.; Wałpuski, B.; Jakubowska, M. Common configurations and challenges in screen-printed enzymatic electrochemical biosensors. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High-Energy Physics Experiments, Wilga, Poland, 3–10 June 2018; SPIE: Bellingham, WA, USA, 2018; p. 7. [Google Scholar]
- Lakhera, P.; Chaudhary, V.; Jha, A.; Singh, R.; Kush, P.; Kumar, P. Recent developments and fabrication of the different electrochemical biosensors based on modified screen printed and glassy carbon electrodes for the early diagnosis of diverse breast cancer biomarkers. Mater. Today Chem. 2022, 26, 101129. [Google Scholar] [CrossRef]
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M. Fundamentals, applications, and future directions of bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jiang, J.; Wu, W. Scalably Nanomanufactured Atomically Thin Materials-Based Wearable Health Sensors. Small Struct. 2022, 3, 2100120. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, A.; Zhao, Z.; Li, M.; Song, Y. Heterogeneous wettability surfaces: Principle, construction, and applications. Small Struct. 2020, 1, 2000028. [Google Scholar] [CrossRef]
- Kaliyaraj Selva Kumar, A.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Zub, K.; Hoeppener, S.; Schubert, U.S. Inkjet printing and 3D printing strategies for biosensing, analytical, and diagnostic applications. Adv. Mater. 2022, 34, 2105015. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhang, L.; Xu, J.; Xiao, X.; Zhang, X. Inkjet-printed flexible sensors: From function materials, manufacture process, and applications perspective. Mater. Today Commun. 2022, 31, 103263. [Google Scholar] [CrossRef]
- Isailović, J.; Vidović, K.; Hočevar, S.B. Simple electrochemical sensors for highly sensitive detection of gaseous hydrogen peroxide using polyacrylic-acid-based sensing membrane. Sens. Actuators B Chem. 2022, 352, 131053. [Google Scholar] [CrossRef]
- Pradela-Filho, L.A.; Gongoni, J.L.; Arantes, I.V.; De Farias, D.M.; Paixão, T.R. Controlling the inkjet printing process for electrochemical (bio) sensors. Adv. Mater. Technol. 2023, 8, 2201729. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Bhuiyan, S.R.A.; Zhu, Y.; Yao, S. Printed Strain Sensors for On-Skin Electronics. Small Struct. 2022, 3, 2100131. [Google Scholar] [CrossRef]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of paper-based devices for reagentless electrochemical (bio) sensor strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guo, Q.; Xiao, J.; Zheng, M.; Zhang, D.; Yang, J. An inkjet-printed smartphone-supported electrochemical biosensor system for reagentless point-of-care analyte detection. Sens. Actuators B Chem. 2021, 346, 130447. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, D.; Guo, Q.; Xiao, J.; Zheng, M.; Yang, J. Study of the Enzyme Activity Change due to Inkjet Printing for Biosensor Fabrication. ACS Biomater. Sci. Eng. 2021, 7, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Bioprinting: Inkjet printing proteins and hybrid cell-containing materials and structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]
- Aghababaie, M.; Foroushani, E.S.; Changani, Z.; Gounani, Z.; Mobarakeh, M.S.; Hadady, H.; Khedri, M.; Maleki, R.; Asadnia, M.; Razmjou, A. Recent Advances In the development of enzymatic paper-based microfluidic biosensors. Biosens. Bioelectron. 2023, 226, 115131. [Google Scholar] [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. npj Flex. Electron. 2018, 2, 30. [Google Scholar] [CrossRef]
- Demuru, S.; Huang, C.-H.; Parvez, K.; Worsley, R.; Mattana, G.; Piro, B.; Noël, V.; Casiraghi, C.; Briand, D. All-inkjet-printed graphene-gated organic electrochemical transistors on polymeric foil as highly sensitive enzymatic biosensors. ACS Appl. Nano Mater. 2022, 5, 1664–1673. [Google Scholar] [CrossRef]
- Basiaga, M.; Paszenda, Z.; Walke, W.; Karasiński, P.; Marciniak, J. Electrochemical Impedance Spectroscopy and corrosion resistance of SiO2 coated cpTi and Ti-6Al-7Nb alloy. In Information Technologies in Biomedicine; Springer: Berlin/Heidelberg, Germany, 2014; Volume 4, pp. 411–420. [Google Scholar]
- Chiang, Y.-C.; Lin, W.-H.; Chang, Y.-C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Noordadi, M.; Mehrnejad, F.; Sajedi, R.H.; Jafari, M.; Ranjbar, B. The potential impact of carboxylic-functionalized multi-walled carbon nanotubes on trypsin: A Comprehensive spectroscopic and molecular dynamics simulation study. PLoS ONE 2018, 13, e0198519. [Google Scholar] [CrossRef] [PubMed]
- Soltman, D.; Subramanian, V. Inkjet-Printed Line Morphologies and Temperature Control of the Coffee Ring Effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Durgan, C.J.; Matthews, D.J.; Motley, J.R.; Tan, X.; Pholsena, K.; Árnadóttir, L.; Castle, J.R.; Jacobs, P.G.; Cargill, R.S. Fabrication of a flexible amperometric glucose sensor using additive processes. ECS J. Solid State Sci. Technol. JSS 2015, 4, P3069. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Buesen, D.; Williams, R.; Henig, J.; Stapf, S.; Mukherjee, K.; Freier, E.; Lubitz, W.; Winkler, M.; Happe, T.; et al. Preventing the coffee-ring effect and aggregate sedimentation by in situ gelation of monodisperse materials. Chem. Sci. 2018, 9, 7596–7605. [Google Scholar] [CrossRef]
- Shen, X.; Ho, C.-M.; Wong, T.-S. Minimal Size of Coffee Ring Structure. J. Phys. Chem. B 2010, 114, 5269–5274. [Google Scholar] [CrossRef]
- Mampallil, D.; Eral, H.B. A review on suppression and utilization of the coffee-ring effect. Adv. Colloid Interface Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef]
- He, B.; Liu, H. Electrochemical biosensor based on pyruvate oxidase immobilized AuNRs@ Cu2O-NDs as electroactive probes loaded poly (diallyldimethylammonium chloride) functionalized graphene for the detection of phosphate. Sens. Actuators B Chem. 2020, 304, 127303. [Google Scholar] [CrossRef]
- de Rooij, M.R. Electrochemical methods: Fundamentals and applications. Anti Corros. Methods Mater. 2003, 50, 5. [Google Scholar] [CrossRef]
- Yao, L.; Teng, J.; Zhu, M.; Zheng, L.; Zhong, Y.; Liu, G.; Xue, F.; Chen, W. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens. Bioelectron. 2016, 85, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Li, Y.; Chen, Y.-Q.; Zhang, X.-B.; Hu, G.-Q. Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes. Biosens. Bioelectron. 2005, 21, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Ogabiela, E.; Adeloju, S.B.; Cui, J.; Wu, Y.; Chen, W. A novel ultrasensitive phosphate amperometric nanobiosensor based on the integration of pyruvate oxidase with highly ordered gold nanowires array. Biosens. Bioelectron. 2015, 71, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ogabiela, E.E.; Hui, J.; Wang, Y.; Zhang, Y.; Tong, L.; Zhang, J.; Adeloju, S.B.; Zhang, X.; Wu, Y. Electrochemical Biosensor based on Pt/Au Alloy Nanowire Arrays for Phosphate Detection. J. Electrochem. Soc. 2015, 162, B62–B67. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Lawal, A.T. Fabrication of a bilayer potentiometric phosphate biosensor by cross-link immobilization with bovine serum albumin and glutaraldehyde. Anal. Chim. Acta 2011, 691, 89–94. [Google Scholar] [CrossRef]
- Gilbert, L.; Jenkins, A.T.A.; Browning, S.; Hart, J.P. Development of an amperometric, screen-printed, single-enzyme phosphate ion biosensor and its application to the analysis of biomedical and environmental samples. Sens. Actuators B Chem. 2011, 160, 1322–1327. [Google Scholar] [CrossRef]
- Lawal, A.T.; Adeloju, S.B. Polypyrrole based amperometric and potentiometric phosphate biosensors: A comparative study B. Biosens. Bioelectron. 2013, 40, 377–384. [Google Scholar] [CrossRef]
- Luo, X.-L.; Xu, J.-J.; Du, Y.; Chen, H.-Y. A glucose biosensor based on chitosan–glucose oxidase–gold nanoparticles biocomposite formed by one-step electrodeposition. Anal. Biochem. 2004, 334, 284–289. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon nanotube screen-printed electrochemical sensors. Analyst 2004, 129, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ludwig, R. Direct electron transfer of enzymes facilitated by cytochromes. ChemElectroChem 2019, 6, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Park, D.-S.; Chang, S.-C.; McNeil, C.J.; Shim, Y.-B. The biosensor based on the pyruvate oxidase modified conducting polymer for phosphate ions determinations. Biosens. Bioelectron. 2006, 21, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Suen, H.-M.E.; Pasvol, G.; Cunnington, A.J. Clinical and laboratory features associated with serum phosphate concentrations in malaria and other febrile illnesses. Malar. J. 2020, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Han, J.; Jang, A.; Bishop, P.L.; Ahn, C.H. A disposable on-chip phosphate sensor with planar cobalt microelectrodes on polymer substrate. Biosens. Bioelectron. 2007, 22, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Talarico, D.; Palleschi, G.; Moscone, D.; Arduini, F. Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate. Anal. Chim. Acta 2016, 919, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Talarico, D.; Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Screen-printed electrode modified with carbon black nanoparticles for phosphate detection by measuring the electroactive phosphomolybdate complex. Talanta 2015, 141, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.C.; Leung, H.F.; Hon, P.Y.; Barford, J.P.; Renneberg, R. A screen-printed biosensor using pyruvate oxidase for rapid determination of phosphate in synthetic wastewater. Appl. Microbiol. Biotechnol. 2005, 66, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.J.; López, J.R.; Correig, X.; Katakis, I. Reagentless carbon paste phosphate biosensors: Preliminary studies. Sens. Actuators B Chem. 1998, 47, 13–20. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B.; Verma, N. Recent advances in phosphate biosensors. Biotechnol. Lett. 2015, 37, 1335–1345. [Google Scholar] [CrossRef]
- Norouzi, P.; Pirali-Hamedani, M.; Faridbod, F.; Ganjali, M.R. Flow Injection Phosphate Biosensor Based on PyOx-MWCNTs Film on a Glassy Carbon Electrode Using FFT Continuous Cyclic Voltammetry. Int. J. Electrochem. Sci. 2010, 5, 1225–1235. [Google Scholar] [CrossRef]
- Gavalas, V.G.; Chaniotakis, N.A. Phosphate biosensor based on polyelectrolyte-stabilized pyruvate oxidase. Anal. Chim. Acta 2001, 427, 271–277. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Zhu, H.; Liu, H.; Chen, Y.; Yang, M.J.C. Dispersing multi-walled carbon nanotubes with water–soluble block copolymers and their use as supports for metal nanoparticles. Carbon 2007, 45, 285–292. [Google Scholar] [CrossRef]
- NB, R.K.; Crasta, V.; Praveen, B.; Kumar, M.J.N.R. Studies on structural, optical and mechanical properties of MWCNTs and ZnO nanoparticles doped PVA nanocomposites. Nanotechnol. Rev. 2015, 4, 457–467. [Google Scholar]
- Vuković, G.D.; Marinković, A.D.; Čolić, M.; Ristić, M.Đ.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 2010, 157, 238–248. [Google Scholar] [CrossRef]
| Electrode | Composition | Linear Range | Reagents Addition | Manufacturing Method | Ref |
|---|---|---|---|---|---|
| Co microelectrodes | e-beam evaporator of Au and Cobalt | 0.01–10 mM | Potassium hydrogen phthalate | Photolithography | [47] |
| GLA/PyOD/CoPC/SPE | PyOD cross-linked with GLA | 2.5–130 µM | MOPS, pyruvic acid, MgSO4, NaCl | Drop cast | [40] |
| CB/SPE | CB | 0.5–100 µM | Molybdate, H2SO4, KCL | Drop cast | [49] |
| CB/SPE | CB and reaction substrate | 10–300 µM | None | Drop cast | [48] |
| PyOD/SPE | Gel-entrapment of PyOD | 7.5–625 µM | Pyruvic acid, FAD, TPP, MgCl2, citrate buffer | Drop cast | [50] |
| PyOD/MWCNT/GCE | Gel-entrapment of PyOD and MWCNT | 1–100 µM | Sodium pyruvate, TPP, FAD, Mg2+ | Drop cast | [53] |
| Polyelectrolyte/ PyOD/CE | Physical adsorption of polyelectrolyte and PyOD on the carbon electrode | 0.05–1.25 mM | Pyruvate and HEPES buffer | Drop cast | [54] |
| Multienzyme/ carbon paste | Three kinds of enzymes and carbon paste electrode | 2–250 mM | NAD+, MgCl2, Os(l,10-phenanthroline-5,6-dione)2Cl2), Tris buffer | Drop cast | [51] |
| AuNWs/cofactor/BSA/PyOD/GLA | Crosslinking of cofactors and PyOD by BSA and GLA | 12.5–1000 µM | Pyruvic acid, MgCl2, citrate buffer | Drop cast | [37] |
| Pt/Au/cofactor/BSA/PyOD/GLA | Crosslinking of cofactors and PyOD by BSA and GLA | 0.248–1.456 mM | Sodium pyruvate, MgCl2, NaOH, citrate buffer | Electrodeposition Drop cast | [38] |
| MWCNT-COOH/ cofactor/substrate/BSA/PyOD/GLA/SPE | Crosslinking of MWCNTs, cofactors, substrate and PyOD by BSA and GLA | 0.2–2 mM | None | Inkjet printing | This work |
| Phosphate Concentration in Sample/mM | Founded Phosphate/mM | Recovery/% | RSD/% |
|---|---|---|---|
| 1.50 | 1.49 | 99.1 | 1.7 |
| 1.25 | 1.24 | 98.9 | 3.3 |
| 1.00 | 1.03 | 103 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Bai, Y.; Niu, H.; Chen, L.; Xiao, J.; Guo, Q.; Jia, P. Enzyme Immobilization by Inkjet Printing on Reagentless Biosensors for Electrochemical Phosphate Detection. Biosensors 2024, 14, 168. https://doi.org/10.3390/bios14040168
Zhang D, Bai Y, Niu H, Chen L, Xiao J, Guo Q, Jia P. Enzyme Immobilization by Inkjet Printing on Reagentless Biosensors for Electrochemical Phosphate Detection. Biosensors. 2024; 14(4):168. https://doi.org/10.3390/bios14040168
Chicago/Turabian StyleZhang, Dongxing, Yang Bai, Haoran Niu, Lingyun Chen, Junfeng Xiao, Qiuquan Guo, and Peipei Jia. 2024. "Enzyme Immobilization by Inkjet Printing on Reagentless Biosensors for Electrochemical Phosphate Detection" Biosensors 14, no. 4: 168. https://doi.org/10.3390/bios14040168
APA StyleZhang, D., Bai, Y., Niu, H., Chen, L., Xiao, J., Guo, Q., & Jia, P. (2024). Enzyme Immobilization by Inkjet Printing on Reagentless Biosensors for Electrochemical Phosphate Detection. Biosensors, 14(4), 168. https://doi.org/10.3390/bios14040168






