A Multienzyme Reaction-Mediated Electrochemical Biosensor for Sensitive Detection of Organophosphorus Pesticides
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Preparation of Fe3O4@Au–Pt
2.3. Preparation of GN–Au NPs
2.4. Colorimetric Assay
2.5. Detection of ETH Using the Double-Enzyme Reaction System
2.6. Detection of ETH in Real Samples
3. Results and Discussion
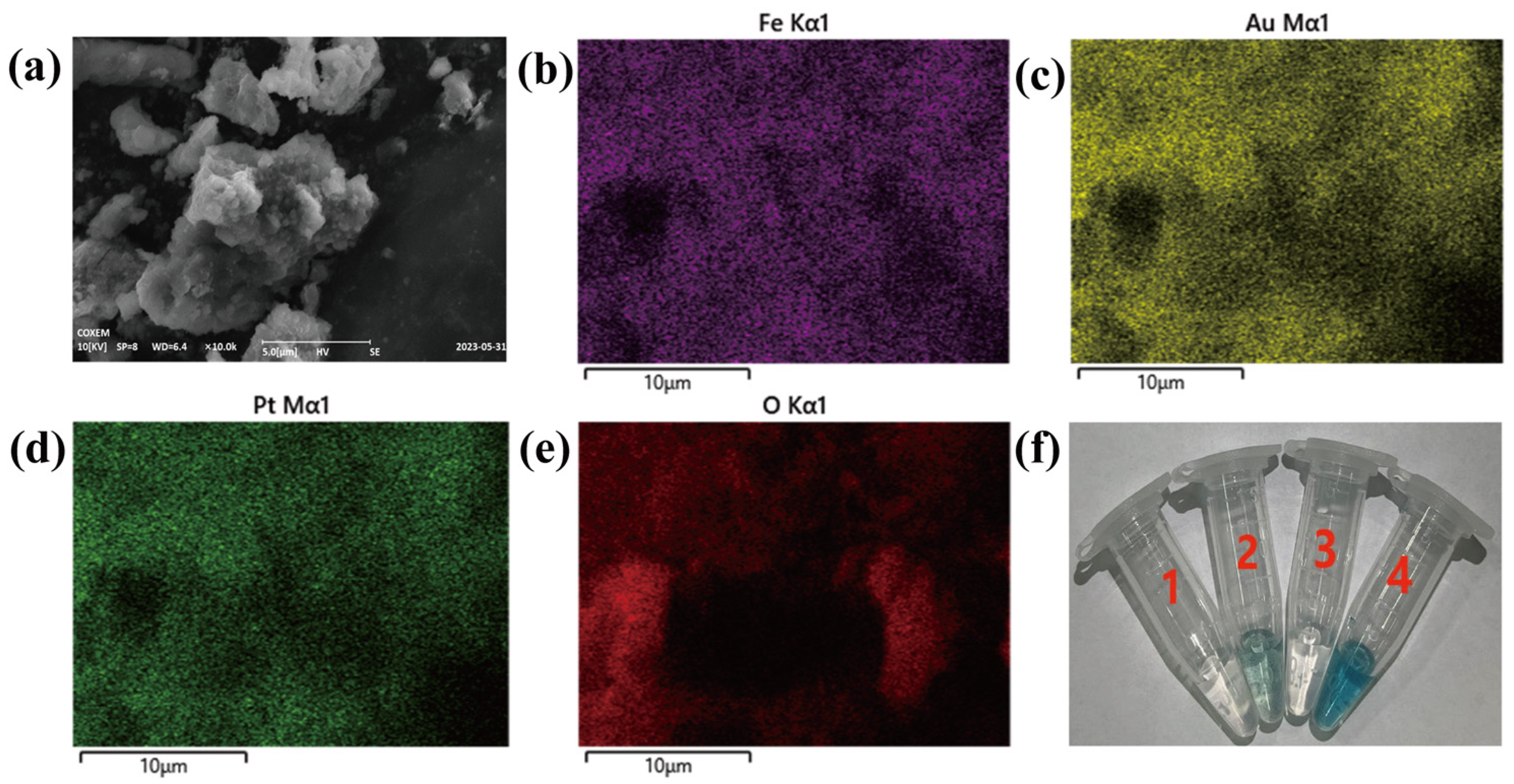
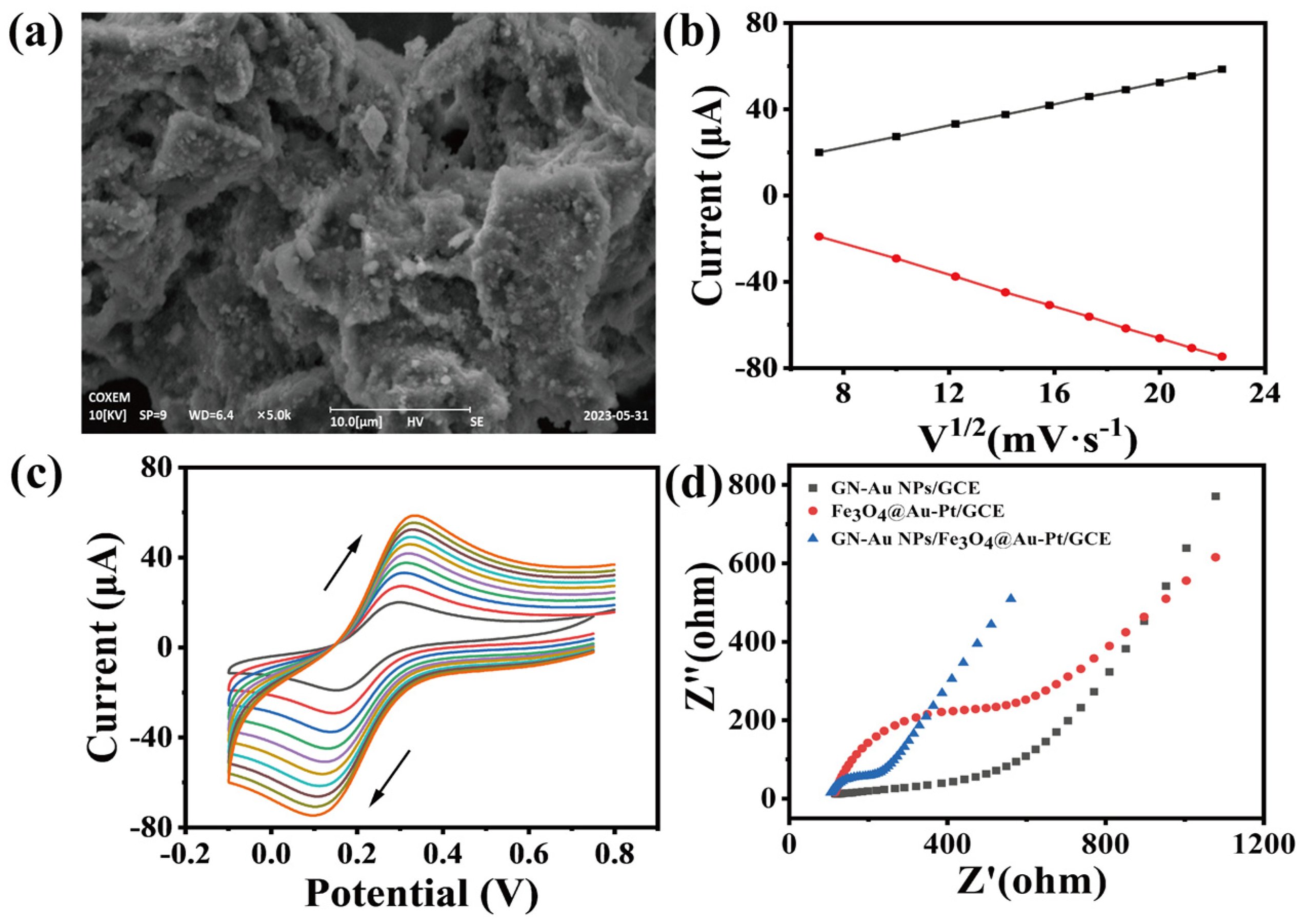
3.1. Characterization of Fe3O4@Au–Pt
3.2. Characterization of GN–Au NPs
3.3. Development of a Graphene Nanozymes Complexes Electrochemical System
3.4. Optimization of the Components in the Reaction System
3.5. Detection of H2O2 by Graphene Complexes Electrochemical System
3.6. Development of MRMEC for ETH
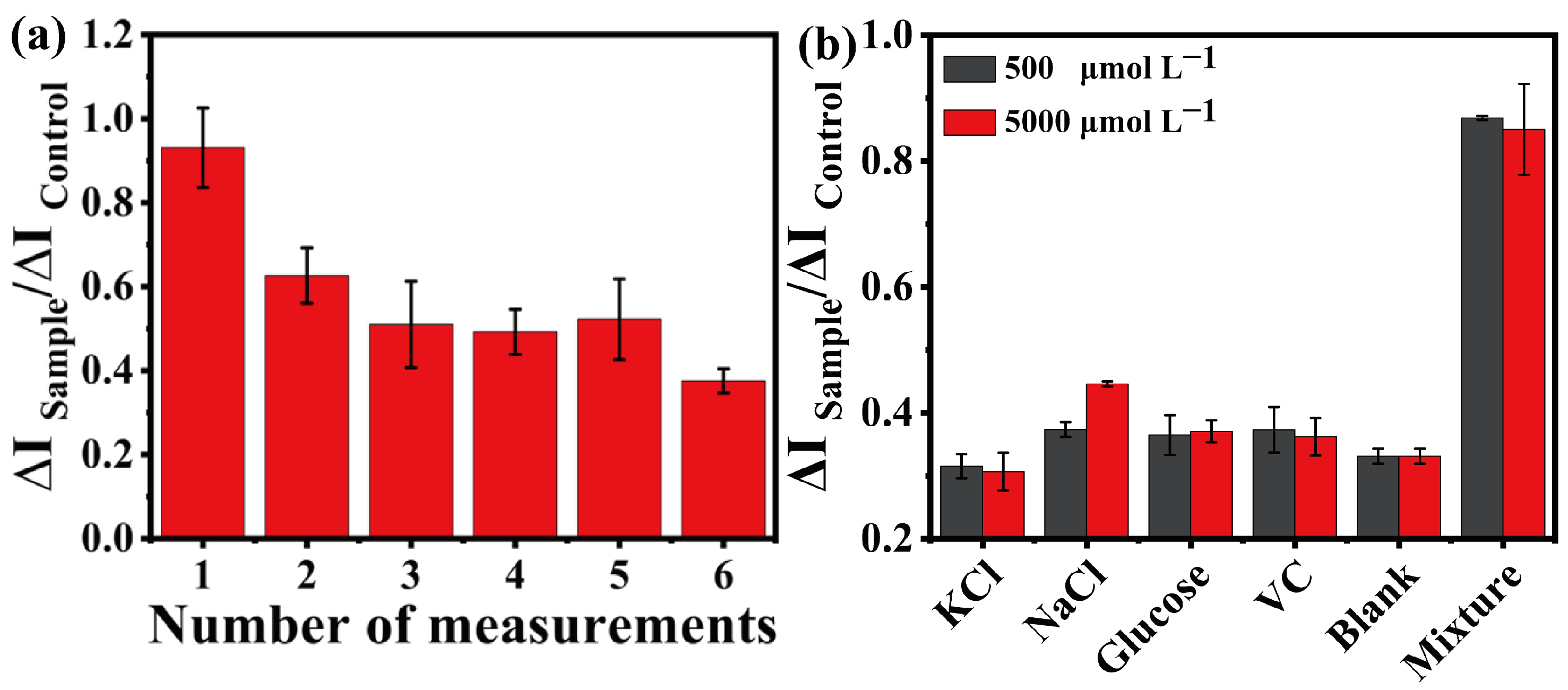
3.7. MRMEC for ETH Detection Performance Studies
3.8. Sample Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Luo, Y.X.; Tan, Y.; Kan, J.Q. Effects of ethephon on ethephon residue and quality properties of chili pepper during pre-harvest ripening. J. Food Sci. Technol.-Mysore 2021, 58, 2098–2108. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Kaur, T.; Gill, M.I.S.; Dhaliwal, H.S.; Ghuman, B.S.; Chahil, B.S. Studies on optimization of ripening techniques for banana. J. Food Sci. Technol.-Mysore 2010, 47, 315–319. [Google Scholar] [CrossRef]
- Yang, X.T.; Zhang, Z.Q.; Joyce, D.; Huang, X.M.; Xu, L.Y.; Pang, X.Q. Characterization of chlorophyll degradation in banana and plantain during ripening at high temperature. Food Chem. 2009, 114, 383–390. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Sun, C.-D.; Zhang, L.-L.; Dai, X.; Xu, C.-J.; Chen, K.-S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. 2010, 126, 229–235. [Google Scholar] [CrossRef]
- Wang, S.Q.; Jin, H.F.; Tang, Q.Q.; Fu, J.; Ren, Z.M.; Peng, C.K.; Shang, L.Q.; Hao, W.D.; Wei, X.T. The effect of ethephon on immune system in male offspring of mice. Environ. Toxicol. Pharmacol. 2017, 49, 119–123. [Google Scholar] [CrossRef]
- Bhadoria, P.; Nagar, M.; Bharihoke, V.; Bhadoria, A.S. Ethephon, an organophosphorous, a Fruit and Vegetable Ripener: Has potential hepatotoxic effects? J. Fam. Med. Prim. Care 2018, 7, 179–183. [Google Scholar] [CrossRef]
- Maragou, N.C.; Balayiannis, G. Determination of Ethephon in Pesticide Formulations by Ion Exchange Chromatography with Indirect Spectrophotometric Detection. Anal. Lett. 2020, 53, 795–806. [Google Scholar] [CrossRef]
- Kröpfl, D.; Schweiger, K.; Wagner, F.S.; Prettner, E. An Alternative Approach for the Detection of Ethephon (2-Chlorethylphosphonic acid) Residues in Apples. Nat. Prod. Commun. 2006, 1, 1934578X0600100408. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Dong, S.J.; Wei, H. Recent Advances on Nanozyme-based Electrochemical Biosensors. Electroanalysis 2023, 35, 38–49. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in electrochemical affinity biosensing. Microchim. Acta 2020, 187, 423. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, H.M.; Chen, S.; Yu, H.T.; Quan, X. Interface Engineering Catalytic Graphene for Smart Colorimetric Biosensing. ACS Nano 2012, 6, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, H.; Chen, D.; Li, F. Protein-Directed Metal Oxide Nanoflakes with Tandem Enzyme-Like Characteristics: Colorimetric Glucose Sensing Based on One-Pot Enzyme-Free Cascade Catalysis. Adv. Funct. Mater. 2018, 28, 1800018. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Torok, V.A.; Hodgson, K.; Xu, Y.; Goodacre, R.; Behera, B.K.; Bansal, V. Ultrasensitive Colorimetric Detection of Murine Norovirus Using NanoZyme Aptasensor. Anal. Chem. 2019, 91, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wu, S.H.; Lu, X.M.; Wu, P.; Liu, J.W. Manganese as a Catalytic Mediator for Photo-oxidation and Breaking the pH Limitation of Nanozymes. Nano Lett. 2019, 19, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.C.; Müller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic Peroxidase-Like Activity. Adv. Funct. Mater. 2010, 21, 501–509. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; de la Escosura-Muñiz, A. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, P.S.; Rangasamy, G.; Shankar, V.U.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 12. [Google Scholar] [CrossRef]
- Chinese Standard GB 23200.16-2016; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. China Standards Press of China: Beijing, China, 2016. Available online: http://down.foodmate.net/standard/sort/3/50187.html (accessed on 13 June 2023).
- Xu, H.; Chen, J.; Zhang, Z.; Hung, C.-T.; Yang, J.; Li, W. In Situ Confinement of Ultrasmall Metal Nanoparticles in Short Mesochannels for Durable Electrocatalytic Nitrate Reduction with High Efficiency and Selectivity. Adv. Mater. 2022, 35, 2207522. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yue, C.C.; Wang, J.; Zhang, Y.H.; Fang, W.H.; Dang, J.Q.; Wu, Y.; Zhao, H.; Li, Z.X. Fe-Ni metal-organic frameworks with prominent peroxidase-like activity for the colorimetric detection of Sn2+ ions. Analyst 2020, 145, 6349–6356. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yin, Y.; Gunasekaran, S. Oxygen-terminated few-layered Ti3C2Tx MXene nanosheets as peroxidase-mimic nanozyme for colorimetric detection of kanamycin. Biosens. Bioelectron. 2022, 218, 114774. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Luo, J.; Jing, L.; Wang, Y.; Shen, H.; Yu, R.; Sun, S.; Xing, Y.; Ming, T.; Liu, M.; et al. Reduced Graphene Oxide and Gold Nanoparticles-Modified Electrochemical Aptasensor for Highly Sensitive Detection of Doxorubicin. Nanomaterials 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding Growth for Size Control of 5−40 nm Diameter Gold Nanoparticles. Langmuir 2001, 17, 6747–7182. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, X.Y.; Chen, K.; Yin, W.M.; Li, Q.; Wang, P.; Lu, Z.C.; Ma, J.; Han, H.Y. Ultrasensitive SERS detection of Bacillus thuringiensis special gene based on Au@Ag NRs and magnetic beads. Biosens. Bioelectron. 2017, 92, 321–327. [Google Scholar] [CrossRef]
- Jin, G.H.; Ko, E.; Kim, M.K.; Tran, V.K.; Son, S.E.; Geng, Y.; Hur, W.; Seong, G.H. Graphene oxide-gold nanozyme for highly sensitive electrochemical detection of hydrogen peroxide. Sens. Actuator B-Chem. 2018, 274, 201–209. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.; Lang, Y.; Jiang, X.; Wu, P. Rationale of 3,3’,5,5’-Tetramethylbenzidine as the Chromogenic Substrate in Colorimetric Analysis. Anal. Chem. 2020, 92, 12400–12406. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry-an update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef]
- Peng, B.; Wang, C.; He, X.; Ma, Y.; Zhou, M.; Ma, X.; Zhao, S.; Fang, Y. A smartphone-assisted ratiometric colorimetric and fluorescent probe for triple-mode determination of nitrite based on MnO2 nanoparticles and carbon quantum dots. Food Chem. 2023, 410, 135151. [Google Scholar] [CrossRef]
- Kong, F.-Z.; Jahan, S.; Zhong, R.; Cao, X.-Y.; Li, W.-L.; Wang, Y.-X.; Xiao, H.; Liu, W.-W.; Cao, C.-X. Electrophoresis Titration Model of a Moving Redox Boundary Chip for a Point-of-Care Test of an Enzyme-Linked Immunosorbent Assay. ACS Sens. 2019, 4, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ju, P.; Zhang, Y.; Jiang, F.; Ding, H.; Sun, C. CoMoO4 nanobelts as efficient peroxidase mimics for the colorimetric determination of H2O2. Microchim. Acta 2020, 187, 424. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Peng, X.Y.; Liu, Z.G.; Dai, Y.; Han, Y.J.; Fan, L.F.; Guo, Y.J. CuO nanorod-decorated hemin-graphene with enhanced peroxidase-mimicking performance for the colorimetric and electrochemical determination of 4-aminophenol with a smartphone. Analyst 2023, 148, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Tvorynska, S.; Barek, J.; Josypčuk, B. Acetylcholinesterase-choline oxidase-based mini-reactors coupled with silver amalgam electrode for amperometric detection of acetylcholine in flow injection analysis. J. Electroanal. Chem. 2020, 860, 113883. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Liu, C.; Chen, X.; Chen, Y. Double-enzymes-mediated Fe2+/Fe3+ conversion as magnetic relaxation switch for pesticide residues sensing. J. Hazard. Mater. 2020, 403, 123619. [Google Scholar] [CrossRef]
- Xiu, W.L.; Zhao, P.N.; Pan, Y.J.; Wang, X.R.; Zhang, L.A.; Ge, S.G.; Yu, J.H. Flexible SERS strip based on HKUST-1(cu)/biomimetic antibodies composite multilayer for trace determination of ethephon. Anal. Chim. Acta 2023, 1253, 9. [Google Scholar] [CrossRef] [PubMed]
- Marín, J.M.; Pozo, O.J.; Beltrán, J.; Hernández, F. An ion-pairing liquid chromatography/tandem mass spectrometric method for the determination of ethephon residues in vegetables. Rapid Commun. Mass Spectrom. 2005, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-K.; Cho, J.-M.; Abd El-Aty, A.M.; Rahman, M.M.; Choi, J.-H.; Seo, Y.-J.; Shin, H.-C.; Shim, J.-H. Simple extraction method using syringe filter for detection of ethephon in tomatoes by negative-ion mode liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2015, 29, 1480–1485. [Google Scholar] [CrossRef]
- Hanot, V.; Joly, L.; Bonnechère, A.; Van Loco, J. Rapid Determination of Ethephon in Grapes by Hydrophilic Interaction Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2014, 8, 524–530. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhai, S.; Wang, M.; He, L.; Peng, D.; Liu, S.; Yang, Y.; Fang, S.; Zhang, H. Electrochemical sensor based on a polyaniline-modified SnO2 nanocomposite for detecting ethephon. Anal. Methods 2015, 7, 4725–4733. [Google Scholar] [CrossRef]
- Wang, B.; He, Q.; Li, G.; Long, Y.; Zhang, G.; Liu, H.; Liu, J. Sensitive Determination of Trace 4-Nitrophenol in Ambient Environment Using a Glassy Carbon Electrode Modified with Formamide-Converted Nitrogen-Doped Carbon Materials. Int. J. Mol. Sci. 2022, 23, 12182. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Ganesh, T.; Lelutiu, N.; Gueorguieva, P.; Dingledine, R. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology 2015, 93, 15–27. [Google Scholar] [CrossRef] [PubMed]



| Methods | Linear Ranges (mol L−1) | LOD (mol L−1) | LOQ (mol L−1) | References |
|---|---|---|---|---|
| SERS with HKUST-1(Cu) 1 | 6.92 × 10−9–6.92 × 10−5 | 9.62 × 10−10 | 2.92 × 10−9 | [37] |
| Ion-pairing LC-MS/MS 2 | 3.30 × 10−8–3.30 × 10−6 | 3.30 × 10−8 | 8.25 × 10−8 | [38] |
| Negative-ion LC-MS/MS 3 | 6.92 × 10−8–6.92 × 10−7 | 2.07 × 10−7 | 6.21 × 10−7 | [39] |
| Hydrophilicity GC-MS/MS 4 | 3.50×10−7–1.3×10−6 | 1.70 × 10−7 | 3.40 × 10−7 | [40] |
| EC with Polyaniline@SnO2 5 | 6.90 × 10−11–3.4 × 10−8 | 3.30 × 10−11 | 1.00 × 10−10 | [41] |
| MRMEC | 1.00 × 10−7–5.00 × 10−4 | 2.01 × 10−9 | 6.00 × 10−9 | This work |
| Sample | Added (μmol L−1) | Found (μmol L−1) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Mangoes | 0.05 | 0.052 ± 0.005 | 104.13 | 9.79 |
| 0.50 | 0.55 ± 0.02 | 110.72 | 2.88 | |
| 5.00 | 5.5 ± 0.8 | 110.59 | 15.97 | |
| 50.00 | 50 ± 2 | 99.64 | 3.24 | |
| 500.00 | 460 ± 20 | 92.18 | 4.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.; Tang, X.; Wen, R.; Xu, C.; Wei, J.; Han, B.; Wu, L. A Multienzyme Reaction-Mediated Electrochemical Biosensor for Sensitive Detection of Organophosphorus Pesticides. Biosensors 2024, 14, 62. https://doi.org/10.3390/bios14020062
Ji C, Tang X, Wen R, Xu C, Wei J, Han B, Wu L. A Multienzyme Reaction-Mediated Electrochemical Biosensor for Sensitive Detection of Organophosphorus Pesticides. Biosensors. 2024; 14(2):62. https://doi.org/10.3390/bios14020062
Chicago/Turabian StyleJi, Chengzhen, Xuemei Tang, Ruiming Wen, Chengdong Xu, Jing Wei, Bingjun Han, and Long Wu. 2024. "A Multienzyme Reaction-Mediated Electrochemical Biosensor for Sensitive Detection of Organophosphorus Pesticides" Biosensors 14, no. 2: 62. https://doi.org/10.3390/bios14020062
APA StyleJi, C., Tang, X., Wen, R., Xu, C., Wei, J., Han, B., & Wu, L. (2024). A Multienzyme Reaction-Mediated Electrochemical Biosensor for Sensitive Detection of Organophosphorus Pesticides. Biosensors, 14(2), 62. https://doi.org/10.3390/bios14020062





