Role of Peroxynitrite in the Pathogenesis of Parkinson’s Disease and Its Fluorescence Imaging-Based Detection
Abstract
1. Introduction
2. Parkinson’s Disease
2.1. Epidemiology of PD
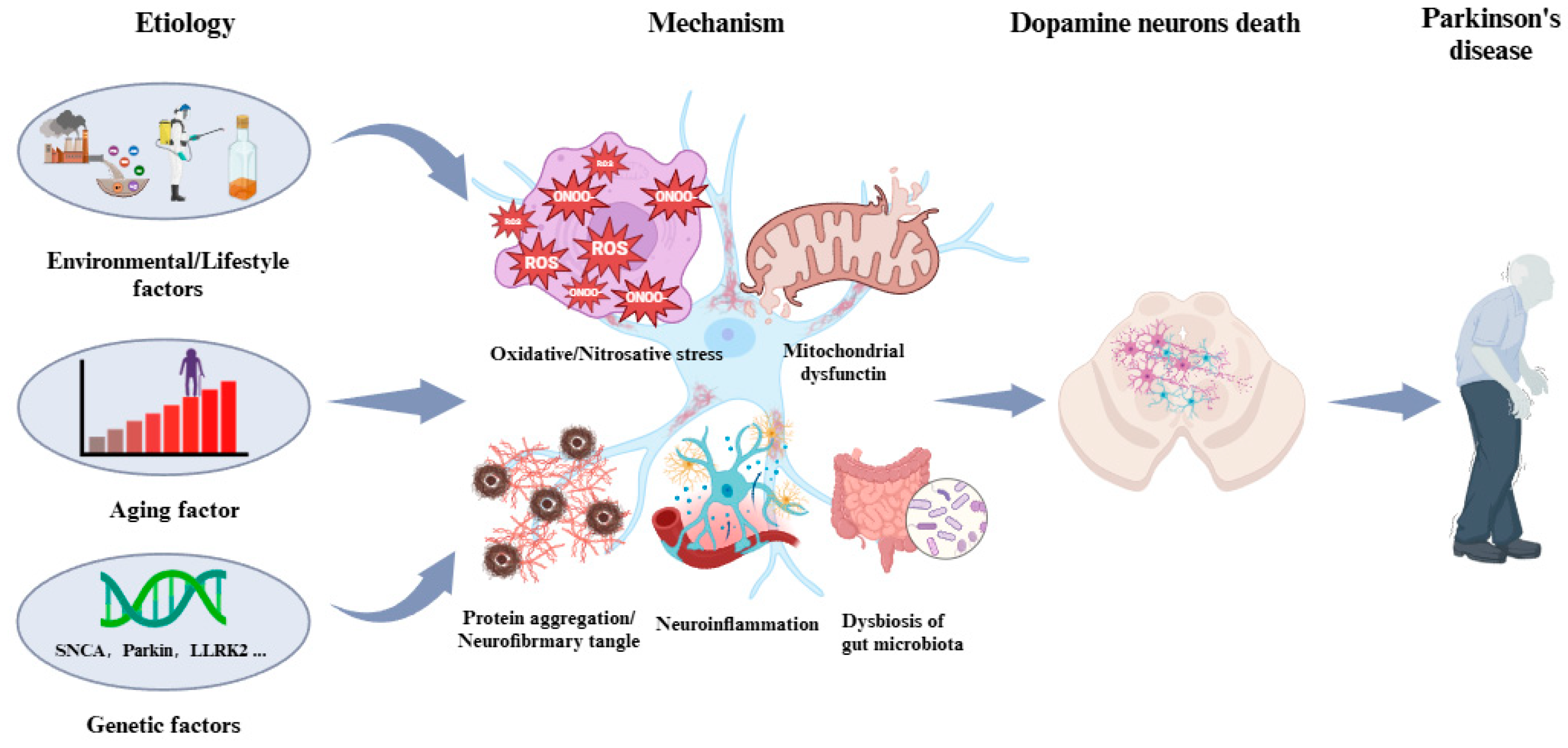
2.2. Manifestation, Etiology, and Pathogenesis of PD
3. Peroxynitrite and Its Involvement in PD
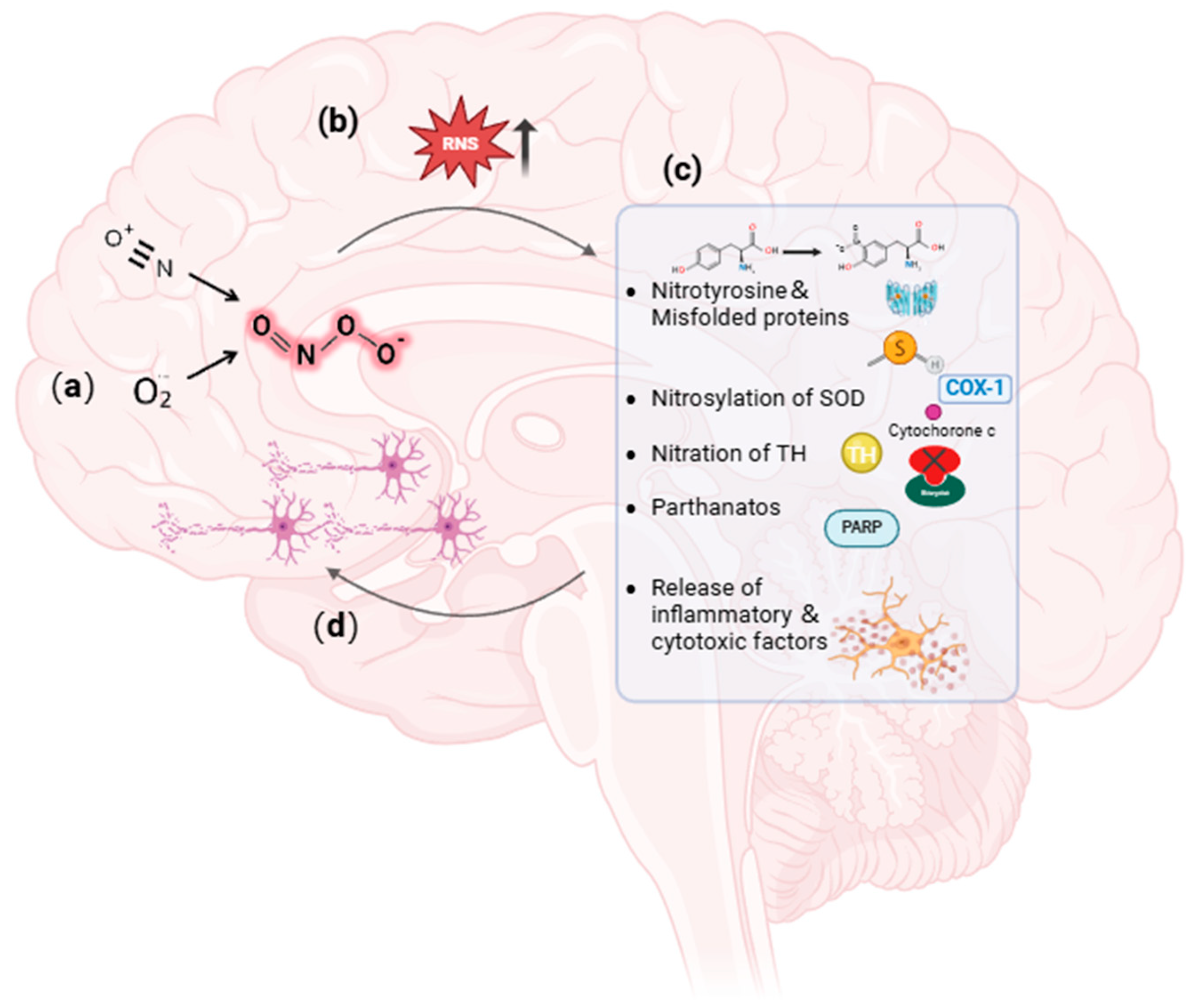
3.1. Profile of Peroxynitrite
3.2. Involvement of Peroxynitrite in PD Pathogenesis
4. Fluorescence Imaging-Based Peroxynitrite Detection
4.1. Boron-Based Peroxynitrite Probes
4.2. C=C- or C=N-Based Peroxynitrite Probes
4.3. Phosphine-Based Peroxynitrite Probes
4.4. 1,8-Naphthimide Derivative-Based Peroxynitrite Probes
4.5. Other Moiety-Based Peroxynitrite Probes
4.6. Nanoparticle-Based Peroxynitrite Detection
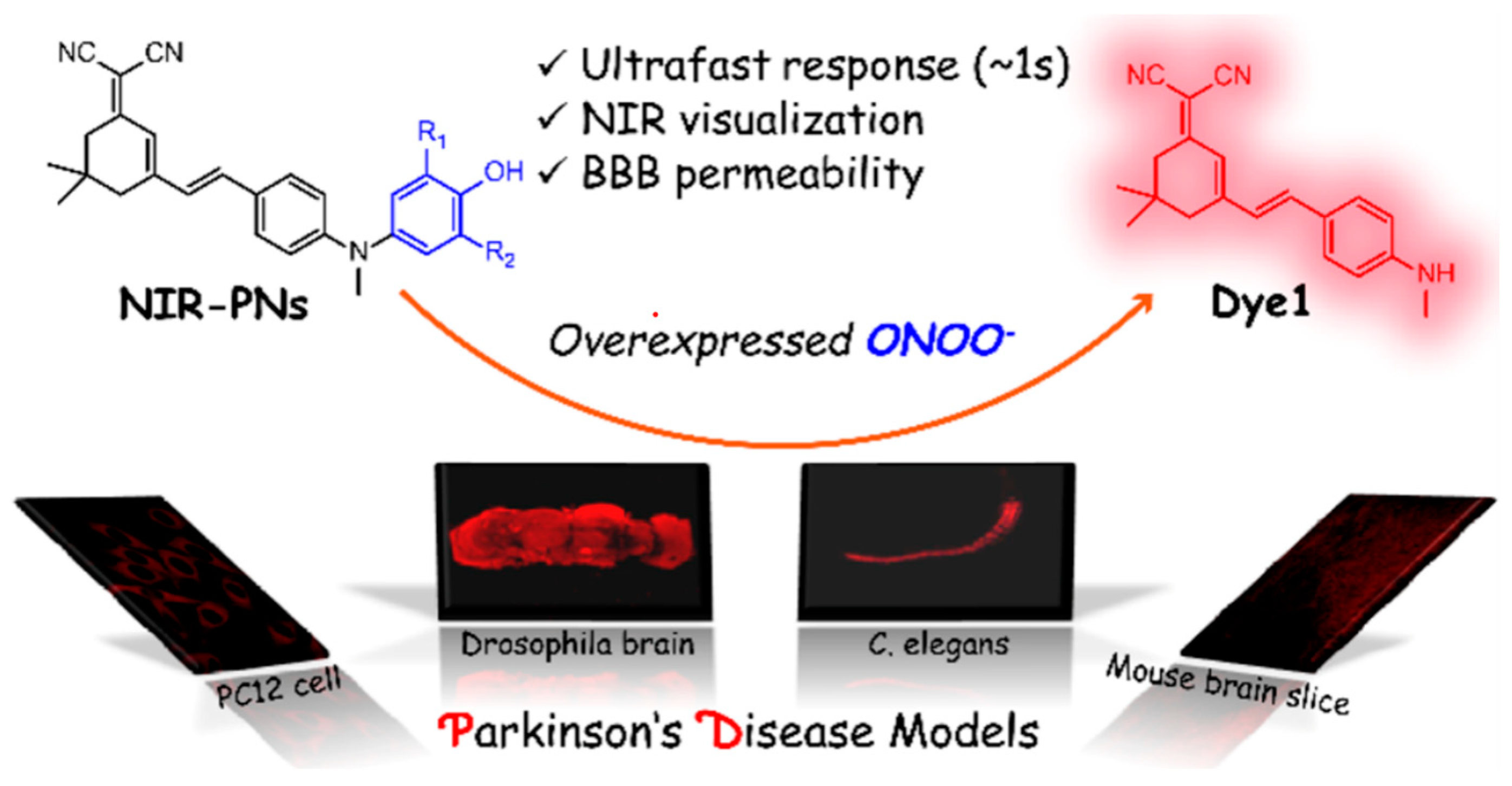
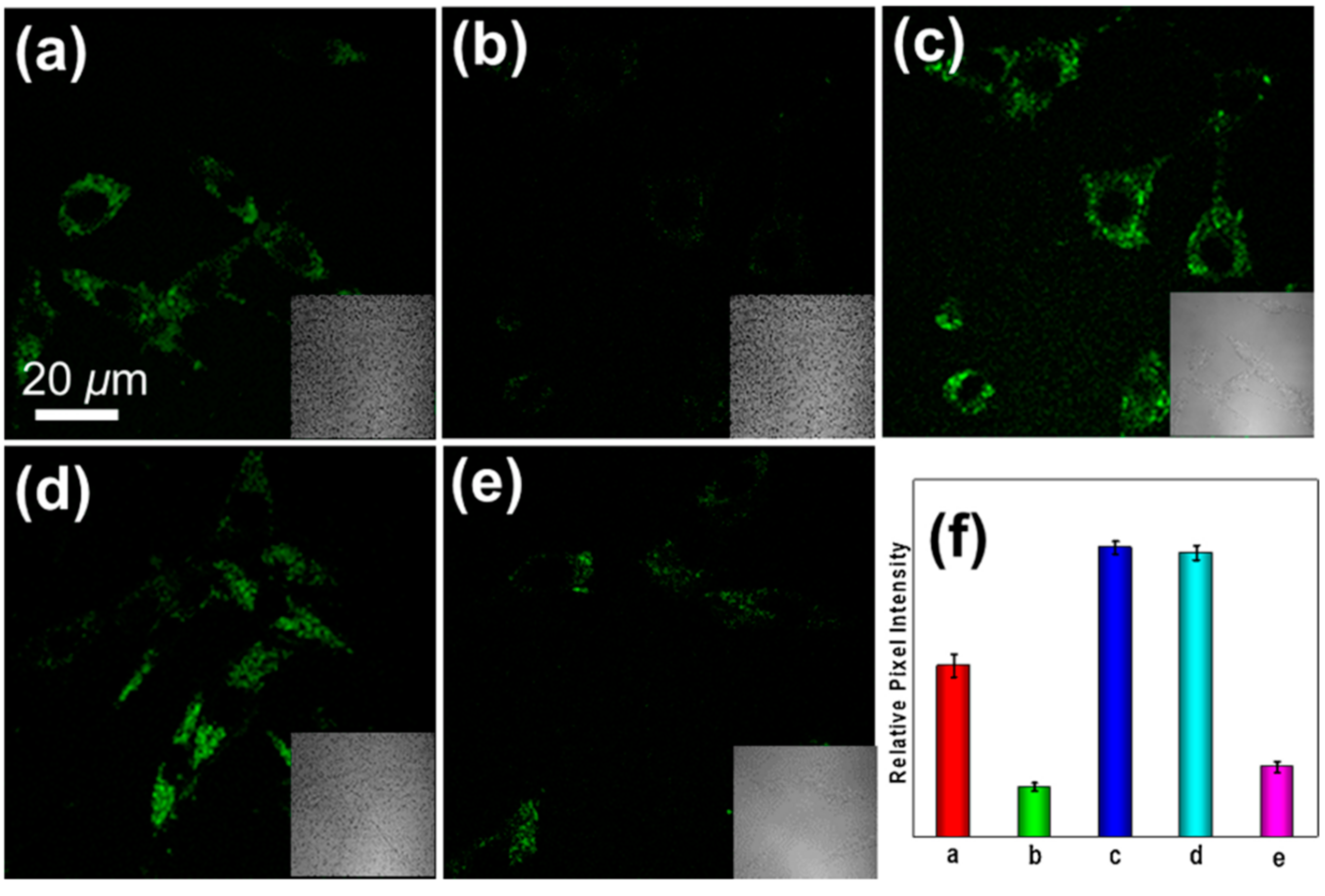
5. Application of Fluorescent Probes in PD Study
6. Conclusions and Prospectives
Author Contributions
Funding
Conflicts of Interest
References
- Guatteo, E.; Berretta, N.; Monda, V.; Ledonne, A.; Mercuri, N.B. Pathophysiological Features of Nigral Dopaminergic Neurons in Animal Models of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4508. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. GBD 2016 Neurology Collaborators Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; et al. GBD 2016 Parkinson’s Disease Collaborators Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yin, P.; Wang, L.; Qu, M.; Kan, G.L.; Zhang, H.; Zhang, Q.; Xiao, Y.; Deng, Y.; Dong, Z.; et al. Prevalence of Parkinson’s Disease: A Community-Based Study in China. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 2940–2944. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A. The Burden of Parkinson’s Disease: A Worldwide Perspective. Lancet Neurol. 2018, 17, 928–929. [Google Scholar] [CrossRef]
- Darweesh, S.K.L.; Raphael, K.G.; Brundin, P.; Matthews, H.; Wyse, R.K.; Chen, H.; Bloem, B.R. Parkinson Matters. J. Park. Dis. 2018, 8, 495–498. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet Lond. Engl. 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Melis, M.; Haehner, A.; Mastinu, M.; Hummel, T.; Tomassini Barbarossa, I. Molecular and Genetic Factors Involved in Olfactory and Gustatory Deficits and Associations with Microbiota in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4286. [Google Scholar] [CrossRef]
- Mantri, S.; Morley, J.F.; Siderowf, A.D. The Importance of Preclinical Diagnostics in Parkinson Disease. Park. Relat. Disord. 2019, 64, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grünewald, A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Day, J.O.; Mullin, S. The Genetics of Parkinson’s Disease and Implications for Clinical Practice. Genes 2021, 12, 1006. [Google Scholar] [CrossRef]
- Tobacco Smoking and the Reduced Risk of Parkinson Disease: A Puzzle of 60 Years—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32371449/ (accessed on 17 August 2024).
- Macklin, E.A.; Ascherio, A.; Schwarzschild, M.A. Effect of Urate-Elevating Inosine on Progression of Early Parkinson Disease-Reply. JAMA 2022, 327, 85–86. [Google Scholar] [CrossRef]
- Kawada, T. Risk Reduction of Parkinson’s Disease by Caffeinated Beverage Consumption. Park. Relat. Disord. 2022, 103, 152. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell. 2019, 18, e13031. [Google Scholar] [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s Disease Pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Lee, S.Y.H.; Yates, N.J.; Tye, S.J. Inflammatory Mechanisms in Parkinson’s Disease: From Pathogenesis to Targeted Therapies. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2022, 28, 485–506. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired Dopamine Metabolism in Parkinson’s Disease Pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kishor Kale, N.; Borah, S.; Shrikant Deokar, S.; et al. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease, and Parkinson’s Disease, Huntington’s Disease and Amyotrophic Lateral Sclerosis—An Updated Review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Stykel, M.G.; Ryan, S.D. Nitrosative Stress in Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A Multifaceted Oxidizing and Nitrating Metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Nauser, T.; Kissner, R.; Rüegger, H. Peroxynitrous Acid: Controversy and Consensus Surrounding an Enigmatic Oxidant. Dalton Trans. Camb. Engl. 2012, 41, 13779–13787. [Google Scholar] [CrossRef]
- Ramdial, K.; Franco, M.C.; Estevez, A.G. Cellular Mechanisms of Peroxynitrite-Induced Neuronal Death. Brain Res. Bull. 2017, 133, 4–11. [Google Scholar] [CrossRef]
- Iova, O.-M.; Marin, G.-E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders—An Overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef]
- Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-Induced Oxidation and Nitration Products of Guanine and 8-Oxoguanine: Structures and Mechanisms of Product Formation. Nitric Oxide Biol. Chem. 2006, 14, 109–121. [Google Scholar] [CrossRef]
- Mahdi, A.; Tengbom, J.; Alvarsson, M.; Wernly, B.; Zhou, Z.; Pernow, J. Red Blood Cell Peroxynitrite Causes Endothelial Dysfunction in Type 2 Diabetes Mellitus via Arginase. Cells 2020, 9, 1712. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Sharma, S.K. Peroxynitrite and Mitochondrial Dysfunction in the Pathogenesis of Parkinson’s Disease. Antioxid. Redox Signal. 2003, 5, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Bachschmid, M.; Beckman, J.S.; Munzel, T.; Ullrich, V. The Impact of Metal Catalysis on Protein Tyrosine Nitration by Peroxynitrite. Biochem. Biophys. Res. Commun. 2004, 317, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, Y.; Furuta, A.; Taniguchi, N.; Yamada, T.; Kira, J.; Iwaki, T. Increased Expression of Manganese Superoxide Dismutase Is Associated with That of Nitrotyrosine in Myopathies with Rimmed Vacuoles. Acta Neuropathol. 2002, 103, 59–65. [Google Scholar] [CrossRef]
- Crow, J.P.; Ye, Y.Z.; Strong, M.; Kirk, M.; Barnes, S.; Beckman, J.S. Superoxide Dismutase Catalyzes Nitration of Tyrosines by Peroxynitrite in the Rod and Head Domains of Neurofilament-L. J. Neurochem. 1997, 69, 1945–1953. [Google Scholar] [CrossRef]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A Versatile Oxidative Stress Biomarker for Major Neurodegenerative Diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef]
- Redondo-Horcajo, M.; Romero, N.; Martínez-Acedo, P.; Martínez-Ruiz, A.; Quijano, C.; Lourenço, C.F.; Movilla, N.; Enríquez, J.A.; Rodríguez-Pascual, F.; Rial, E.; et al. Cyclosporine A-Induced Nitration of Tyrosine 34 MnSOD in Endothelial Cells: Role of Mitochondrial Superoxide. Cardiovasc. Res. 2010, 87, 356–365. [Google Scholar] [CrossRef]
- Quijano, C.; Castro, L.; Peluffo, G.; Valez, V.; Radi, R. Enhanced Mitochondrial Superoxide in Hyperglycemic Endothelial Cells: Direct Measurements and Formation of Hydrogen Peroxide and Peroxynitrite. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3404–H3414. [Google Scholar] [CrossRef][Green Version]
- McCarty, M.F.; Lerner, A. Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3624. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.; Deng, R.; Shen, J. Pros and Cons of Current Approaches for Detecting Peroxynitrite and Their Applications. Biomed. J. 2014, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, W.; Wang, X.; Yang, F.; Xie, L.; Shen, J.; Brimble, M.A.; Xiao, Q.; Yao, S.Q. Fluorescent Probes for Bioimaging of Potential Biomarkers in Parkinson’s Disease. Chem. Soc. Rev. 2021, 50, 1219–1250. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Zhang, D.; Zhu, M. Fluorescent Materials with Excellent Biocompatibility and Their Application in Bio-Sensing, Bio-Imaging. Biosensors 2023, 13, 906. [Google Scholar] [CrossRef]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, S.; Zhai, Z.; Wang, K.; Liu, X.; Xiao, H.; Zhuo, S.; Liu, Y. Recent Progress of Small-Molecule Ratiometric Fluorescent Probes for Peroxynitrite in Biological Systems. Chemistry 2022, 28, e202200828. [Google Scholar] [CrossRef]
- Li, M.; Han, H.; Zhang, H.; Song, S.; Shuang, S.; Dong, C. Boronate Based Sensitive Fluorescent Probe for the Detection of Endogenous Peroxynitrite in Living Cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 243, 118683. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Song, W.; Zhong, W.; Sun, T.; Zhu, J.; Wang, J. A Novel Borate Fluorescent Probe for Rapid Selective Intracellular Peroxynitrite Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119398. [Google Scholar] [CrossRef]
- Li, M.; Han, H.; Song, S.; Shuang, S.; Dong, C. AIE-Based Fluorescent Boronate Probe and Its Application in Peroxynitrite Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 120044. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, Y.; Song, S.; Shuang, S.; Dong, C. A Bifunctional Fluorescence Probe for Dual-Channel Detecting of Mitochondrial Viscosity and Endogenous/Exogenous Peroxynitrite. Bioorganic Chem. 2022, 119, 105484. [Google Scholar] [CrossRef] [PubMed]
- Tian, H., Jr.; Guo, C.; Hu, X.-L.; Wang, J.-B.; Zang, Y.; James, T.D.; Li, J.; He, X.-P. Human Serum Albumin-based Supramolecular Host-guest Boronate Probe for Enhanced Peroxynitrite Sensing. Org. Biomol. Chem. 2023, 21, 4661–4666. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Ran, Y.; He, Y.; Lu, X.; Wang, J.; Zhao, W.; Zhang, J. Near-Infrared Fluorescence Probe for Visualizing Fluctuations of Peroxynitrite in Living Cells and Inflammatory Mouse Models. Chemosensors 2023, 11, 316. [Google Scholar] [CrossRef]
- Xu, Z.; Qian, J.; Ge, Y.; Wang, Y.; Chen, H. A Two-Photon Fluorescent Probe for the Visual Detection of Peroxynitrite in Living Cells and Zebrafish. Molecules 2022, 27, 4858. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Li, M.; Dong, C.; Shuang, S. Multifunctional Mitochondria-Targeting Near-Infrared Fluorescent Probe for Viscosity, ONOO-, Mitophagy, and Bioimaging. ACS Biomater. Sci. Eng. 2023, 9, 3581–3589. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, X.; Hu, X.; Xu, L.; Liu, X.; Gao, G.; Wang, R.; Liang, G. A Chemiluminescent Probe for Imaging Peroxynitrite in Inflammatory Cells and Tissues. Anal. Chem. 2023, 95, 6496–6500. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Y.; Tan, S.; Jiang, X.; Chen, Z.; Yu, Q.; Chen, H.; Zhuang, Y.; Zeng, H.; Fu, X.; et al. Multiscale Imaging of Peroxynitrite in Gliomas with a Blood-brain Barrier Permeable Probe Reveals Its Potential as a Biomarker and Target for Glioma Treatment. Biosens. Bioelectron. 2023, 236, 115415. [Google Scholar] [CrossRef]
- Weber, M.; Yamada, N.; Tian, X.; Bull, S.D.; Minoshima, M.; Kikuchi, K.; Mackenzie, A.B.; James, T.D. Sensing Peroxynitrite in Different Organelles of Murine RAW264.7 Macrophages with Coumarin-Based Fluorescent Probes. Front. Chem. 2020, 8, 39. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Li, P.; Fu, Q. Ratiometric Optical Probes for Biosensing. Theranostics 2023, 13, 2632–2656. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small Molecule-Based Ratiometric Fluorescence Probes for Cations, Anions, and Biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Q.; Gao, M.-J.; Guo, J.-S.; Wang, Y.-H.; Wei, R.; Meng, Y.-L.; Kang, Y.-F. A Highly Selective Probe for Ratiometric Imaging Peroxynitrite in Living Cells and in Vivo. Bioorganic Chem. 2022, 128, 106055. [Google Scholar] [CrossRef]
- Zhang, J.; Kan, J.; Sun, Y.; Won, M.; Kim, J.H.; Zhang, W.; Zhou, J.; Qian, Z.; Kim, J.S. Nanoliposomal Ratiometric Fluorescent Probe toward ONOO- Flux. ACS Appl. Bio Mater. 2021, 4, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Shu, W.; Kang, H.; Duan, Q.; Zhang, X.; Liang, C.; Gao, M.; Xu, L.; Jing, J.; Zhang, X. A Deep Red Ratiometric Fluorescent Probe for Accurate Detection of Peroxynitrite in Mitochondria. Anal. Chim. Acta 2022, 1203, 339652. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Shu, W.; Han, R.; Kang, H.; Zhang, X.; Jing, J.; Zhang, R.; Zhang, X. A Xanthene-Based Fluorescent Probe for Detection of Peroxynitrite in Living Cells and Zebrafish. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 277, 121264. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, H.; Wu, J.; Cheng, Z.; Yu, F. Construction of a Mitochondria-Endoplasmic Reticulum Dual-Targeted Red-Emitting Fluorescent Probe for Imaging Peroxynitrite in Living Cells and Zebrafish. Chem. Asian J. 2022, 17, e202200388. [Google Scholar] [CrossRef]
- Liu, Z.; Mo, S.; Hao, Z.; Hu, L. Recent Progress of Spectroscopic Probes for Peroxynitrite and Their Potential Medical Diagnostic Applications. Int. J. Mol. Sci. 2023, 24, 12821. [Google Scholar] [CrossRef]
- Shen, Y.; Dai, L.; Zhang, Y.; Li, H.; Chen, Y.; Zhang, C. A Novel Pyridinium-Based Fluorescent Probe for Ratiometric Detection of Peroxynitrite in Mitochondria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117762. [Google Scholar] [CrossRef]
- Gu, B.; Liu, C.; Wu, Y.; Zhang, C.; Shen, Y.; Liu, M. Application of a Colorimetric and Near-Infrared Fluorescent Probe in Peroxynitrite Detection and Imaging in Living Cells. ACS Omega 2020, 5, 27530–27535. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, D. Selective Detection of Peroxynitrite in Living Cells by a Near-Infrared Diphenyl Phosphinate-Based Dicyanoisophorone Probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 244, 118890. [Google Scholar] [CrossRef]
- Sonawane, P.M.; Lee, W.; Kim, Y.; Roychaudhury, A.; Bhosale, V.K.; Kim, D.; Park, H.-S.; Kim, C.-H.; Churchill, D.G. Phosphinate-Benzoindocyanin Fluorescent Probe for Endogenous Mitochondrial Peroxynitrite Detection in Living Cells and Gallbladder Access in Inflammatory Zebrafish Animal Models. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120568. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Wu, C.; Zhang, C.; He, S.; Tang, S.; Li, H.; Shen, Y. A Morpholino Hydrazone-based Lysosome-targeting Fluorescent Probe With Fast Response and High Sensitivity for Imaging Peroxynitrite in Living Cells. Spectrochim Acta A Mol Biomol Spectrosc 2021, 262, 120100. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wu, R.; Wang, X.; Qin, G.; Wu, F.; Wang, C.; Chen, M.; Wang, N.; Wang, Q.; Cao, D. Highly Sensitive Benzothiazole-based Chemosensors for Detection and Bioimaging of Peroxynitrite in Living Cells. RSC Adv. 2022, 12, 27933–27939. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dong, C.; Wang, Y.; Liu, Q.; Wei, H.; Zhao, B.; Xu, X.; Dong, B.; Fan, C. A Near-Infrared Fluorescent Probe with Remarkably Large Stokes Shift for Specifical Imaging of Peroxynitrite Fluctuations in Hela Cells. Bioorganic Chem. 2023, 141, 106866. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Q.; Yan, M.; Zhang, C.; Yuan, H.; He, W. Activity-Based Fluorescent Molecular Logic Gate Probe for Dynamic Tracking of Mitophagy Induced by Oxidative Stress. Anal. Chem. 2021, 93, 3502–3509. [Google Scholar] [CrossRef]
- Cui, Y.; Han, S.; Zhang, J.; Wang, X. A Ratiometric Fluorescent Nanoprobe for Ultrafast Imaging of Peroxynitrite in Living Cells. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2022, 27, 595–603. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.; Rees, T.W.; Liao, X.; Yan, X.; Chen, Y.; Ji, L.; Chao, H. Lysosome-Targeting Iridium(III) Probe with Near-Infrared Emission for the Visualization of NO/O2•- Crosstalk via In Vivo Peroxynitrite Imaging. Anal. Chem. 2020, 92, 6003–6009. [Google Scholar] [CrossRef]
- Ren, J.; Du, Z.; Zhang, W.; Zhang, R.; Song, B.; Yuan, J. Development of a Fluorescein Modified Ruthenium(II) Complex Probe for Lysosome-Targeted Ratiometric Luminescence Detection and Imaging of Peroxynitrite in Living Cells. Anal. Chim. Acta 2022, 1205, 339784. [Google Scholar] [CrossRef]
- Wu, Y.; Han, H.-H.; He, L.; Li, L.; Zang, Y.; Li, J.; He, X.-P.; Ding, Y.; Cao, W.; James, T.D. Selective Detection of Peroxynitrite Using an Isatin Receptor and a Naphthalimide Fluorophore. Chem. Commun. Camb. Engl. 2023, 59, 5051–5054. [Google Scholar] [CrossRef]
- Han, Y.; Luo, C.; Quan, Z.; Li, H.; Sun, S.; Xu, Y. New “Destruction Seek to Survive” Strategy Based on a Serum Albumin Assembly with a Squaraine Molecule for the Detection of Peroxynitrite. Anal. Chem. 2023, 95, 7278–7285. [Google Scholar] [CrossRef]
- Yan, J.-L.; Liu, S.-S.; Wu, W.-N.; Zhao, X.-L.; Fan, Y.-C.; Wang, Y.; Xu, Z.-H. A Dihydro-benzo[4,5]imidazo[1,2-c]quinazoline-based Probe with Aggregation-induced Ratiometric Emission for the Ratiometric Fluorescent Detection of Peroxynitrite in Living Cells and Zebrafish. Anal Methods. 2023, 15, 5311–5315. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, Z.; Nong, L.; Cheng, J.; Lin, W. A Therapeutic Probe for Detecting and Inhibiting ONOO- in Senescent Cells. J. Mater. Chem. B 2023, 11, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Zhao, Z. Using Nanoliposomes to Construct a FRET-Based Ratiometric Fluorescent Probe for Sensing Intracellular pH Values. Anal. Chem. 2016, 88, 12380–12385. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hou, L.; Li, Z.; Lin, T.; Tian, J.; Zhao, S. A Mitochondria-targeted Ratiometric Fluorescent Nanoprobe for Imaging of Peroxynitrite in Living Cells. Talanta 2021, 231, 122421. [Google Scholar] [CrossRef]
- Shao, J.; Sun, S.; Zhan, D.; Pan, L.; Min, G.; Li, X.; Huang, K.; Chen, W.; Yang, L.; Liu, X.-Y.; et al. Phycocyanin - Carbon Dots Nanoprobe for the Ratiometric Fluorescence Determination of Peroxynitrite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 275, 121177. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, L.L.; Han, H.H.; He, X.P.; Cao, W.; James, T.D. Selective FRET Nano Probe Based on Carbon Dots and Naphthalimide-isatin for the Ratiometric Detection of Peroxynitrite in Drug-induced Liver Injury. Chem. Sci. 2023, 15, 757–764. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, J.; Ji, C.-L.; Shaibani, M.S.S.; Li, Z.; Lim, K.; Zhang, C.-W.; Li, L.; Liu, Z. Ultrafast Detection of Peroxynitrite in Parkinson’s Disease Models Using a Near-Infrared Fluorescent Probe. Anal. Chem. 2020, 92, 4038–4045. [Google Scholar] [CrossRef]
- Yan, M.; Fang, H.; Wang, X.; Xu, J.; Zhang, C.; Xu, L.; Li, L. A Two-Photon Fluorescent Probe for Visualizing Endoplasmic Reticulum Peroxynitrite in Parkinson’s Disease Models. Sens. Actuators B Chem. 2021, 328, 129003. [Google Scholar] [CrossRef]
- Shao, T.; Xu, X.; Wang, L.; Shen, Y.; Zhao, J.; Li, H.; Zhang, D.; Du, W.; Bai, H.; Peng, B.; et al. A Novel α-Ketoamide Reactivity-Based Two-Photon Fluorogenic Probe for Visualizing Peroxynitrite in Parkinson’s Disease Models. J. Innov. Opt. Health Sci. 2023, 16, 2250039. [Google Scholar] [CrossRef]
- Kang, H.; Shu, W.; Yu, J.; Gao, M.; Han, R.; Jing, J.; Zhang, R.; Zhang, X. A Near-Infrared Fluorescent Probe for Ratiometric Imaging Peroxynitrite in Parkinson’s Disease Model. Sens. Actuators B Chem. 2022, 359, 131393. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Wu, X.; Kou, J.; Feng, T.; Zhang, R.; Xu, C.; Kong, F.; Tang, B. A Sequentially Activated Probe for Imaging of Superoxide Anion and Peroxynitrite in PC12 Cells under Oxidative Stress. Anal. Chem. 2024, 96, 7138–7144. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, G.; Niu, Y.; Xu, C.; Li, Y.; Zhang, J.; Jiao, X.; Wang, X.; Tang, B. Dual-Channel Imaging of Amyloid-β Plaques and Peroxynitrite To Illuminate Their Correlations in Alzheimer’s Disease Using a Unimolecular Two-Photon Fluorescent Probe. Anal. Chem. 2021, 93, 15088–15095. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, Y.; Liu, G.; Zhao, Y.; Liu, J.; Li, Y.; Zhang, J.; Jiao, X.; Wang, X.; Tang, B. Two-Photon Fluorescence Imaging of the Cerebral Peroxynitrite Stress in Alzheimer’s Disease. Chem. Commun. Camb. Engl. 2022, 58, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, L.; Gong, J.; Xiong, J.; Zi, S.; Xie, H.; Zhang, F.; Mao, Z.; Liu, Z.; Kim, J.S. An Activity-Based Fluorescent Probe for Imaging Fluctuations of Peroxynitrite (ONOO-) in the Alzheimer’s Disease Brain. Angew. Chem. Int. Ed Engl. 2022, 61, e202206894. [Google Scholar] [CrossRef]
- Chen, S.; Huang, W.; Tan, H.; Yin, G.; Chen, S.; Zhao, K.; Huang, Y.; Zhang, Y.; Li, H.; Wu, C. A Large Stokes Shift NIR Fluorescent Probe for Visual Monitoring of Mitochondrial Peroxynitrite during Inflammation and Ferroptosis and in an Alzheimer’s Disease Model. Analyst 2023, 148, 4331–4338. [Google Scholar] [CrossRef]
| Serial Number | Probe Structure | Excitation/Emission (nm) | Working Mechanism/Application | Reference |
|---|---|---|---|---|
| 1 (FAM) |  | 580/625 | Boron-based/cells | Dong et al. [50] |
| 2 (L) |  | 421/500 | Boron-based/cells | Zhu et al. [51] |
| 3 (ADB) |  | 362/440 | Boron-based/cells | Dong et al. [52] |
| 4 (PV) | 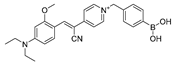 | 447/507 | Boron-based/cells and mice | Dong et al. [53] |
| 5 (TCM-2) |  | -/560 | Boron-based/cells | He et al. [54] |
| 6 (BDP-ENE-S-Py+) |  | 620/640,900 | Boron-based/cells, mice | Zhang et al. [55] |
| 7 (HDBT-ONOO−) | 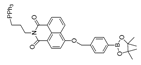 | 372,450/558 | Boron-based/cells, zebrafish | Chen et al. [56] |
| 8 (P-1) | 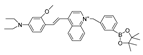 | 358/570 | Boron-based/cells, zebrafish | Shuang et al. [57] |
| 9 (B-PD) | 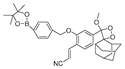 | - | Boron-based/cells, zebrafish | Liang et al. [58] |
| 10 (NOSTracker) |  | 645/696 | Boron-based/cells, mice | Li et al. [59] |
| 11 (CM) | 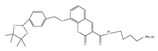 | 340/400 | Boron-based/cells | James et al. [60] |
| 12 (CL) | 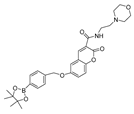 | 340/395 | Boron-based/cells | James et al. [60] |
| 13 (CE) |  | 340/405 | Boron-based/cells | James et al. [60] |
| 14 (JQ-2) |  | 461/569,657 | Boron-based/cells, zebrafish | Kang et al. [63] |
| 15 (NRF) |  | 462/700 | C=C-based/cells, mice | Kim et al. [64] |
| 16 (MXMP) | 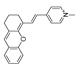 | 390,520/530,660 | C=C-based/cells | Zhang et al. [65] |
| 17 (DMX) | 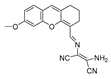 | 475/510 | C=N-based/cells, zebrafish | Zhang et al. [66] |
| 18 (MCSA) | 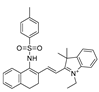 | 540/635 | C=N-based/cells, zebrafish. | Yu et al. [67] |
| 19 (COUS) |  | 363,586/484,723 | C=C-based/cells | Hu et al. [68] |
| 20 (CPC) | 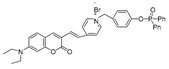 | 503/538,643 | Phosphine-based/cells | Zhang et al. [69] |
| 21 (AN-DP) | 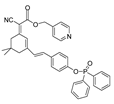 | 454/670 | Phosphine-based/cells. | Liu et al. [70] |
| 22 (NR-ONOO) | 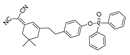 | 400/560 | Phosphine-based/cells | Ma et al. [71] |
| 23 (BICBzDP) | 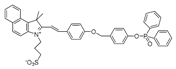 | 549/576 | Phosphine-based/cells, zebrafish | Churchill et al. [72] |
| 24 (BMP) |  | 427/515 | Phosphine-based/cells | Shen et al. [73] |
| 25 (BS1) | 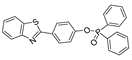 | 365/430 | Phosphine-based/cells | Cao et al. [74] |
| 26 (XPC) | 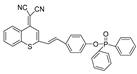 | 550/750 | Phosphine-based/cells | Fan et al. [75] |
| 27 (NC-NP530/460) |  | 405/460,530 | 1,8-naphthimide derivative-based/cells | Wang et al. [77] |
| 28 (Ir-NIR) | 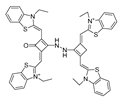 | 405/702 | MAB-based/cells | Chao et al. [78] |
| 29 (Ru-FL-ONOO) | 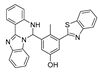 | 490/635 | Fluorescein-phenylhydrazine-based/cells, zebrafish | Yuan et al. [79] |
| 30 (DSPE-PEG/HN-I) |  | 448/563 | 1-methylindoline-2,3-dione/cells | James et al. [80] |
| 31 (SQDC) | 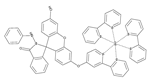 | 630/685 | Squaraine-based/cells | Xu et al. [81] |
| 32 (probe 1) |  | 365/485,545 | Dihydroquinoline-based/cells, mice | Xu et al. [82] |
| 33 (FLASN) |  | 405/525 | Flavonol-based/cells, zebrafish, mice | Lin et al. [83] |
| 34 (CD-N-I) | 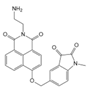 | 245,350/400 | CDs-based/cells | James et al. [88] |
| 35 (NIR-PN1) |  | 511/670 | Dicyanoisophorone-based/cells, drosophila, C. elegans, mice | Liu et al. [89] |
| 36 (ER-PN) | 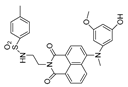 | 450/540 | AP-based/cells, C. elegans | Li et al. [90] |
| 37 (DFlu) | 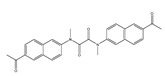 | 313/490 | Naphthylamine ketone-based/cells, zebrafish | Li et al. [91] |
| 38 (K-ONOO) | 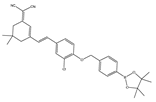 | 405,525/570,678 | Borate ester-based/cells, zebrafish | Zhang et al. [92] |
| 39 (DHX-SP) | 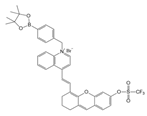 | 470/560 | Phenylborate and pyridinium-based/cells | Tang et al. [93] |
| 40 (NATP) |  | 390,450/565 | Oxindole/cells, mice | Tang et al. [94] |
| 41 (BTNPO) | 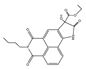 | 380/506 | Oxindole/cells, mice | Tang et al. [95] |
| 42 (Rd-DPA3) |  | 564/698 | Azanediyldiphenol-based/cells, mice | Kim et al. [96] |
| 43 (DCO-POT) |  | 500/670 | C=C/cells, zebrafish | Wu et al. [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Chen, F.; Zhang, C.; Kang, Y.; Yang, Y.; Zhang, C. Role of Peroxynitrite in the Pathogenesis of Parkinson’s Disease and Its Fluorescence Imaging-Based Detection. Biosensors 2024, 14, 506. https://doi.org/10.3390/bios14100506
Lv J, Chen F, Zhang C, Kang Y, Yang Y, Zhang C. Role of Peroxynitrite in the Pathogenesis of Parkinson’s Disease and Its Fluorescence Imaging-Based Detection. Biosensors. 2024; 14(10):506. https://doi.org/10.3390/bios14100506
Chicago/Turabian StyleLv, Jiye, Feiyu Chen, Changchan Zhang, Yubing Kang, Yan Yang, and Chengwu Zhang. 2024. "Role of Peroxynitrite in the Pathogenesis of Parkinson’s Disease and Its Fluorescence Imaging-Based Detection" Biosensors 14, no. 10: 506. https://doi.org/10.3390/bios14100506
APA StyleLv, J., Chen, F., Zhang, C., Kang, Y., Yang, Y., & Zhang, C. (2024). Role of Peroxynitrite in the Pathogenesis of Parkinson’s Disease and Its Fluorescence Imaging-Based Detection. Biosensors, 14(10), 506. https://doi.org/10.3390/bios14100506




