1. Introduction
For centuries, hypersaline water bodies worldwide have been revered for their therapeutic benefits, yet comprehensive information on water composition, dynamics, and correlations with healing properties remains limited [
1,
2,
3].
Cojocna Balneary Resort in Cluj County, Transylvania, Romania, comprising two hypersaline lakes, the Bathing Lake, also known as Török Lake (L1), and the Great Lake (L2,
Figure 1), is an example of a natural hypersaline water resource and an excellent study environment. The lakes are located on former salt mines that have collapsed and filled with water, resulting in the dissolution of salt from halite bedrock by underground springs in salt mine pits. Currently, the bathing lakes are popular touristic and balneotherapeutic destinations in the metropolitan proximity area of Cluj-Napoca, Romania, not only in summertime but also during the cold season, due to the indoor heated saltwater pool, supplied with water from the L2, along with the newly settled infrastructure for balneotherapeutic procedures.
The Cojocna lakes comprise a cluster of interconnected bodies of water, whose bathymetric and morphometric characteristics were investigated in the early 1970s for the first time by Romanian geographer Teodor Pinzaru and published in 1971 in the local Studia Geographia Journal of Babes-Bolyai University from Romania. The publication [
4] is only available as a print copy in the university library and highlights that Lakes 1 and 2 were partly set up for bathing and spa-therapeutic treatment. Later, Serban et al. [
5] showed that the salt lakes from Cojocna revealed very active dynamics, and the reduction of the lake depth is about 1 m in 10 years (see insertion in
Figure 1), and the salinity differs from one lake to another.
The region’s geological history attributes Cojocna to a Miocene-age marine salt deposit [
6,
7], giving rise to unique hypersaline ecosystems with distinct properties [
6,
8,
9]. Several physicochemical properties associated with microbial diversity and mud analysis of these lakes have been investigated [
7,
8,
9,
10,
11,
12], and different opinions about salinity, electrical conductivity, pH, and microbial communities are found in these point studies. Despite their reputation, the therapeutic properties of Cojocna’s salt lakes remain largely undocumented, primarily due to a lack of comprehensive information on the chemical composition and dynamics of these waters. Secondly, there is a notable absence of substantial medical evidence demonstrating the curative effects on patients. While standard physicochemical and biological parameters are available for regulatory purposes in the indoor facility of the resort, there is a notable absence of basic information on outdoor water bodies for the public.
The previously reported salinity levels showed variability, ranging from 31.4 ppt for L1 and 31.2 ppt for L2 [
9] to the other reported value of 101.01 g/L [
10]. The high salinity of these lakes creates a favorable environment for hosting halophilic microorganisms represented by archaea, bacteria, and eukarya that require high salinity conditions to survive, hence their name “salt-loving.” They are commonly found in hypersaline waters and require sodium ions for their growth and metabolism, typically in salt concentrations greater than 3%, equivalent to the average ocean level, which has about 3.5% NaCl [
13]. Alexe et al. [
8] reported that the surface waters of hypersaline lakes, with salinity levels exceeding 10%, are dominated by halophilic communities.
The majority of halophilic microorganisms, including bacteria and archaea, produce photosynthetic pigments, which, in dense populations of halophiles, generate the pink, red, or purple color of water bodies [
14]. However, Cojocna lakes never showed the coloration associated with algal blooming, their visual appearance ranging from dark, opaque blue-green to dark green or even black. The abundance and diversity of microorganisms in these lakes are remarkable, with Lake 1 being observed to always be greener and darker than Lake 2, suggesting a higher concentration of photosynthetic microorganisms, although both lakes benefit from similar climatic conditions. These visual and empiric observations triggered questions about their potentially distinct health benefits. Thus, the frequent public question, “Which lake is better for bathing for my health condition?” remains unanswered due to the lack of scientific information.
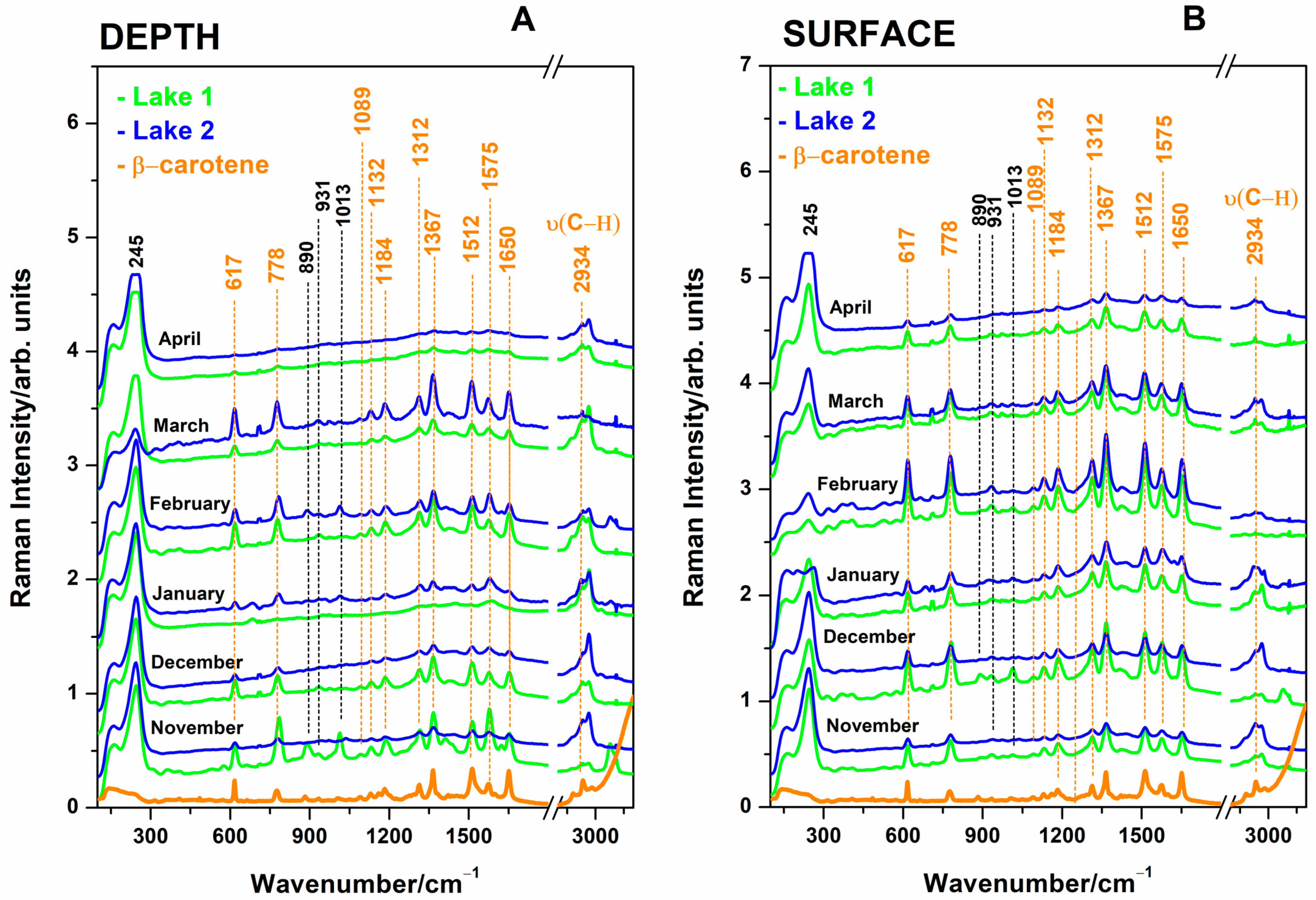
The present study employing Raman and SERS study of lake waters refers to the winter months only, to exclude the anthropogenic influence through touristic and balneary exploitation during the warm months, from May to October.
This paper introduces an innovative approach, using normal Raman spectroscopy with 532 nm laser line excitation to measure the raw salt water spectra from the first-meter surface layer, which is the only one exposed to bathing cure. Further, SERS analyses of waters are conducted, aiming to prospect for sensitive detection of organic compounds and to investigate any correlation between Raman and SERS data. Usually, in salty environmental waters, normal Raman spectra exhibit a typical sulfate band [
12] at 979 cm
−1 assigned to the sulfate stretching mode [
12] among the water bands. Other molecular anions are rarely detectable due to their low concentration and high fluorescence background in water. But excitation with the 532 nm line may produce resonance Raman spectra of carotenoids from photosynthetic microorganisms, thus reflecting the microorganism population. Investigating correlations between these spectroscopic Raman and SERS data sets might reveal additional insight into the inorganic/organic balance of waters without laborious extraction and separation procedures, which require high chemical consumption. Raman and SERS data are analyzed in conjunction with the pH and conductivity measurements during the monitoring period to address the monthly composition and dynamics of surface waters.
Figure 1.
Geographic location of salt lakes L1 and L2 from Cojocna Băi resort, Romania (
A–
C), as snapped from Google Maps, and photographs of the lakes taken in April 2023, as indicated (
D,
E). Insertions in B are adapted from Serban et al., 2005 [
5], and show the dynamic reduction of lake depth between 1969 and 2001, with about 1 m in 10 years.
Figure 1.
Geographic location of salt lakes L1 and L2 from Cojocna Băi resort, Romania (
A–
C), as snapped from Google Maps, and photographs of the lakes taken in April 2023, as indicated (
D,
E). Insertions in B are adapted from Serban et al., 2005 [
5], and show the dynamic reduction of lake depth between 1969 and 2001, with about 1 m in 10 years.
Many studies have demonstrated the usefulness of Raman spectroscopy for the identification and characterization of pigments and biomarkers produced by microorganisms [
15,
16,
17,
18,
19,
20,
21,
22], although debates regarding unambiguous species detection remain due to their Raman complex signature dependencies on many environmental factors as well as the experimental conditions. An interesting recent pilot study employing confocal Raman microscopy in situ reported by Wieser et al. (2023) [
22] highlighted the importance of chemometric tools to be able to accurately characterize the biological dynamics of algae material on different culture days for algae exploitation, demonstrating significant differences along the algal growing time and detecting chemical changes in cells without extraction and separation needed. Such an approach would also be suitable for monitoring the algal-rich environmental water bodies on a larger scale. The drawback is still the implementation of such an automatic approach that requires a robust chemometric component to become user-accessible.
The SERS technique has so far been less employed to study environmental water bodies [
23]. Although apparently abundant literature linking “SERS + environmental waters” is available, addressing a wide range of pollutants, pharmaceuticals, dyes, toxins [
24,
25,
26], or other harmful substances spiked in waters and subsequently detected and eventually quantified for limited concentration ranges, or addressing novel nanostructured substrates with increased enhancement factors, SERS is yet blamed for a lack of interlaboratory operability, reproducibility, and standardization procedures that are not yet recognized as an agreed technique for monitoring programs. Obviously, a lack of data sets regarding SERS in environmental waters does not allow for realistic conclusions on SERS operability for such monitoring purposes.
In this paper, we focus on the SERS of salt lake waters, with multiple goals: (i) to assess the capability of Raman spectroscopy to provide real-time and in situ monitoring information directly beneficial for the ongoing monitoring of the salt lakes, to capture changes in the composition and dynamics of the lake waters as they occur; (ii) to link raw Raman data of waters with their corresponding SERS signature; (iii) to investigate potential correlations between classical physicochemical parameters and Raman/SERS output of waters; and (iv) to draw conclusions on the relevance of such a monitoring approach for transferability to stakeholders related to balneary treatment in salt waters. These objectives faced scant knowledge of the salt lake properties, just to mention that the only salinity data in the existing literature was contradictory [
9,
10].
To the best of our knowledge, this is the first SERS pilot monitoring study for environmental water bodies.
2. Material and Methods
2.1. Silver Colloid Synthesis and Characterization
Materials required for the preparation of the colloid, the silver nitrate, and the sodium citrate were purchased from Sigma-Aldrich. As the SERS active surface, a sodium citrate silver colloid prepared according to the standard procedure reported by Lee and Meisel [
27] was employed. Briefly, 45 mg of silver nitrate was dissolved in 150 mL of triply distilled water and brought to a boil. A solution of 1% sodium citrate (5 mL) and 100 mL of pure water were added to the boiling solution and allowed to continue boiling for an hour. The resulting AgNPs stock has been freshly characterized using UV-VIS electronic spectroscopy and electron microscopy techniques (SEM and TEM), and their usual features in terms of absorbance, morphology, and size distribution have been in full agreement (
Supplementary Information, Figures S1 and S2) with previously reported and used similar AgNPs stocks [
23,
24,
25,
26,
28]. The Raman spectrum of blank colloid showed only a weak band of water, as expected. AgNP stocks were freshly prepared at the beginning of the study and carefully preserved in dark and cold conditions for SERS monthly monitoring experiments on lake waters. The SERS sampling protocol followed a similar approach by adding 10 µL of raw lake water sample to 500 µL of colloidal AgNPs and measuring them immediately within three replicated independent samples from each water batch.
2.2. Sample Collection and Preparation
Raw water samples from the two adjacent salt lakes have been collected monthly in triplicate from each lake, one sample from 1 m depth and two samples from surface waters (about 15 cm depth) during the cold season from November 2022 to April 2023, resulting in 36 raw water samples (2 lakes × 3 samples per lake × 6 months). The bathing water level only (first meter depth) was taken into account since this layer is the only one accessible for balneary bathing due to its high salinity.
Water pH, temperature, and electrical conductivity were measured in situ at the time of collection. Lake water was sampled in a 500 mL vial (three per lake) and immediately transported to the laboratory and stored in cold and dark conditions, while subsamples were prepared for Raman and SERS immediate measurements (same day). We obtained written permission for experimental sampling through a collaboration contract signed with the authorities responsible for the management of the Balneary Lakes at Cojocna Resort.
The micro-Raman spectra were recorded from liquid water samples by drop coating deposition Raman (DCDR). Droplets of 10 µL from each water sample were placed on a hydrophobic, Teflon-coated stainless steel µ-RIMTM slide from BioTools.
The SERS samples were made by adding 10 µL of raw water samples to 500 µL of silver colloidal solution and measured in glass vials of 2 mL with a cap. The SERS samples were measured in triplicate with a portable instrument.
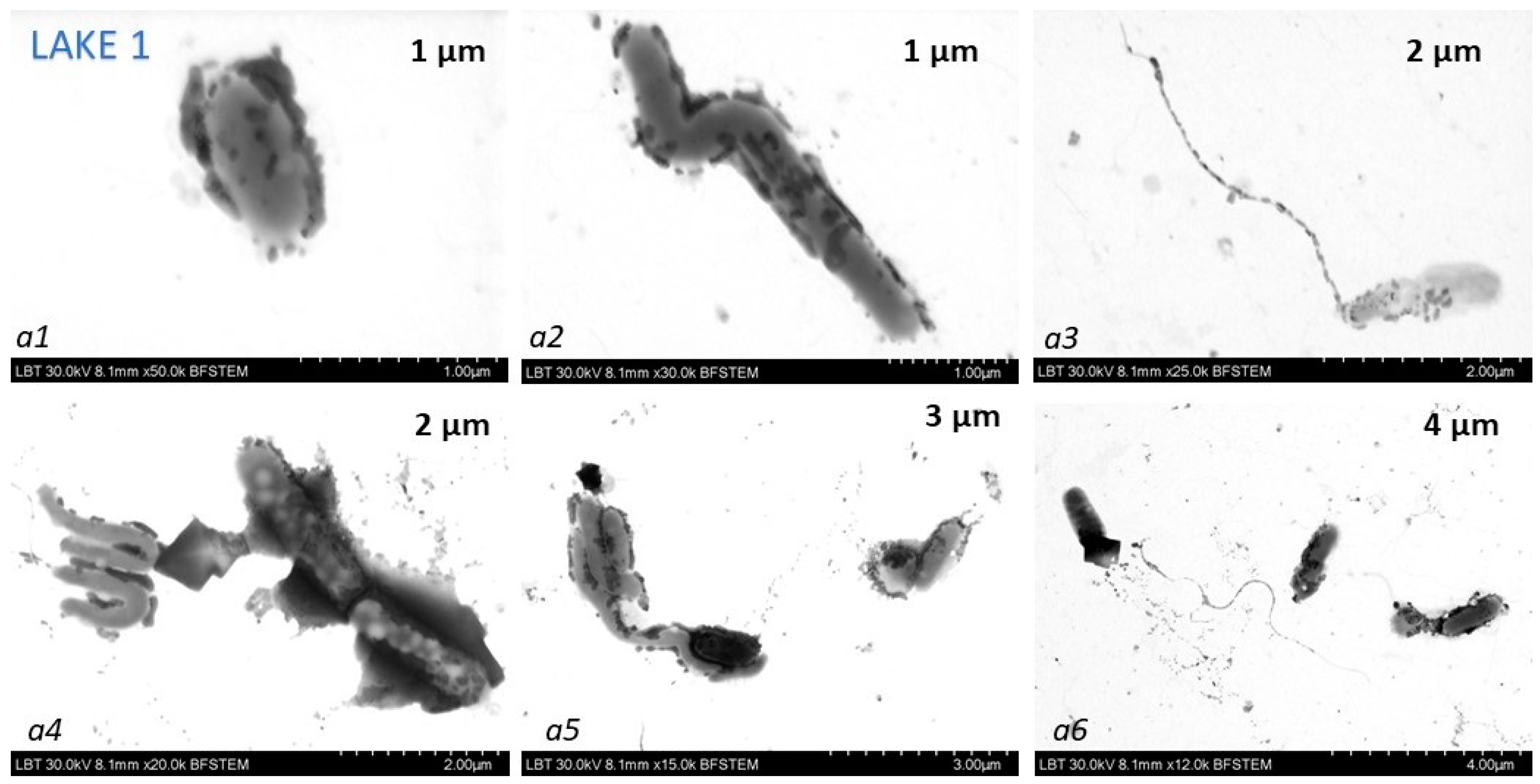
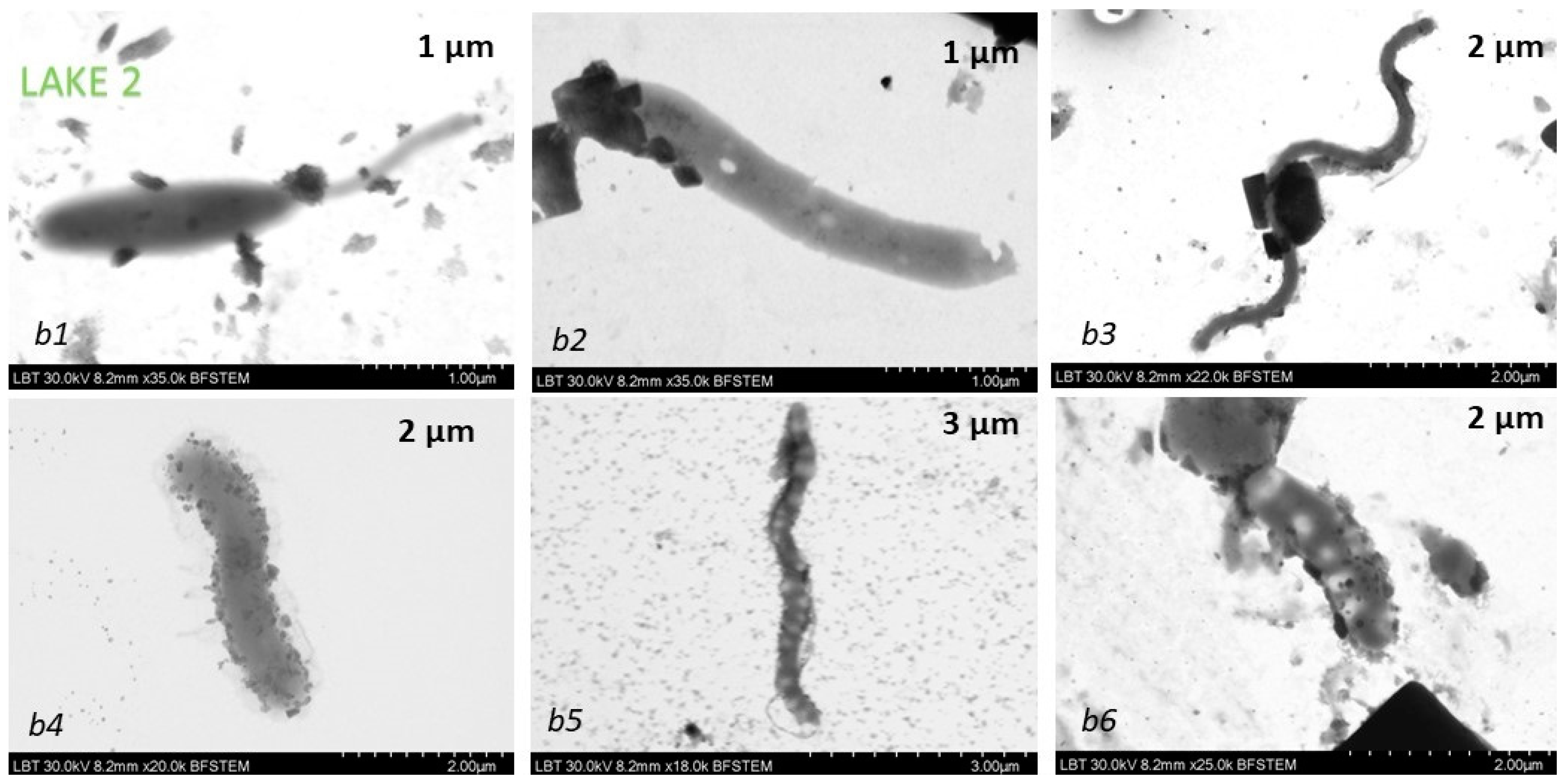
The samples collected in April were used for TEM measurements. For TEM measurements of the same SERS samples, we used probes prepared in a similar way to the SERS samples, from which 7 µL suspension droplets were deposited onto 300 mesh carbon-coated copper grids.
2.3. Instrumentation
The electrical conductivity and pH of the samples were measured on site with HQ40d equipment (HACH USA, multi-analyzer). The conductivity resolution measuring range is 0.01 µS/cm - 0.1 mS/cm and the pH resolution is 0.001/0.01/0.1 pH.
A Renishaw InVia Reflex confocal Raman system has been employed for micro-Raman spectra acquisition. A Cobolt diode-pumped solid-state (DPSS) air-cooled laser operating at 532 nm has been employed for Raman excitation (100 mW) via a Leica research-grade microscope with a 5× objective. Detection was achieved with a RenCam CCD detector with 1024 × 256 pixels and a spectral resolution of 0.5 cm−1.
The optical absorbance of the obtained silver nanoparticles and collected water samples was measured using a UV-Vis Shimadzu 1900 spectrophotometer.
The SERS spectra were recorded using a rapid Wasatch Photonics fully modular Raman Spectrometer (WP 532-A-SR-IC) with a 532 nm line for excitation and an output power of 10 mW. SERS spectra were recorded in triplicate in the spectral range 100–3200 cm−1 with a spectral resolution of 14 cm−1. The experiments were conducted three times using different samples prepared from the same batch. All the measurements have been performed in triplicate at room temperature.
Transmission electron microscopy in conjunction with energy dispersive spectroscopy (TEM-EDX) was done on a Hitachi SU8230, Japan, cold field emission scanning transmission electron microscope.
2.4. Data Processing and Statistical Analysis
Data processing has been achieved using OriginPro 2021b, OriginLab Corporation, Northampton, MA, USA. Averaged raw Raman spectra were calculated from three independent subsamples of each case and further used for analytical purposes. For surface waters, the averaged spectral data of the two diametral sampling locations on each lake was further used for the statistical analysis. To this averaged Raman spectrum, another one has been added, referring to the "depth" of the water (1 m). Thus, 2 lakes × 2 samples each × 6 months resulted in a total of 24 sample data sets for statistics. To calculate the Pearson correlation coefficient from the Raman datasheet, the band intensity at 981 cm−1 attributable to sulfate stretching mode has been used, while for SERS spectra, the 245 cm−1 strong and omnipresent signal attributable to Ag-Cl (due to the chloride ions-induced aggregation of AgNPs) has been considered, both being relevant for the “inorganic” spectral signature of waters. The strong SERS signal at 1512 cm−1 attributable to β-carotene C=C stretching mode was used as being relevant for the organic, microorganism-generated spectral counterpart. These spectral band intensity values were analyzed regarding any correlation within Raman-SERS as well as each Raman technique with the sample pH and electrical conductivity values. These parameter values were measured three times for each lake at the water collection point and further used as averaged values for each date of collection.
The statistical analysis, including correlation analysis and principal components analysis (PCA), was conducted in the R programming language version 4.2.1. For this purpose, the package ggcorrplot library version 0.1.4.1 was used to visualize Pearson’s correlation coefficients among various physio-chemical properties (electrical conductivity and pH) and Raman and SERS signals of six-month-collected water samples [
29]. PCA was performed using the FactoMineR package version 2.8 [
29,
30]. Results from the PCA were finally visualized using the factoextra library version 1.0.7 [
31]. The PCA analyses for differentiation of the two distinct lakes were conducted on a dataset comprising the calculated SERS relative intensity values of the characteristic band at 245 cm
−1 and the β-carotene C=C stretching mode at 1512 cm
−1. The PCA analyses conducted to discriminate physicochemical properties with SERS involved the intensity ratio I
245/I
1512 and the measured electrical conductivity and pH values of the water samples.
For all statistical analyses, the raw datasets, without normalization and background subtraction procedures, were used. For comparative SESR data presentation, spectra were background subtracted and normalized to units.
4. Discussion
4.1. Statistical Correlation between Physicochemical Properties and Raman and SERS Signals of Lakes
The Pearson correlation coefficient was employed to facilitate a comprehensive investigation of the relationship between the Raman and SERS spectral data of two lake water samples and their respective physicochemical parameters (electrical conductivity, pH). This analysis aimed to elucidate how changes in relevant peak intensity within the Raman and SERS spectra correlate with fluctuations in pH and electrical conductivity over a six month timeline.
We identified pairs that displayed significant correlations, whether positive or negative. A positive correlation indicates that two variables change simultaneously in the same direction, while a negative correlation suggests that they alternate in opposite directions. We considered correlation values above 0.5 as sufficiently significant for our analysis.
Figure 7 illustrates the correlation coefficients between the Raman signal at 981 cm
−1 of the sulfate and the measured pH and electrical conductivity, respectively, as well as the correlation between the Raman signal at 981 cm
−1 and the SERS signal at 245 cm
−1 of the Cl
− band. In Lake 1, a significant correlation is evident between the Raman signal at 981 cm
−1 and the physicochemical parameters (
Figure 7A,C). This suggests a strong positive linear relationship between Raman data and electrical conductivity values in both surface and deep water samples and the pH on the surface, but a negative correlation with the pH in depth. Conversely, in Lake 2, surface pH values negatively correlate with depth Raman data (
Figure 7B), while a positive correlation is observed between depth Raman data on both depth and surface electrical conductivity (
Figure 7D). Notably, no significant correlation is seen between the surface Raman data and pH, or electrical conductivity, in Lake 2.
A comprehensive examination of the association between Raman spectral data at 981 cm
−1 and SERS data at 245 cm
−1, shown in
Figure 7E,F, reveals distinct correlation patterns. In Lake 1, there is a strong negative correlation between surface Raman data and SERS data at depth, while a good positive correlation is evident between depth Raman data and surface SERS. In Lake 2, a strong positive correlation is observed across all combinations when exploring the relationship between Raman and SERS spectral data.
Figure 8 displays the correlation coefficients between the SERS signals at 245 cm
−1 of Cl
− band and at 1512 cm
−1 of β-carotene band along with the measured pH and electrical conductivity.
The analyses revealed distinct patterns for the two lakes. Lake 1 showed a consistent positive correlation between the Raman data and physicochemical parameters, while Lake 2 exhibited a more complex relationship. In summary, the relationship between the Raman and SERS spectral data of the two lakes, along with their corresponding physicochemical parameters, exhibits a complex and distinctive pattern. The correlation analysis conducted for Lake 1 revealed a predictable relationship between the Raman spectral data at 981 cm−1, the physicochemical parameters, and the SERS spectral data at 245 cm−1. Similarly, significant correlations were noted for Lake 2 between the depth Raman spectral data and pH and electrical conductivity. These findings emphasize the complex and distinct nature of the relationship between spectral data and physicochemical parameters in the two lakes. The variations in the correlations underscore the unique dynamics governing the interplay between Raman and SERS data with pH and electrical conductivity, highlighting the significance of monitoring the physicochemical parameters of salt waters. These parameters act as essential indicators of the complex interactions between chemical constituents and environmental factors, emphasizing the need for tailored interpretations and considerations for each lake water system. The samples collected within a six-month timeline offered relevant water quality parameters that can be used in monitoring living (micro-) organisms and their ecosystems.
In Lake 1, a strong correlation is observed between the SERS signal at 1512 cm
−1 from depths and the physicochemical parameters (
Figure 8A,C). Notably, the depth SERS spectral data shows a negative correlation with surface pH data and depth electrical conductivity. Conversely, in Lake 2, a significant positive correlation is seen between the pertinent parameters and the SERS data at 1512 cm
−1 (
Figure 8B,D). Specifically, surface SERS data correlates strongly with surface pH, while depth SERS spectral data correlates inversely with depth pH values. Furthermore, a good negative correlation is noted between surface SERS data and surface electrical conductivity, as well as between depth SERS spectral data and depth electrical conductivity. These findings perfectly support the well-known physical situations when excessive AgNP aggregation hampers SERS detection of organic molecular species efficiency (high anions concentration, high conductivity, low SERS signal).
In Lake 1, a good correlation is also evident between the SERS spectral band at 245 cm
−1 and the physicochemical parameters. However, no significant correlation is observed in the case of Lake 2. Further analysis in Lake 1 shows an inverse linear relationship between depth SERS spectral data and surface pH values and between surface SERS data and depth pH data (
Figure 8E). Contrastingly, electrical conductivity data indicates a negative correlation between depth SERS spectral data and depth conductivity and a positive correlation between surface SERS spectral data and surface conductivity (
Figure 8G). However, in the case of Lake 2, no notable correlation values are observed between the SERS data and the pH values (
Figure 8F,H).
Based on the results from the correlation coefficient analyses (
Figure 8), distinct patterns between the SERS signals are observed at 245 cm
−1 for the Cl
− band and at 1512 cm
−1 for the carotenoid band, along with measured pH and electrical conductivity in the two lakes. These parameters reflect the overall health of the aquatic ecosystem, and changes in parameters over the long term provide valuable data for studying trends and patterns in water quality, the presence of contaminants, or changes in the overall health of aquatic ecosystems.
The Pearson correlation coefficient analyses allowed for a comprehensive investigation of the spectral data of the two lakes and their respective physicochemical parameters, such as electrical conductivity and pH. The results underscore the complexity of interactions between nanostructures used as SERS amplifier substrates and the unique dynamics of the water samples investigated. It is important to note that, due to the small sample volume, the correlation coefficients calculated here are subjected to sensitivity fluctuations due to the background signal. Although optimized to record the best signal-to-background SERS data, external factors influencing higher background, such as living organism movement in a spotted droplet, cannot be avoided. Hence, other relationships between spectral data and physiochemical properties may be lost in the background or underestimated. By using statistical analysis, this drawback is reduced considerably, and in the following, we report on the unbiased, multivariate analysis of the spectral data by PCA.
4.2. Relative Band Intensities of Carotenoids Analysis with PCA of Two Lakes
PCA, or principal component analysis, is a widely used statistical technique for transforming a high-dimensional dataset into a lower-dimensional representation while retaining most of the relevant information. The goal of PCA is to find a set of orthogonal axes, called principal components (PCs), that capture the maximum variance in the data. These components are ranked in order of importance, with the first component explaining the most variance. By projecting the data onto a subset of PCs, PCA allows us to reduce the number of features or variables while minimizing the loss of information. This can serve as a mechanism to reveal patterns in multidimensional data that would otherwise remain hidden.
For this purpose, we conducted PCA on a dataset comprising the SERS relative intensities of β-carotene on 13 bands (2972, 2930, 2882, 1651, 1574, 1512, 1365, 1311, 1184, 1133, 1085, 775, and 615 cm−1). The dataset was constructed in the following manner: the observations correspond to the relative band intensities on each individual band for both of the lakes respectively. The variables correspond to the depth profile at each month of sampling.
The first two PCs managed to capture a total of 77.8% of the total explained variance (
Figure 9). The distinct separation observed in the first principal component highlighted pronounced differences in the β-carotene band intensities between the two lakes, signifying potential variations in the composition or environmental factors influencing its distribution. These 13 band characteristics of SERS bands of β-carotene at sub-micromole concentrations [
33] are specific to cyanobacteria. The higher number of cyanobacteria results in more intense SERS bands of β-carotene, so higher SERS bands mean a higher abundance of cyanobacteria.
Since the SERS signature profiles of β-carotene bands display distinct profiles among the two lakes (given the observations at various months and depth profiles throughout the year), we further investigated which sampling parameters contributed the most to the variance. The different month-depth sampling covariates that influence the first principal components the most are surface–November, depth–March, depth–February, depth–November, surface–December, and depth–January. Conversely, the second principal component has seen its highest contributions from surface–January, surface–March, depth–December, surface–February, and surface–April. We observed no dominant month or sampling profiles that strongly contribute to the L1-L2 band differentiation, since all months were well represented on the first two principal components.
4.3. PCA Analysis of Physics-Chemical Properties with SERS Signals of Lakes
We conducted a PCA analysis using the physicochemical parameters in order to assess whether they can also be used as discriminant markers for differentiating samples from L1 and L2. In this case, the first 2 PCs capture 67.8% of the total explained variance for this dataset (
Figure 10). PC2 seems to roughly correspond with the variance stemming from the changing of seasons—samples from the spring months tend to be on the lower side of the y-axis, while samples from the winter months mostly localize on the upper side.
Similar to our previous PCA, we investigated the association between variables and the first principal components. The conductivity, pH, and SERS relative band intensity all contributed equally to the differentiation, with no dominance associated with either deep or surface sampling origin.
5. Conclusions
In this monitoring experiment, we employed nanotechnology-based techniques and instrumentation to explore the capability of Raman and SERS techniques to draw pertinent information regarding organic/inorganic content and dyanmics in two salt lakes used for balneotherapy. These measurements help to define the overall therapeutic properties concerning the dynamics of inorganic salt ions, contributing to a comprehensive understanding of the ecosystem’s health.
Here we present sensitive data relying on surface enhanced Raman scattering (SERS) signal monitoring of the molecular signature of salt lake water composition during the winter months (November 2022 to February 2023). AgNPs aggregation and SERS activity are strongly correlated with the presence of anions, primarily Cl− and SO42− directly detected, but also others, and their concentration and synergic effect, while the presence and balance of nutrients are crucial for halophile microorganisms’ proliferation. Algal productivity is often correlated to levels of anions and their ratio, as well as with other water ions and other micronutrients. The anions characteristic of saltwater bodies are strong aggregation agents for colloidal silver nanoparticles generally used in surface-enhanced Raman scattering. To record reliable SERS spectra and get insight into the organic chemical content, the SERS technique required careful optimization of the sampling protocol regarding the volume ratio of added salt water and AgNPs. This dependence provides valuable insight into the aggregation dynamics of AgNPs in relation to the concentration of the anion and, consequently, the overall SERS signature. SERS data for water samples from two salt lakes are discussed and compared in a monitoring study aiming to trace organic changes associated with microbial metabolites.
We found significant spectral differences between water samples in two lakes, as well as significant chemical changes from one month to another. The SERS reproducibility in terms of band position and relative intensity was remarkable and not expected, and this feature was interpreted in terms of ubiquitous cyanobacteria presence in water droplets, which prompted the typical submicromole concentration SERS signal of β-carotene, an abundant carotenoid in cyanobacteria. As a matter of fact, we expected to record the SERS signature of aromatic substances resulting from microbial metabolites and decomposition, which are responsible for mud formation, as well as the possible inflow of aromatic residues either from the microbial community or from the presence of Anthropocene species at distinct levels within this timeline. A few exceptions from the regular SERS pattern of carotenoid were observed, such as the appearance of additional SERS bands at 2979 cm−1 in one or two cases, which, however, suggested that the multiplexed SERS pattern comes from multiple contributions, primarily cyanobacteria and possible other organic contributions. Overall, the Raman and SERS data on the salt lake’s water, perfectly compatible with in situ monitoring initiatives, clearly highlighted and differentiated two adjacent salt water bodies in terms of both organic and inorganic composition and dynamics, although both water bodies benefit from identical climate conditions and anthropogenic influence, specifically for balneary resort exploitation. Organic/inorganic balance is a crucial characteristic for decision-making regarding any forthcoming therapeutic effect or potential investigation of the hypersaline water bathing approach.